Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize
Abstract
1. Introduction
2. Results
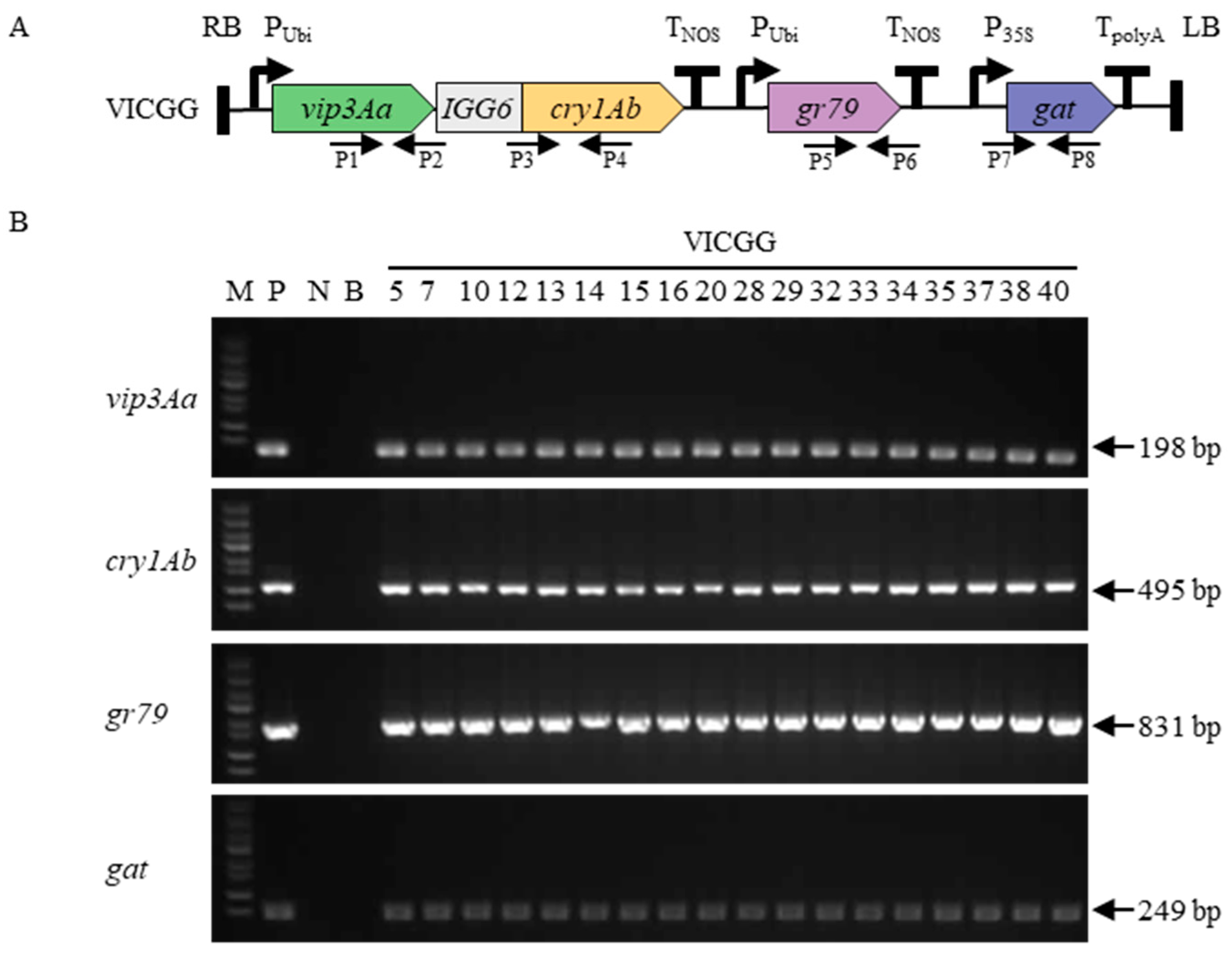
2.1. Maize Transformation and Identification of Transgenic Maize Plants
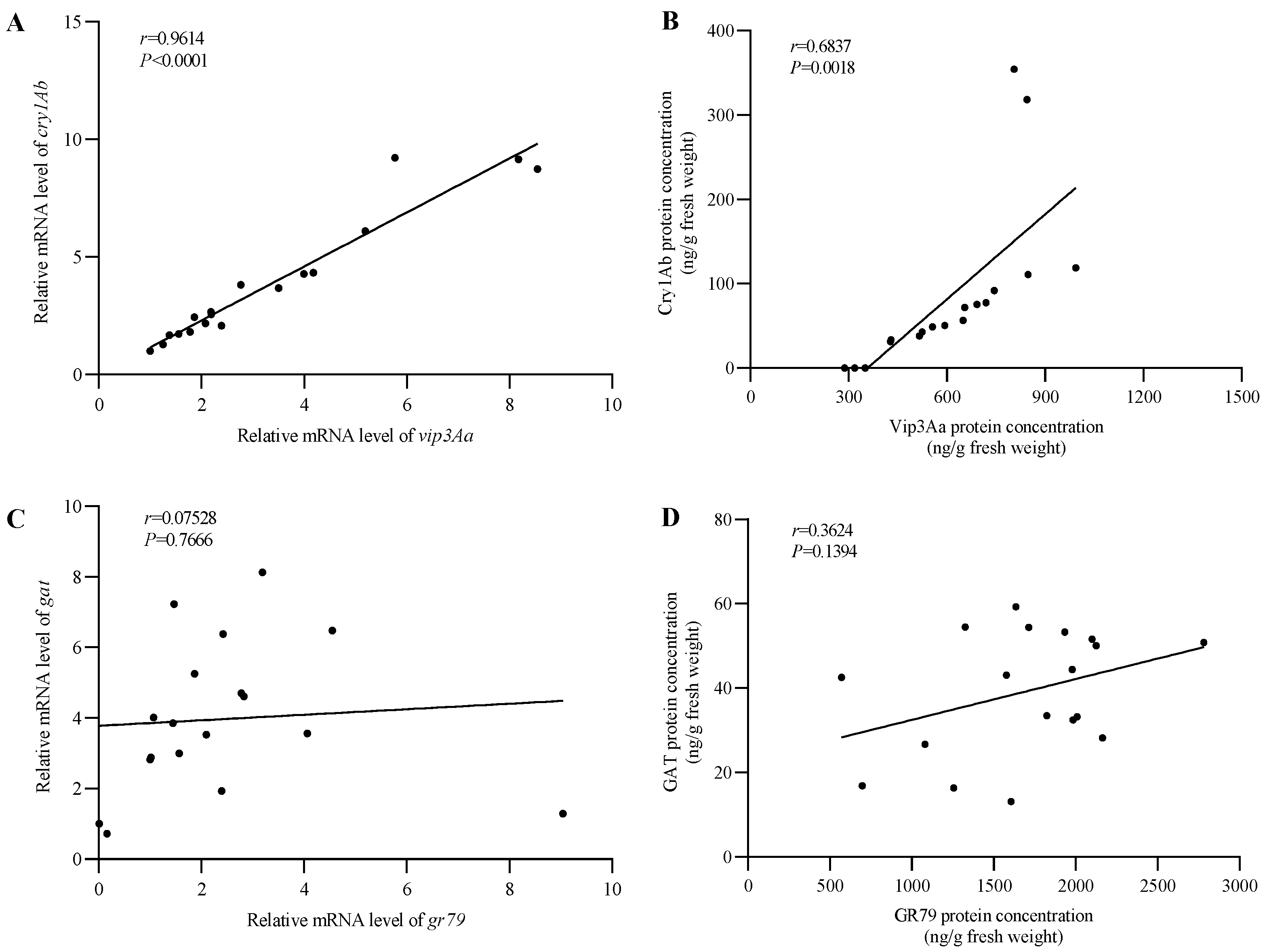
2.2. Quantitative Analysis of Protein Content and Transcription Level in Transgenic Maize Plants
2.3. The Transcript and Protein Forms of the IGG6-Based Bicistron
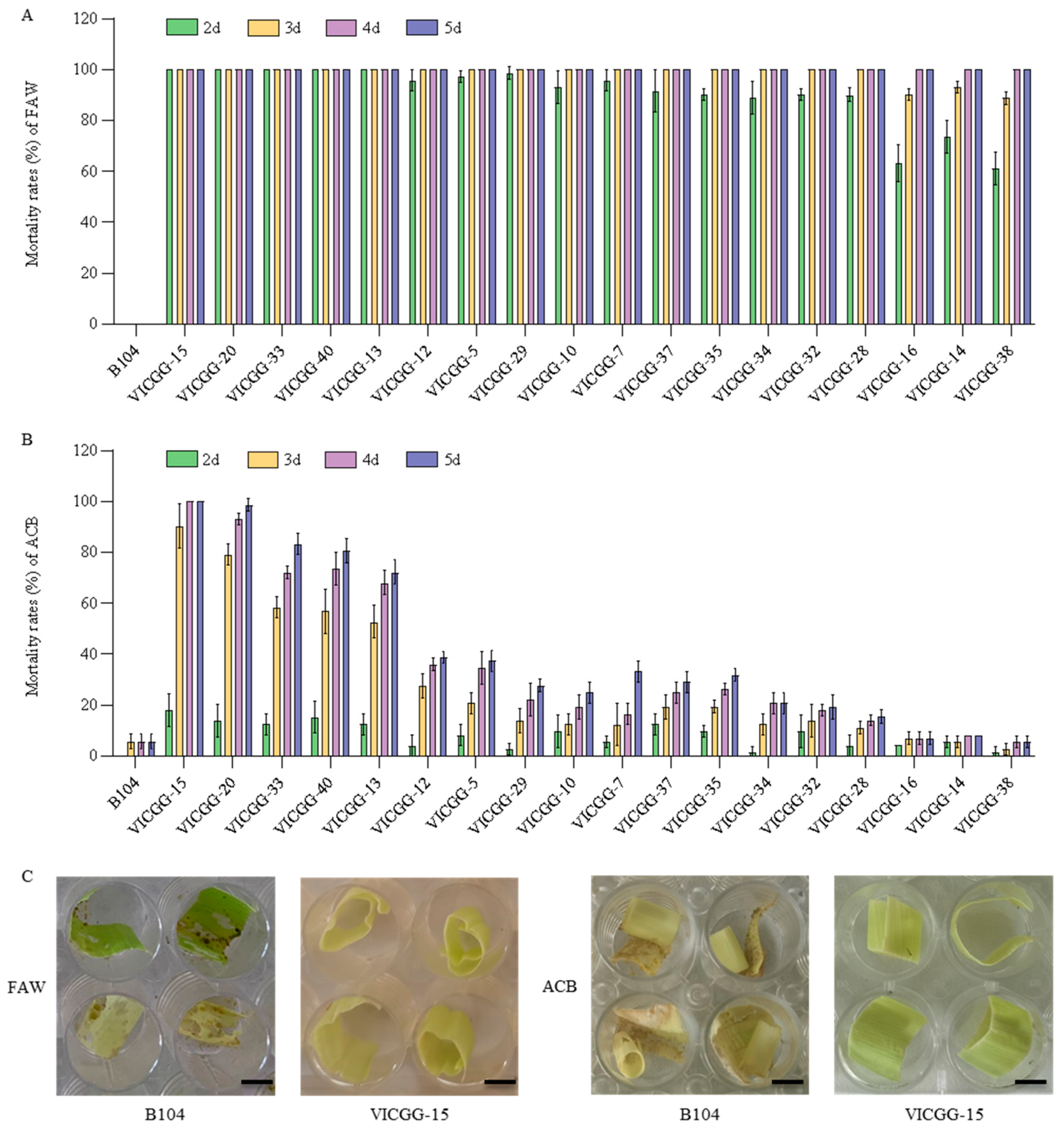
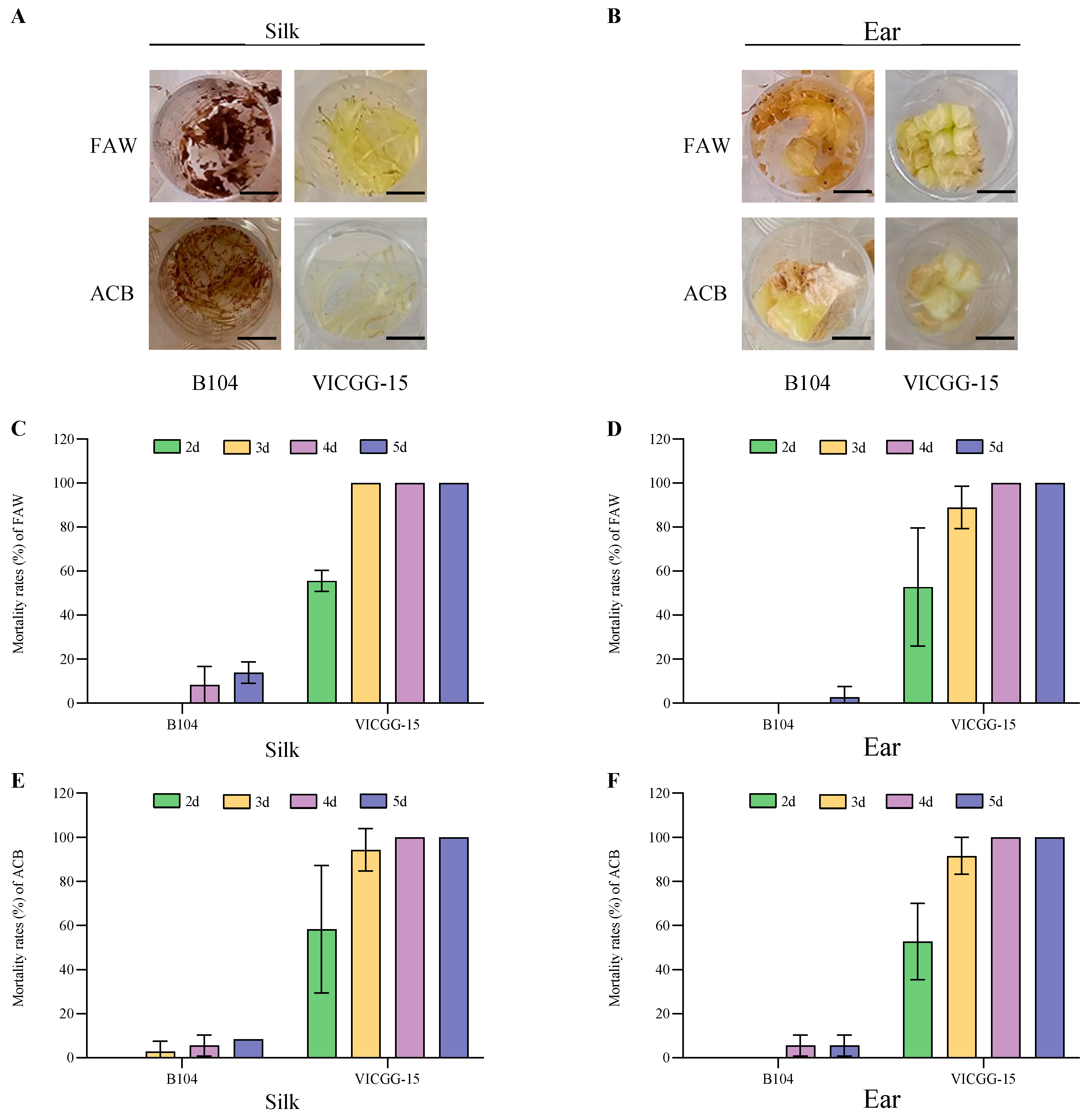
2.4. Bioassay of VICGG Transgenic Maize Lines
2.5. Glyphosate Tolerance Analysis and Agronomic Traits Investigation of Transgenic Maize VICGG-15
3. Discussion
3.1. Mechanism and Efficiency of IGG6-Based Bicistronic System
3.2. Application Prospect of IGG6 in GM Crops
4. Materials and Methods
4.1. Construction of Vectors
4.2. Maize Transformation
4.3. Molecular Analysis of Transgenic Maize Plants
4.4. Laboratory Bioassays
4.5. Field Bioassays
4.6. Glyphosate Tolerance Analysis
4.7. Agronomic Traits Investigation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | full name |
| Bt | Bacillus thuringiensis |
| ELISA | enzyme-linked immunosorbent assay |
| FAW | fall armyworm |
| ACB | Asian corn borer |
| GM | genetically modified |
| IRESs | internal ribosomal entry sites |
| CDS | coding sequence |
| HR | high resistant |
| HS | high susceptible |
| WAT | weeks after treatment |
References
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA Brief No. 55; ISAAA: Ithaca, NY, USA, 2019; ISBN 978-1-892456-69-9. [Google Scholar]
- Douglas, E.; Halpin, C. Gene Stacking. In Molecular Techniques in Crop Improvement, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 613–629. ISBN 978-90-481-2966-9. [Google Scholar]
- Minskaia, E.; Ryan, M.D. Protein Coexpression Using FMDV 2A: Effect of “Linker” Residues. BioMed Res. Int. 2013, 2013, 291730. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, A.L.; Workman, C.J.; Wang, Y.; Vignali, K.M.; Dilioglou, S.; Vanin, E.F.; Vignali, D.A. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 2004, 22, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, A.L.; Vignali, D.A. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin. Biol. Ther. 2005, 5, 627–638. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Feuer, G.; Day, S.L.; Wrzesinski, S.; Planelles, V. Multigene lentiviral vectors based on differential splicing and translational control. Mol. Ther. 2001, 4, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Marchisio, M.A. Synthetic polycistronic sequences in eukaryotes. Synth. Syst. Biotechnol. 2021, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-H.; Liang, Y.S.; Jung, H.; Ahn, M.-J.; Suh, S.-C.; Kweon, S.-J.; Kim, D.-H.; Kim, Y.-M.; Kim, J.-K. Application of two bicistronic systems involving 2A and IRES sequences to the biosynthesis of carotenoids in rice endosperm. Plant Biotechnol. J. 2010, 8, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, S.M.; Chien, A.C.; Lee, C.G.L. Exploiting internal ribosome entry sites in gene therapy vector design. Curr. Gene Ther. 2004, 4, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.L.; Mitra, A. Expressing multiple genes in a single open reading frame with the 2A region of foot-and-mouth disease virus as a linker. Mol. Breed. 2002, 9, 191–199. [Google Scholar] [CrossRef]
- Yue, Q.; Meng, J.; Qiu, Y.; Yin, M.; Zhang, L.; Zhou, W.; An, Z.; Liu, Z.; Yuan, Q.; Sun, W.; et al. A polycistronic system for multiplexed and precalibrated expression of multigene pathways in fungi. Nat. Commun. 2023, 14, 4267. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Chen, L.; Li, Y.; Bills, G.F.; Zhang, X.Y.; Xiang, M.C.; Li, S.J.; Che, Y.S.; Wang, C.S.; Niu, X.M.; et al. Functional Operons in Secondary Metabolic Gene Clusters in Glarea lozoyensis (Fungi, Ascomycota, Leotiomycetes). mBio 2015, 6, e00703-15. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yue, Q.; Miao, L.; Li, S.; Tian, J.; Si, W.; Zhang, L.; Yang, W.; Zhou, X.; Zhang, J.; et al. A novel nucleic acid linker for multi-gene expression enhances plant and animal synthetic biology. Plant J. 2024, 118, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, P.; Li, X.; Wen, N.; Wang, Y.; Lu, W.; Lin, M.; Lang, Z. In maize, co-expression of GAT and GR79-EPSPS provides high glyphosate resistance, along with low glyphosate residues. aBIOTECH 2023, 4, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Verbruggen, H.; Li, T.; Zhu, J.; Chen, Z.; He, H.Q.; Bao, S.X.; Sun, J.H. Identification of polycistronic transcriptional units and non-canonical introns in green algal chloroplasts based on long-read RNA sequencing data. BMC Genom. 2021, 22, 298. [Google Scholar] [CrossRef]
- Gallaher, S.D.; Craig, R.J.; Ganesan, I.; Purvine, S.O.; Mccorkle, S.R.; Grimwood, J.; Strenkert, D.; Davidi, L.; Roth, M.S.; Jeffers, T.L.; et al. Widespread polycistronic gene expression in green algae. Proc. Natl. Acad. Sci. USA 2021, 118, e2017714118. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Tseng, E.; Salamov, A.; Zhang, J.; Meng, X.; Zhao, Z.; Kang, D.; Underwood, J.; Grigoriev, I.V.; Figueroa, M.; et al. Widespread Polycistronic Transcripts in Fungi Revealed by Single-Molecule mRNA Sequencing. PLoS ONE 2015, 10, e0132628. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. Termination and post-termination events in eukaryotic translation. Adv. Protein Chem. Struct. Biol. 2012, 86, 45–93. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Pushing the limits of the scanning mechanism for initiation of translation. Gene 2002, 299, 1–34. [Google Scholar] [CrossRef]
- Urwin, P.; Yi, L.; Martin, H.; Atkinson, H.; Gilmartin, P.M. Functional characterization of the EMCV IRES in plants. Plant J. 2000, 24, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Faure, G.; Laass, S.; Kolbe, E.; Seitz, K.; Wehrheim, C.; Wolf, Y.I.; Koonin, E.V.; Soppa, J. Translational coupling via termination-reinitiation in archaea and bacteria. Nat. Commun. 2019, 10, 4006. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef]
- Ryan, M.D.; King, A.M.; Thomas, G.P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991, 72, 2727–2732. [Google Scholar] [CrossRef] [PubMed]
- Frame, B.R.; Shou, H.X.; Chikwamba, R.K.; Zhang, Z.Y.; Xiang, C.B.; Fonger, T.M.; Pegg, S.E.K.; Li, B.C.; Nettleton, D.S.; Pei, D.Q. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002, 129, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Lang, Z.; Zhang, J.; Zhu, L.; Huang, D. Chloroplast-targeted expression of the codon-optimized truncated cry1Ah gene in transgenic tobacco confers a high level of protection against insects. Plant Cell Rep. 2013, 32, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1248.5-2006; Rules for Evaluation of Maize for Resistance to Pests Part 5: Rules for Evaluation of Maize for Resistance to Asian Corn Borer. Ministry of Agriculture of the PRC: Beijing, China, 2006.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lv, W.; Yue, Q.; Wen, N.; Wang, Y.; Lang, Z.; Xu, W.; Li, S. Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize. Int. J. Mol. Sci. 2024, 25, 13408. https://doi.org/10.3390/ijms252413408
Chen Y, Lv W, Yue Q, Wen N, Wang Y, Lang Z, Xu W, Li S. Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize. International Journal of Molecular Sciences. 2024; 25(24):13408. https://doi.org/10.3390/ijms252413408
Chicago/Turabian StyleChen, Yuxiao, Wenjie Lv, Qun Yue, Ning Wen, Yinxiao Wang, Zhihong Lang, Wei Xu, and Shengyan Li. 2024. "Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize" International Journal of Molecular Sciences 25, no. 24: 13408. https://doi.org/10.3390/ijms252413408
APA StyleChen, Y., Lv, W., Yue, Q., Wen, N., Wang, Y., Lang, Z., Xu, W., & Li, S. (2024). Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize. International Journal of Molecular Sciences, 25(24), 13408. https://doi.org/10.3390/ijms252413408






