Bovine Lactoferrin Enhances Toll-like Receptor 7 Response in Plasmacytoid Dendritic Cells and Modulates Cellular Immunity
Abstract
1. Introduction
2. Results
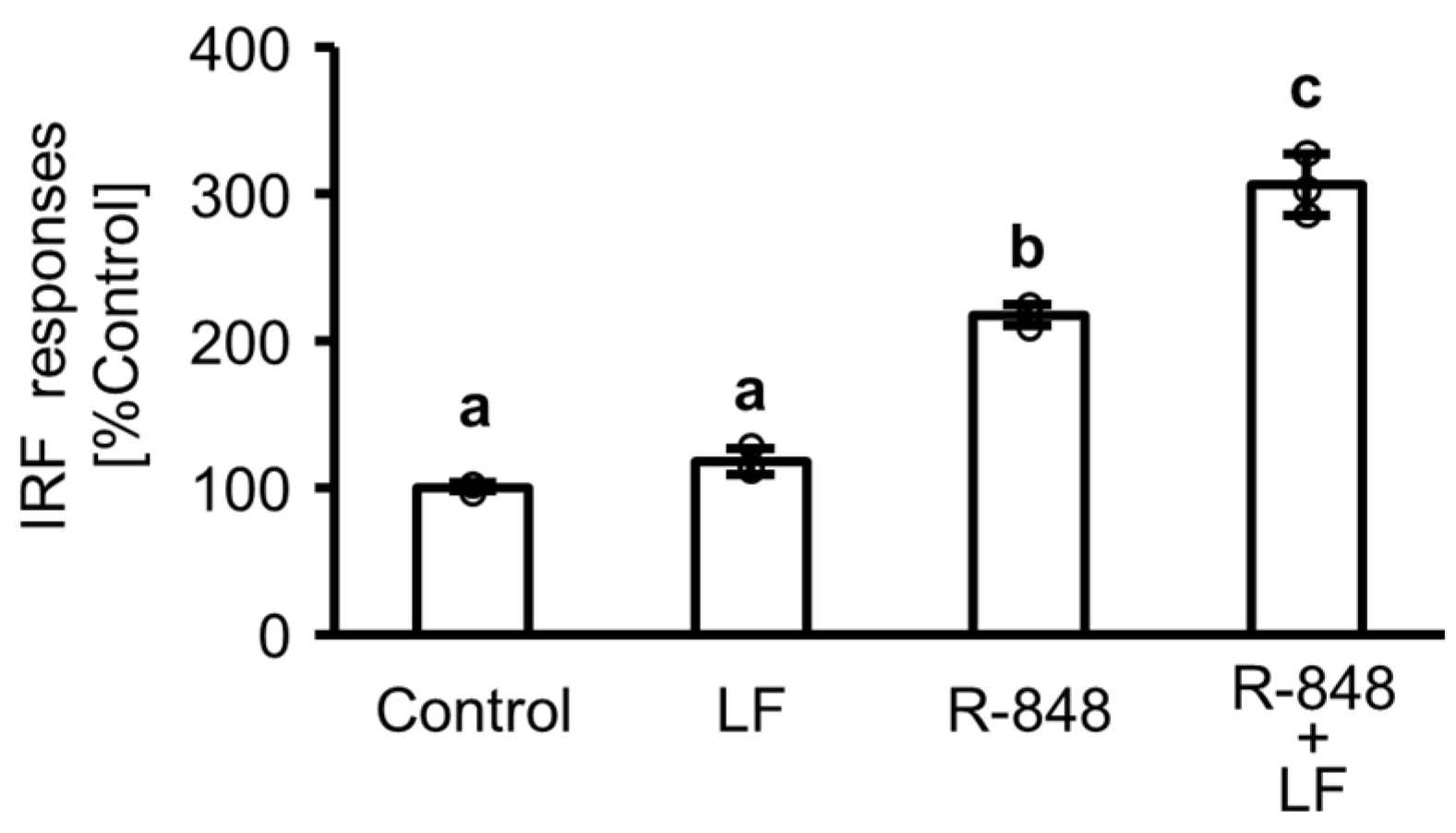
2.1. Response of Interferon (IFN) Regulatory Factor (IRF) Followed by Activation of IFN-Sensitive Response Element (ISRE) in Toll-like Receptor (TLR) 7 Reporter THP-1 Cells
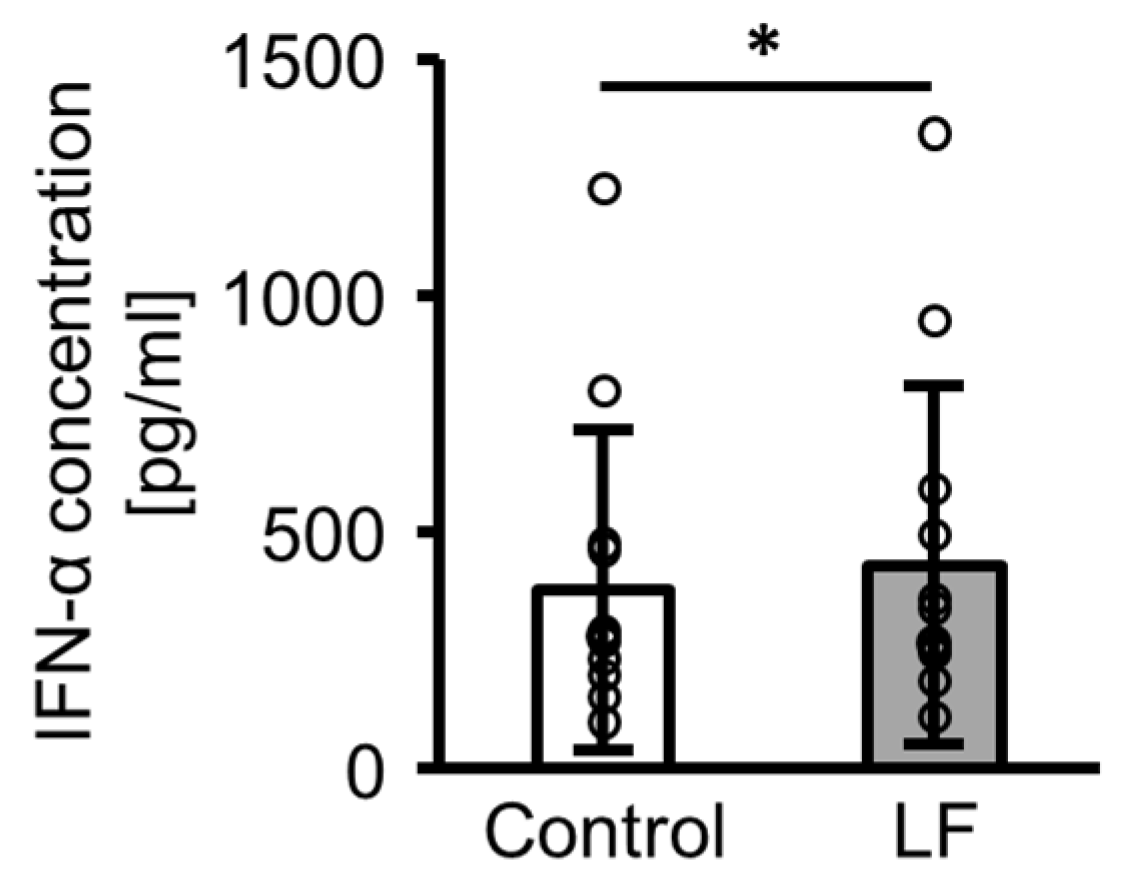
2.2. IFN-α Production from Peripheral Blood Mononuclear Cells (PBMCs) Following Lactoferrin (LF) Treatment
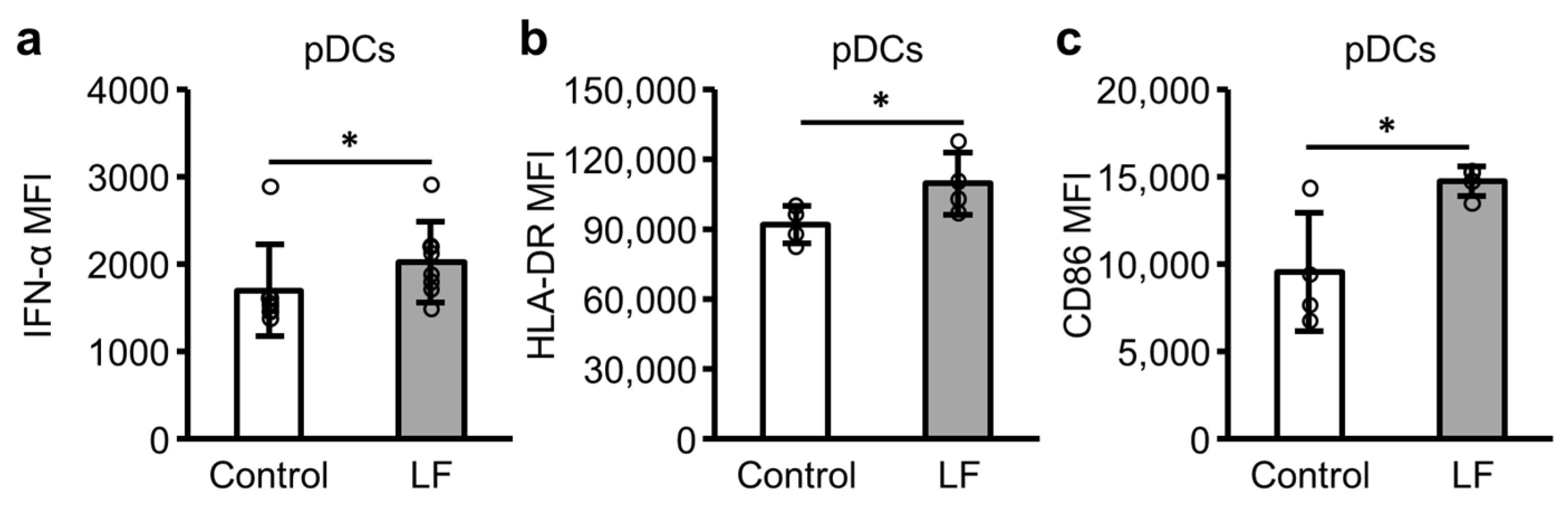
2.3. Plasmacytoid Dendritic Cell (pDC) Activity in PBMCs Following LF Treatment
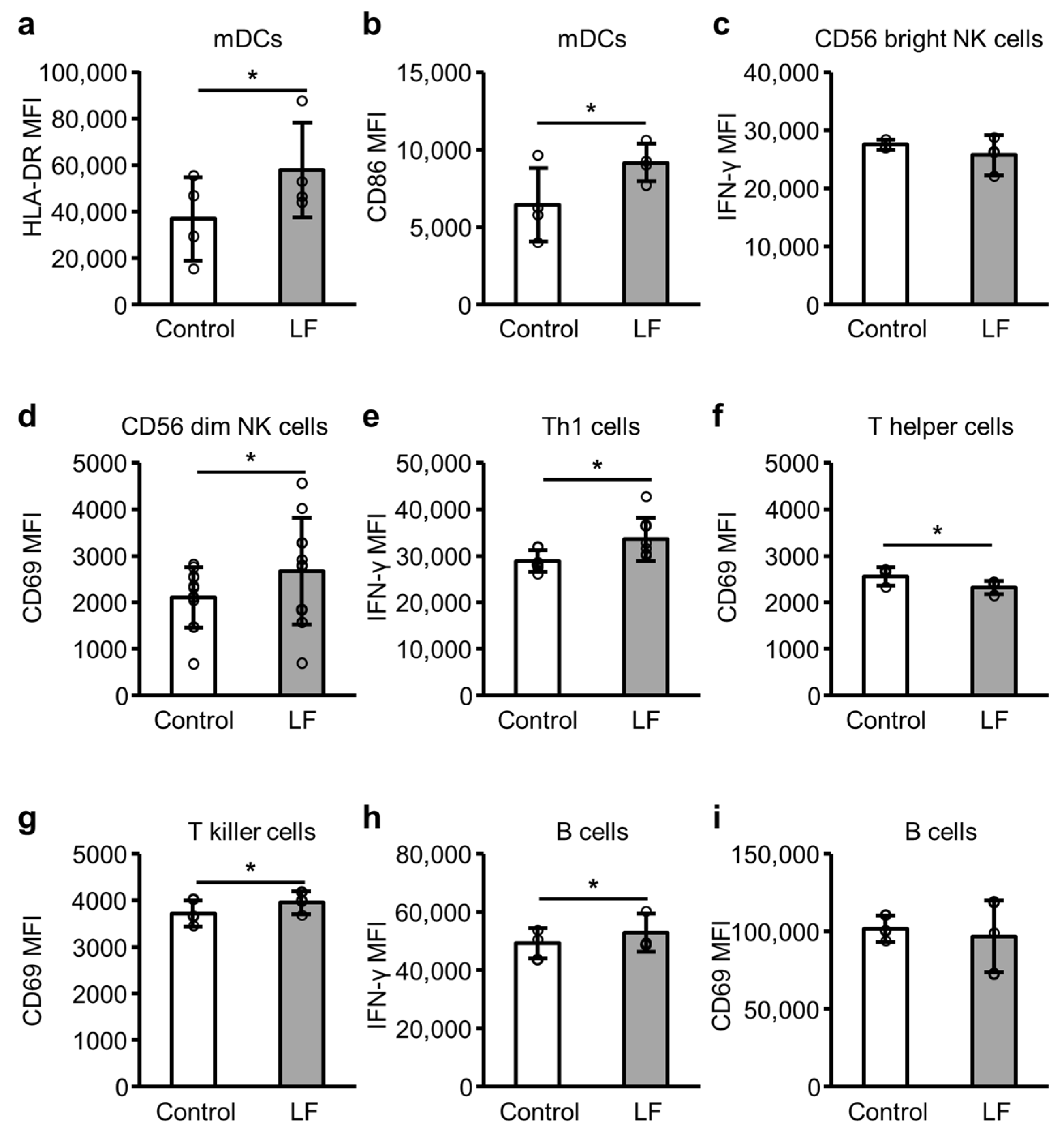
2.4. Immune Cell Activity Following LF Treatment
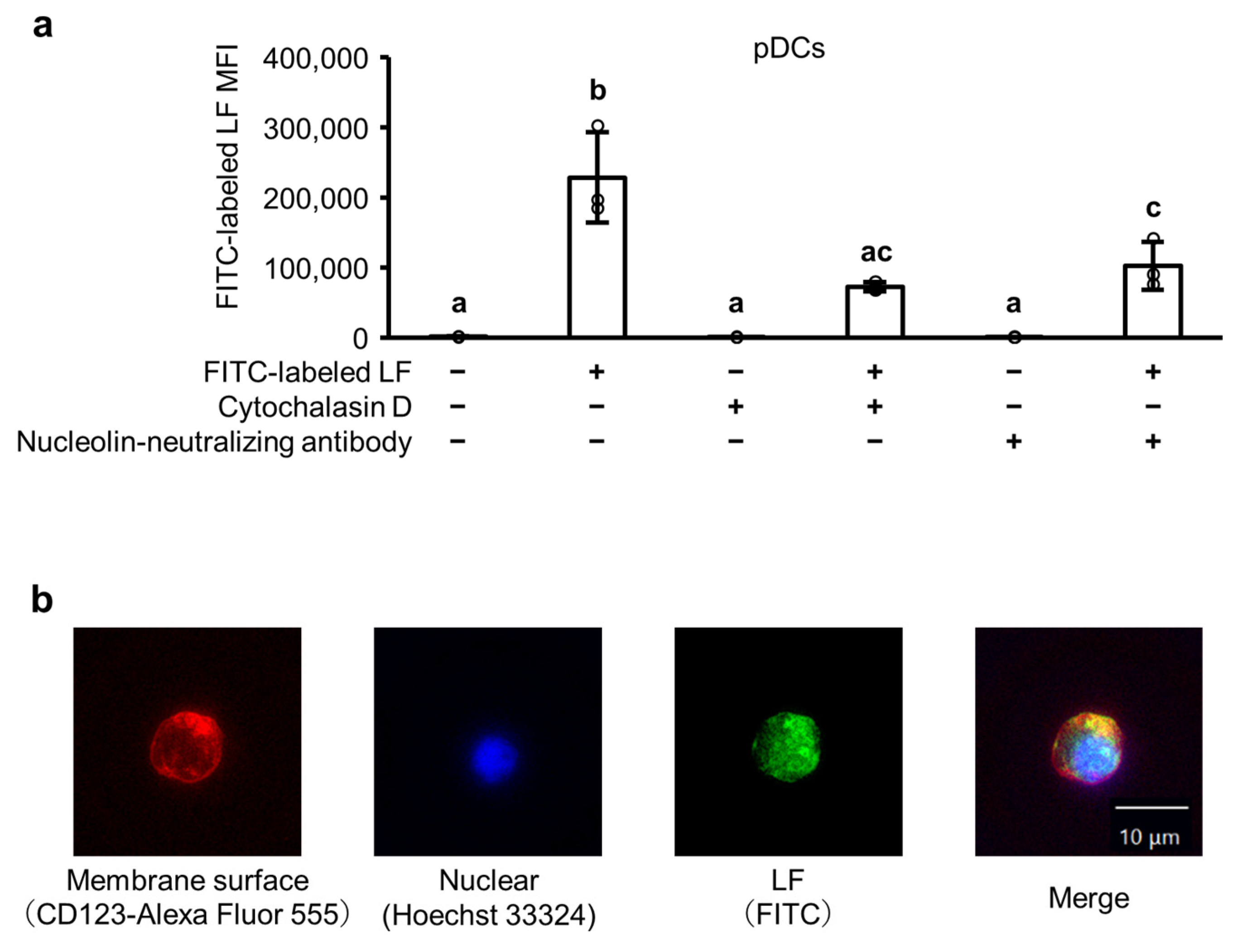
2.5. The Cellular Uptake of LF in pDCs
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Materials
4.3. IRF Response Assay
4.4. Preparation of PBMCs
4.5. PBMC Culture
4.6. Flow Cytometry
4.7. Isolated pDC Culture and Fluorescence Microscopy Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Nagarkar, D.R.; Bowman, E.R.; Schneider, D.; Gosangi, B.; Lei, J.; Zhao, Y.; McHenry, C.L.; Burgens, R.V.; Miller, D.J.; et al. Role of Double-Stranded RNA Pattern Recognition Receptors in Rhinovirus-Induced Airway Epithelial Cell Responses. J. Immunol. 2009, 183, 6989–6997. [Google Scholar] [CrossRef] [PubMed]
- Grellet, E.; L’Hôte, I.; Goulet, A.; Imbert, I. Replication of the Coronavirus Genome: A Paradox among Positive-Strand RNA Viruses. J. Biol. Chem. 2022, 298, 101923. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Wertz, G.W. The Genome of Respiratory Syncytial Virus Is a Negative-Stranded RNA That Codes for at Least Seven mRNA Species. J. Virol. 1982, 43, 150–157. [Google Scholar] [CrossRef]
- Pawełczyk, M.; Kowalski, M.L. The Role of Human Parainfluenza Virus Infections in the Immunopathology of the Respiratory Tract. Curr. Allergy Asthma Rep. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslund, T.I.; Liljeström, P.; Weber, F.; Reis E Sousa, C. RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5’-Phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Thompson, S.R.; Sarnow, P. Enterovirus 71 Contains a Type I IRES Element That Functions When Eukaryotic Initiation Factor eIF4G Is Cleaved. Virology 2003, 315, 259–266. [Google Scholar] [CrossRef]
- Lande, R.; Gilliet, M. Plasmacytoid Dendritic Cells: Key Players in the Initiation and Regulation of Immune Responses: Plasmacytoid Dendritic Cells. Ann. N.Y. Acad. Sci. 2010, 1183, 89–103. [Google Scholar] [CrossRef]
- Perng, Y.-C.; Lenschow, D.J. ISG15 in Antiviral Immunity and Beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-Five Years of Research on Bovine Lactoferrin Applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef]
- Kuhara, T.; Yamauchi, K.; Tamura, Y.; Okamura, H. Oral Administration of Lactoferrin Increases NK Cell Activity in Mice via Increased Production of IL-18 and Type I IFN in the Small Intestine. J. Interferon Cytokine Res. 2006, 26, 489–499. [Google Scholar] [CrossRef]
- Arciniega-Martínez, I.M.; Campos-Rodríguez, R.; Drago-Serrano, M.E.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Reséndiz-Albor, A.A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. 2016, 64, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Miyakawa, M.; Tada, A.; Oda, H.; Motobayashi, H.; Iwabuchi, S.; Tamura, S.; Tanaka, M.; Hashimoto, S. Lactoferrin and Its Digestive Peptides Induce Interferon-α Production and Activate Plasmacytoid Dendritic Cells Ex Vivo. Biometals 2023, 36, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, M.; Kubo, S.; Tada, A.; Konno, N.; Oda, H.; Tanaka, M.; Saito, S.; Aoki, Y.; Yamada, K. Effects of Bovine Lactoferrin on Subjective Gastrointestinal Symptoms Related to Physical Conditions in Healthy Subjects―A Randomized, Double—Blind, Placebo—Controlled Trial―. Jpn. Pharmacol. Ther. 2021, 49, 2187–2193. [Google Scholar]
- Oda, H.; Kubo, S.; Tada, A.; Yago, T.; Sugita, C.; Yoshida, H.; Toida, T.; Tanaka, M.; Kurokawa, M. Effects of Bovine Lactoferrin on the Maintenance of Respiratory and Systemic Physical Conditions in Healthy Adults—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 3959. [Google Scholar] [CrossRef]
- Anders, H.-J.; Lichtnekert, J.; Allam, R. Interferon-α and -β in Kidney Inflammation. Kidney Int. 2010, 77, 848–854. [Google Scholar] [CrossRef]
- Postal, M.; Vivaldo, J.F.; Fernandez-Ruiz, R.; Paredes, J.L.; Appenzeller, S.; Niewold, T.B. Type I Interferon in the Pathogenesis of Systemic Lupus Erythematosus. Curr. Opin. Immunol. 2020, 67, 87–94. [Google Scholar] [CrossRef]
- Miyakawa, M.; Oda, H.; Shin, K.; Sugita, C.; Yoshida, H.; Tanaka, M.; Sonoda, T.; Kurokawa, M. Effects of Lactoferrin on Gene Expression of Type Ⅰ Interferon in HT-29 Cells and Subjective Symptoms Related to Physical Condition in Healthy Adults. Jpn. Pharmacol. Ther. 2021, 49, 1501–1506. [Google Scholar]
- Tewary, P.; Dela Rosa, G.; Rodriguez, L.; Riboldi, E.; Shirota, H.; Patrek, K.; Klinman, D.; Yang, D.; Oppenheim, J. Lactoferrin Inhibits DNA-Induced IFN-Alpha Production by Human Plasmacytoid Dendritic Cells (111.4). J. Immunol. 2011, 186, 111.4. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, Y.; Zhang, Y.; Li, Y.; Guo, Y.; He, Y.; Wang, J.; Lian, J.; Hao, C.; Moorman, J.P.; et al. Role of A20 in Interferon-α-mediated Functional Restoration of Myeloid Dendritic Cells in Patients with Chronic Hepatitis C. Immunology 2014, 143, 670–678. [Google Scholar] [CrossRef]
- Benlahrech, A.; Donaghy, H.; Rozis, G.; Goodier, M.; Klavinskis, L.; Gotch, F.; Patterson, S. Human NK Cell Up-Regulation of CD69, HLA-DR, Interferon γ Secretion and Cytotoxic Activity by Plasmacytoid Dendritic Cells Is Regulated through Overlapping but Different Pathways. Sensors 2009, 9, 386–403. [Google Scholar] [CrossRef]
- Brinkmann, V.; Geiger, T.; Alkan, S.; Heusser, C.H. Interferon Alpha Increases the Frequency of Interferon Gamma-Producing Human CD4+ T Cells. J. Exp. Med. 1993, 178, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- De Goër De Herve, M.-G.; Durali, D.; Dembele, B.; Giuliani, M.; Tran, T.-A.; Azzarone, B.; Eid, P.; Tardieu, M.; Delfraissy, J.-F.; Taoufik, Y. Interferon-Alpha Triggers B Cell Effector 1 (Be1) Commitment. PLoS ONE 2011, 6, e19366. [Google Scholar] [CrossRef] [PubMed]
- Kranzer, K.; Bauer, M.; Lipford, G.B.; Heeg, K.; Wagner, H.; Lang, R. CpG-oligodeoxynucleotides Enhance T-cell Receptor-triggered Interferon-γ Production and Up-regulation of CD69 via Induction of Antigen-presenting Cell-derived Interferon Type I and Interleukin-12. Immunology 2000, 99, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Tzvetkov, E.; Pereira, A.; Wu, Y.; Kasar, S.; Przetak, M.M.; Vlach, J.; Niewold, T.B.; Jensen, M.A.; Okitsu, S.L. TLR7 and TLR8 Differentially Activate the IRF and NF-κB Pathways in Specific Cell Types to Promote Inflammation. ImmunoHorizons 2020, 4, 93–107. [Google Scholar] [CrossRef]
- He, X.-S.; Draghi, M.; Mahmood, K.; Holmes, T.H.; Kemble, G.W.; Dekker, C.L.; Arvin, A.M.; Parham, P.; Greenberg, H.B. T Cell–Dependent Production of IFN-γ by NK Cells in Response to Influenza A Virus. J. Clin. Invest. 2004, 114, 1812–1819. [Google Scholar] [CrossRef]
- Marzio, R.; Mauël, J.; Betz-Corradin, S. CD69 and Regulatiof the Immune Function. Immunopharmacol. Immunotoxicol. 1999, 21, 565–582. [Google Scholar] [CrossRef]
- Tel, J.; Lambeck, A.J.A.; Cruz, L.J.; Tacken, P.J.; De Vries, I.J.M.; Figdor, C.G. Human Plasmacytoid Dendritic Cells Phagocytose, Process, and Present Exogenous Particulate Antigen. J. Immunol. 2010, 184, 4276–4283. [Google Scholar] [CrossRef]
- Kitagawa, S.; Matsuda, T.; Washizaki, A.; Murakami, H.; Yamamoto, T.; Yoshioka, Y. Elucidation of the Role of Nucleolin as a Cell Surface Receptor for Nucleic Acid-Based Adjuvants. npj Vaccines 2022, 7, 115. [Google Scholar] [CrossRef]
- Gardner, J.K.; Mamotte, C.D.; McGonigle, T.; Dye, D.E.; Jackaman, C.; Nelson, D.J. Lipid-Laden Partially-Activated Plasmacytoid and CD4−CD8α+ Dendritic Cells Accumulate in Tissues in Elderly Mice. Immun. Ageing 2014, 11, 11. [Google Scholar] [CrossRef][Green Version]
- Severa, M.; Diotti, R.A.; Etna, M.P.; Rizzo, F.; Fiore, S.; Ricci, D.; Iannetta, M.; Sinigaglia, A.; Lodi, A.; Mancini, N.; et al. Differential Plasmacytoid Dendritic Cell Phenotype and Type I Interferon Response in Asymptomatic and Severe COVID-19 Infection. PLoS Pathog. 2021, 17, e1009878. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Praženicová, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef]
- Legrand, D.; Vigié, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.; Carpentier, M.; Briand, J.; Mazurier, J.; Hovanessian, A.G. Surface Nucleolin Participates in Both the Binding and Endocytosis of Lactoferrin in Target Cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Tonello, F.; Massimino, M.L.; Peggion, C. Nucleolin: A Cell Portal for Viruses, Bacteria, and Toxins. Cell. Mol. Life Sci. 2022, 79, 271. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; To, M.; Saruta, J.; Sato, C.; Yamamoto, Y.; Kondo, Y.; Shimizu, T.; Kamata, Y.; Tsukinoki, K. Salivary Lactoferrin Is Transferred into the Brain via the Sublingual Route. Biosci. Biotechnol. Biochem. 2017, 81, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric Digestion of Bovine Lactoferrin In Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of Oral Administration Mode on the Efficacy of Commercial Bovine Lactoferrin against Iron and Inflammatory Homeostasis Disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of Lactoferrin Transportation into Blood Circulation from Intestine via Lymphatic Pathway in Adult Rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef]
- Palomares, O.; Rückert, B.; Jartti, T.; Kücüksezer, U.C.; Puhakka, T.; Gomez, E.; Fahrner, H.B.; Speiser, A.; Jung, A.; Kwok, W.W.; et al. Induction and Maintenance of Allergen-Specific FOXP3+ Treg Cells in Human Tonsils as Potential First-Line Organs of Oral Tolerance. J. Allergy Clin. Immunol. 2012, 129, 510–520.e9. [Google Scholar] [CrossRef]
- Yrlid, U.; Milling, S.W.F.; Miller, J.L.; Cartland, S.; Jenkins, C.D.; MacPherson, G.G. Regulation of Intestinal Dendritic Cell Migration and Activation by Plasmacytoid Dendritic Cells, TNF-α and Type 1 IFNs after Feeding a TLR7/8 Ligand. J. Immunol. 2006, 176, 5205–5212. [Google Scholar] [CrossRef]
- Shin, O.S.; Isberg, R.R.; Akira, S.; Uematsu, S.; Behera, A.K.; Hu, L.T. Distinct Roles for MyD88 and Toll-Like Receptors 2, 5, and 9 in Phagocytosis of Borrelia burgdorferi and Cytokine Induction. Infect. Immun. 2008, 76, 2341–2351. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Huang, S.-T.; Wu, J.-C.; Chu, T.-H.; Huang, S.-C.; Lee, C.-C.; Tai, M.-H. Novel HDGF/HIF-1α/VEGF Axis in Oral Cancer Impacts Disease Prognosis. BMC Cancer 2019, 19, 1083. [Google Scholar] [CrossRef]
| Age (Years) | Total | Men | Women |
|---|---|---|---|
| 20–29 | 3 | 1 | 2 |
| 30–39 | 8 | 5 | 3 |
| 40–49 | 4 | 3 | 1 |
| 50–59 | 1 | 1 | 0 |
| Total | 16 | 10 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yago, T.; Tada, A.; Kubo, S.; Oda, H.; Iwabuchi, S.; Tanaka, M.; Hashimoto, S. Bovine Lactoferrin Enhances Toll-like Receptor 7 Response in Plasmacytoid Dendritic Cells and Modulates Cellular Immunity. Int. J. Mol. Sci. 2024, 25, 13369. https://doi.org/10.3390/ijms252413369
Yago T, Tada A, Kubo S, Oda H, Iwabuchi S, Tanaka M, Hashimoto S. Bovine Lactoferrin Enhances Toll-like Receptor 7 Response in Plasmacytoid Dendritic Cells and Modulates Cellular Immunity. International Journal of Molecular Sciences. 2024; 25(24):13369. https://doi.org/10.3390/ijms252413369
Chicago/Turabian StyleYago, Takumi, Asuka Tada, Shutaro Kubo, Hirotsugu Oda, Sadahiro Iwabuchi, Miyuki Tanaka, and Shinichi Hashimoto. 2024. "Bovine Lactoferrin Enhances Toll-like Receptor 7 Response in Plasmacytoid Dendritic Cells and Modulates Cellular Immunity" International Journal of Molecular Sciences 25, no. 24: 13369. https://doi.org/10.3390/ijms252413369
APA StyleYago, T., Tada, A., Kubo, S., Oda, H., Iwabuchi, S., Tanaka, M., & Hashimoto, S. (2024). Bovine Lactoferrin Enhances Toll-like Receptor 7 Response in Plasmacytoid Dendritic Cells and Modulates Cellular Immunity. International Journal of Molecular Sciences, 25(24), 13369. https://doi.org/10.3390/ijms252413369








