The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment
Abstract
1. Introduction
2. The Resistance System in Ferroptosis
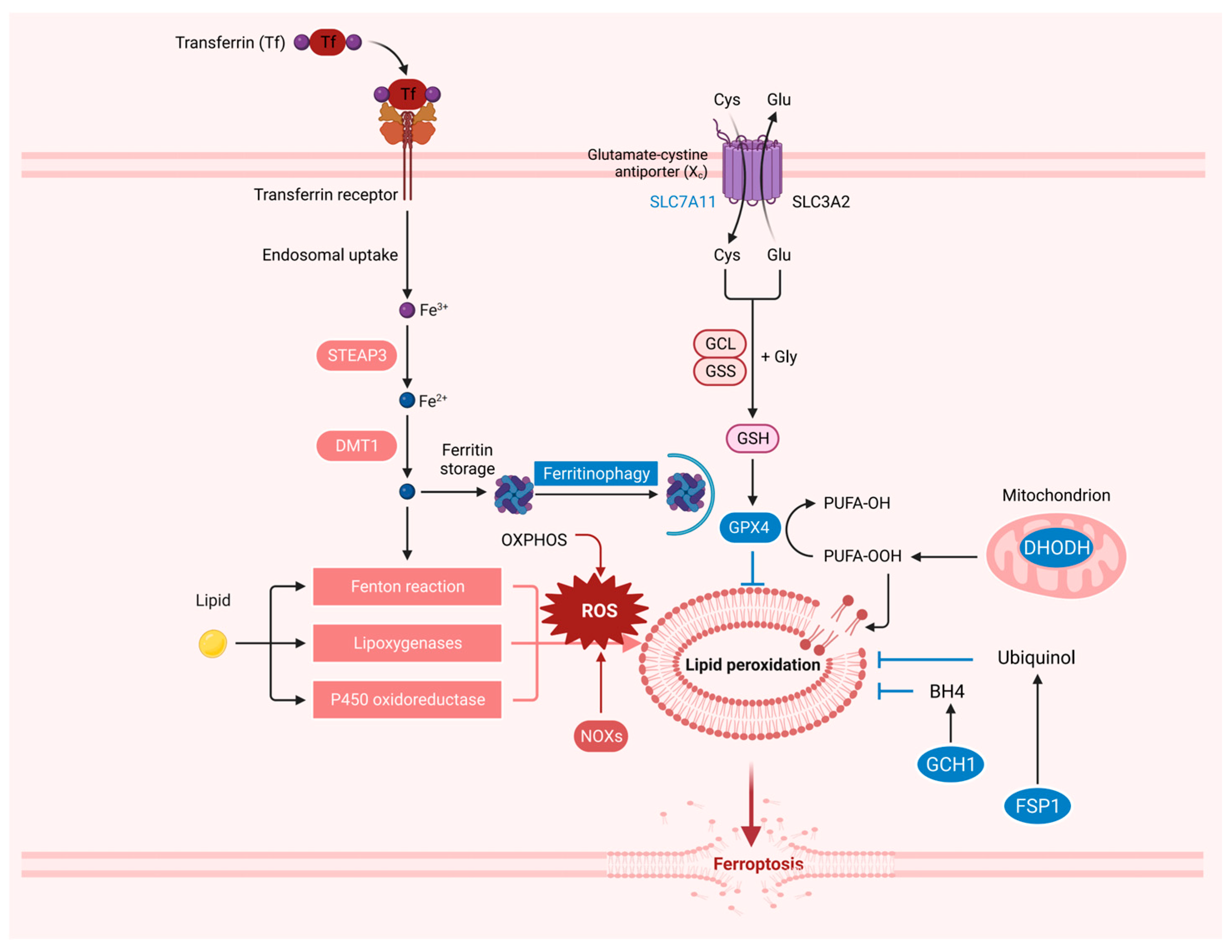
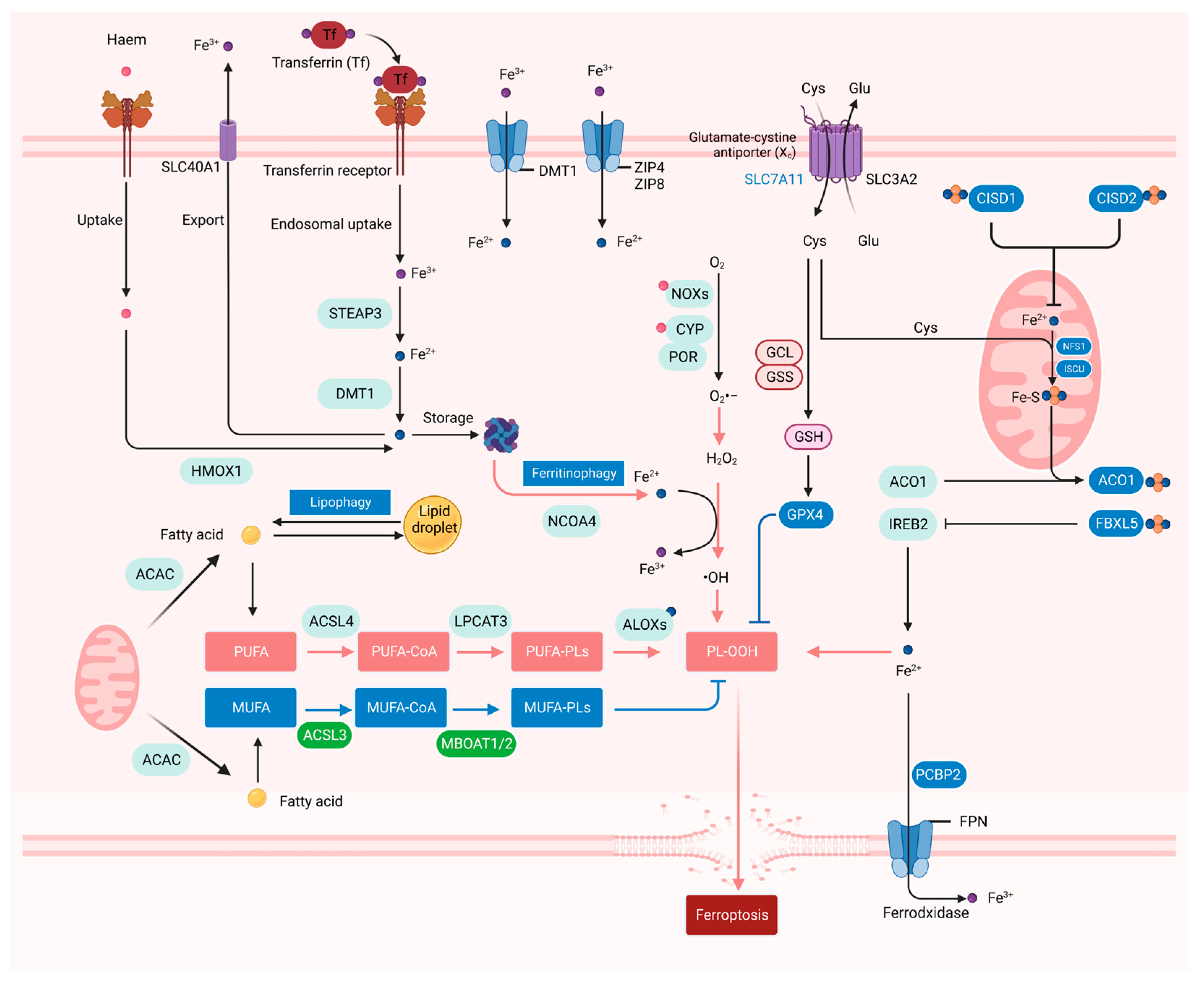
3. The Iron, ROS, and Lipid Metabolism in Ferroptosis
4. Ferroptosis in the Progression of Pancreatic Cancer
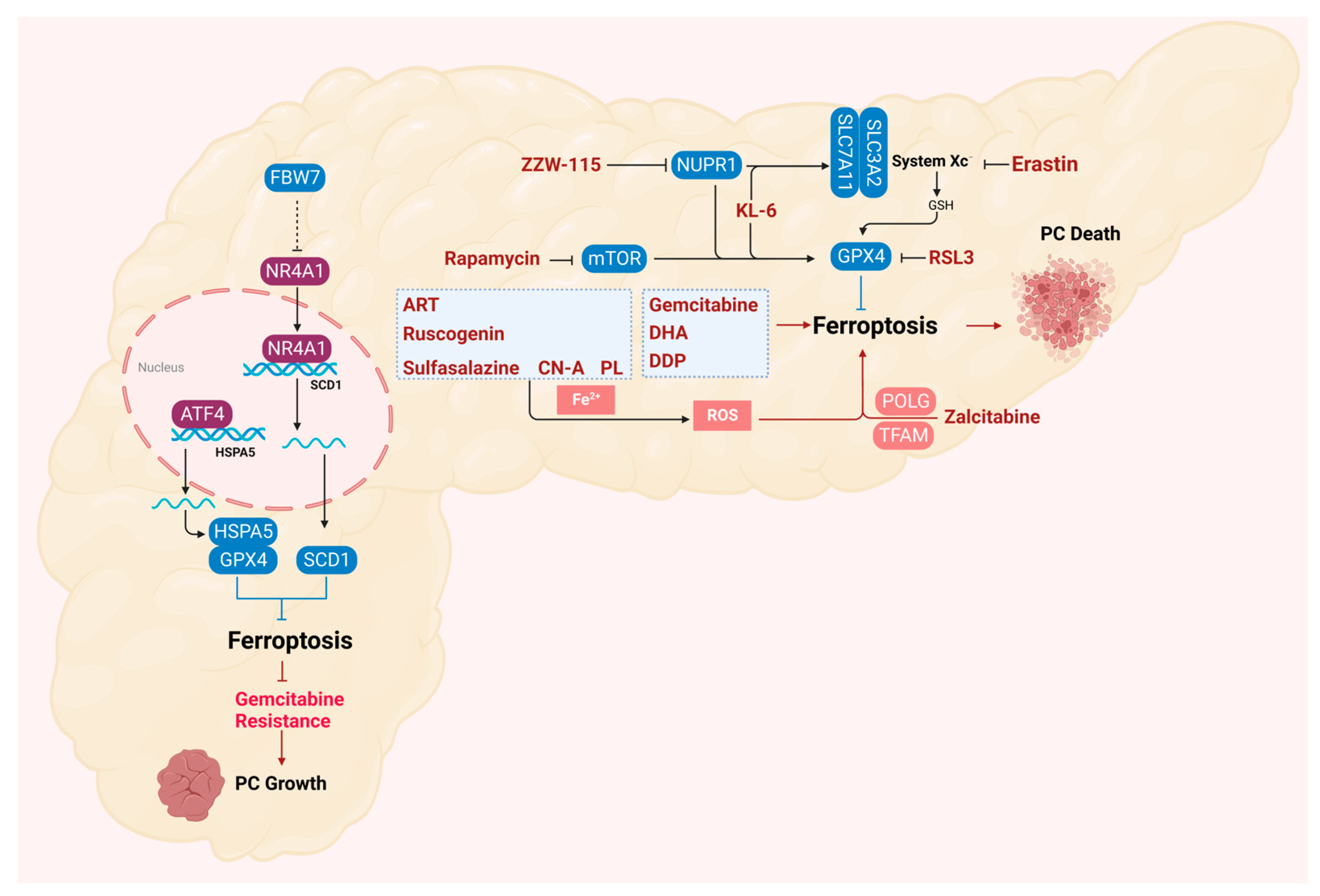
5. Ferroptosis in the Treatment of Pancreatic Cancer
6. Ferroptosis in the Drug Resistance of Pancreatic Cancer
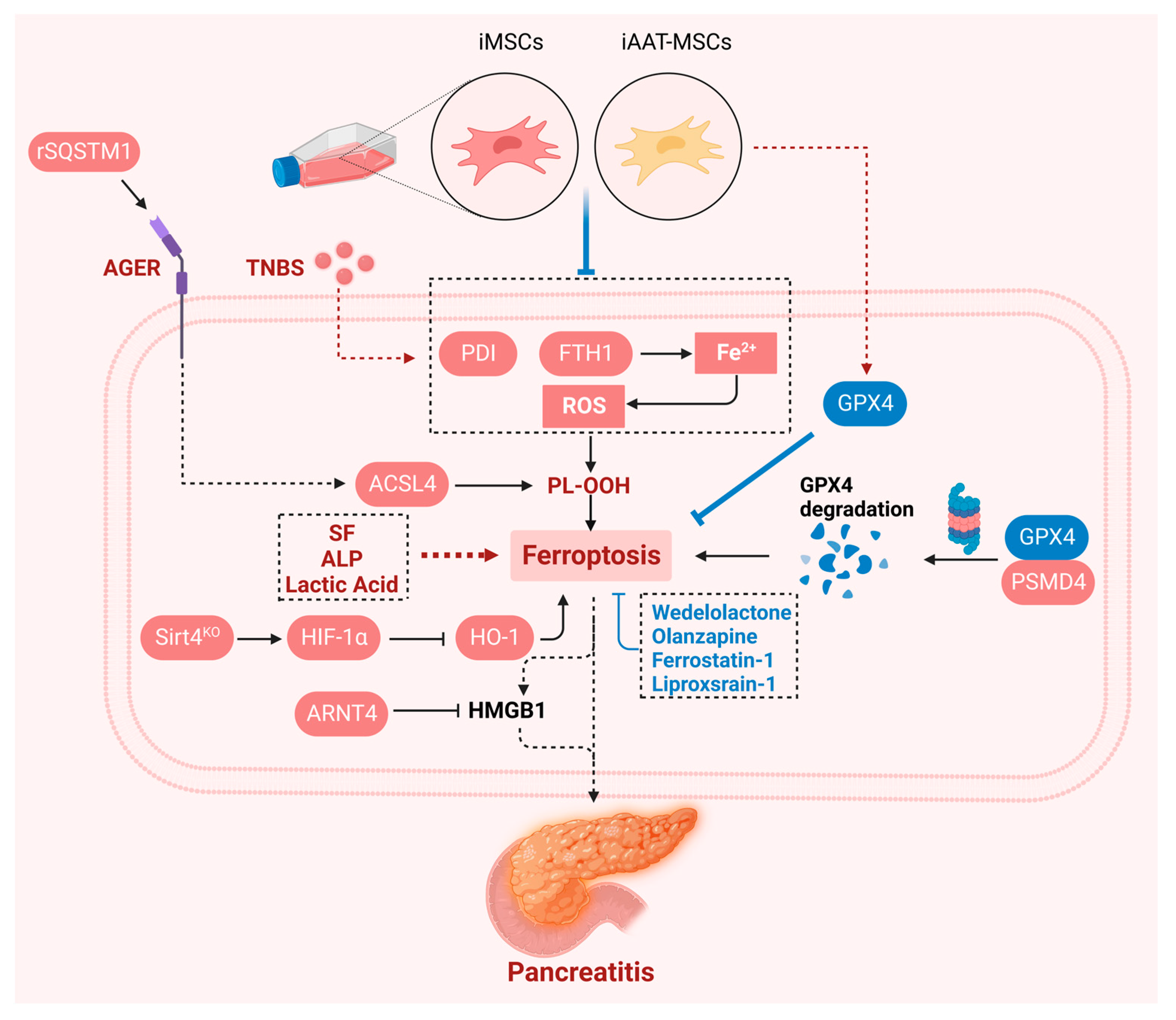
7. Ferroptosis in Pancreatitis
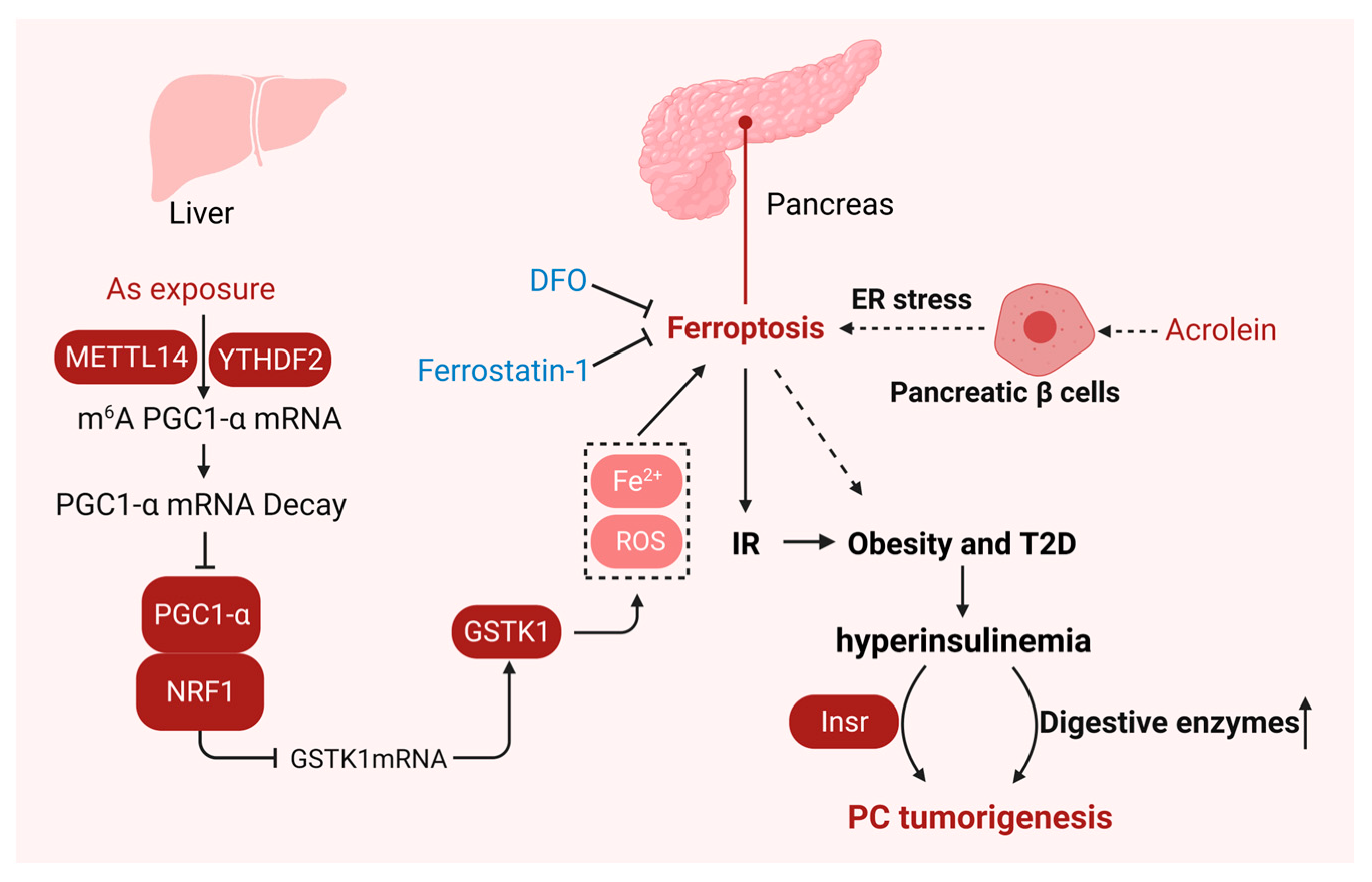
8. Ferroptosis in Insulin Resistance
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Dhasmana, S.; Kotnala, S.; Laskar, P.; Khan, S.; Haque, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. CEACAM7 expression contributes to early events of pancreatic cancer. J. Adv. Res. 2024, 55, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Maisonneuve, P.; Lowenfels, A.B. Epidemiology of pancreatic cancer: An overview. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef]
- Zhen, D.B.; Rabe, K.G.; Gallinger, S.; Syngal, S.; Schwartz, A.G.; Goggins, M.G.; Hruban, R.H.; Cote, M.L.; McWilliams, R.R.; Roberts, N.J.; et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: A PACGENE study. Genet. Med. 2015, 17, 569–577. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Bartoli, M.; Barat, M.; Dohan, A.; Gaujoux, S.; Coriat, R.; Hoeffel, C.; Cassinotto, C.; Chassagnon, G.; Soyer, P. CT and MRI of pancreatic tumors: An update in the era of radiomics. Jpn. J. Radiol. 2020, 38, 1111–1124. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, R.; Guo, K.; Ren, S.; Zhang, Y.; Lu, Z.; Tian, L.; Li, T.; Chen, X.; Wang, Z. Potential Metabolite Biomarkers for Early Detection of Stage-I Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 744667. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Huang, Q.; Ding, Y.; Fang, C.; Wang, H.; Kong, L. The Emerging Role of Ferroptosis in Sepsis, Opportunity or Challenge? Infect. Drug Resist. 2023, 16, 5551–5562. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The crosstalk between ferroptosis and anti-tumor immunity in the tumor microenvironment: Molecular mechanisms and therapeutic controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef]
- Damiescu, R.; Efferth, T.; Dawood, M. Dysregulation of different modes of programmed cell death by epigenetic modifications and their role in cancer. Cancer Lett. 2024, 584, 216623. [Google Scholar] [CrossRef]
- Hao, M.; Jiang, Y.; Zhang, Y.; Yang, X.; Han, J. Ferroptosis regulation by methylation in cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188972. [Google Scholar] [CrossRef]
- He, R.; Liu, Y.; Fu, W.; He, X.; Liu, S.; Xiao, D.; Tao, Y. Mechanisms and cross-talk of regulated cell death and their epigenetic modifications in tumor progression. Mol. Cancer 2024, 23, 267. [Google Scholar] [CrossRef]
- Kong, J.; Lyu, H.; Ouyang, Q.; Shi, H.; Zhang, R.; Xiao, S.; Guo, D.; Zhang, Q.; Chen, X.Z.; Zhou, C.; et al. Insights into the Roles of Epigenetic Modifications in Ferroptosis. Biology 2024, 13, 122. [Google Scholar] [CrossRef]
- Veglia Tranchese, R.; Battista, S.; Cerchia, L.; Fedele, M. Ferroptosis in Cancer: Epigenetic Control and Therapeutic Opportunities. Biomolecules 2024, 14, 1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fleishman, J.S.; Cheng, S.; Wang, W.; Wu, F.; Wang, Y.; Wang, Y. Epigenetic modification of ferroptosis by non-coding RNAs in cancer drug resistance. Mol. Cancer 2024, 23, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, X.; Feng, X.; Jiang, D.S. Effects of DNA, RNA, and Protein Methylation on the Regulation of Ferroptosis. Int. J. Biol. Sci. 2023, 19, 3558–3575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, J.; Wu, S.; Fleishman, J.S.; Li, Y.; Xu, Y.; Zou, W.; Wang, J.; Feng, Y.; Chen, J.; et al. Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases. Signal Transduct. Target. Ther. 2023, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xue, C.; Li, M.; Wei, J.; Zheng, L.; Chen, S.; Duan, Y.; Deng, H.; Tang, F.; Xiong, W.; et al. Ferroptosis: A critical mechanism of N(6)-methyladenosine modification involved in carcinogenesis and tumor progression. Sci. China Life Sci 2024, 67, 1119–1132. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Le, J.; Sun, Y.; Dian, Y.; Yao, L.; Xiong, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis: Mechanisms and therapeutic targets. MedComm (2020) 2024, 5, e70010. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, L.; Makitie, A.A.; Florek, E.; Czarnywojtek, A.; Saba, N.F.; Ferlito, A. Iron, Ferroptosis, and Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 15127. [Google Scholar] [CrossRef]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kolle, P.; Tschoep, K.; Issels, R.D.; Daniel, P.T.; et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Hu, Y.; Wu, Z.; Li, Y.; Kong, M.; Kang, Z.; Zuoyuan, B.; Yang, Z. TFRC upregulation promotes ferroptosis in CVB3 infection via nucleus recruitment of Sp1. Cell Death Dis. 2022, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Kroemer, G.; Klionsky, D.J.; Zeh, H.J.; Kang, R.; Wang, J.; Tang, D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Bai, Y.; Li, K.; Liu, N.; Xu, Y.; Dal, E.; Wang, Y.; Lin, R.; Wang, H.; Liu, Z.; et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist. Updates 2023, 68, 100960. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, Z.; Wu, R.; Chen, X.; Yu, C.; Stockwell, B.; Kroemer, G.; Kang, R.; Tang, D. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 2023, 15, eadg3049. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Zeh, H.J.; Kang, R.; Bai, L.; Tang, D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 2020, 11, 6339. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef]
- Liu, X.; Olszewski, K.; Zhang, Y.; Lim, E.W.; Shi, J.; Zhang, X.; Zhang, J.; Lee, H.; Koppula, P.; Lei, G.; et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020, 22, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-) : Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Bai, L.; Zhi, L.; Yang, Y.; Zhao, Q.; Chen, C.; Qi, Y.; Gao, W.; He, W.; et al. RBMS1 regulates lung cancer ferroptosis through translational control of SLC7A11. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free. Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Speckmann, B.; Bidmon, H.J.; Pinto, A.; Anlauf, M.; Sies, H.; Steinbrenner, H. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J. Biol. Chem. 2011, 286, 10764–10772. [Google Scholar] [CrossRef]
- Stefely, J.A.; Pagliarini, D.J. Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem. Sci. 2017, 42, 824–843. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Morré, D.J.; Morré, D.M. Non-mitochondrial coenzyme Q. BioFactors 2011, 37, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Carew, N.T.; Schmidt, H.M.; Yuan, S.; Galley, J.C.; Hall, R.; Altmann, H.M.; Hahn, S.A.; Miller, M.P.; Wood, K.C.; Gabris, B.; et al. Loss of cardiomyocyte CYB5R3 impairs redox equilibrium and causes sudden cardiac death. J. Clin. Investig. 2022, 132, e147120. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Li, R.; Liu, Y.; Zhou, L.; Wang, C.; Lv, C.; Gao, L.; Cui, D. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J. Exp. Clin. Cancer Res. 2022, 41, 307. [Google Scholar] [CrossRef]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Muller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kossl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef]
- Douglas, G.; Hale, A.B.; Crabtree, M.J.; Ryan, B.J.; Hansler, A.; Watschinger, K.; Gross, S.S.; Lygate, C.A.; Alp, N.J.; Channon, K.M. A requirement for Gch1 and tetrahydrobiopterin in embryonic development. Dev. Biol. 2015, 399, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Wu, L.L.; Yang, L.; Yang, L.; Wang, J. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023, 14, 519. [Google Scholar] [CrossRef]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, H.; Graham, E.T.; Deik, A.A.; Eaton, J.K.; Wang, W.; Sandoval-Gomez, G.; Clish, C.B.; Doench, J.G.; Schreiber, S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020, 16, 302–309. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef]
- Theil, E.C. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Shen, Y.; Li, H.; Rausch, W.D.; Huang, X. Iron Dyshomeostasis and Ferroptosis: A New Alzheimer’s Disease Hypothesis? Front. Aging Neurosci. 2022, 14, 830569. [Google Scholar] [CrossRef]
- Brown, C.W.; Amante, J.J.; Chhoy, P.; Elaimy, A.L.; Liu, H.; Zhu, L.J.; Baer, C.E.; Dixon, S.J.; Mercurio, A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell 2019, 51, 575–586.e4. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Xu, Y.; Sang, L.; Liu, X.; Li, Y. Ferroptosis, pyroptosis and necroptosis in acute respiratory distress syndrome. Cell Death Discov. 2023, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- To, T.L.; Cuadros, A.M.; Shah, H.; Hung, W.H.W.; Li, Y.; Kim, S.H.; Rubin, D.H.F.; Boe, R.H.; Rath, S.; Eaton, J.K.; et al. A Compendium of Genetic Modifiers of Mitochondrial Dysfunction Reveals Intra-organelle Buffering. Cell 2019, 179, 1222–1238.e17. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Aldrovandi, M.; Fedorova, M.; Conrad, M. Juggling with lipids, a game of Russian roulette. Trends Endocrinol. Metab. 2021, 32, 463–473. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Pandey, A.V.; Fluck, C.E. NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020, 16, 644–652. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, Y.; Zhou, L.; Yan, Y.; Liu, N.; Zhang, R.; Huang, Y.; Wang, M.; Tang, Y.; Ali, D.W.; et al. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy 2021, 17, 3175–3195. [Google Scholar] [CrossRef]
- Zhou, C.; Yi, C.; Yi, Y.; Qin, W.; Yan, Y.; Dong, X.; Zhang, X.; Huang, Y.; Zhang, R.; Wei, J.; et al. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/beta-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol. Cancer 2020, 19, 118. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Kremer, D.M.; Nelson, B.S.; Lin, L.; Yarosz, E.L.; Halbrook, C.J.; Kerk, S.A.; Sajjakulnukit, P.; Myers, A.; Thurston, G.; Hou, S.W.; et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat. Commun. 2021, 12, 4860. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Mo, S.; Zhang, Y.; Mao, X.; Chen, J.; Liu, Y.; Tong, W.-M.; Lu, Z.; Yu, S.; et al. Targeting N-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma. Cell Death Differ. 2023, 30, 1988–2004. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, B.; Chen, M. Partner of NOB1 homolog transcriptionally activated by E2F transcription factor 1 promotes the malignant progression and inhibits ferroptosis of pancreatic cancer. Chin. J. Physiol. 2023, 66, 388–399. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Jia, J.; Hu, C.; Lu, J.; Li, J.; Xu, H.; Fang, J.; Feng, D.; Wang, L.; Chen, Y. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene 2022, 41, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Marinis, J.M.; Beal, A.M.; Savadkar, S.; Wu, Y.; Khan, M.; Taunk, P.S.; Wu, N.; Su, W.; Wu, J.; et al. RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer. Cancer Cell 2018, 34, 757–774 e757. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Zeng, L.; Li, J.; Klionsky, D.J.; Kroemer, G.; Jiang, J.; Tang, D.; Kang, R. DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy 2022, 18, 2036–2049. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Dong, H.; Niu, K.; Zhang, W.; Feng, K.; Yang, R.; Zhang, Y. Crosstalk of ferroptosis regulators and tumor immunity in pancreatic adenocarcinoma: Novel perspective to mRNA vaccines and personalized immunotherapy. Apoptosis 2023, 28, 1423–1435. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, F.; Zou, C.; Bao, T.; Wu, M.; Yang, D.; Bu, S. The construction and analysis of a ferroptosis-related gene prognostic signature for pancreatic cancer. Aging 2021, 13, 10396–10414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Liu, S.; Huang, W.; Gu, J.; Zhao, Z.; Qin, H.; Luo, L.; Yang, J.; Fang, Y.; et al. Alteration of tumor-associated macrophage subtypes mediated by KRT6A in pancreatic ductal adenocarcinoma. Aging 2020, 12, 23217–23232. [Google Scholar] [CrossRef]
- Ansari, D.; Gustafsson, A.; Andersson, R. Update on the management of pancreatic cancer: Surgery is not enough. World J. Gastroenterol. 2015, 21, 3157–3165. [Google Scholar] [CrossRef]
- Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Tang, D. Cell death in pancreatic cancer: From pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 804–823. [Google Scholar] [CrossRef]
- Yang, G.; Guan, W.; Cao, Z.; Guo, W.; Xiong, G.; Zhao, F.; Feng, M.; Qiu, J.; Liu, Y.; Zhang, M.Q.; et al. Integrative Genomic Analysis of Gemcitabine Resistance in Pancreatic Cancer by Patient-derived Xenograft Models. Clin. Cancer Res. 2021, 27, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Liu, J.; Kang, R.; Tang, D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef]
- Liu, J.; Song, X.; Kuang, F.; Zhang, Q.; Xie, Y.; Kang, R.; Kroemer, G.; Tang, D. NUPR1 is a critical repressor of ferroptosis. Nat. Commun. 2021, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhao, B.; Guan, X.; Dong, J.; Ying, J. A benzochalcone derivative synchronously induces apoptosis and ferroptosis in pancreatic cancer cells. PeerJ 2023, 11, e16291. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef]
- Song, Z.; Xiang, X.; Li, J.; Deng, J.; Fang, Z.; Zhang, L.; Xiong, J. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol. Rep. 2020, 43, 516–524. [Google Scholar] [CrossRef]
- Chen, G.; Guo, G.; Zhou, X.; Chen, H. Potential mechanism of ferroptosis in pancreatic cancer. Oncol. Lett. 2020, 19, 579–587. [Google Scholar] [CrossRef]
- Yang, N.D.; Tan, S.H.; Ng, S.; Shi, Y.; Zhou, J.; Tan, K.S.; Wong, W.S.; Shen, H.M. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J. Biol. Chem. 2014, 289, 33425–33441. [Google Scholar] [CrossRef]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Torii, S.; Shintoku, R.; Kubota, C.; Yaegashi, M.; Torii, R.; Sasaki, M.; Suzuki, T.; Mori, M.; Yoshimoto, Y.; Takeuchi, T.; et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 2016, 473, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Daher, B.; Parks, S.K.; Durivault, J.; Cormerais, Y.; Baidarjad, H.; Tambutte, E.; Pouyssegur, J.; Vucetic, M. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019, 79, 3877–3890. [Google Scholar] [CrossRef]
- Ye, Z.; Zhuo, Q.; Hu, Q.; Xu, X.; Mengqi, L.; Zhang, Z.; Xu, W.; Liu, W.; Fan, G.; Qin, Y.; et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021, 38, 101807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Q.; Sun, X.; Zeh, H.J., 3rd; Lotze, M.T.; Kang, R.; Tang, D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free. Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Li, Y.; Ren, X.; Zhou, Y.; Hu, W.; Zhou, C.; Jing, Q.; Yang, C.; Wang, L.; et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021, 12, 705. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Q.; Li, W.; Li, M.; Dong, S.; Peng, Y.; Yin, J.; Lu, Y.; Liu, L.; Zhao, Q. The Role of Ferroptosis Signature in Overall Survival and Chemotherapy of Pancreatic Adenocarcinoma. DNA Cell Biol. 2022, 41, 116–127. [Google Scholar] [CrossRef]
- Engle, D.D.; Tiriac, H.; Rivera, K.D.; Pommier, A.; Whalen, S.; Oni, T.E.; Alagesan, B.; Lee, E.J.; Yao, M.A.; Lucito, M.S.; et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science 2019, 364, 1156–1162. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef]
- Pinho, A.V.; Chantrill, L.; Rooman, I. Chronic pancreatitis: A path to pancreatic cancer. Cancer Lett. 2014, 345, 203–209. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Liu, J.; Kang, R.; Tang, D. The circadian clock protects against ferroptosis-induced sterile inflammation. Biochem. Biophys. Res. Commun. 2020, 525, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, J.; Zou, B.; Li, C.; Zeh, H.J.; Kang, R.; Kroemer, G.; Huang, J.; Tang, D. Trypsin-Mediated Sensitization to Ferroptosis Increases the Severity of Pancreatitis in Mice. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, T.; Li, J.; Yu, P.; Mei, Y.; Li, M.; Qi, X.; Liu, F. Serum iron fluctuations link ferroptosis process with mortality and prognosis of acute pancreatitis. iScience 2023, 26, 107774. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Y.; Cao, F.; Wang, G.; Li, F. An in-Depth Exploration of the Genetic Interaction Network Between Ferroptosis and Acute Pancreatitis. J. Inflamm. Res. 2023, 16, 4425–4439. [Google Scholar] [CrossRef]
- Ma, D.; Li, C.; Jiang, P.; Jiang, Y.; Wang, J.; Zhang, D. Inhibition of Ferroptosis Attenuates Acute Kidney Injury in Rats with Severe Acute Pancreatitis. Dig. Dis. Sci. 2021, 66, 483–492. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, H.; Mei, C.; Cui, M.; He, Q.; Wang, Q.; Li, D.; Song, Y.; Li, J.; Chen, S.; et al. Sirtuin4 alleviates severe acute pancreatitis by regulating HIF-1alpha/HO-1 mediated ferroptosis. Cell Death Dis. 2023, 14, 694. [Google Scholar] [CrossRef]
- Fan, R.; Sui, J.; Dong, X.; Jing, B.; Gao, Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free. Radic. Biol. Med. 2021, 173, 29–40. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, J.; Xiong, Q.; Yu, H.; Du, H. Secondary iron overload induces chronic pancreatitis and ferroptosis of acinar cells in mice. Int. J. Mol. Med. 2023, 51, 9. [Google Scholar] [CrossRef]
- Pan, Z.; Van den Bossche, J.L.; Rodriguez-Aznar, E.; Janssen, P.; Lara, O.; Ates, G.; Massie, A.; De Paep, D.L.; Houbracken, I.; Mambretti, M.; et al. Pancreatic acinar cell fate relies on system x(C)(-) to prevent ferroptosis during stress. Cell Death Dis. 2023, 14, 536. [Google Scholar] [CrossRef]
- Shoeibi, S.; Green, E.; Wei, H.; Gou, W.; Strange, C.; Wang, H. Immortalized Mesenchymal Stromal Cells Overexpressing Alpha-1 Antitrypsin Protect Acinar Cells from Apoptotic and Ferroptotic Cell Death. J. Cell. Mol. Med. 2024, 28, e70093. [Google Scholar] [CrossRef]
- Yang, L.; Ye, F.; Liu, J.; Klionsky, D.J.; Tang, D.; Kang, R. Extracellular SQSTM1 exacerbates acute pancreatitis by activating autophagy-dependent ferroptosis. Autophagy 2023, 19, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Fang, X.; Zhang, Y.; Wei, J.; Zhang, Y.; Tian, J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: Mechanisms and therapeutic opportunities. Cell Death Dis. 2023, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Ramkumar, K.M. Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic beta-cells in diabetes. Apoptosis 2023, 28, 958–976. [Google Scholar] [CrossRef]
- Bruni, A.; Pepper, A.R.; Pawlick, R.L.; Gala-Lopez, B.; Gamble, A.F.; Kin, T.; Seeberger, K.; Korbutt, G.S.; Bornstein, S.R.; Linkermann, A.; et al. Ferroptosis-inducing agents compromise in vitro human islet viability and function. Cell Death Dis. 2018, 9, 595. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress- related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Liu, S.; Zhang, Y.; Qiu, T.; Jiang, L.; Bai, J.; Yao, X.; Wang, N.; Yang, G.; et al. m(6)A methylation-mediated PGC-1alpha contributes to ferroptosis via regulating GSTK1 in arsenic-induced hepatic insulin resistance. Sci. Total Environ. 2023, 905, 167202. [Google Scholar] [CrossRef]
- Yang, J.; Waldron, R.T.; Su, H.Y.; Moro, A.; Chang, H.H.; Eibl, G.; Ferreri, K.; Kandeel, F.R.; Lugea, A.; Li, L.; et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G675–G687. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef]
- Toledo, F.G.S.; Chari, S.; Yadav, D. Understanding the Contribution of Insulin Resistance to the Risk of Pancreatic Cancer. Am. J. Gastroenterol. 2021, 116, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 2010, 21, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Graubard, B.I.; Chari, S.; Limburg, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005, 294, 2872–2878. [Google Scholar] [CrossRef]
- Zhang, A.M.Y.; Xia, Y.H.; Lin, J.S.H.; Chu, K.H.; Wang, W.C.K.; Ruiter, T.J.J.; Yang, J.C.C.; Chen, N.; Chhuor, J.; Patil, S.; et al. Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammation. Cell Metab. 2023, 35, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ding, C.; Wang, R.; Qiu, J.; Liu, Y.; Tao, J.; Luo, W.; Weng, G.; Yang, G.; Zhang, T. E3 ubiquitin ligase RBCK1 confers ferroptosis resistance in pancreatic cancer by facilitating MFN2 degradation. Free. Radic. Biol. Med. 2024, 221, 136–154. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Q.; Liu, Y.; Wu, W. Oncogenic KRAS Promotes Ferroptosis in Pancreatic Cancer through Regulation of the FOSL1-TFRC Axis. Pancreas 2024. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Cai, Y.; Yu, H.; Cao, G.; Dai, E.; Kang, R.; Tang, D.; Hu, N.; Han, L. OSGIN1 promotes ferroptosis resistance by directly enhancing GCLM activity. Biochem. Biophys. Res. Commun. 2024, 740, 151015. [Google Scholar] [CrossRef]
- He, Z.; Zheng, D.; Li, F.; Chen, L.; Wu, C.; Zeng, Z.; Yu, C. TMOD3 accelerated resistance to immunotherapy in KRAS-mutated pancreatic cancer through promoting autophagy-dependent degradation of ASCL4. Drug Resist. Updates 2024, 78, 101171. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Zhang, Y.; Yang, Y.; Yang, Y.; Zu, G.; Yu, X.; Chen, W.; Qin, Y.; Xu, X.; et al. IL15RA-STAT3-GPX4/ACSL3 signaling leads to ferroptosis resistance in pancreatic cancer. Acta Biochim. Biophys. Sin. 2024. [Google Scholar] [CrossRef]
- Qu, T.; Cha, L.; Liu, H.; Tian, L.; Hu, X.; Zou, H.; Feng, Y.; Sun, C.; Cao, J.; Guo, W.; et al. Circ_0005397 inhibits ferroptosis of pancreatic cancer cells by up-regulating PCBP2 through KAT6A/H3K9Ac. FASEB J. 2024, 38, e70028. [Google Scholar] [CrossRef]
- Feng, L.; Chen, L.; Wang, W.; Wei, Q.; Chen, M.; Jiang, X.; Hu, S.; Wu, Y.; Duan, L.; Zhu, L.; et al. PRMT6-mediated ADMA promotes p62 phase separation to form a negative feedback loop in ferroptosis. Theranostics 2024, 14, 4090–4106. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Xiao, M.; Liang, C.; Hua, J.; Liu, J.; Wang, W.; Yu, X.; Meng, Q.; Shi, S. ARID3A enhances chemoresistance of pancreatic cancer via inhibiting PTEN-induced ferroptosis. Redox Biol. 2024, 73, 103200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Chen, H.Y.; Lai, X.Q. ELAVL1-dependent SOAT2 exacerbated the pancreatitis-like cellular injury of AR42J cells induced by hyperstimulation with caerulein. Kaohsiung J. Med. Sci. 2024, e12911. [Google Scholar] [CrossRef]
- Dong, X.; Luo, W.; Wang, Y.; Zhu, Q.; Yuan, C.; Xiao, W.; Gong, W.; Lu, G.; Shi, X.; Li, J. Role and mechanism of myonectin in severe acute pancreatitis: A crosstalk between skeletal muscle and pancreas. Skelet. Muscle 2024, 14, 29. [Google Scholar] [CrossRef]
- Cao, R.; Feng, Z.; Mo, J.; Wu, J.; Li, J.; Li, W.; Wang, Z.; Ma, Q.; Wu, Z.; Zhou, C. Pharmacological inhibition of SREBP1 suppresses pancreatic cancer growth via inducing GPX4-mediated ferroptosis. Cell. Signal. 2024, 124, 111381. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Kroemer, G.; Tang, D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021, 28, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; O’Prey, J.; Morton, J.P.; Nixon, C.; MacKay, G.; Mrowinska, A.; Au, A.; Rai, T.S.; Zheng, L.; Ridgway, R.; et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 2013, 504, 296–300. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Ren, Z.; Li, Y.; Zou, W.; Chen, J.; Wang, H. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist. Updates 2023, 66, 100916. [Google Scholar] [CrossRef]
- Miao, Y.D.; Quan, W.; Dong, X.; Gan, J.; Ji, C.F.; Wang, J.T.; Zhang, F. A bibliometric analysis of ferroptosis, necroptosis, pyroptosis, and cuproptosis in cancer from 2012 to 2022. Cell Death Discov. 2023, 9, 129. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Sun, Q.; Li, Y.; Zhou, C.; Sun, C. Susceptibility of acute myeloid leukemia cells to ferroptosis and evasion strategies. Front. Mol. Biosci. 2023, 10, 1275774. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, M.; Li, Y.; Shen, M.; Kong, D.; Shao, J.; Ding, H.; Tan, S.; Chen, A.; Zhang, F.; et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 2020, 16, 1482–1505. [Google Scholar] [CrossRef] [PubMed]

| Gene Regulators | ||||||
|---|---|---|---|---|---|---|
| Name | Mechanism or Target | Driver or Suppressor | Study Type | Outcomes | Indication | References |
| GPX4 | Targeting lipid metabolism | Suppressor | KC mice | High-iron diets or Gpx4 depletion promoted 8-OHG release and thus activated the TMEM173/STING pathway and subsequent macrophage infiltration Kras-driven PDAC in mice | Pancreatic cancer | [38] |
| KRAS | Targeting lipid metabolism | Driver | KRASG12D mice | KRASG12D promoted macrophages to switch to M2-like phenotype via STAT3-dependent fatty acid oxidation | Pancreatic cancer | [35] |
| SLC7A11 | Targeting oxidative stress | Suppressor | KPC mice | The import of oxidized cysteine (cystine) via system xC− is a critical dependency of pancreatic cancer | Pancreatic cancer | [87] |
| GOT1 | Targeting iron homeostasis | Suppressor | PDAC cell lines | GOT1 inhibition represses mitochondrial metabolism and promotes a catabolic state | Pancreatic cancer | [88] |
| B3GNT3 | Targeting oxidative stress | Suppressor | PDAC cell lines | B3GNT3 catalyzes the glycosylation of 4F2hc, stabilizes the 4F2hc protein, and enhances the interaction between 4F2hc and xCT | Pancreatic cancer | [89] |
| PNO1 | Unclassified | Suppressor | PDAC cell lines | Knockdown of PNO1 promotes ferroptosis in PANC-1 cells | Pancreatic cancer | [90] |
| DCN | Unclassified | Unclassified | PDAC cell lines, HT1080, HeLa | DCN released by ferroptotic cells activates AGER-dependent tumor-protective immune response | Pancreatic cancer, etc. | [94] |
| HMGB1 | Unclassified | Unclassified | PDAC cell lines, HT1080 | HMGB1 released by ferroptotic cells activates AGER-dependent inflammation in macrophages | Pancreatic cancer, etc. | [95] |
| RBCK1 | Targeting oxidative stress | Suppressor | PDAC cell lines | RBCK1 promotes proteasome degradation of MFN2, resulting in reduced ROS production and lipid peroxidation | Pancreatic cancer | [145] |
| FOSL1 | Targeting oxidative stress | Driver | PDAC cell lines | KRAS/FOSL1/TFRC axis promoted the PDAC cells vulnerable to alteration of the iron level in the tumor microenvironment | Pancreatic cancer | [146] |
| OSGIN1 | Targeting oxidative stress | Suppressor | PDAC cell lines | OSGIN1 directly enhanced GCLM activity | Pancreatic cancer | [147] |
| TMOD3 | Targeting lipid metabolism | Driver | PDAC cell lines, BALB/c nude mice and C57BL/6 mice | TMOD3 facilitated autophagic degradation of ACSL4 | Pancreatic cancer | [148] |
| IL15 | Targeting lipid metabolism | Suppressor | PANC-1 and SW1990 cell lines, BALB/c nude mice | IL15 activates IL15RA-STAT3 axis to promote GPX4 and ACSL3 | Pancreatic cancer | [149] |
| circ_0005397 | Targeting oxidative stress | Suppressor | PDAC cell lines | Circ_0005397 promotes PCBP2 expression through KAT6 A and H3K9 ac | Pancreatic cancer | [150] |
| PRMT6 | Targeting oxidative stress | Suppressor | PDAC cell lines | PRMT6 mediates the asymmetric dimethylarginine modification of p62 to facilitate its phase separation, preventing Keap1 from activating Nrf2 signaling and inhibiting ferroptosis | Pancreatic cancer | [151] |
| ARID3A | Targeting lipid metabolism | Suppressor | PDAC cell lines | The inhibition of ARID3A attenuates transcriptional repression of PTEN, leading to GPX4 depletion and increased lipid peroxidation | Pancreatic cancer progress and chemosensitivity | [152] |
| FBW7 | Targeting lipid metabolism | Driver | PDAC cell lines | FBW7 inhibits the expression of SCD1 by inhibiting NR4A1 | Chemosensitivity | [113] |
| ELAVL1 | Targeting oxidative stress | Driver | Rat pancreatic exocrine cells | ELAVL1-dependent SOAT2 exacerbated pancreatic exocrine cell injury | Pancreatitis | [153] |
| ARNTL | Targeting oxidative stress | Suppressor | Acute pancreatitis model mice induced by l-arginine | ARNTL prevents experimental acute pancreatitis by blocking ferroptosis-mediated release of HMGB1 | Acute pancreatitis | [121] |
| SIRT4 | Targeting oxidative stress | Acute pancreatitis model mice induced by l-arginine | SIRT4 regulates the expression of ferroptosis-related proteins by mediating HIF-1α/HO-1 pathway | Acute pancreatitis | [126] | |
| SQSTM1 | Targeting lipid metabolism | Driver | Acute pancreatitis model mice induced by caerulein | Recombinant SQSTM1 protein increases the expression of AGER-dependent ACSL4 | Acute pancreatitis | [131] |
| C1QTNF5 | Targeting iron homeostasis | Driver | SAP mouse model through pancreatic duct ligation (PDL) | Myonectin promoted iron-accumulation-induced ferroptosis leading to acinar cell necrosis | Severe acute pancreatitis | [154] |
| Agents | ||||||
| Name | Mechanism or Target | Driver or Suppressor | Study type | Outcomes | Indication | References |
| Gemcitabine | Driver | PDAC cell lines | Gemcitabine time-dependently increased GPX4 protein expression and GPX4 activity | Chemosensitivity | [114] | |
| Zalcitabine | Targeting lipid metabolism | Driver | PDAC cell lines | Zalcitabine induced mitochondrial DNA stress and contributed to macroautophagy/autophagy-dependent ferroptotic cell death via lipid peroxidation | Pancreatic cancer | [103] |
| ZZW-115 | Targeting iron homeostasis | Suppressor | PDAC cell lines, knockout mice | ZZW-115 inhibited completely the translocation of NUPR1 from the cytoplasm to the nucleus by competing with importins | Pancreatic cancer | [104] |
| KL-6 | Unclassified | Unclassified | PDAC cell lines | KL-6 promoted lipid oxidation in a dose-dependent manner | Pancreatic cancer | [105] |
| Piperlongumine/Cotylenin A/Sulfasalazine | Unclassified | Unclassified | PDAC cell lines | Piperlongumine induced ROS production | Pancreatic cancer | [106] |
| Ruscogenin | Targeting iron homeostasis | Driver | PDAC cell lines | Ruscogenin increased intracellular ferrous concentration and ROS production | Pancreatic cancer | [107] |
| Artesunate | Targeting iron homeostasis | Driver | PDAC cell lines | ART promotes the lysosomal degradation of ferritin | Pancreatic cancer | [108] |
| DHA | Targeting oxidative stress | Driver | PDAC cell lines | Combination therapy with DHA and cisplatin increased mitochondrial-derived ROS accumulation | Chemosensitivity | [116] |
| Fatostatin | Targeting lipid metabolism | Driver | BxPC-3 and MIAPaCa-2 cell lines, BALB/c nude mice | Fatostatin inhibits SREBP1 transcription-mediated GPX4 upregulation | Pancreatic cancer | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, H.; Kong, J.; Chen, J.; Zhang, R.; Xiao, S.; Guo, D.; Zhang, Q.; Chen, X.-Z.; Tang, J.; Zhou, C. The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment. Int. J. Mol. Sci. 2024, 25, 13334. https://doi.org/10.3390/ijms252413334
Lyu H, Kong J, Chen J, Zhang R, Xiao S, Guo D, Zhang Q, Chen X-Z, Tang J, Zhou C. The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment. International Journal of Molecular Sciences. 2024; 25(24):13334. https://doi.org/10.3390/ijms252413334
Chicago/Turabian StyleLyu, Hao, Jinghua Kong, Jiasi Chen, Rui Zhang, Shuai Xiao, Dong Guo, Qi Zhang, Xing-Zhen Chen, Jingfeng Tang, and Cefan Zhou. 2024. "The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment" International Journal of Molecular Sciences 25, no. 24: 13334. https://doi.org/10.3390/ijms252413334
APA StyleLyu, H., Kong, J., Chen, J., Zhang, R., Xiao, S., Guo, D., Zhang, Q., Chen, X.-Z., Tang, J., & Zhou, C. (2024). The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment. International Journal of Molecular Sciences, 25(24), 13334. https://doi.org/10.3390/ijms252413334







