Systemic and Cardiac Microvascular Dysfunction in Hypertension
Abstract
1. Introduction
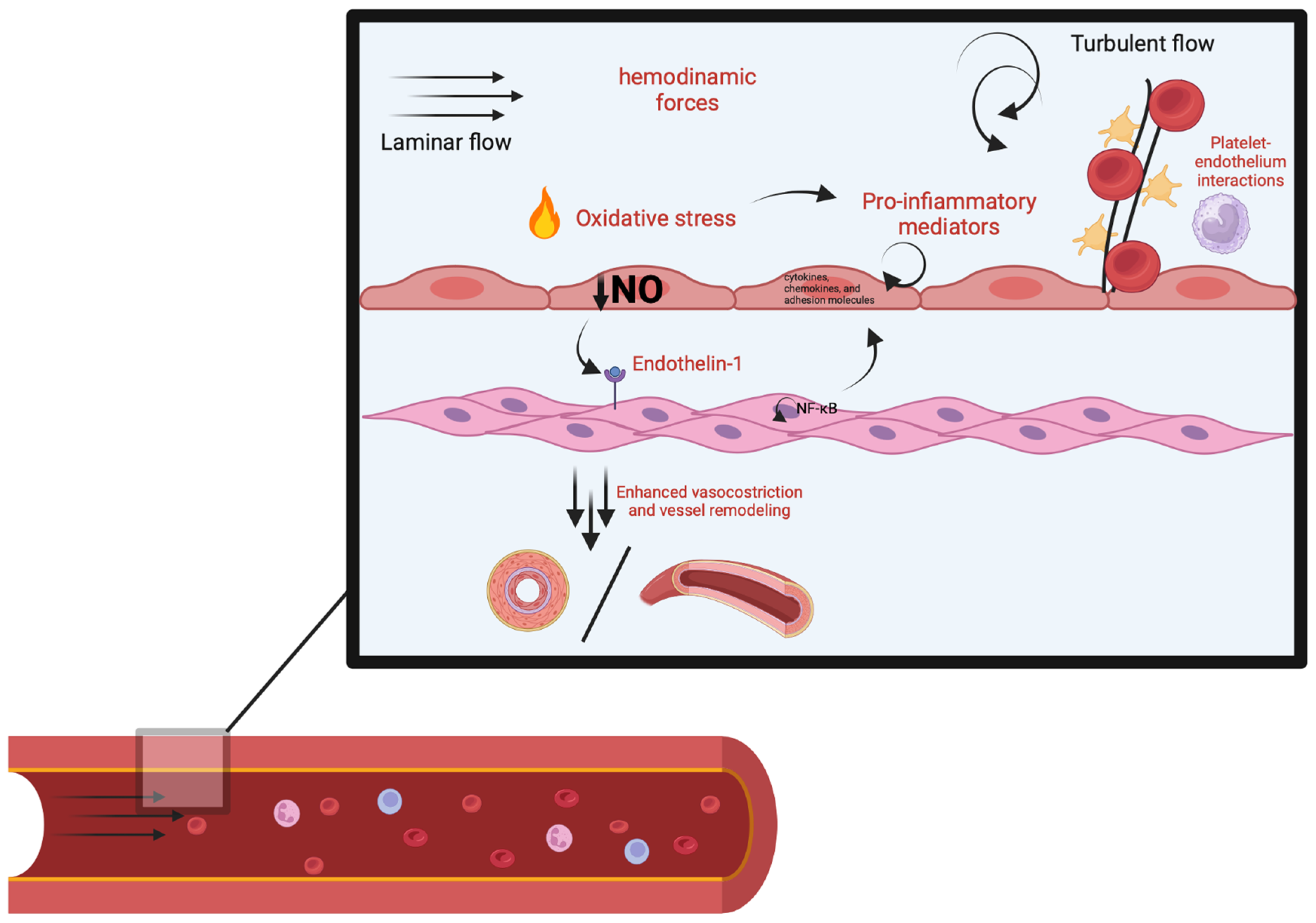
2. Vascular Changes
3. Molecular Mechanism
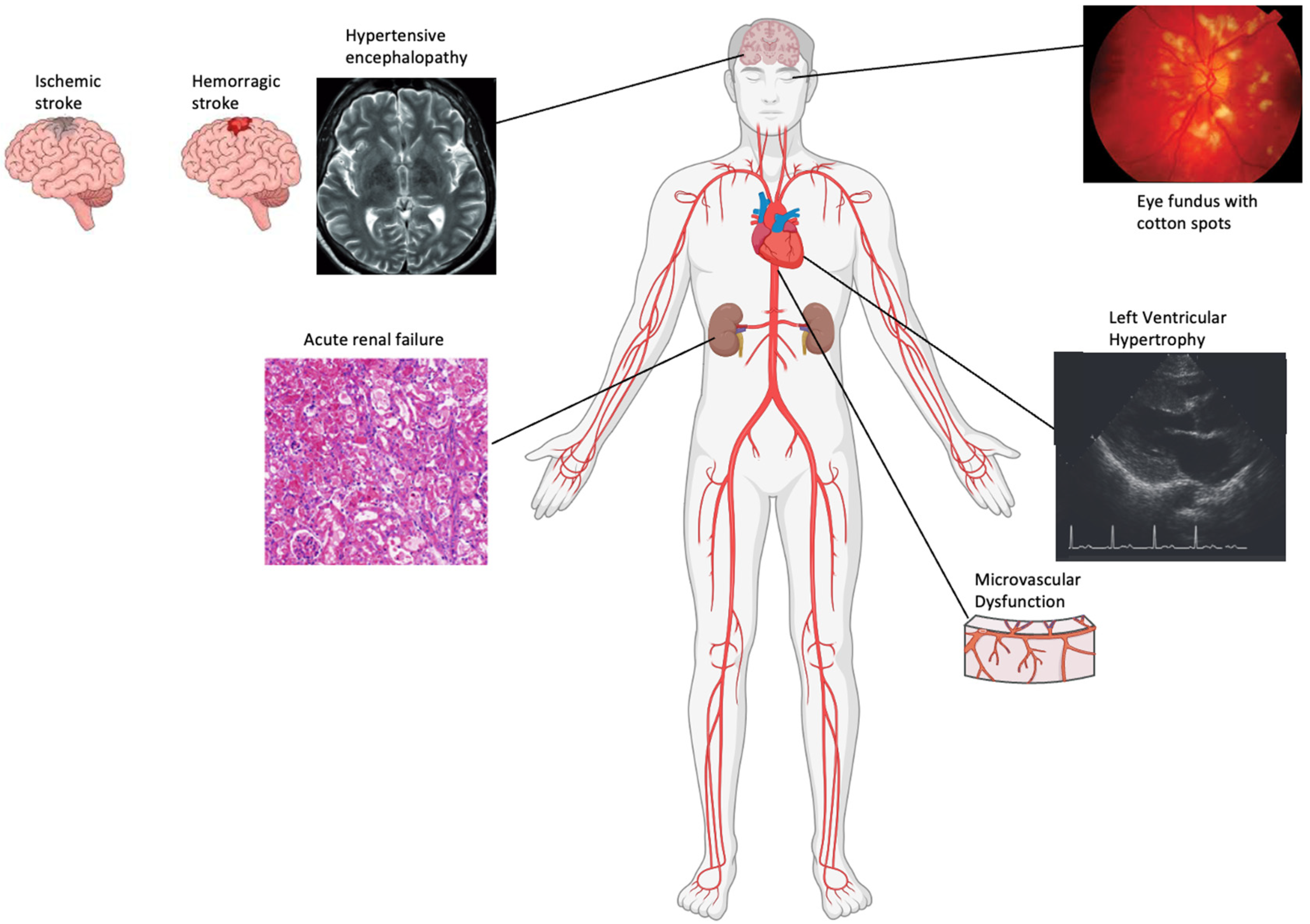
4. Methods to Evaluate Vascular Structural Alterations
4.1. Kidney
4.2. Brain
4.3. Retina
4.4. Heart
5. Conclusions
Funding
Conflicts of Interest
References
- Bateman, R.M.; Sharpe, M.D.; Ellis, C.G. Bench-to-bedside review: Microvascular dysfunction in sepsis—Hemodynamics, oxygen transport, and nitric oxide. Crit. Care. 2003, 7, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Bright. Cases and Observations Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. Med. Chir. Rev. 1836, 25, 23–25. [Google Scholar]
- Johnson, G. On certain points in the Anatomy and Pathology of Bright’s Disease of the Kidney. 2. On the Influence of the Minute Blood-vessels upon the Circulation. Med. Chir. Trans. 1868, 51, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Ewald, C.A. Ueber die Veränderungen kleiner Gefässe bei Morbus Brightii und die darauf bezüglichen Theorien. Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1877, 71, 453–499. [Google Scholar] [CrossRef]
- Folkow, B. Physiological aspects of primary hypertension. Physiol. Rev. 1982, 62, 347–504. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Aalkjaer, C. Structure and function of small arteries. Physiol. Rev. 1990, 70, 921–961. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Guelfi, D.; Muiesan, M.L.; Valentini, U.; Cimino, A.; Girelli, A.; Rodella, L.; Bianchi, R.; Sleiman, I.; et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 2001, 103, 1238–1244. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Porteri, E.; Rizzoni, D.; Corbellini, C.; La Boria, E.; Boari, G.E.M.; Pilu, A. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension 2011, 58, 29–36. [Google Scholar] [CrossRef]
- Osman, Y.; Imam, Y.Z.; Salem, K.; Al-Hail, H.; Uthman, B.; Deleu, D. Isolated Brainsteam involvement in a patient with Hypertensive encephalopathy. Case Rep. Neurol. Med. 2013, 2013, 540947. [Google Scholar]
- Naved, B.A.; Bonventre, J.V.; Hubbell, J.A.; Hukriede, N.A.; Humphreys, B.D.; Kesselman, C.; Valerius, M.T.; McMahon, A.P.; Shankland, S.J.; Wertheim, J.A.; et al. Kidney repair regeneration: Perspectives of the NIDDK (Re)Building a Kidney consortium. Kidney Int. 2022, 101, 845–853. [Google Scholar] [CrossRef]
- Seitun, S.; Massobrio, L.; Rubegni, A.; Nesti, C.; Morelli, M.C.; Boccalini, S.; Pregliasco, A.G.; Budaj, I.; Deferrari, L.; Rosa, G.M.; et al. MELAS Syndrome with Cardiac Involvement: A Multimodality Imaging Approach. Case Rep. Cardiol. 2016, 2016, 1490181. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.G. Cotton-Wool Spots May Challenge Diagnosis. Rev. Ophthalmol. 2004. Available online: https://www.reviewofophthalmology.com/article/cotton-wool-spots-may-challenge-diagnosis (accessed on 21 November 2024).
- Christensen, K.L.; Mulvany, M.J. Location of resistance arteries. J. Vasc. Res. 2001, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.I.; Ambrosio, G.; Pries, A.R.; Struijker-Boudier, H.A.J. Microcirculation in hypertension: A new target for treatment? Circulation 2001, 104, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J. Myogenic response gradient in an arteriolar network. Am. J. Physiol. Circ. Physiol. 1993, 264 Pt 2, H2168–H2179. [Google Scholar] [CrossRef]
- Mulvany, M.J. Small artery structure: Time to take note? Am. J. Hypertens. 2007, 20, 853–854. [Google Scholar] [CrossRef][Green Version]
- Simon, G. Pathogenesis of structural vascular changes in hypertension. J. Hypertens. 2004, 22, 3–10. [Google Scholar] [CrossRef]
- Mulvany, M.J. Small artery remodeling in hypertension. Curr. Hypertens. Rep. 2002, 4, 49–55. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J. Endothelial Dysfunction and Vascular Remodeling in Hypertension. In Pediatric Hypertension; Flynn, J.T., Ingelfinger, J.R., Brady, T.M., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rodríguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Pedrinelli, R.; Spessot, M.; Salvetti, A. Reactive hyperemia during short-term blood flow and pressure changes in the hypertensive forearm. J. Hypertens. 1990, 8, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, M.; Davidsson, S.; Hjemdahl, P.; Melcher, A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiol. Scand. 1996, 158, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Pu, Q.; De Ciuceis, C.; Savoia, C.; Virdis, A.; Neves, M.F.; Touyz, R.M.; Schiffrin, E.L. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension 2004, 43, 399–404. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Deng, L.Y. Structure and function of resistance arteries of hypertensive patients treated with a beta-blocker or a calcium channel antagonist. J. Hypertens. 1996, 14, 1247–1255. [Google Scholar] [CrossRef]
- Boari, G.E.M.; Rizzardi, N.; de Ciuceis, C.; Platto, C.; Paiardi, S.; Porteri, E.; Paini, A.; Salvetti, M.; Muiesan, M.L.; Rizzoni, D.; et al. Determinants of the structure of resistance-sized arteries in hypertensive patients. Blood Press. 2008, 17, 204–211. [Google Scholar] [CrossRef]
- Bonacci, E.; Santacroce, N.; D’Amico, N.; Mattace, R. Nail-fold capillaroscopy in the study of microcirculation in elderly hypertensive patients. Arch. Gerontol. Geriatr. 1996, 22 (Suppl. 1), 79–83. [Google Scholar] [CrossRef]
- Harper, R.N.; Moore, M.A.; Marr, M.C.; Watts, L.E.; Hutchins, P.M. Arteriolar rarefaction in the conjunctiva of human essential hypertensives. Microvasc. Res. 1978, 16, 369–372. [Google Scholar] [CrossRef]
- Shore, A.C. Capillaroscopy and the measurement of capillary pressure. Br. J. Clin. Pharmacol. 2000, 50, 501–513. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Rossini, C.; Porteri, E.; La Boria, E.; Corbellini, C.; Mittempergher, F.; Betta, E.D.; Petroboni, B.; Sarkar, A.; Agabiti-Rosei, C.; et al. Circulating endothelial progenitor cells, microvascular density and fibrosis in obesity before and after bariatric surgery. Blood Press. 2013, 22, 165–172. [Google Scholar] [CrossRef]
- Kara, A.; Akin, S.; dos Reis Miranda, D.; Struijs, A.; Caliskan, K.; van Thiel, R.J.; Dubois, E.A.; de Wilde, W.; Zijlstra, F.; Gommers, D.; et al. Microcirculatory assessment of patients under VA-ECMO. Crit. Care 2016, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Massey, M.J.; Hou, P.C.; Filbin, M.; Wang, H.; Ngo, L.; Huang, D.T.; Aird, W.C.; Novack, V.; Trzeciak, S.; Yealy, D.M.; et al. Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit. Care 2018, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.A.; Camici, P.G. Myocardial Blood Flow Measurement by PET: Technical Aspects and Clinical Applications. J. Nucl. Med. 2005, 46, 75–88. [Google Scholar] [PubMed]
- Hozumi, T.; Yoshida, K.; Ogata, Y.; Akasaka, T.; Asami, Y.; Takagi, T.; Morioka, S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 1998, 97, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Marble, S.J.; Barron, H.V.; Braunwald, E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002, 105, 1909–1913. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Ryan, K.A.; Mesley, R.; Marble, S.J.; McCabe, C.H.; Van de Werf, F.; Braunwald, E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000, 101, 125–130. [Google Scholar] [CrossRef]
- Agati, L.; Tonti, G.; Galiuto, L.; Di Bello, V.; Funaro, S.; Madonna, M.P.; Garramone, B.; Magri, F. Quantification methods in contrast echocardiography. Eur. J. Echocardiogr. 2005, 6 (Suppl. 2), S14–S20. [Google Scholar] [CrossRef]
- Jerosch-Herold, M.; Wilke, N.; Stillman, A.E.; Wilson, R.F. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med. Phys. 1998, 25, 73–84. [Google Scholar] [CrossRef]
- Durante, A.; Laricchia, A.; Benedetti, G.; Esposito, A.; Margonato, A.; Rimoldi, O.; De Cobelli, F.; Colombo, A.; Camici, P.G. Identification of High-Risk Patients After ST-Segment-Elevation Myocardial Infarction: Comparison Between Angiographic and Magnetic Resonance Parameters. Circ. Cardiovasc. Imaging 2017, 10, e005841. [Google Scholar] [CrossRef]
- Ruilope, L.M. Renal Damage in Hypertension. Eur. J. Cardiovasc. Prev. Rehabil. 1995, 2, 40–44. [Google Scholar] [CrossRef]
- Ito, S. Cardiorenal syndrome: An evolutionary point of view. Hypertension 2012, 60, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.A. Hypertensive vascular disease; description and natural history. J. Chronic Dis. 1955, 1, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.T.; Bak, A.A.A.; Diercks, G.F.H.; Grobbee, D.E.; Crijns, H.J.G.M.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E.; Prevend Study Group. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Bakris, G.L. Renal function and target organ damage in hypertension. Eur. Heart J. 2011, 32, 1599–1604. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nangaku, M. Endothelial Dysfunction: The Secret Agent Driving Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 3. [Google Scholar] [CrossRef]
- Prewitt, R.L.; Chen, I.I.H.; Dowell, R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 1982, 243, H243–H251. [Google Scholar] [CrossRef]
- Serné, E.H.; Gans, R.O.B.; Ter Maaten, J.C.; Tangelder, G.J.; Donker, A.J.M.; Stehouwer, C.D.A. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 2001, 38, 238–242. [Google Scholar] [CrossRef]
- Querfeld, U.; Mak, R.H.; Pries, A.R. Microvascular disease in chronic kidney disease: The base of the iceberg in cardiovascular comorbidity. Clin. Sci. 2020, 134, 1333–1356. [Google Scholar] [CrossRef]
- Virdis, A.; Savoia, C.; Grassi, G.; Lembo, G.; Vecchione, C.; Seravalle, G.; Taddei, S.; Volpe, M.; Rosei, E.A.; Rizzoni, D. Evaluation of microvascular structure in humans: A “state-of-the-art” document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J. Hypertens. 2014, 32, 2120–2129. [Google Scholar] [CrossRef]
- Kelsen, S.; Hall, J.E.; Chade, A.R. Endothelin—A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am. J. Physiol. Renal Physiol. 2011, 301, F218–F225. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; de Miguel, L.S.; Petrimpol, M.; Humar, R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr. Opin. Pharmacol. 2007, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chatziantoniou, C.; Boffa, J.J.; Tharaux, P.L.; Flamant, M.; Ronco, P.; Dussaule, J.C. Progression and regression in renal vascular and glomerular fibrosis. Int. J. Exp. Pathol. 2004, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]
- Webb, A.J.S.; Werring, D.J. New Insights into Cerebrovascular Pathophysiology and Hypertension. Stroke 2022, 53, 1054–1064. [Google Scholar] [CrossRef]
- Iadecola, C.; Gottesman, R.F. Neurovascular and Cognitive Dysfunction in Hypertension. Circ. Res. 2019, 124, 1025–1044. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Harrison, D.G.; Figueroa, C.A.; Lacolley, P.; Laurent, S. Central Artery Stiffness in Hypertension and Aging: A Problem with Cause and Consequence. Circ. Res. 2016, 118, 379–381. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Mechanisms of remodelling of small arteries, antihypertensive therapy and the immune system in hypertension. Clin. Investig. Med. 2015, 38, E394–E402. [Google Scholar] [CrossRef]
- Naessens, D.M.P.; de Vos, J.; Richard, E.; Wilhelmus, M.M.M.; Jongenelen, C.A.M.; Scholl, E.R.; van der Wel, N.N.; Heijst, J.A.; Teunissen, C.E.; Strijkers, G.J.; et al. Effect of long-term antihypertensive treatment on cerebrovascular structure and function in hypertensive rats. Sci. Rep. 2023, 13, 3481. [Google Scholar] [CrossRef]
- Chan, S.L.; Baumbach, G.L. Deficiency of Nox2 prevents angiotensin II-induced inward remodeling in cerebral arterioles. Front. Physiol. 2013, 4, 51179. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Lammie, G.A. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002, 12, 358–370. [Google Scholar] [PubMed]
- Ter Telgte, A.; Van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; De Leeuw, F.E. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.; Gao, F.Q.; Noor, R.; Kiss, A.; Balasubramaniam, G.; Au, K.; Rogaeva, E.; Masellis, M.; Black, S.E. Collagenosis of the Deep Medullary Veins: An Underrecognized Pathologic Correlate of White Matter Hyperintensities and Periventricular Infarction? J. Neuropathol. Exp. Neurol. 2017, 76, 299–312. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kirkpatrick, A.C.; Csiszar, A.; Prodan, C.I. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef]
- Poels, M.M.F.; Ikram, M.A.; Van Der Lugt, A.; Hofman, A.; Niessen, W.J.; Krestin, G.P.; Breteler, M.M.; Vernooij, M.W. Cerebral microbleeds are associated with worse cognitive function: The Rotterdam Scan Study. Neurology 2012, 78, 326–333. [Google Scholar] [CrossRef]
- Reboussin, D.M.; Pajewski, N.M.; Williamson, J.D. Analysis of Long-term Benefits of Intensive Blood Pressure Control-Reply. JAMA 2019, 322, 170. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Carare, R.O.; Aldea, R.; Agarwal, N.; Bacskai, B.J.; Bechman, I.; Boche, D.; Bu, G.; Bulters, D.; Clemens, A.; Counts, S.E.; et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group-part of Vascular Professional Interest Area (PIA): Cerebrovascular disease and the failure of elimination of Amyloid-β from the brain and retina with age and Alzheimer’s disease-Opportunities for Therapy. Alzheimers Dement. 2020, 12, e12053. [Google Scholar]
- Mortensen, K.N.; Sanggaard, S.; Mestre, H.; Lee, H.; Kostrikov, S.; Xavier, A.L.R.; Gjedde, A.; Benveniste, H.; Nedergaard, M. Impaired Glymphatic Transport in Spontaneously Hypertensive Rats. J. Neurosci. 2019, 39, 6365–6377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Ye, Y.; Wang, S.; Jiaerken, Y.; Hong, H.; Li, K.; Zeng, Q.; Luo, X.; Xu, X.; et al. The Influence of Demographics and Vascular Risk Factors on Glymphatic Function Measured by Diffusion Along Perivascular Space. Front. Aging Neurosci. 2021, 13, 693787. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Dupuis, F.; Atkinson, J.; Limiñana, P.; Chillon, J.M. Captopril improves cerebrovascular structure and function in old hypertensive rats. Br. J. Pharmacol. 2005, 144, 349–356. [Google Scholar] [CrossRef]
- Strandgaard, S. Autoregulation of cerebral blood flow in hypertensive patients: The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 1976, 53, 720–727. [Google Scholar] [CrossRef]
- Pearson, A.C.S.; Subramanian, A.; Schroeder, D.R.; Findlay, J.Y. Effect of Antihypertensive Treatment on Cerebral Blood Flow in Older Adults: A Systematic Review and Meta-Analysis. Hypertension 2022, 79, 1067–1078. [Google Scholar]
- Sforza, M.; Bianchini, E.; Alivernini, D.; Salvetti, M.; Pontieri, F.E.; Sette, G. The impact of cerebral vasomotor reactivity on cerebrovascular diseases and cognitive impairment. J. Neural Transm. 2022, 129, 1321–1330. [Google Scholar] [CrossRef]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Delles, C.; Michelson, G.; Harazny, J.; Oehmer, S.; Hilgers, K.F.; Schmieder, R.E. Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 2004, 35, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Hoth, K.F.; Tate, D.F.; Poppas, A.; Forman, D.E.; Gunstad, J.; Moser, D.J.; Paul, R.H.; Jefferson, A.L.; Haley, A.P.; Cohen, R.A. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke 2007, 38, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Nezu, T.; Hosomi, N.; Aoki, S.; Kubo, S.; Araki, M.; Mukai, T.; Takahashi, T.; Maruyama, H.; Higashi, Y.; Matsumoto, M. Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertens. Res. 2015, 38, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Brandner, D.D.; Le, P.; McNeal, D.W.; Gong, X.; Dou, H.; Fulton, D.J.; Beller, A.; Ngyuen, T.; Larson, E.B.; et al. Vasodilator Dysfunction and Oligodendrocyte Dysmaturation in Aging White Matter. Ann. Neurol. 2018, 83, 142. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Mitchell, P. The eye in hypertension. Lancet 2007, 369, 425–435. [Google Scholar] [CrossRef]
- Hayreh, S.S. Hypertensive retinopathy. Ophthalmologica 2002, 216, 232–240. [Google Scholar]
- Wilkinson, C.P.; Ferris, F.L.I.I.I.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, T.Y. Diabetic retinopathy and systemic vascular complications. Prog. Retin. Eye Res. 2008, 27, 161–176. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Crea, F. Primary coronary microvascular dysfunction: Clinical presentation, pathophysiology, and management. Circulation 2010, 121, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Brush, J.E.; Cannon, R.O.; Schenke, W.H.; Bonow, R.O.; Leon, M.B.; Maron, B.J.; Epstein, S.E. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N. Engl. J. Med. 1988, 319, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Rame, J.E.; Khera, A.; Murphy, S.A.; Canham, R.M.; Peshock, R.M.; de Lemos, J.A.; Drazner, M.H. Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation. Hypertension 2007, 49, 1385–1391. [Google Scholar] [CrossRef]
- Drazner, M.H. The progression of hypertensive heart disease. Circulation 2011, 123, 327–334. [Google Scholar] [CrossRef]
- Rubin, S.; Cremer, A.; Boulestreau, R.; Rigothier, C.; Kuntz, S.; Gosse, P. Malignant hypertension: Diagnosis, treatment and prognosis with experience from the Bordeaux cohort. J. Hypertens. 2019, 37, 316–324. [Google Scholar] [CrossRef]
- Gosse, P.; Coulon, P.; Papaioannou, G.; Litalien, J.; Lemetayer, P. Impact of malignant arterial hypertension on the heart. J. Hypertens. 2011, 29, 798–802. [Google Scholar] [CrossRef]
- Prejbisz, A.; Klisiewicz, A.; Januszewicz, A.; Lenders, J.W.; Pręgowska-Chwała, B.; Jóźwik-Plebanek, K.; Michałowska, I.; Januszewicz, M.; Andziak, P.; Hoffman, P.; et al. 22-Year-old patient with malignant hypertension associated with primary aldosteronism. J. Hum. Hypertens. 2013, 27, 138–140. [Google Scholar] [CrossRef]
- Misumi, I.; Fukushima, M.; Wada, K.; Urata, J.; Sato, K.; Nagano, M.; Tsujita, K. A case of malignant hypertension with multi-organ injury. Radiol. Case Rep. 2022, 17, 455–461. [Google Scholar] [CrossRef]
- Shantsila, A.; Dwivedi, G.; Shantsila, E.; Butt, M.; Beevers, D.G.; Lip, G.Y. A comprehensive assessment of cardiac structure and function in patients with treated malignant phase hypertension: The West Birmingham Malignant Hypertension project. Int. J. Cardiol. 2013, 167, 67–72. [Google Scholar] [CrossRef]
- Drazner, M.H. The transition from hypertrophy to failure: How certain are we? Circulation 2005, 112, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.D.; Edvardsen, T.; Lai, S.; Castillo, E.; Pan, L.; Jerosch-Herold, M.; Sinha, S.; Kronmal, R.; Arnett, D.; Crouse, J.R., III; et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: The Multi-Ethnic Study of Atherosclerosis. Circulation 2005, 112, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, K.; Yamanaga, K.; Komura, N.; Sakamoto, K.; Miyazaki, T.; Ishii, M.; Tabata, N.; Akasaka, T.; Sueta, D.; Arima, Y.; et al. Impact of left ventricular hypertrophy on impaired coronary microvascular dysfunction. Int. J. Cardiol. 2015, 187, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Brown, J.M.; Bajaj, N.S.; Chandra, A.; Divakaran, S.; Weber, B.; Bibbo, C.F.; Hainer, J.; Taqueti, V.R.; Dorbala, S.; et al. Hypertensive coronary microvascular dysfunction: A subclinical marker of end organ damage and heart failure. Eur. Heart J. 2020, 41, 2366–2375. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Yang, E.H.; Rihal, C.S.; Prasad, A.; Lennon, R.J.; Best, P.J.; Lerman, L.O.; Lerman, A. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur. Heart J. 2010, 31, 936–942. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Cristell, N.; Cianflone, D.; Durante, A.; Ammirati, E.; Vanuzzo, D.; Banfi, M.; Calori, G.; Latib, A.; Crea, F.; Marenzi, G.; et al. High-sensitivity C-reactive protein is within normal levels at the very onset of first ST-segment elevation acute myocardial infarction in 41% of cases: A multiethnic case-control study. J. Am. Coll. Cardiol. 2011, 58, 2654–2661. [Google Scholar] [CrossRef]
- Twine, C.P. The Relationship Between CRP and MACE: Controversial and Confounded. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 234. [Google Scholar] [CrossRef]
| Aspect | Key Characteristics | Pathophysiological Consequences |
|---|---|---|
| Oxidative Stress |
|
|
| Endothelial Dysfunction |
|
|
| Inflammatory Response |
|
|
| Fibrosis and ECM Remodeling |
|
|
| VSMC Dysfunction |
|
|
| Angiotensin II Activation |
|
|
| Rarefaction |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante, A.; Mazzapicchi, A.; Baiardo Redaelli, M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int. J. Mol. Sci. 2024, 25, 13294. https://doi.org/10.3390/ijms252413294
Durante A, Mazzapicchi A, Baiardo Redaelli M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. International Journal of Molecular Sciences. 2024; 25(24):13294. https://doi.org/10.3390/ijms252413294
Chicago/Turabian StyleDurante, Alessandro, Alessandro Mazzapicchi, and Martina Baiardo Redaelli. 2024. "Systemic and Cardiac Microvascular Dysfunction in Hypertension" International Journal of Molecular Sciences 25, no. 24: 13294. https://doi.org/10.3390/ijms252413294
APA StyleDurante, A., Mazzapicchi, A., & Baiardo Redaelli, M. (2024). Systemic and Cardiac Microvascular Dysfunction in Hypertension. International Journal of Molecular Sciences, 25(24), 13294. https://doi.org/10.3390/ijms252413294








