Synthesis and hLDHA Inhibitory Activity of New Stiripentol-Related Compounds of Potential Use in Primary Hyperoxaluria
Abstract
1. Introduction
2. Results and Discussion
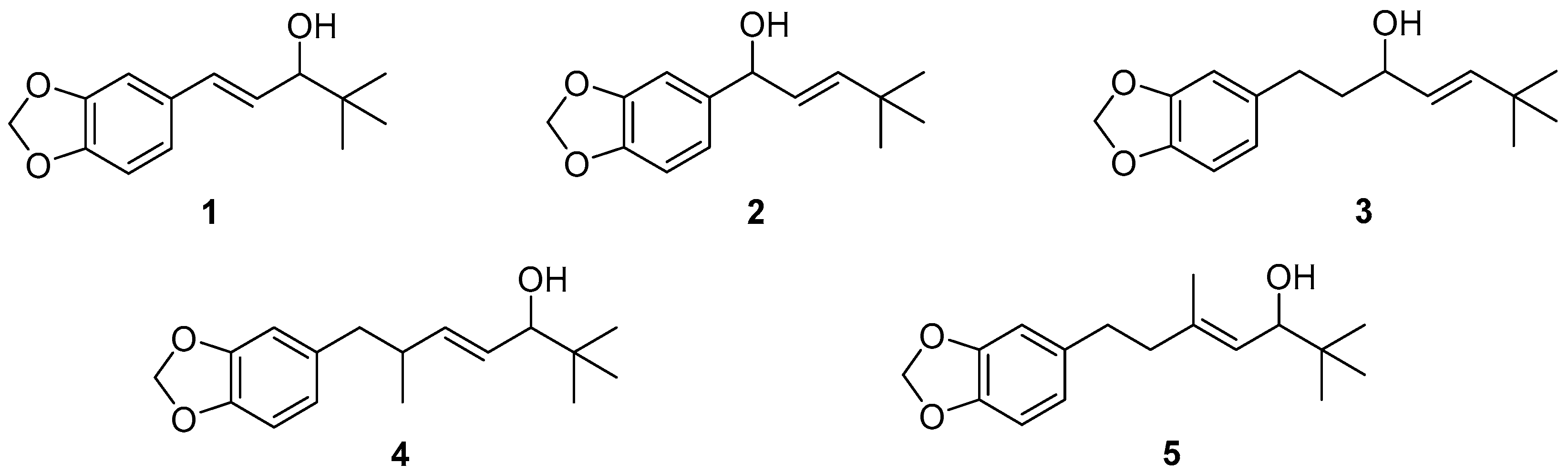
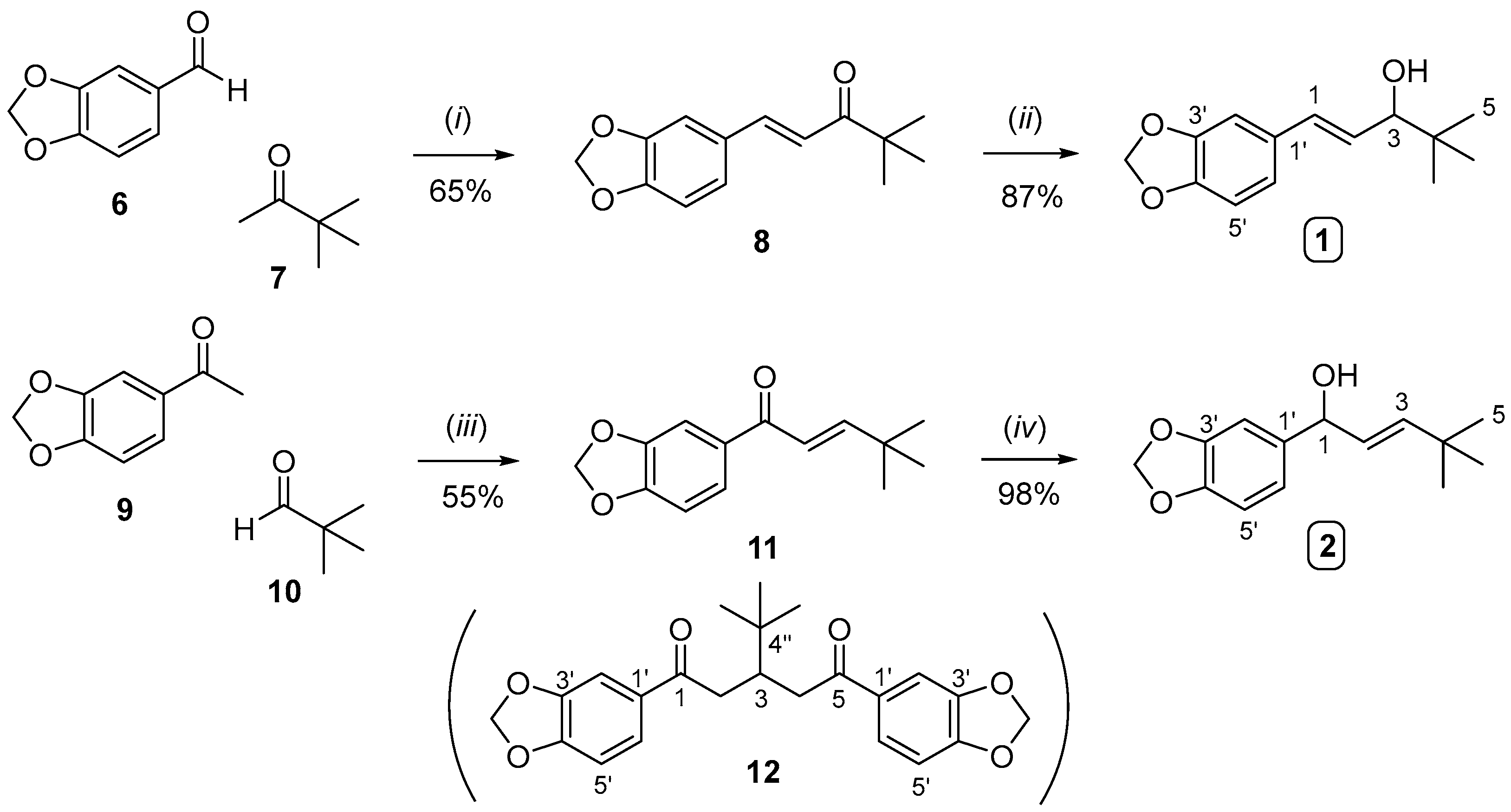
2.1. Synthesis of Stiripentol (STP, 1) and Iso-Stiripentol (Iso-STP, 2)
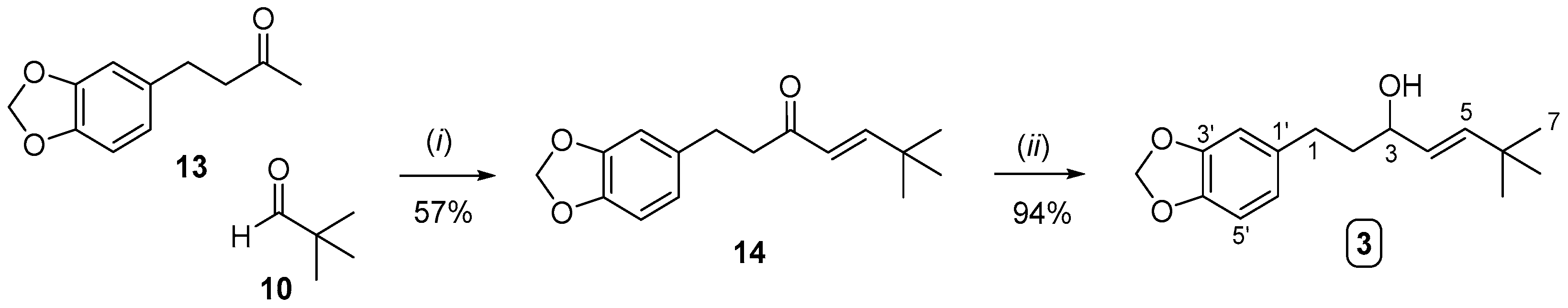
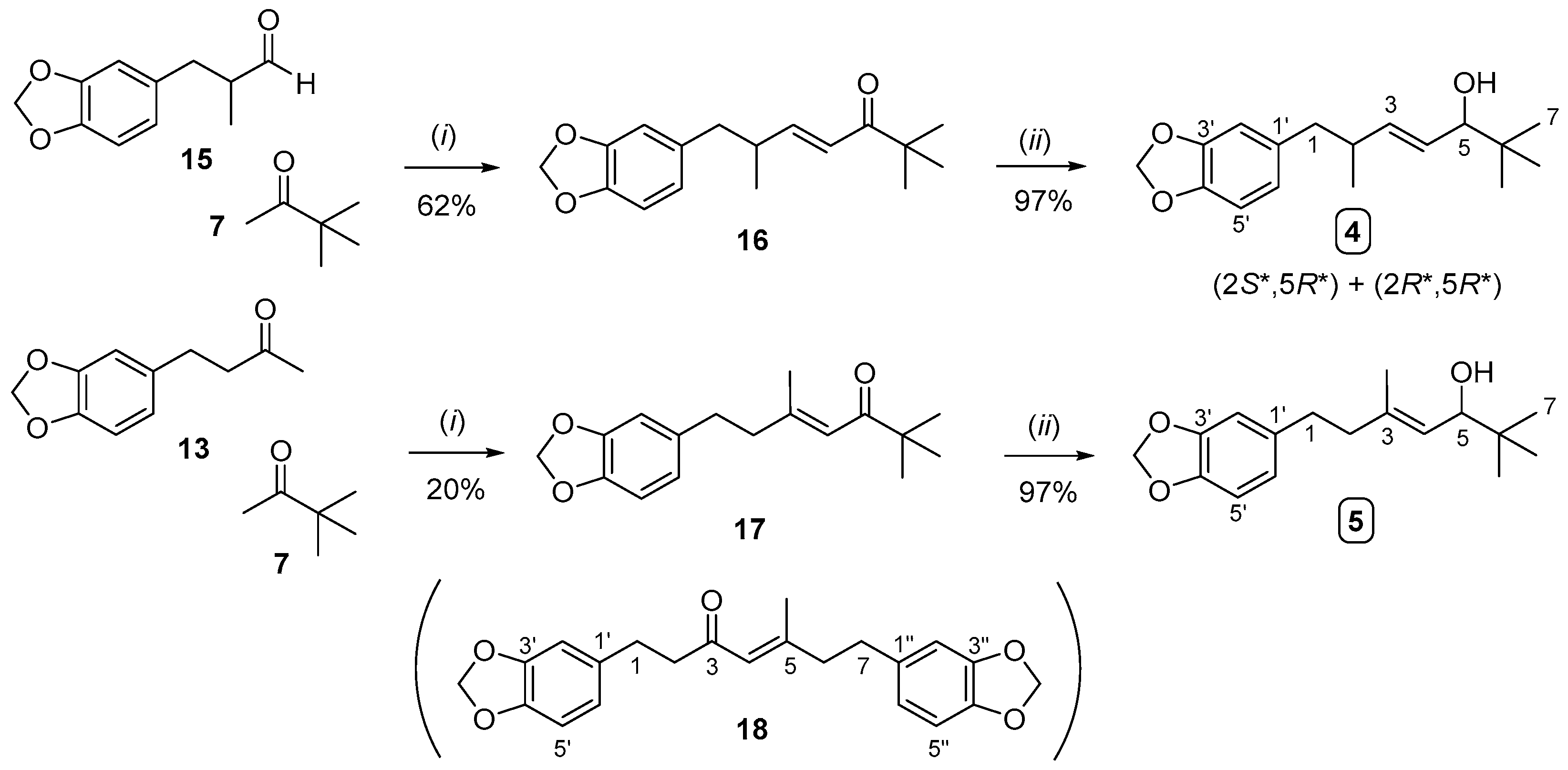
2.2. Synthesis of Compound 3 (Analogue to Iso-STP) and Compounds 4 and 5 (Analogues to STP)
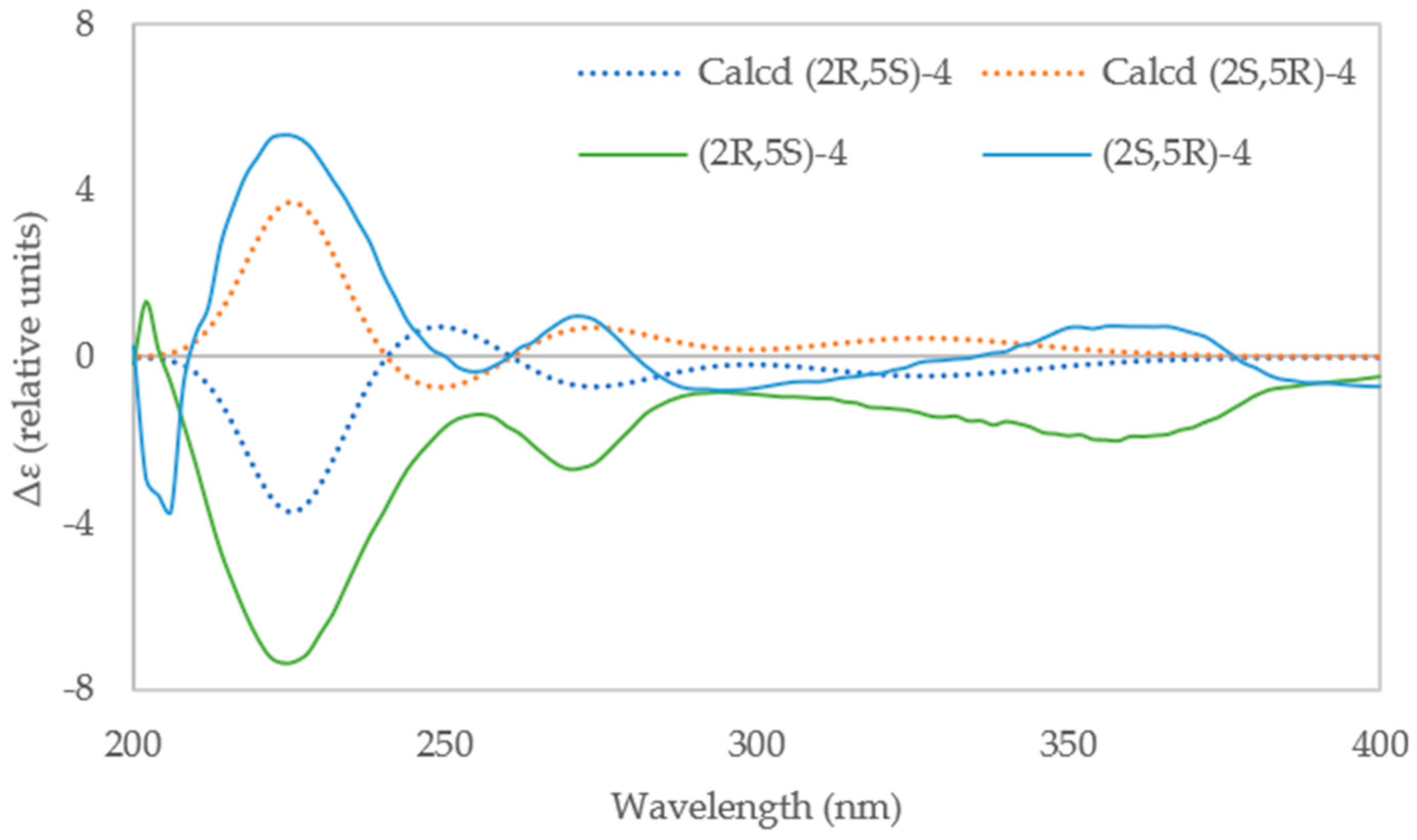
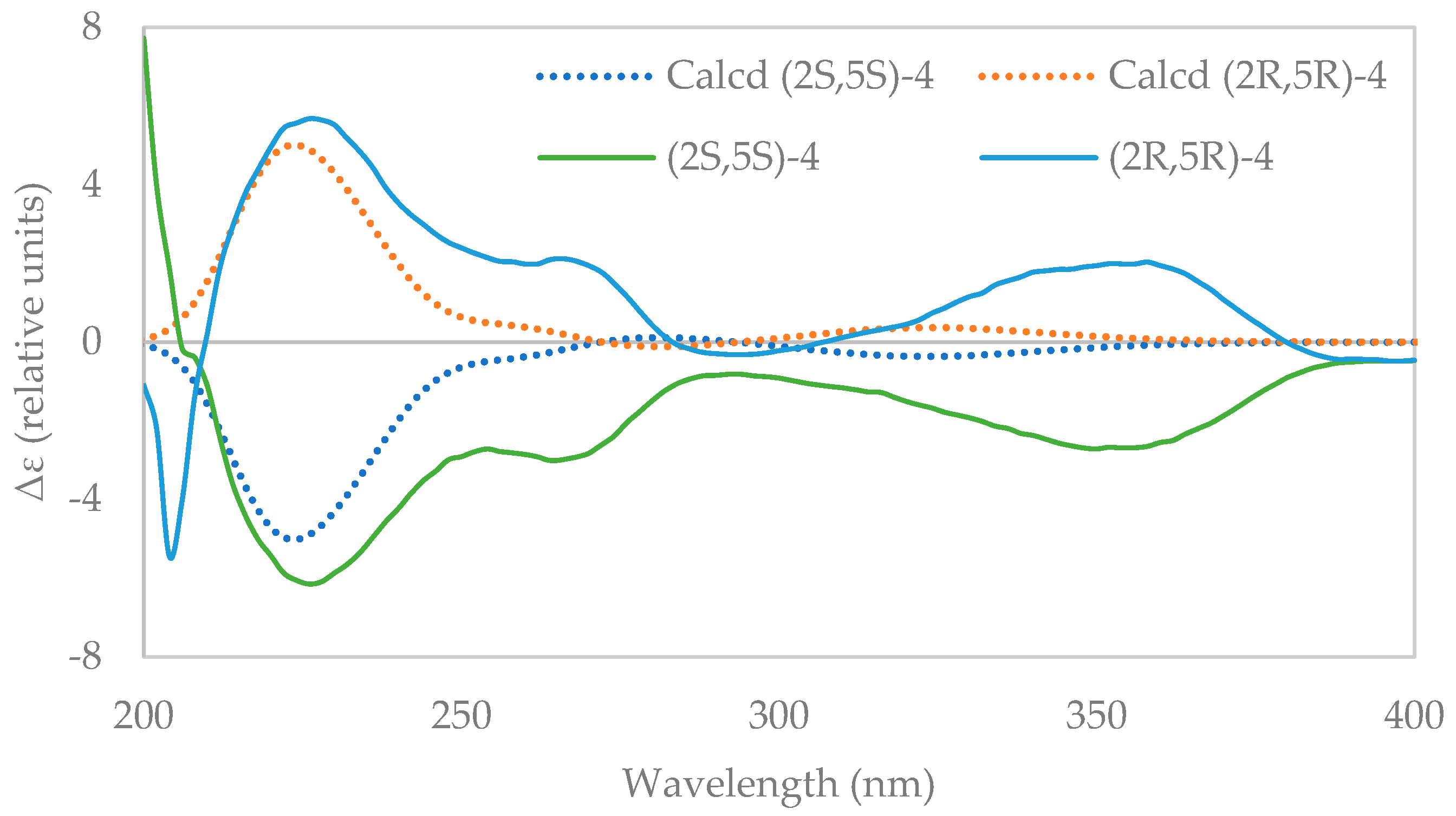
2.3. Resolution of Racemates 1, 3, and 4 into Their Enantiomers and Determination of Absolute Configuration
2.4. Inhibitory Activity of Racemates 1–5 and Selected Enantiomerically Pure Compounds Against hLDHA
3. Materials and Methods
3.1. General Experimental Methods
3.2. Synthesis of Stiripentol (1)
3.2.1. Synthesis of Compound 8
3.2.2. Synthesis of Stiripentol (1) from Compound 8
3.3. Synthesis of Iso-Stiripentol (2)
3.3.1. Synthesis of Compound 11
3.3.2. Synthesis of Iso-Stiripentol (2) from Compound 11
3.4. Synthesis of Compound 3
3.4.1. Synthesis of Compound 14
3.4.2. Synthesis of Compound 3 from Compound 14
3.5. Synthesis of Compound 4
3.5.1. Synthesis of Compound 16
3.5.2. Synthesis of Compound 4 from Compound 16
3.6. Synthesis of Compound 5
3.6.1. Synthesis of Compound 17
3.6.2. Synthesis of Compound 5 from Compound 17
3.7. Resolution of Racemates and Optical Rotatory Power Analysis
3.8. Esterification of (−)-3 with (−)-(2R)- and (+)-(2S)-MTPA Chloride (Mosher’s Method)
3.9. Computational Details of ECD Calculation
3.10. Human Lactate Dehydrogenase a Enzymatic Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, D.; Zhang, P.; Chen, Y.; Liu, X.; Chen, Y. Efficacy of pharmacological treatments for Dravet syndrome: Systematic review and network meta-analysis. Seizure 2024, 117, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Stiripentol: A review in Dravet syndrome. Drugs 2019, 79, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Dravet, C. Les epilepsies graves de l’enfant. Vie Méd. 1978, 8, 543–548. [Google Scholar]
- Fan, H.-C.; Yang, M.-T.; Lin, L.-C.; Chiang, K.-L.; Chen, C.-M. Clinical and genetic features of Dravet syndrome: A prime example of the role of precision medicine in genetic epilepsy. Int. J. Mol. Sci. 2024, 25, 31. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Zhao, X.; Li, B. Dravet syndrome: Advances in etiology, clinical presentation, and treatment. Epilepsy Res. 2022, 188, 107041. [Google Scholar] [CrossRef]
- Vallet, F.M.J. Anxiolytic and Muscle Relaxant 4,4-Dimethyl-1-(3,4-Methylenedioxyphenyl)-1-Pentenes. Patent FR2173691 28 February 1972. [Google Scholar]
- Gao, C.; Pielas, M.; Jiao, F.; Mei, D.; Wang, X.; Kotulska, K.; Jozwiak, S. Epilepsy in Dravet syndrome: Current and future therapeutic opportunities. J. Clin. Med. 2023, 12, 2532. [Google Scholar] [CrossRef]
- Bacq, A.; Depaulis, A.; Castagne, V.; Le Guern, M.-E.; Wirrell, E.C.; Verleye, M. An update on stiripentol mechanisms of action: A narrative review. Adv. Ther. 2024, 41, 1351–1371. [Google Scholar] [CrossRef]
- Cellini, B. A molecular journey on the pathogenesis of primary hyperoxaluria. Curr. Opin. Nephrol. Hypertens. 2024, 33, 398–404. [Google Scholar] [CrossRef]
- Shee, K.; Stoller, M.L. Perspectives in primary hyperoxaluria—Historical, current and future clinical interventions. Nat. Rev. Urol. 2022, 19, 137–146. [Google Scholar] [CrossRef]
- Bacchetta, J.; Lieske, J.C. Primary hyperoxaluria type 1: Novel therapies at a glance. Clin. Kidney J. 2022, 15, i17–i22. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Deepthi, B.; Kamath, N.; Iyengar, A.; Thomas, C.C.; Uthup, S.; Saha, A.; Mathew, G.; Agarwal, I.; Tiewsoh, K.; et al. Clinical characteristics, genetic profile and short-term outcomes of children with primary hyperoxaluria type 2: A nationwide experience. Pediatr. Nephrol. 2024, 39, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; Raza, A.; Aziz, T.; Khaliq, S. HOGA1 gene pathogenic variants in primary hyperoxaluria type III: Spectrum of pathogenic sequence variants, and phenotypic association. Hum. Mutat. 2022, 43, 1757–1779. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Harvey, E.; Milliner, D.S.; Frishberg, Y.; Sas, D.J.; Calle, J.; Copelovitch, L.; Penniston, K.L.; Saland, J.; Somers, M.J.G.; et al. Diagnosis and management of primary hyperoxalurias: Best practices. Pediatr. Nephrol. 2024, 39, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Fargue, S.; Bourdain, C.A. Primary hyperoxaluria type 1: Pathophysiology and genetics. Clin. Kidney J. 2022, 15, i4–i8. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Lumasiran: A review in primary hyperoxaluria type 1. Drugs 2024, 84, 219–226. [Google Scholar] [CrossRef]
- Gang, X.; Liu, F.; Mao, J. Lumasiran for primary hyperoxaluria type 1: What we have learned? Front. Pediatr. 2022, 10, 1052625. [Google Scholar] [CrossRef]
- Syed, Y.Y. Nedosiran: First approval. Drugs 2023, 83, 1729–1733. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, J.; Shah, M.; Migliorati, J.M.; Tawfik, S.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X. Nedosiran, a candidate siRNA drug for the treatment of primary hyperoxaluria: Design, development, and clinical studies. ACS Pharmacol. Transl. Sci. 2022, 5, 1007–1016. [Google Scholar] [CrossRef]
- Granchi, C.; Paterni, I.; Rani, R.; Minutolo, F. Small-molecule inhibitors of human LDH5. Future Med. Chem. 2013, 5, 1967–1991. [Google Scholar] [CrossRef]
- Fiume, L.; Manerba, M.; Vettraino, M.; Di Stefano, G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med. Chem. 2014, 6, 429–445. [Google Scholar] [CrossRef]
- Martin-Higueras, C.; Torres, A.; Salido, E. Molecular therapy of primary hyperoxaluria. J. Inherit. Metab. Dis. 2017, 40, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Moya-Garzon, M.D.; Gomez-Vidal, J.A.; Alejo-Armijo, A.; Altarejos, J.; Rodriguez-Madoz, J.R.; Fernandes, M.X.; Salido, E.; Salido, S.; Diaz-Gavilan, M. Small molecule-based enzyme inhibitors in the treatment of primary hyperoxalurias. J. Pers. Med. 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Le Dudal, M.; Huguet, L.; Perez, J.; Vandermeersch, S.; Bouderlique, E.; Tang, E.; Martori, C.; Chemaly, N.; Nabbout, R.; Haymann, J.-P.; et al. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J. Clin. Investig. 2019, 129, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Violier, P.; Boyer, O.; Berthaud, R.; Dorval, G. Treatment with stiripentol in a patient with primary hyperoxaluria type 1: Lesson for the clinical nephrologist. J. Nephrol. 2022, 35, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Kempf, C.; Pfau, A.; Holle, J.; Müller-Schlüter, K.; Bufler, P.; Knauf, F.; Müller, D. Stiripentol fails to lower plasma oxalate in a dialysis-dependent PH1 patient. Pediatr. Nephrol. 2020, 35, 1787–1789. [Google Scholar] [CrossRef]
- Martin-Higueras, C.; Feldkötter, M.; Hoppe, B. Is stiripentol truly effective for treating primary hyperoxaluria? Clin. Kidney J. 2021, 14, 442–444. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, W.; Zhou, J.; Huang, Q.; Zeng, G. Navigating the evolving landscape of primary hyperoxaluria: Traditional management defied by the rise of novel molecular drugs. Biomolecules 2024, 14, 511. [Google Scholar] [CrossRef]
- Clinical Trials, National Library of Medicine, National Center for Biotechnology Information. Evaluation of the Efficacy and Safety of Stiripentol in Patients 6 Years and Older with Primary Hyperoxaluria Type 1, 2 or 3 (CRYSTAL). Available online: https://clinicaltrials.gov/study/NCT06465472?cond=hyperoxaluria&term=stiripentol&rank=2 (accessed on 31 October 2024).
- Cellini, B.; Baum, M.A.; Frishberg, Y.; Groothoff, J.W.; Harris, P.C.; Hulton, S.A.; Knauf, F.; Knight, J.; Lieske, J.C.; Lowther, W.T.; et al. Opportunities in primary and enteric hyperoxaluria at the cross-roads between the clinic and laboratory. Kidney Int. Rep. 2024, 9, 3083–3096. [Google Scholar] [CrossRef]
- Wu, M.-C.; Liang, Y.-F.; Jurca, T.; Yap, G.P.A.; Leung, T.-F.; Ong, T.-G. Reactive dicarbon as a flexible ligand for transition-metal coordination and catalysis. J. Am. Chem. Soc. 2022, 144, 12996–13005. [Google Scholar] [CrossRef]
- Diaz, I.; Salido, S.; Nogueras, M.; Cobo, J. Design and synthesis of new pyrimidine-quinolone hybrids as novel hLDHA inhibitors. Pharmaceuticals 2022, 15, 792. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Cuadrado, C.; Altarejos, J.; Fernandes, M.X.; Salido, E.; Diaz-Gavilan, M.; Salido, S. Lactate dehydrogenase A inhibitors with a 2,8-dioxabicyclo[3.3.1]nonane scaffold: A contribution to molecular therapies for primary hyperoxalurias. Bioorg. Chem. 2022, 129, 106127. [Google Scholar] [CrossRef] [PubMed]
- Salido, S.; Alejo-Armijo, A.; Parola, A.J.; Sebastian, V.; Alejo, T.; Irusta, S.; Arruebo, M.; Altarejos, J. Chitosan derivatives as nanocarriers for hLDHA inhibitors delivery to hepatic cells: A selective strategy for targeting primary hyperoxaluria diseases. Int. J. Pharm. 2022, 627, 122224. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Vidal, J.; Ruiz-Martos, L.; Salido, S.; Altarejos, J. Proanthocyanidins in pruning wood extracts of four European plum (Prunus domestica L.) cultivars and their hLDHA inhibitory activity. Chem. Biodivers. 2023, 20, e202200931. [Google Scholar] [CrossRef] [PubMed]
- Salido, S.; Alejo-Armijo, A.; Altarejos, J. Synthesis and hLDH inhibitory activity of analogues to natural products with 2,8-dioxabicyclo[3.3.1]nonane scaffold. Int. J. Mol. Sci. 2023, 24, 9925. [Google Scholar] [CrossRef]
- Diaz, I.; Salido, S.; Nogueras, M.; Cobo, J. Synthesis of ethyl pyrimidine-quinolincarboxylates selected from virtual screening as enhanced lactate dehydrogenase (LDH) inhibitors. Int. J. Mol. Sci. 2024, 25, 9744. [Google Scholar] [CrossRef]
- Fernandez-Mimbrera, M.A.; Salido, S.; Marchal, J.A.; Alejo-Armijo, A. Tracking selective internalization and intracellular dynamics of modified chitosan polymeric micelles of interest in primary hyperoxaluria diseases. ACS Omega 2024, 9, 39503–39512. [Google Scholar] [CrossRef]
- Stevens, J.S.; Al-Awqati, Q. Lactate dehydrogenase 5: Identification of a druggable target to reduce oxaluria. J. Clin. Investig. 2019, 129, 2201–2204. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Rodriguez-Rodriguez, B.; Martin-Higueras, C.; Franco-Montalban, F.; Fernandes, M.X.; Gomez-Vidal, J.A.; Pey, A.L.; Salido, E.; Diaz-Gavilan, M. New salicylic acid derivatives, double inhibitors of glycolate oxidase and lactate dehydrogenase, as effective agents decreasing oxalate production. Eur. J. Med. Chem. 2022, 237, 114396. [Google Scholar] [CrossRef]
- Bononi, G.; Di Bussolo, V.; Tuccinardi, T.; Minutolo, F.; Granchi, C. A patent review of lactate dehydrogenase inhibitors (2014-present). Expert Opin. Ther. Pat. 2024, 34, 1121–1135. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: A promising approach for cancer chemotherapy. J. Med. Chem. 2016, 59, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Woodford, M.R.; Chen, V.Z.; Backe, S.J.; Bratslavsky, G.; Mollapour, M. Structural and functional regulation of lactate dehydrogenase-A in cancer. Future Med. Chem. 2020, 12, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Lee, E.-J.; Park, W.; Ha, K.-T.; Chung, H.-S. Natural compounds as lactate dehydrogenase inhibitors: Potential therapeutics for lactate dehydrogenase inhibitors-related diseases. Front. Pharmacol. 2023, 14, 1275000. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.S.; Leenaraj, D.R.; Ghabbour, H.A.; Joe, I.H.; Attia, M.I. Spectroscopic identification, structural features, Hirshfeld surface analysis and molecular docking studies on stiripentol: An orphan antiepileptic drug. J. Mol. Struct. 2019, 1180, 110–118. [Google Scholar] [CrossRef]
- Vashchenko, V.; Kutulya, L.; Krivoshey, A. Simple and effective protocol for Claisen-Schmidt condensation of hindered cyclic ketones with aromatic aldehydes. Synthesis 2007, 2007, 2125–2134. [Google Scholar] [CrossRef]
- Borner, C.; Dennis, M.R.; Sinn, E.; Woodward, S. Copper-catalysed asymmetric 1,4-addition of organozinc compounds to linear aliphatic enones using 2,2′-dihydroxy 3,3′-dithioether derivatives of 1,1′-binaphthalene. Eur. J. Org. Chem. 2001, 13, 2435–2446. [Google Scholar] [CrossRef]
- Shen, D.D.; Levy, R.H.; Savitch, J.L.; Boddy, A.V.; Tombret, F.; Lepage, F. Comparative anticonvulsant potency and pharmacokinetics of (+)- and (-)-enantiomers of stiripentol. Epilepsy Res. 1992, 12, 29–36. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.; Rashed, M.; Cui, D.; Tombret, F.; Botte, H.; Lepage, F.; Levy, R.H.; Baillie, T.A. Metabolic chiral inversion of stiripentol in the rat. I. Mechanistic studies. Drug Metab. Dispos. 1994, 22, 544–553. Available online: https://dmd.aspetjournals.org/content/22/4/544 (accessed on 31 October 2024).
- Jacobsen, E.E.; Anthonsen, T.; El-Behairy, M.F.; Sundby, E.; Aboul-Enein, M.N.; Attia, M.I.; Abd, A.; Amin, K.M.; Abdel-Rehim, M. Lipase catalysed kinetic resolution of stiripentol. Int. J. Chem. 2012, 4, 7–13. [Google Scholar] [CrossRef]
- El-Behairy, M.F.; Sundby, E. Synthesis of the antiepileptic (R)-stiripentol by a combination of lipase catalyzed resolution and alkene metathesis. Tetrahedron Asymmetry 2013, 24, 285–289. [Google Scholar] [CrossRef]
- Saleh, O.A.; El-Behairy, M.F.; Badawey, A.M.; El-Azzouny, A.A.; Aboul-Enein, H.Y. Analysis of stiripentol enantiomers on several chiral stationary phases: A comparative study. Chromatographia 2015, 78, 267–271. [Google Scholar] [CrossRef]
- Dreher, S.D.; Katz, T.J.; Lam, K.-C.; Rheingold, A.L. Application of the Russig-Laatsch reaction to synthesize a bis[5]helicene chiral pocket for asymmetric catalysis. J. Org. Chem. 2000, 65, 815–822. [Google Scholar] [CrossRef]
- Castro, J.M.; Linares-Palomino, P.J.; Salido, S.; Altarejos, J.; Nogueras, M.; Sanchez, A. Enantiospecific synthesis, separation and olfactory evaluation of all diastereomers of a homologue of the sandalwood odorant Polysantol®. Tetrahedron 2005, 61, 11192–11203. [Google Scholar] [CrossRef]
- Ohtani, I.I.; Hotta, K.; Ichikawa, Y.; Isobe, M. Application of modified Mosher’s method to α-aromatic secondary alcohols. Exception of the rule and conformational analyses. Chem. Lett. 1995, 24, 513–514. [Google Scholar] [CrossRef]
- Junior, F.M.S.; Junior, J.M.B. Absolute configuration from chiroptical spectroscopy. In Chiral Separations and Stereochemical Elucidation. Fundamentals, Methods, and Applications; Cass, Q., Tiritan, M.E., Junior, J.M.B., Barreiro, J.C., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 551–591. [Google Scholar] [CrossRef]
- Bader, A.; Tuccinardi, T.; Granchi, C.; Martinelli, A.; Macchia, M.; Minutolo, F.; De Tommasi, N.; Braca, A. Phenylpropanoids and flavonoids from Phlomis kurdica as inhibitors of human lactate dehydrogenase. Phytochemistry 2015, 116, 262–268. [Google Scholar] [CrossRef]
- Kamitaki, B.K.; Minacapelli, C.D.; Zhang, P.; Wachuku, C.; Gupta, K.; Catalano, C.; Rustgi, V. Drug-induced liver injury associated with antiseizure medications from the FDA Adverse Event Reporting System (FAERS). Epilepsy Behav. 2021, 117, 107832. [Google Scholar] [CrossRef]
- Tran, A.; Treluyer, J.-M.; Rey, E.; Barbet, J.; Ferracci, G.; d’Athis, P.; Vincent, J.; Pons, G. Protective effect of stiripentol on acetaminophen-induced hepatotoxicity in rat. Toxicol. Appl. Pharmacol. 2001, 170, 145–152. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Q.; Zhang, Y.; Liu, T. Adverse events associated with stiripentol in children aged 0–17 years: An analysis of a real-world pharmacovigilance database. Epilepsy Behav. 2024, 161, 110073. [Google Scholar] [CrossRef]
- Armarego, W.L.F. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Burlington, UK, 2017. [Google Scholar]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.02, Wallingford, CT. 2016. Available online: https://gaussian.com/g16new/ (accessed on 6 November 2024).
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumloeffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rupiani, S.; Buonfiglio, R.; Manerba, M.; Di Ianni, L.; Vettraino, M.; Giacomini, E.; Masetti, M.; Falchi, F.; Di Stefano, G.; Roberti, M.; et al. Identification of N-acylhydrazone derivatives as novel lactate dehydrogenase A inhibitors. Eur. J. Med. Chem. 2015, 101, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Billiard, J.; Dennison, J.B.; Briand, J.; Annan, R.S.; Chai, D.; Colón, M.; Dodson, C.S.; Gilbert, S.A.; Greshock, J.; Jing, J.; et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013, 1, 19. [Google Scholar] [CrossRef] [PubMed]

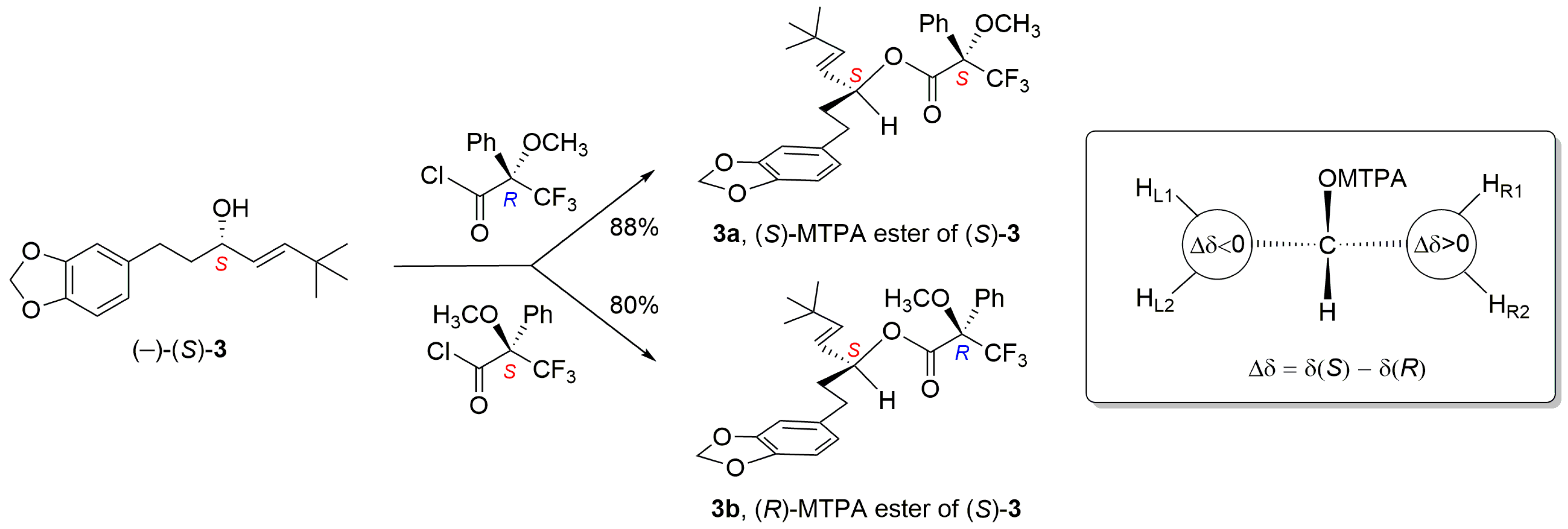
| Proton | δ(S) (Ester 3a) (ppm) | δ(R) (Ester 3b) (ppm) | ∆δSR = δ(S) − δ(R) | |
|---|---|---|---|---|
| ppm | Hz (400 MHz) | |||
| O-CH2-O | 5.92 | 5.92 | 0 | 0 |
| 2′ | 6.58 | 6.63 | −0.05 | −20 |
| 5′ | 6.71 | 6.72 | −0.01 | −4 |
| 6′ | 6.53 | 6.58 | −0.05 | −20 |
| 1 | 2.41–2.52 (2.47) | 2.49–2.62 (2.56) | −0.09 | −36 |
| 2 | 1.81–2.02 (1.92) | 1.85–2.08 (1.97) | −0.05 | −20 |
| 3 | 5.46 | 5.42 | +0.04 | +16 |
| 4 | 5.38 | 5.23 | +0.15 | +60 |
| 5 | 5.89 | 5.83 | +0.06 | +24 |
| 6a, 6b, 7 | 1.01 | 0.99 | +0.02 | +8 |
| Compound | % Inhibition a (hLDHA) | IC50 (μM) b (hLDHA) | R2 |
|---|---|---|---|
| Stiripentol ((±)-1) | 9.9 ± 2.3 | >500 | |
| (−)-(S)-1 | 34.0 ± 2.1 | >500 | |
| (+)-(R)-1 | 38.4 ± 1.6 | >500 | |
| iso-Stiripentol ((±)-2) | 21.1 ± 3.5 | >500 | |
| (±)-3 | 70.0 ± 3.0 | 125.3 ± 19.0 | 0.98 |
| (−)-(S)-3 | ND c | 213.7 ± 13.9 | 0.96 |
| (+)-(R)-3 | ND c | 166.3 ± 16.9 | 0.99 |
| (±)-(2S*,5R*)-4 | 56.3 ± 8.2 | 199.8 ± 23.8 | 0.98 |
| (−)-(2R,5S)-4 | ND c | 101.7 ± 6.7 | 0.99 |
| (+)-(2S,5R)-4 | ND c | 128.4 ± 12.4 | 0.97 |
| (±)-(2R*,5R*)-4 | 71.7 ± 2.2 | 129.6 ± 15.3 | 0.98 |
| (+)-(2R,5R)-4 | ND c | 179.7 ± 10.5 | 0.95 |
| (−)-(2S,5S)-4 | ND c | 149.3 ± 10.4 | 0.97 |
| (±)-5 | 46.4 ± 2.8 | 200.5 ± 13.9 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-Molina, M.; Ortega-Vidal, J.; Molina-Canteras, J.; Cobo, J.; Altarejos, J.; Salido, S. Synthesis and hLDHA Inhibitory Activity of New Stiripentol-Related Compounds of Potential Use in Primary Hyperoxaluria. Int. J. Mol. Sci. 2024, 25, 13266. https://doi.org/10.3390/ijms252413266
Rico-Molina M, Ortega-Vidal J, Molina-Canteras J, Cobo J, Altarejos J, Salido S. Synthesis and hLDHA Inhibitory Activity of New Stiripentol-Related Compounds of Potential Use in Primary Hyperoxaluria. International Journal of Molecular Sciences. 2024; 25(24):13266. https://doi.org/10.3390/ijms252413266
Chicago/Turabian StyleRico-Molina, Mario, Juan Ortega-Vidal, Juan Molina-Canteras, Justo Cobo, Joaquín Altarejos, and Sofía Salido. 2024. "Synthesis and hLDHA Inhibitory Activity of New Stiripentol-Related Compounds of Potential Use in Primary Hyperoxaluria" International Journal of Molecular Sciences 25, no. 24: 13266. https://doi.org/10.3390/ijms252413266
APA StyleRico-Molina, M., Ortega-Vidal, J., Molina-Canteras, J., Cobo, J., Altarejos, J., & Salido, S. (2024). Synthesis and hLDHA Inhibitory Activity of New Stiripentol-Related Compounds of Potential Use in Primary Hyperoxaluria. International Journal of Molecular Sciences, 25(24), 13266. https://doi.org/10.3390/ijms252413266







