Sugar Transport and Signaling in Shoot Branching
Abstract
1. Sugar Transport from Source to Sinks
1.1. Long-Distance Transport of Sugars from Source to Sink Organs
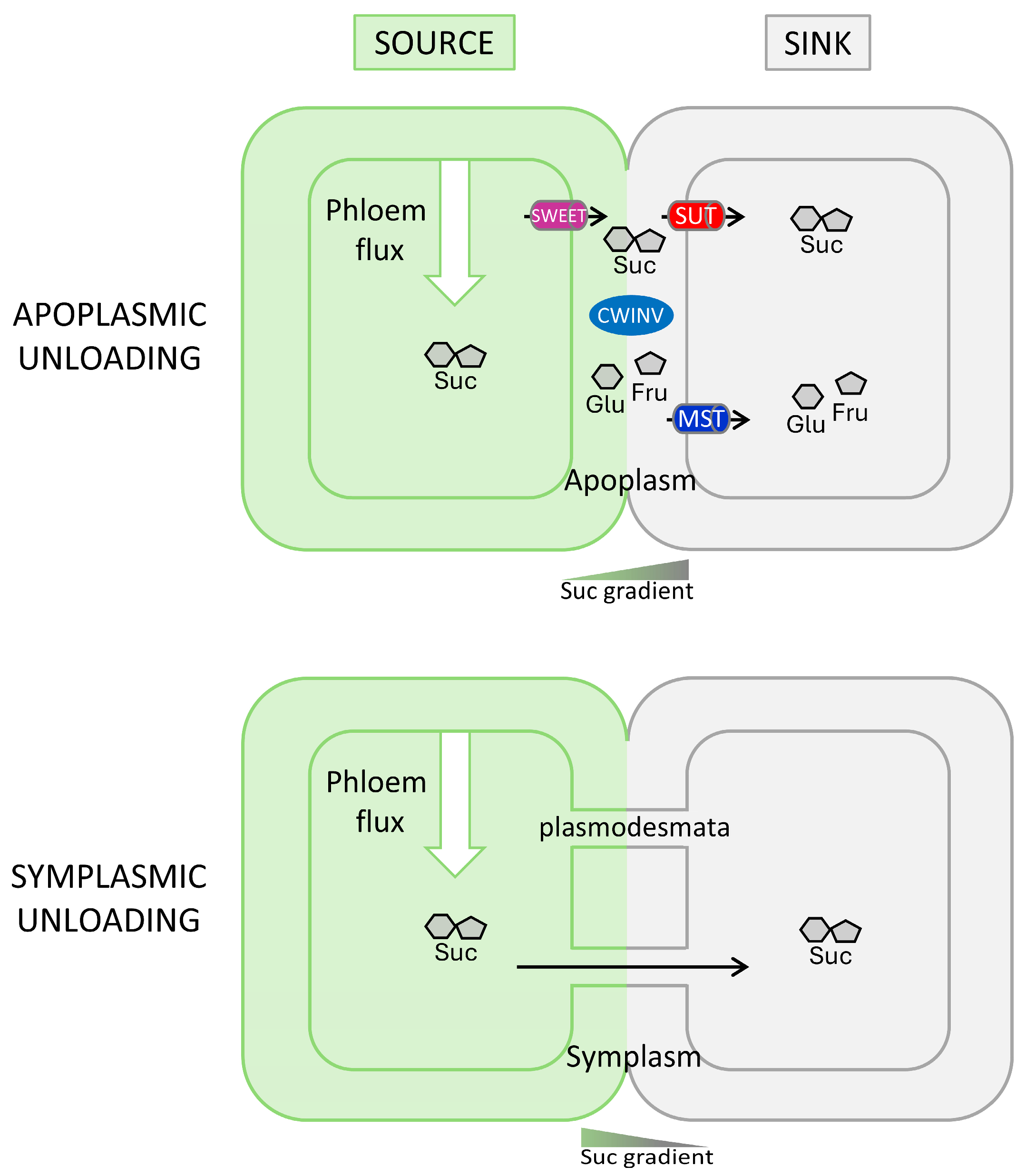
1.2. A Spatio-Temporal Control of Sugar-Unloading Pathways
2. Axillary Bud as a Major Carbon Sink
2.1. Axillary Bud, a Critical Carbon Sink for Plant Branching and Organismal Development
2.2. Structure, Establishment, and Activity of Axillary Vegetative Bud
3. Sugar Supply Towards Axillary Bud
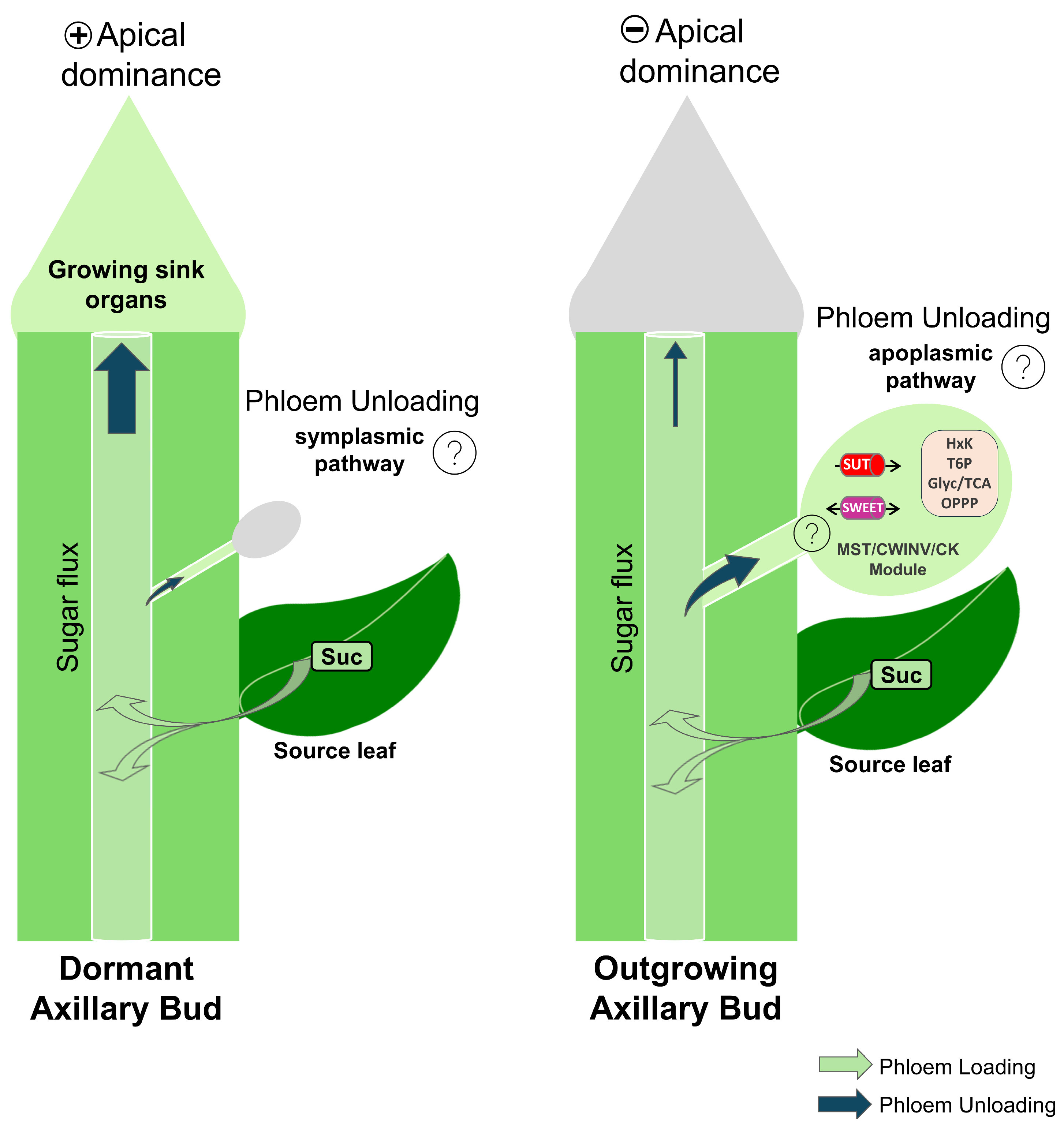
3.1. Sugar Supply During the Transition from Dormant to Active Buds
3.2. Sugar Metabolism Activation in Growing Axillary Buds
3.3. A Possible Switch from Symplasmic to Apoplasmic Unloading Pathways During Axillary Bud Developmental Transition
4. Sugar as a Signal Molecule with a Potential Role in the Establishment of Axillary Bud Sink Strength
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Turgeon, R. Mechanisms of phloem loading. Curr. Opin. Plant Biol. 2018, 43, 71–75. [Google Scholar] [CrossRef]
- van Bel, A.J.E. Transport phloem: Low profile, high impact. Plant Physiol. 2003, 131, 1509–1510. [Google Scholar]
- Patrick, J.W. PHLOEM UNLOADING: Sieve Element Unloading and Post-Sieve Element Transport. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 191–222. [Google Scholar] [CrossRef]
- Turgeon, R.; Wolf, S. Phloem Transport: Cellular Pathways and Molecular Trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221. [Google Scholar] [CrossRef]
- Pegler, J.L.; Grof, C.P.; Patrick, J.W. Sugar loading of crop seeds—A partnership of phloem, plasmodesmal and membrane transport. New Phytol. 2023, 239, 1584–1602. [Google Scholar] [CrossRef]
- Weber, H.; Borisjuk, L.; Heim, U.; Sauer, N.; Wobus, U. A role for sugar transporters during seed development: Molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 1997, 9, 895–908. [Google Scholar] [CrossRef]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular physiology of legume seed development. Annu. Rev. Plant Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef]
- Morin, A.; Kadi, F.; Porcheron, B.; Vriet, C.; Maurousset, L.; Lemoine, R.; Pourtau, N.; Doidy, J. Genome-wide identification of invertases in Fabaceae, focusing on transcriptional regulation of Pisum sativum invertases in seed subjected to drought. Physiol. Plant. 2022, 174, e13673. [Google Scholar] [CrossRef] [PubMed]
- Weschke, W.; Panitz, R.; Sauer, N.; Wang, Q.; Neubohn, B.; Weber, H.; Wobus, U. Sucrose transport into barley seeds: Molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J. 2000, 21, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Godt, D.; Roitsch, T. The developmental and organ specific expression of sucrose cleaving enzymes in sugar beet suggests a transition between apoplasmic and symplasmic phloem unloading in the tap roots. Plant Physiol. Biochem. 2006, 44, 656–665. [Google Scholar] [CrossRef]
- Milne, R.J.; Grof, C.P.; Patrick, J.W. Mechanisms of phloem unloading: Shaped by cellular pathways, their conductances and sink function. Curr. Opin. Plant Biol. 2018, 43, 8–15. [Google Scholar] [CrossRef]
- Carpaneto, A.; Geiger, D.; Bamberg, E.; Sauer, N.; Fromm, J.; Hedrich, R. Phloem-localized, Proton-coupled Sucrose Carrier ZmSUT1 Mediates Sucrose Efflux under the Control of the Sucrose Gradient and the Proton Motive Force. J. Biol. Chem. 2005, 280, 21437–21443. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Johnson, M.E.; Krentz, A.D.; Grof, C.P.; Perroux, J.M.; Ward, J.M. Arabidopsis Sucrose Transporter AtSUCHigh-Affinity Transport Activity, Intragenic Control of Expression, and Early Flowering Mutant Phenotype. Plant Physiol. 2006, 143, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2013, 201, 1150–1155. [Google Scholar] [CrossRef]
- Durand, M.; Porcheron, B.; Hennion, N.; Maurousset, L.; Lemoine, R.; Pourtau, N. Water Deficit Enhances C Export to the Roots in Arabidopsis thaliana Plants with Contribution of Sucrose Transporters in Both Shoot and Roots. Plant Physiol. 2016, 170, 1460–1479. [Google Scholar] [CrossRef]
- Hennion, N.; Durand, M.; Vriet, C.; Doidy, J.; Maurousset, L.; Lemoine, R.; Pourtau, N. Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol. Plant. 2018, 165, 44–57. [Google Scholar] [CrossRef]
- Paniagua, C.; Sinanaj, B.; Benitez-Alfonso, Y. Plasmodesmata and their role in the regulation of phloem unloading during fruit development. Curr. Opin. Plant Biol. 2021, 64, 102145. [Google Scholar] [CrossRef]
- Braun, D.M. Phloem Loading and Unloading of Sucrose: What a Long, Strange Trip from Source to Sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2013, 65, 1713–1735. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, X.-L.; Wang, X.-F.; Xia, G.-H.; Pan, Q.-H.; Fan, R.-C.; Wu, F.-Q.; Yu, X.-C.; Zhang, D.-P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, I.F. Tansley Review No. 27 The control of carbon partitioning in plants. New Phytol. 1990, 116, 341–381. [Google Scholar] [CrossRef] [PubMed]
- Marcelis, L. Sink strength as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 1996, 47, 1281–1291. [Google Scholar] [CrossRef]
- Hageman, A.; Van Volkenburgh, E. Sink Strength Maintenance Underlies Drought Tolerance in Common Bean. Plants 2021, 10, 489. [Google Scholar] [CrossRef]
- Ho, L.C. Metabolism and Compartmentation of Imported Sugars in Sink Organs in Relation to Sink Strength. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Sussex, I.M.; Kerk, N.M. The evolution of plant architecture. Curr. Opin. Plant Biol. 2001, 4, 33–37. [Google Scholar] [CrossRef]
- Guo, W.; Chen, L.; Herrera-Estrella, L.; Cao, D.; Tran, L.-S.P. Altering Plant Architecture to Improve Performance and Resistance. Trends Plant Sci. 2020, 25, 1154–1170. [Google Scholar] [CrossRef]
- Boumaza, R.; Huché-Thélier, L.; Demotes-Mainard, S.; Le Coz, E.; Leduc, N.; Pelleschi-Travier, S.; Qannari, E.M.; Sakr, S.; Santagostini, P.; Symoneaux, R.; et al. Sensory profiles and preference analysis in ornamental horticulture: The case of the rosebush. Food Qual. Prefer. 2010, 21, 987–997. [Google Scholar] [CrossRef]
- Garbez, M.; Galopin, G.; Sigogne, M.; Favre, P.; Demotes-Mainard, S.; Symoneaux, R. Assessing the visual aspect of rotating virtual rose bushes by a labeled sorting task. Food Qual. Prefer. 2015, 40, 287–295. [Google Scholar] [CrossRef]
- Ishka, M.R.; Julkowska, M. Tapping into the plasticity of plant architecture for increased stress resilience. F1000Research 2023, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Rameau, C.; Wijerathna-Yapa, A. Lessons from a century of apical dominance research. J. Exp. Bot. 2023, 74, 3903–3922. [Google Scholar] [CrossRef]
- Lifschitz, E.; Eviatar, T.; Rozman, A.; Shalit, A.; Goldshmidt, A.; Amsellem, Z.; Alvarez, J.P.; Eshed, Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 6398–6403. [Google Scholar] [CrossRef]
- Iwata, H.; Gaston, A.; Remay, A.; Thouroude, T.; Jeauffre, J.; Kawamura, K.; Oyant, L.H.; Araki, T.; Denoyes, B.; Foucher, F. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 2011, 69, 116–125. [Google Scholar] [CrossRef]
- Randoux, M.; Davière, J.; Jeauffre, J.; Thouroude, T.; Pierre, S.; Toualbia, Y.; Perrotte, J.; Reynoird, J.; Jammes, M.; Oyant, L.H.; et al. RoKSN, a floral repressor, forms protein complexes with RoFD and RoFT to regulate vegetative and reproductive development in rose. New Phytol. 2013, 202, 161–173. [Google Scholar] [CrossRef]
- Goetz, M.; Rabinovich, M.; Smith, H.M. The role of auxin and sugar signaling in dominance inhibition of inflorescence growth by fruit load. Plant Physiol. 2021, 187, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- León-Burgos, A.F.; Sáenz, J.R.R.; Quinchua, L.C.I.; Toro-Herrera, M.A.; Unigarro, C.A.; Osorio, V.; Balaguera-López, H.E. Increased fruit load influences vegetative growth, dry mass partitioning, and bean quality attributes in full-sun coffee cultivation. Front. Sustain. Food Syst. 2024, 8, 1379207. [Google Scholar] [CrossRef]
- Haim, D.; Shalom, L.; Simhon, Y.; Shlizerman, L.; Kamara, I.; Morozov, M.; Albacete, A.; Rivero, R.M.; Sadka, A. Alternate bearing in fruit trees: Fruit presence induces polar auxin transport in citrus and olive stem and represses IAA release from the bud. J. Exp. Bot. 2020, 72, 2450–2462. [Google Scholar] [CrossRef]
- Drummond, R.S.; Janssen, B.J.; Luo, Z.; Oplaat, C.; Ledger, S.E.; Wohlers, M.W.; Snowden, K.C. Environmental Control of Branching in Petunia. Plant Physiol. 2015, 168, 735–751. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Amoroso, T.; Ulloa, M.G.U.; Morel, P.; Sakr, S. Genotype × Light Quality Interaction on Rose Architecture. Agronomy 2020, 10, 913. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Amoroso, T.; Dubuc, B.; Citerne, S.; Perez-Garcia, M.-D.; Sakr, S. Involvement of sugar and abscisic acid in the genotype-specific response of rose to far-red light. Front. Plant Sci. 2022, 13, 929029. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.; Sanchez, R.; Deregibus, V. The effect of plant density on tillering: The involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ. Exp. Bot. 1986, 26, 365–371. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Burson, B.L.; Finlayson, S.A. Phytochrome B Represses Teosinte Branched1 Expression and Induces Sorghum Axillary Bud Outgrowth in Response to Light Signals. Plant Physiol. 2006, 140, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome Regulation of Branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef]
- González-Grandío, E.; Poza-Carrión, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1 Promotes Axillary Bud Dormancy in Response to Shade in Arabidopsis. Plant Cell 2013, 25, 834–850. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Huché-Thélier, L.; Morel, P.; Boumaza, R.; Guérin, V.; Sakr, S. Temporary water restriction or light intensity limitation promotes branching in rose bush. Sci. Hortic. 2013, 150, 432–440. [Google Scholar] [CrossRef]
- Corot, A.; Roman, H.; Douillet, O.; Autret, H.; Perez-Garcia, M.-D.; Citerne, S.; Bertheloot, J.; Sakr, S.; Leduc, N.; Demotes-Mainard, S. Cytokinins and Abscisic Acid Act Antagonistically in the Regulation of the Bud Outgrowth Pattern by Light Intensity. Front. Plant Sci. 2017, 8, 1724. [Google Scholar] [CrossRef]
- Girault, T.; Bergougnoux, V.; Combes, D.; Viemont, J.; Leduc, N. Light controls shoot meristem organogenic activity and leaf primordia growth during bud burst in Rosa sp. Plant Cell Environ. 2008, 31, 1534–1544. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Andrieu, B.; Struik, P.C. Cessation of Tillering in Spring Wheat in Relation to Light Interception and Red: Far-red Ratio. Ann. Bot. 2006, 97, 649–658. [Google Scholar] [CrossRef]
- Beveridge, C.A.; Weller, J.L.; Singer, S.R.; Hofer, J.M. Axillary Meristem Development. Budding Relationships between Networks Controlling Flowering, Branching, and Photoperiod Responsiveness. Plant Physiol. 2003, 131, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Li-Marchetti, C.; Le Bras, C.; Relion, D.; Citerne, S.; Huché-Thélier, L.; Sakr, S.; Morel, P.; Crespel, L. Genotypic differences in architectural and physiological responses to water restriction in rose bush. Front. Plant Sci. 2015, 06, 355. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; George, G.; Ongaro, V.; Williamson, L.; Willetts, B.; Ljung, K.; McCulloch, H.; Leyser, O. Auxin and Strigolactone Signaling Are Required for Modulation of Arabidopsis Shoot Branching by Nitrogen Supply. Plant Physiol. 2014, 166, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G.; Thangavelu, M.; Dong-Il, K. A possible role of cytokinin in mediating long-distance nitrogen signaling in the promotion of sylleptic branching in hybrid poplar. J. Plant Physiol. 2006, 163, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cai, T.; Luo, Y.; Wang, Z. Optimizing plant density and nitrogen application to manipulate tiller growth and increase grain yield and nitrogen-use efficiency in winter wheat. PeerJ 2019, 7, e6484. [Google Scholar] [CrossRef]
- Li, X.; Xia, K.; Liang, Z.; Chen, K.; Gao, C.; Zhang, M. MicroRNA393 is involved in nitrogen-promoted rice tillering through regulation of auxin signal transduction in axillary buds. Sci. Rep. 2016, 6, 32158. [Google Scholar] [CrossRef]
- Huang, W.; Bai, G.; Wang, J.; Zhu, W.; Zeng, Q.; Lu, K.; Sun, S.; Fang, Z. Two Splicing Variants of OsNPF7.7 Regulate Shoot Branching and Nitrogen Utilization Efficiency in Rice. Front. Plant Sci. 2018, 9, 300. [Google Scholar] [CrossRef]
- Tian, G.; Gao, L.; Kong, Y.; Hu, X.; Xie, K.; Zhang, R.; Ling, N.; Shen, Q.; Guo, S. Improving rice population productivity by reducing nitrogen rate and increasing plant density. PLoS ONE 2017, 12, e0182310. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Boumaza, R.; Demotes-Mainard, S.; Canet, A.; Symoneaux, R.; Douillet, O.; Guérin, V. Nitrogen deficiency increases basal branching and modifies visual quality of the rose bushes. Sci. Hortic. 2011, 130, 325–334. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, H.; Yu, C.; Luo, N.; Yan, J.; Zheng, S.; Hu, Q.; Zhang, D.; Kou, L.; Meng, X.; et al. Low phosphorus promotes NSP1–NSP2 heterodimerization to enhance strigolactone biosynthesis and regulate shoot and root architecture in rice. Mol. Plant 2023, 16, 1811–1831. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of Strigolactones to the Inhibition of Tiller Bud Outgrowth under Phosphate Deficiency in Rice. Plant Cell Physiol. 2010, 51, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fioreze, S.L.; Castoldi, G.; Pivetta, L.A.; Fernandes, D.M.; Büll, L.T. Tillering of two wheat genotypes as affected by phosphorus levels. Acta Sci. Agron. 2012, 34, 331–338. [Google Scholar] [CrossRef]
- Dong, Y.; Aref, R.; Forieri, I.; Schiel, D.; Leemhuis, W.; Meyer, C.; Hell, R.; Wirtz, M. The plant TOR kinase tunes autophagy and meristem activity for nutrient stress-induced developmental plasticity. Plant Cell 2022, 34, 3814–3829. [Google Scholar] [CrossRef]
- Martin, C.A.; Stutz, J.C.; Kimball, B.A.; Idso, S.B.; Akey, D.H. Growth and Topological Changes of Citrus limon (L.) Burm. f. ‘Eureka’ in Response to High Temperatures and Elevated Atmospheric Carbon Dioxide. J. Am. Soc. Hortic. Sci. 1995, 120, 1025–1031. [Google Scholar] [CrossRef]
- Swiegers, H.W.; Karpinska, B.; Hu, Y.; Dodd, I.C.; Botha, A.-M.; Foyer, C.H. The Effects of High CO2 and Strigolactones on Shoot Branching and Aphid–Plant Compatibility Control in Pea. Int. J. Mol. Sci. 2022, 23, 12160. [Google Scholar] [CrossRef]
- Grbić, V.; Bleecker, A.B. Axillary meristem development in Arabidopsis thaliana. Plant J. 2000, 21, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiao, Y. Control of cell fate during axillary meristem initiation. Cell. Mol. Life Sci. 2019, 77, 2343–2354. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, Y. Axillary meristem initiation—A way to branch out. Curr. Opin. Plant Biol. 2018, 41, 61–66. [Google Scholar] [CrossRef]
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.-R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Jönsson, H.; Heisler, M.G.; Shapiro, B.E.; Meyerowitz, E.M.; Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. USA 2006, 103, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Besnard, F.; Refahi, Y.; Morin, V.; Marteaux, B.; Brunoud, G.; Chambrier, P.; Rozier, F.; Mirabet, V.; Legrand, J.; Lainé, S.; et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2013, 505, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Giulini, A.; Wang, J.; Jackson, D. Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 2004, 430, 1031–1034. [Google Scholar] [CrossRef]
- Wang, M.; Le Gourrierec, J.; Jiao, F.; Demotes-Mainard, S.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Crespel, L.; Bertheloot, J.; Chen, J.; et al. Convergence and Divergence of Sugar and Cytokinin Signaling in Plant Development. Int. J. Mol. Sci. 2021, 22, 1282. [Google Scholar] [CrossRef]
- Skylar, A.; Sung, F.; Hong, F.; Chory, J.; Wu, X. Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev. Biol. 2011, 351, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.L.; Formosa-Jordan, P.; Malivert, A.; Margalha, L.; Confraria, A.; Feil, R.; Lunn, J.E.; Jönsson, H.; Landrein, B.; Baena-González, E. Sugar signaling modulates SHOOT MERISTEMLESS expression and meristem function in Arabidopsis. Proc. Natl. Acad. Sci. USA 2024, 121, e2408699121. [Google Scholar] [CrossRef]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef]
- Scofield, S.; Dewitte, W.; Nieuwland, J.; Murray, J.A. The Arabidopsis homeobox gene SHOOT MERISTEMLESS has cellular and meristem-organisational roles with differential requirements for cytokinin and CYCD3 activity. Plant J. 2013, 75, 53–66. [Google Scholar] [CrossRef]
- Yanai, O.; Shani, E.; Dolezal, K.; Tarkowski, P.; Sablowski, R.; Sandberg, G.; Samach, A.; Ori, N. Arabidopsis KNOXI Proteins Activate Cytokinin Biosynthesis. Curr. Biol. 2005, 15, 1566–1571. [Google Scholar] [CrossRef]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble Carbohydrates Regulate Auxin Biosynthesis via PIF Proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Kean-Galeno, T.; Lopez-Arredondo, D.; Herrera-Estrella, L. The Shoot Apical Meristem: An Evolutionary Molding of Higher Plants. Int. J. Mol. Sci. 2024, 25, 1519. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, J. Aspects Particuliers de la Morphogenèse Caulinaire des Végétaux Ligneux et Introduction à Leur Étude Quantitative; IRSIA, Universitaires de Bruxelles: Bruxelles, Belgium, 1987. [Google Scholar]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, Para, and Ecodormancy: Physiological Terminology and Classification for Dormancy Research. HortScience 1987, 22, 701. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Doust, A.N. Activation of apoplastic sugar at the transition stage may be essential for axillary bud outgrowth in the grasses. Front. Plant Sci. 2022, 13, 1023581. [Google Scholar] [CrossRef]
- Tarancón, C.; González-Grandío, E.; Oliveros, J.C.; Nicolas, M.; Cubas, P. A conserved carbon starvation response underlies bud dormancy in woody and herbaceous species. Front. Plant Sci. 2017, 8, 788. [Google Scholar] [CrossRef]
- Pasternak, T.; Kircher, S.; Palme, K.; Pérez-Pérez, J.M. Regulation of early seedling establishment and root development in Arabidopsis thaliana by light and carbohydrates. Planta 2023, 258, 1–15. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends Plant Sci. 2021, 27, 379–390. [Google Scholar] [CrossRef]
- Barnola, P.; Crochet, A.; Payan, E.; Gendraud, M.; Lavarenne, S. Modifications du métabolisme énergétique et de la perméabilité dans le bourgeon apical et l’axe sous-jacent au cours de l’arrêt de croissance momentané de jeunes plants de chêne. Physiol. Vég. 1986, 24, 307–314. [Google Scholar]
- Marquat, C.; Vandamme, M.; Gendraud, M.; Pétel, G. Dormancy in vegetative buds of peach: Relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Sci. Hortic. 1999, 79, 151–162. [Google Scholar] [CrossRef]
- Maurel, K.; Leite, G.B.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef]
- Girault, T.; Abidi, F.; Sigogne, M.; Pelleschi-Travier, S.; Boumaza, R.; Sakr, S.; Leduc, N. Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ. 2010, 33, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Alaoui-Sossé, B.; Ricaud, S.; Barnola, P.; Dizengremel, P. Rhythmic growth and carbon allocation in Quercus robur. Sucrose metabolizing enzymes in leaves. Physiol. Plant. 1996, 96, 667–673. [Google Scholar] [CrossRef]
- Maurel, K.; Sakr, S.; Gerbe, F.; Guilliot, A.; Bonhomme, M.; Rageau, R.; Pétel, G. Sorbitol uptake is regulated by glucose through the hexokinase pathway in vegetative peach-tree buds. J. Exp. Bot. 2004, 55, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Decourteix, M.; Alves, G.; Bonhomme, M.; Peuch, M.; Baaziz, K.B.; Brunel, N.; Guilliot, A.; Rageau, R.; Améglio, T.; Pétel, G.; et al. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: Their potential role in early events of growth resumption. Tree Physiol. 2008, 28, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2009, 30, 89–102. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Chandler, P.M.; Swain, S.M.; King, R.W.; Richards, R.A.; Spielmeyer, W. Inhibition of Tiller Bud Outgrowth in the tin Mutant of Wheat Is Associated with Precocious Internode Development. Plant Physiol. 2012, 160, 308–318. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Mullet, J.E. Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ. 2015, 38, 1471–1478. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Mullet, J.E. Transcriptome Profiling of Tiller Buds Provides New Insights into PhyB Regulation of Tillering and Indeterminate Growth in Sorghum. Plant Physiol. 2016, 170, 2232–2250. [Google Scholar] [CrossRef]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef]
- Chatfield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000, 24, 159–169. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S.; et al. Insight into the Role of Sugars in Bud Burst Under Light in the Rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Rabot, A.; Laloi, M.; Mortreau, E.; Sigogne, M.; Leduc, N.; Lemoine, R.; Sakr, S.; Vian, A.; Pelleschi-Travier, S. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ. 2011, 34, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Dierck, R.; De Keyser, E.; De Riek, J.; Dhooghe, E.; Van Huylenbroeck, J.; Prinsen, E.; Van Der Straeten, D. Change in Auxin and Cytokinin Levels Coincides with Altered Expression of Branching Genes during Axillary Bud Outgrowth in Chrysanthemum. PLoS ONE 2016, 11, e0161732. [Google Scholar] [CrossRef] [PubMed]
- Salam, B.B.; Malka, S.K.; Zhu, X.; Gong, H.; Ziv, C.; Teper-Bamnolker, P.; Ori, N.; Jiang, J.; Eshel, D. Etiolated Stem Branching Is a Result of Systemic Signaling Associated with Sucrose Level. Plant Physiol. 2017, 175, 734–745. [Google Scholar] [CrossRef]
- Fichtner, F.; Barbier, F.F.; Feil, R.; Watanabe, M.; Annunziata, M.G.; Chabikwa, T.G.; Höfgen, R.; Stitt, M.; Beveridge, C.A.; Lunn, J.E. Trehalose 6-phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.). Plant J. 2017, 92, 611–623. [Google Scholar] [CrossRef]
- Moreno-Pachon, N.M.; Mutimawurugo, M.-C.; Heynen, E.; Sergeeva, L.; Benders, A.; Blilou, I.; Hilhorst, H.W.M.; Immink, R.G.H. Role of Tulipa gesneriana TEOSINTE BRANCHED1 (TgTB1) in the control of axillary bud outgrowth in bulbs. Plant Reprod. 2017, 31, 145–157. [Google Scholar] [CrossRef]
- Wang, M.; Pérez-Garcia, M.-D.; Davière, J.-M.; Barbier, F.; Ogé, L.; Gentilhomme, J.; Voisine, L.; Péron, T.; Launay-Avon, A.; Clément, G.; et al. Outgrowth of the axillary bud in rose is controlled by sugar metabolism and signalling. J. Exp. Bot. 2021, 72, 3044–3060. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Salam, B.B.; Barbier, F.; Danieli, R.; Teper-Bamnolker, P.; Ziv, C.; Spíchal, L.; Aruchamy, K.; Shnaider, Y.; Leibman, D.; Shaya, F.; et al. Sucrose promotes stem branching through cytokinin. Plant Physiol. 2021, 185, 1708–1721. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.; Govindarajulu, R.; Feil, R.; Siddoway, M.L.; Nielsen, T.; Lunn, J.E.; Hawkins, J.; Whipple, C.; Chuck, G. The regulatory landscape of a core maize domestication module controlling bud dormancy and growth repression. Nat. Commun. 2019, 10, 3810. [Google Scholar] [CrossRef]
- Otori, K.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci. Biotechnol. Biochem. 2017, 81, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.D.J. Apical Dominance. Annu. Rev. Plant Physiol. 1975, 26, 341–367. [Google Scholar] [CrossRef]
- Kebrom, T.H. A Growing Stem Inhibits Bud Outgrowth—The Overlooked Theory of Apical Dominance. Front. Plant Sci. 2017, 8, 1874. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007, 581, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Xue, X.; Wang, J.; Shukla, D.; Cheung, L.S.; Chen, L.-Q. When SWEETs Turn Tweens: Updates and Perspectives. Annu. Rev. Plant Biol. 2022, 73, 379–403. [Google Scholar] [CrossRef]
- Saint-Oyant, L.H.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef]
- Peng, Q.; Cai, Y.; Lai, E.; Nakamura, M.; Liao, L.; Zheng, B.; Ogutu, C.; Cherono, S.; Han, Y. The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple. BMC Plant Biol. 2020, 20, 191. [Google Scholar] [CrossRef]
- Zhen, Q.; Fang, T.; Peng, Q.; Liao, L.; Zhao, L.; Owiti, A.; Han, Y.; Zhen, Q.; Fang, T.; Peng, Q.; et al. Developing gene-tagged molecular markers for evaluation of genetic association of apple SWEET genes with fruit sugar accumulation. Hortic. Res. 2018, 5, 14. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Lin, I.W.; Qu, X.-Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A Cascade of Sequentially Expressed Sucrose Transporters in the Seed Coat and Endosperm Provides Nutrition for the Arabidopsis Embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Wang, S.; Yokosho, K.; Guo, R.; Whelan, J.; Ruan, Y.-L.; Ma, J.F.; Shou, H. The Soybean Sugar Transporter GmSWEET15 Mediates Sucrose Export from Endosperm to Early Embryo. Plant Physiol. 2019, 180, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.-S. SWEET 11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Peng, B.; Song, A.; Jiang, J.; Chen, F. Sugar Transporter, CmSWEET17, Promotes Bud Outgrowth in Chrysanthemum Morifolium. Genes 2020, 11, 26. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef]
- Kanno, Y.; Oikawa, T.; Chiba, Y.; Ishimaru, Y.; Shimizu, T.; Sano, N.; Koshiba, T.; Kamiya, Y.; Ueda, M.; Seo, M. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat. Commun. 2016, 7, 13245. [Google Scholar] [CrossRef]
- Fichtner, F.; Barbier, F.F.; Annunziata, M.G.; Feil, R.; Olas, J.J.; Mueller-Roeber, B.; Stitt, M.; Beveridge, C.A.; Lunn, J.E. Regulation of shoot branching in arabidopsis by trehalose 6-phosphate. New Phytol. 2020, 229, 2135–2151. [Google Scholar] [CrossRef] [PubMed]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef]
- Schneider, A.; Godin, C.; Boudon, F.; Demotes-Mainard, S.; Sakr, S.; Bertheloot, J. Light Regulation of Axillary Bud Outgrowth Along Plant Axes: An Overview of the Roles of Sugars and Hormones. Front. Plant Sci. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins Are Initial Targets of Light in the Control of Bud Outgrowth. Plant Physiol. 2016, 172, 489–509. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; E Koch, K.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Li, C.; Huang, D.; Huang, Y.; Zhang, W.; Pan, X.; Li, W.; Chen, J.; Li, C.; et al. Genome-wide identification of SWEET gene family and the sugar transport function of three candidate genes during female flower bud induction stage of Juglans sigillata Dode. Plant Physiol. Biochem. 2024, 217, 109288. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Lu, C.; Nie, P.; Hu, M.; Zhou, X.; Chen, X.; Wang, W. Predominantly symplastic phloem unloading of photosynthates maintains efficient starch accumulation in the cassava storage roots (Manihot esculenta Crantz). BMC Plant Biol. 2021, 21, 318. [Google Scholar] [CrossRef]
- Paterlini, A.; Dorussen, D.; Fichtner, F.; van Rongen, M.; Delacruz, R.; Vojnović, A.; Helariutta, Y.; Leyser, O. Callose accumulation in specific phloem cell types reduces axillary bud growth in Arabidopsis thaliana. New Phytol. 2021, 231, 516–523. [Google Scholar] [CrossRef]
- van der Schoot, C.; Rinne, P.L. Dormancy cycling at the shoot apical meristem: Transitioning between self-organization and self-arrest. Plant Sci. 2011, 180, 120–131. [Google Scholar] [CrossRef]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in Plant Metabolism and Development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef]
- Jang, J.C.; León, P.; Zhou, L.; Sheen, J. Hexokinase as a sugar sensor in higher plants. Plant Cell 1997, 9, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Cao, D.; Fichtner, F.; Weiste, C.; Perez-Garcia, M.; Caradeuc, M.; Le Gourrierec, J.; Sakr, S.; Beveridge, C.A. HEXOKINASE1 signalling promotes shoot branching and interacts with cytokinin and strigolactone pathways. New Phytol. 2021, 231, 1088–1104. [Google Scholar] [CrossRef]
- Wang, M.; Le Moigne, M.-A.; Bertheloot, J.; Crespel, L.; Perez-Garcia, M.-D.; Ogé, L.; Demotes-Mainard, S.; Hamama, L.; Davière, J.-M.; Sakr, S. BRANCHED1: A Key Hub of Shoot Branching. Front. Plant Sci. 2019, 10, 76. [Google Scholar] [CrossRef]
- Otori, K.; Tanabe, N.; Tamoi, M.; Shigeoka, S.; Otori, K.; Tanabe, N.; Tamoi, M.; Shigeoka, S. Sugar Transporter Protein 1 (STP1) contributes to regulation of the genes involved in shoot branching via carbon partitioning in Arabidopsis. Biosci. Biotechnol. Biochem. 2018, 83, 472–481. [Google Scholar] [CrossRef]
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar availability suppresses the auxin-induced strigolactone pathway to promote bud outgrowth. New Phytol. 2019, 225, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Barbier, F.F.; Zhao, J.; Zafar, S.A.; Uzair, M.; Sun, Y.; Fang, J.; Perez-Garcia, M.; Bertheloot, J.; Sakr, S.; et al. Sucrose promotes D53 accumulation and tillering in rice. New Phytol. 2021, 234, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chabikwa, T.; Barbier, F.; A Dun, E.; Fichtner, F.; Dong, L.; Kerr, S.C.; A Beveridge, C. Auxin-independent effects of apical dominance induce changes in phytohormones correlated with bud outgrowth. Plant Physiol. 2023, 192, 1420–1434. [Google Scholar] [CrossRef]
- Roitsch, T.; Ehneß, R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000, 32, 359–367. [Google Scholar] [CrossRef]
- Werner, T.; Holst, K.; Pörs, Y.; Guivarc’H, A.; Mustroph, A.; Chriqui, D.; Grimm, B.; Schmülling, T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008, 59, 2659–2672. [Google Scholar] [CrossRef]
- McIntyre, K.E.; Bush, D.R.; Argueso, C.T. Cytokinin Regulation of Source-Sink Relationships in Plant-Pathogen Interactions. Front. Plant Sci. 2021, 12, 677585. [Google Scholar] [CrossRef]
- Li, Y.-M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.-S. The Molecular Regulation of Carbon Sink Strength in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An Update on the Signals Controlling Shoot Branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Dun, E.A.; Ferguson, B.J.; Beveridge, C.A. Apical Dominance and Shoot Branching. Divergent Opinions or Divergent Mechanisms? Plant Physiol. 2006, 142, 812–819. [Google Scholar] [CrossRef]
- Oparka, K.; Duckett, C.; Prior, D.; Fisher, D. Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 1994, 6, 759–766. [Google Scholar] [CrossRef]

| Factors | Processes | Species | References |
|---|---|---|---|
| Developmental stages | Apical dominance | Several species (Arabidopsis, pea, tomato, Petunia, rose, etc.) | For review, see [33] |
| Flowering | Tomato and tobacco | [34] | |
| Rose and strawberry | [35,36] | ||
| Fruits load | Arabidopsis | [37] | |
| Coffee | [38] | ||
| Olive and Citrus | [39] | ||
| Light | Light quality | Petunia | [40] |
| Rose | [41,42] | ||
| Dallis and ryegrass | [43] | ||
| Sorghum | [44,45] | ||
| Arabidopsis | [46] | ||
| Light intensity | Rose | [41,47,48,49] | |
| Spring wheat | [50] | ||
| Photoperiod | Pea | [51] | |
| Water stress | Water supply | Rose Arabidopsis | [47,52] [18] |
| Mineral nutrition | Nitrogen nutrition | Arabidopsis | [53] |
| Poplar | [54] | ||
| Rice | [55,56,57,58] | ||
| Rose | [59] | ||
| Phosphorus nutrition | Rice | [60,61] | |
| Wheat | [62] | ||
| Sulfur nutrition | Arabidopsis | [63] | |
| Temperature | High temperature | Citrus lemon | [64] |
| CO2 | High CO2 | Pea | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doidy, J.; Wang, Y.; Gouaille, L.; Goma-Louamba, I.; Jiang, Z.; Pourtau, N.; Le Gourrierec, J.; Sakr, S. Sugar Transport and Signaling in Shoot Branching. Int. J. Mol. Sci. 2024, 25, 13214. https://doi.org/10.3390/ijms252313214
Doidy J, Wang Y, Gouaille L, Goma-Louamba I, Jiang Z, Pourtau N, Le Gourrierec J, Sakr S. Sugar Transport and Signaling in Shoot Branching. International Journal of Molecular Sciences. 2024; 25(23):13214. https://doi.org/10.3390/ijms252313214
Chicago/Turabian StyleDoidy, Joan, Yuhui Wang, Léo Gouaille, Ingrid Goma-Louamba, Zhengrong Jiang, Nathalie Pourtau, José Le Gourrierec, and Soulaiman Sakr. 2024. "Sugar Transport and Signaling in Shoot Branching" International Journal of Molecular Sciences 25, no. 23: 13214. https://doi.org/10.3390/ijms252313214
APA StyleDoidy, J., Wang, Y., Gouaille, L., Goma-Louamba, I., Jiang, Z., Pourtau, N., Le Gourrierec, J., & Sakr, S. (2024). Sugar Transport and Signaling in Shoot Branching. International Journal of Molecular Sciences, 25(23), 13214. https://doi.org/10.3390/ijms252313214







