Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Ziziphora Essential Oil—Bioactive Componenet of KAO–ZEO Gel Formulation
2.2. Antimicrobial Activity of KAO, ZEO and CLEANED CLAY
2.3. Results of Formulation Assessment
2.4. Antimicrobial Activity of ZEO–KAO Mixture
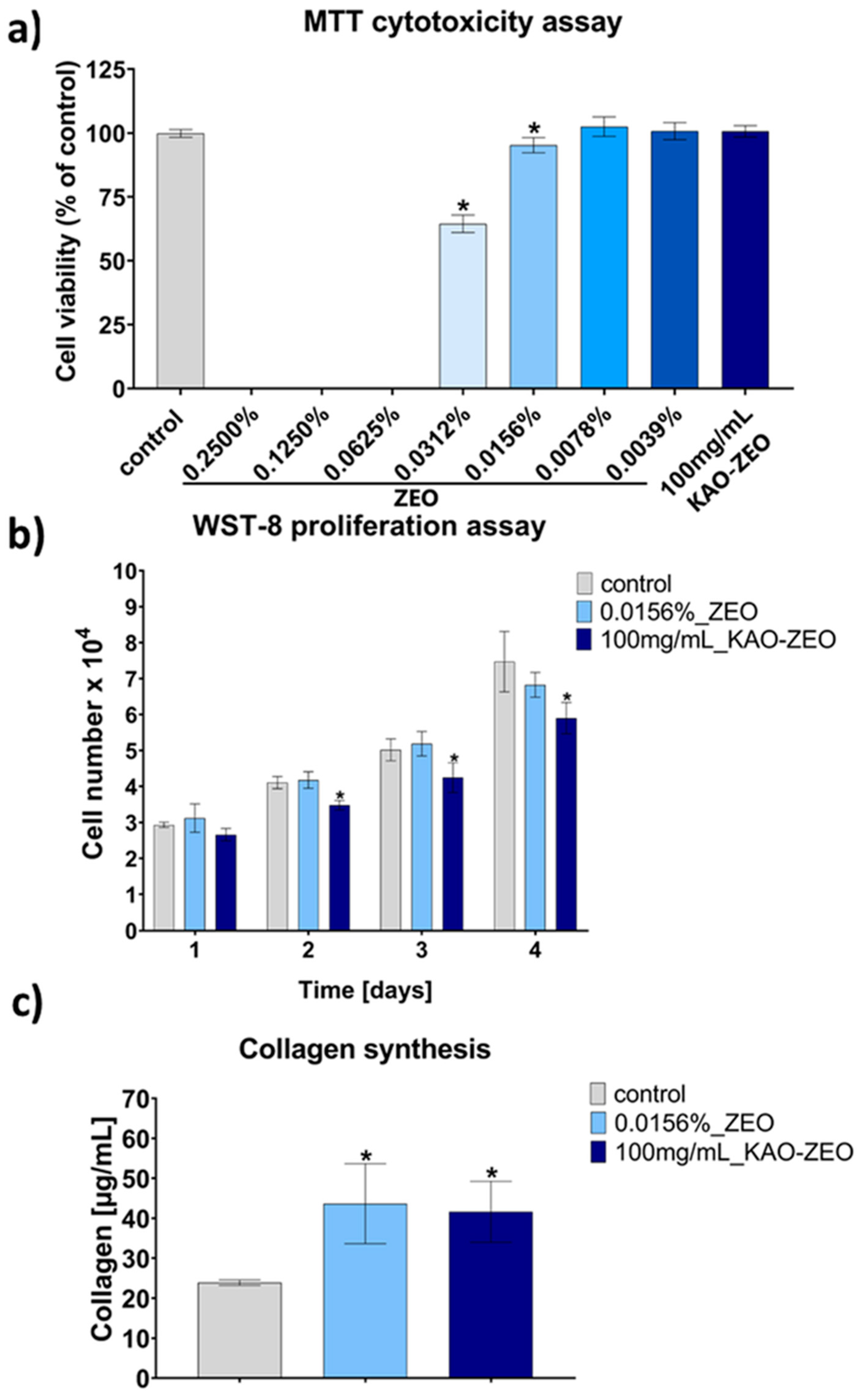
2.5. Evaluation of the Cellular Response to ZEO and KAO–ZEO In Vitro
3. Materials and Methods
3.1. Micronized Kaolinite Clay Powder Preparation
3.2. Plant Material
3.3. Gel Ingredients
3.4. GC–MS
3.5. Kaolin–Ziziphora Essential Oil (KAO–ZEO) Therapeutic Mix Preparation
3.6. Formulation Assessment
3.7. Antimicrobial Activity Testing
3.8. Evaluation of the Cellular Response to ZEO and KAO–ZEO In Vitro
3.8.1. Cell Culture Experiments
3.8.2. Screening Cytotoxicity Test
3.8.3. Cell Proliferation Assessment
3.8.4. Evaluation of Collagen Synthesis
3.8.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Bayır, A.; Eryılmaz, M.; Demirbilek, M.; Denkbaş, E.B.; Arzıman, I.; Durusu, M. Comparison of the topical haemostatic efficacy of nano-micro particles of clinoptilolite and kaolin in a rat model of haemorrhagic injury. Eur. J. Trauma Emerg. Surg. 2016, 42, 77–866, Erratum in Eur. J. Trauma Emerg. Surg. 2016, 42, 793. [Google Scholar] [CrossRef] [PubMed]
- Christidis, G.; Knapp, C.; Venieri, D.; Gounaki, I.; Elgy, C.; Valsami-Jones, E.; Photos-Jones, E. The interweaving roles of mineral and microbiome in shaping the antibacterial activity of archaeological medicinal clays. J. Ethnopharmacol. 2020, 260, 112894. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wang, Z.; Huang, Z.; Xie, Z.; Xia, L.; Zhang, Y. Clays and Wound Healing. Materials 2024, 17, 1691. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-Montmorillonite Composite Sponge for Safe and Effective Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef]
- Long, M.; Liu, Q.; Wang, D.; Wang, J.; Zhang, Y.; Tang, A.; Liu, N.; Bui, B.; Chen, W.; Yang, H. A new nanoclay-based bifunctional hybrid fiber membrane with hemorrhage control and wound healing for emergency self-rescue. Mater. Today Adv. 2021, 12, 100190. [Google Scholar] [CrossRef]
- Šmejkal, K.; Malaník, M.; Zhaparkulova, K.; Sakipova, Z.; Ibragimova, L.; Ibadullaeva, G.; Žemlička, M. Kazakh Ziziphora Species as Sources of Bioactive Substances. Molecules 2016, 21, 826. [Google Scholar] [CrossRef]
- Ivkin, D.; Zhaparkulova, K.; Olusheva, I.; Serebryakov, E.; Smirnov, S.; Semivelichenko, E.; Grishina, A.Y.; Karpov, A.; Eletckaya, E.; Kozhanova, K.; et al. Chemical composition and cardiotropic activity of Ziziphora clinopodioides subsp. bungeana (Juz.) Rech.f. J. Ethnopharmacol. 2023, 315, 116660. [Google Scholar] [CrossRef]
- Zhaparkulova, K.; Karaubayeva, A.; Sakipova, Z.; Biernasiuk, A.; Gaweł-Bęben, K.; Laskowski, T.; Kusniyeva, A.; Omargali, A.; Bekezhanova, T.; Ibragimova, L.; et al. Multidirectional Characterization of Phytochemical Profile and Health-Promoting Effects of Ziziphora bungeana Juz. Extracts. Molecules 2022, 27, 8994. [Google Scholar] [CrossRef]
- Sonboli, A.; Mirjalili, M.H.; Hadian, J.; Ebrahimi, S.N.; Yousefzadi, M. Antibacterial activity and composition of the essential oil of Ziziphora clinopodioides subsp. bungeana (Juz.) Rech. f. from Iran. Z. Naturforschung C J. Biosci. 2006, 61, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Nabokov, N.P.; Sukhanov, A.E.; Naumov, A.I. Alekseev deposits as a new raw-materials base for high-quality kaolins. Glas. Ceram. 1965, 22, 584–586. [Google Scholar] [CrossRef]
- Karaubayeva, A.A.; Omarova, R.A.; Sakipova, Z.B.; Ibragimova, L.N.; Espenbetov, A.A.; Ibadullaeva, G.S.; Gladukh, E.V.; Turgumbayeva, A.A. Physical-chemical investigations of clay minerals of the Alekseyevskoye field in the Republic of Kazakhstan. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 3042–3055. [Google Scholar]
- Kovzalenko, V.A.; Sarsenbay, G.; Sadykov, N.M.-K.; Imangalieva, L.M. Kaolins-Substandard Aluminosilicate Materials. Integr. Use Miner. Raw Mater. 2015, 3, 32–37. Available online: https://kims-imio.kz/wp-content/uploads/2018/03/kims-34-39.pdf (accessed on 21 September 2024).
- Zhaparkulova, K.; Karaybayeva, A.; Nussirbekova, A.; Kurbanov, G.; Seitaliyeva, A.; Ibragimova, L.; Sakipova, Z.; Satbayeva, E.; Ross, S. Biological Activity and Preclinical Study of Toxcicologycal Action of the Essential Oil of Ziziphora bungeana Juz. from Kazakhstan. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 275–280. [Google Scholar]
- Kazeminia, M.; Gandomi, H.; Koohi, M.K.; Noori, N.; Khanjari, A.; Ehterami, A. Optimization of Ziziphora clinopodioides L. essential oil nanoencapsulation in chitosan nanocomplex by response surface methodology. Int. J. Biol. Macromol. 2024, 265, 131114. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Pavela, R.; Bonacucina, G.; Baldassarri, C.; Spinozzi, E.; Torresi, J.; Petrelli, R.; Morshedloo, M.R.; Maggi, F.; Benelli, G.; et al. Development, characterization, insecticidal and sublethal effects of Bunium persicum and Ziziphora clinopodioides-based essential oil nanoemulsions on Culex quinquefasciatus. Ind. Crops Prod. 2022, 186, 115249. [Google Scholar] [CrossRef]
- Khankeshizadeh, S.S.; Rahman, A.; Naderi, F.; Khorshidian, N.; Mohammadi, M. Ziziphora tenuior L. Essential Oil as a Bio-Based Antimicrobial and Plant-Based Preservative for Increasing the Shelf Life of Mayonnaise. J. Food Qual. 2024, 2024, 6774892. [Google Scholar] [CrossRef]
- Hazrati, S.; Govahi, M.; Sedaghat, M.; Beyraghdar; Kashkooli, A. A comparative study of essential oil profile, antibacterial and antioxidant activities of two cultivated Ziziphora species (Z. clinopodioides and Z. tenuior). Ind. Crops Prod. 2020, 157, 112942. [Google Scholar] [CrossRef]
- Huang, X.; Hu, B.; Zhang, X.; Fan, P.; Chen, Z.; Wang, S. Recent advances in the application of clay-containing hydrogels for hemostasis and wound healing. Expert Opin. Drug Deliv. 2024, 21, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. The antibacterial effect of Ziziphora clinopodioides essential oil and nisin against Salmonella typhimurium and Staphylococcus aureus in doogh, a yoghurt-based Iranian drink. Vet. Res. Forum 2016, 7, 213–219. [Google Scholar]
- Ovincy, C.; Babel, S.; Baral, S.; Poudel, S.; Jain, S. Clay Therapy in Wound Healing: A Brief Review of the Literature. J. Wound Manag. Res. 2024, 20, 1–8. [Google Scholar] [CrossRef]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial Activity of Aluminum in Clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef]
- Hao, W.; Flynn, S.L.; Kashiwabara, T.; Alam, S.; Bandara, S.; Swaren, L.; Robbins, L.J.; Alessi, D.S.; Konhauser, K.O. The impact of ionic strength on the proton reactivity of clay minerals. Chem. Geol. 2019, 529, 119294. [Google Scholar] [CrossRef]
- Lindqvist, L.M.; Tandoc, K.; Topisirovic, I.; Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev. 2018, 48, 104–111. [Google Scholar] [CrossRef]
- Dolfi, S.C.; Chan, L.L.-Y.; Qiu, J.; Tedeschi, P.M.; Bertino, J.R.; Hirshfield, K.M.; Oltvai, Z.N.; Vazquez, A. The metabolic demands of cancer cells are coupled to their size and protein synthesis rates. Cancer Metab. 2013, 1, 20. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, M.J.; Park, M.; Ahn, B.; Yu, W.; An, S.; An, B. Essential oils extracted from nine different plants exhibit differential effects on skin antioxidation and elasticity. FEBS Open Bio 2024, 14, 613–625. [Google Scholar] [CrossRef]
- Salem, M.A.; Manaa, E.G.; Osama, N.; Aborehab, N.M.; Ragab, M.F.; Haggag, Y.A.; Ibrahim, M.T.; Hamdan, D.I. Coriander (Coriandrum sativum L.) essential oil and oil-loaded nano-formulations as an anti-aging potentiality via TGFβ/SMAD pathway. Sci. Rep. 2022, 12, 6578. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hwang, E.; Seo, S.A.; Cho, J.-G.; Yang, J.-E.; Yi, T.-H. Eucalyptus globulus extract protects against UVB-induced photoaging by enhancing collagen synthesis via regulation of TGF-β/Smad signals and attenuation of AP-1. Arch. Biochem. Biophys. 2018, 637, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The Use of Some Clay Minerals as Natural Resources for Drug Carrier Applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Sadhra, S.; Kurmi, O.; Mohammed, N.; Foulds, I. Protection afforded by controlled application of a barrier cream: A study in a workplace setting. Br. J. Dermatol. 2014, 171, 813–818. [Google Scholar] [CrossRef]
- Schliemann, S.; Petri, M.; Elsner, P. Preventing irritant contact dermatitis with protective creams: Influence of the application dose. Contact Dermat. 2014, 70, 19–26. [Google Scholar] [CrossRef]
- Williams, L.B. Geomimicry: Harnessing the antibacterial action of clays. Clay Miner. 2017, 52, 1–24. [Google Scholar] [CrossRef][Green Version]
- Abdel-Khalek, N.A.; Selim, K.A.; Mohammed, S.E.; El-Hendawy, H.H.; Elbaz, R.M. Interaction between kaolinite and Staphylococcus gallinarum bacteria. J. Min. World Express 2014, 3, 46–52. [Google Scholar] [CrossRef]
- Bianchi, T.; Wolcott, R.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rita, Z.R.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef]
- Standard of Organization ST RK 1509-1910-02-GP-02-2015; Purified Clay. NJSC Asfendiyarov Kazakh National Medical University: Almaty, Republic of Kazakhstan, 2015.
- Okińczyc, P.; Widelski, J.; Nowak, K.; Radwan, S.; Włodarczyk, M.; Kuś, P.M.; Susniak, K.; Korona-Głowniak, I. Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts. Molecules 2024, 29, 437. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-12; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 1999.
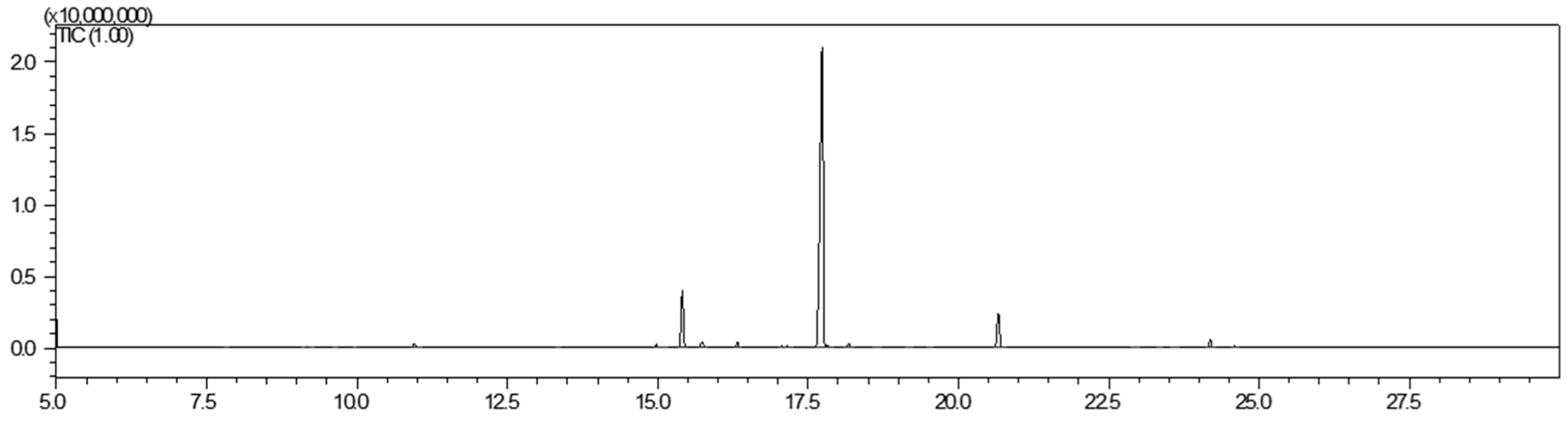

| No | RT | RIexp | RIdb | Area (%) | Name |
|---|---|---|---|---|---|
| 1 | 7.821 | 931 | 936 | 0.19 | α-pinene |
| 2 | 9.094 | 971 | 978 | 0.09 | β-pinene |
| 3 | 9.249 | 976 | 973 | 0.25 | Sabinene |
| 4 | 9.636 | 988 | 987 | 0.16 | β-myrcene |
| 5 | 9.960 | 998 | 979 | 0.11 | 3-octanol |
| 6 | 10.955 | 1028 | 1018 | 0.73 | Limonene |
| 7 | 11.040 | 1031 | 1024 | 0.15 | 1,8-cineol |
| 8 | 13.344 | 1100 | 1082 | 0.11 | Linalool |
| 9 | 14.977 | 1152 | 1150 | 0.58 | Borneol |
| 10 | 15.118 | 1156 | 1136 | 0.12 | Menthone |
| 11 | 15.408 | 1165 | 1146 | 10.28 | iso-menthone |
| 12 | 15.734 | 1175 | 1179 | 1.26 | Isopulegone |
| 13 | 16.321 | 1194 | 1176 | 1.06 | Isomenthol |
| 14 | 17.160 | 1221 | 1220 | 0.36 | p-isopropylbenzaldehyd |
| 15 | 17.734 | 1240 | 1216 | 72.98 | Pulegone |
| 16 | 19.514 | 1300 | 1278 | 0.13 | Carvacrol |
| 17 | 20.665 | 1340 | 1318 | 6.96 | Piperitenone |
| 18 | 22.937 | 1421 | 1421 | 0.29 | Caryophyllene |
| Chemicals Microorganism | ZEO | KAO | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| Gram-positive bacteria | ||||
| S. aureus ATCC 25923 | 5 | 10 | 10 | >20 |
| S. aureus ATCC BA1707 | 5 | 10 | 10 | >20 |
| S. epidermidis ATCC 12228 | 5 | 10 | 10 | >20 |
| M. luteus ATCC 10240 | 2.5 | 5 | 5 | 20 |
| B. cereus ATCC 10876 | 5 | 20 | 10 | >20 |
| E. faecalis ATCC 29212 | 5 | 20 | 10 | >20 |
| S. pneumoniae ATCC 49619 | 1.25 | 1.25 | 5 | 5 |
| S. pyogenes ATCC 19615 | 2.5 | 2.5 | 5 | 5 |
| Gram-negative bacteria | ||||
| S. typhimurium ATCC 14028 | 5 | 10 | 10 | >20 |
| E. coli ATCC 25922 | 2.5 | 2.5 | 10 | 20 |
| P. mirabilis ATCC 12453 | 5 | 5 | 10 | >20 |
| K. pneumoniae ATCC 13883 | 5 | 10 | 10 | >20 |
| P. aeruginosa ATCC 9027 | 5 | 5 | 10 | 20 |
| M. catarrhalis ATCC 25238 | 0.313 | 0.313 | 2.5 | 10 |
| Yeasts | ||||
| C. glabrata ATCC 90030 | 2.5 | 5 | 10 | 10 |
| C. albicans ATCC 102231 | 1.25 | 2.5 | 5 | 10 |
| C. parapsilosis ATCC 22019 | 2.5 | 5 | 5 | 10 |
| C. krusei ATCC 14243 | 1.25 | 2.5 | 5 | 10 |
| C. auris CDC B11903 | 1.25 | 2.5 | 5 | 10 |
| Chemicals Microorganism | KAO–ZEO Mixture | ZEO Mixture | KAO Mixture | Excipients |
|---|---|---|---|---|
| Gram-positive bacteria | ||||
| S. aureus ATCC 25923 | 25 | >100 | 25 | >100 |
| S. aureus ATCC BA1707 | 25 | >100 | 25 | >100 |
| S. aureus ATCC 43300 | 0.32 | >100 | 0.32 | >100 |
| S. epidermidis ATCC 12228 | 0.16 | 50 | 25 | >100 |
| M. luteus ATCC 10240 | 0.32 | 0.32 | 0.32 | >100 |
| E. faecalis ATCC 29212 | 0.32 | >100 | 0.32 | >100 |
| S. pyogenes ATCC 19615 | >100 | >100 | >100 | >100 |
| Gram-negative bacteria | ||||
| E. coli ATCC 25922 | 25 | >100 | 25 | >100 |
| P. aeruginosa ATCC 9027 | 50 | >100 | 50 | >100 |
| Yeasts | ||||
| C. glabrata ATCC 90030 | 25 | >100 | 100 | >100 |
| C. albicans ATCC 102231 | 0.32 | >100 | 100 | >100 |
| C. parapsilosis ATCC 22019 | 0.08 | 50 | 100 | >100 |
| Ingredient | Content (%; w/w) |
|---|---|
| white micronized kaolinite | 30 |
| Zizphora essential oil | 0.25 |
| ammonium acryldimethyltaurate/N-vinyl pyrrolidone copolymer | 0.5 |
| xanthan gum | 0.5 |
| polyglyceryl 4-caprate | 0.5 |
| synthetic amorphous magnesium aluminosilicate | 0.6 |
| glycerin | 10.0 |
| water | 57.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaubayeva, A.A.; Bekezhanova, T.; Zhaparkulova, K.; Susniak, K.; Sobczynski, J.; Kazimierczak, P.; Przekora, A.; Skalicka-Wozniak, K.; Kulinowski, Ł.; Glowniak-Lipa, A.; et al. Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds. Int. J. Mol. Sci. 2024, 25, 13192. https://doi.org/10.3390/ijms252313192
Karaubayeva AA, Bekezhanova T, Zhaparkulova K, Susniak K, Sobczynski J, Kazimierczak P, Przekora A, Skalicka-Wozniak K, Kulinowski Ł, Glowniak-Lipa A, et al. Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds. International Journal of Molecular Sciences. 2024; 25(23):13192. https://doi.org/10.3390/ijms252313192
Chicago/Turabian StyleKaraubayeva, Aigerim A., Tolkyn Bekezhanova, Karlygash Zhaparkulova, Katarzyna Susniak, Jan Sobczynski, Paulina Kazimierczak, Agata Przekora, Krystyna Skalicka-Wozniak, Łukasz Kulinowski, Anna Glowniak-Lipa, and et al. 2024. "Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds" International Journal of Molecular Sciences 25, no. 23: 13192. https://doi.org/10.3390/ijms252313192
APA StyleKaraubayeva, A. A., Bekezhanova, T., Zhaparkulova, K., Susniak, K., Sobczynski, J., Kazimierczak, P., Przekora, A., Skalicka-Wozniak, K., Kulinowski, Ł., Glowniak-Lipa, A., Sakipova, Z. B., & Korona-Głowniak, I. (2024). Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds. International Journal of Molecular Sciences, 25(23), 13192. https://doi.org/10.3390/ijms252313192









