Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape
Abstract
1. Introduction
2. Results and Discussion
2.1. Plant Growth-Promoting Effects
2.2. Physico-Chemical Analysis of Rhizosphere Soil
2.3. Bacterial Community
2.3.1. Alpha Diversity
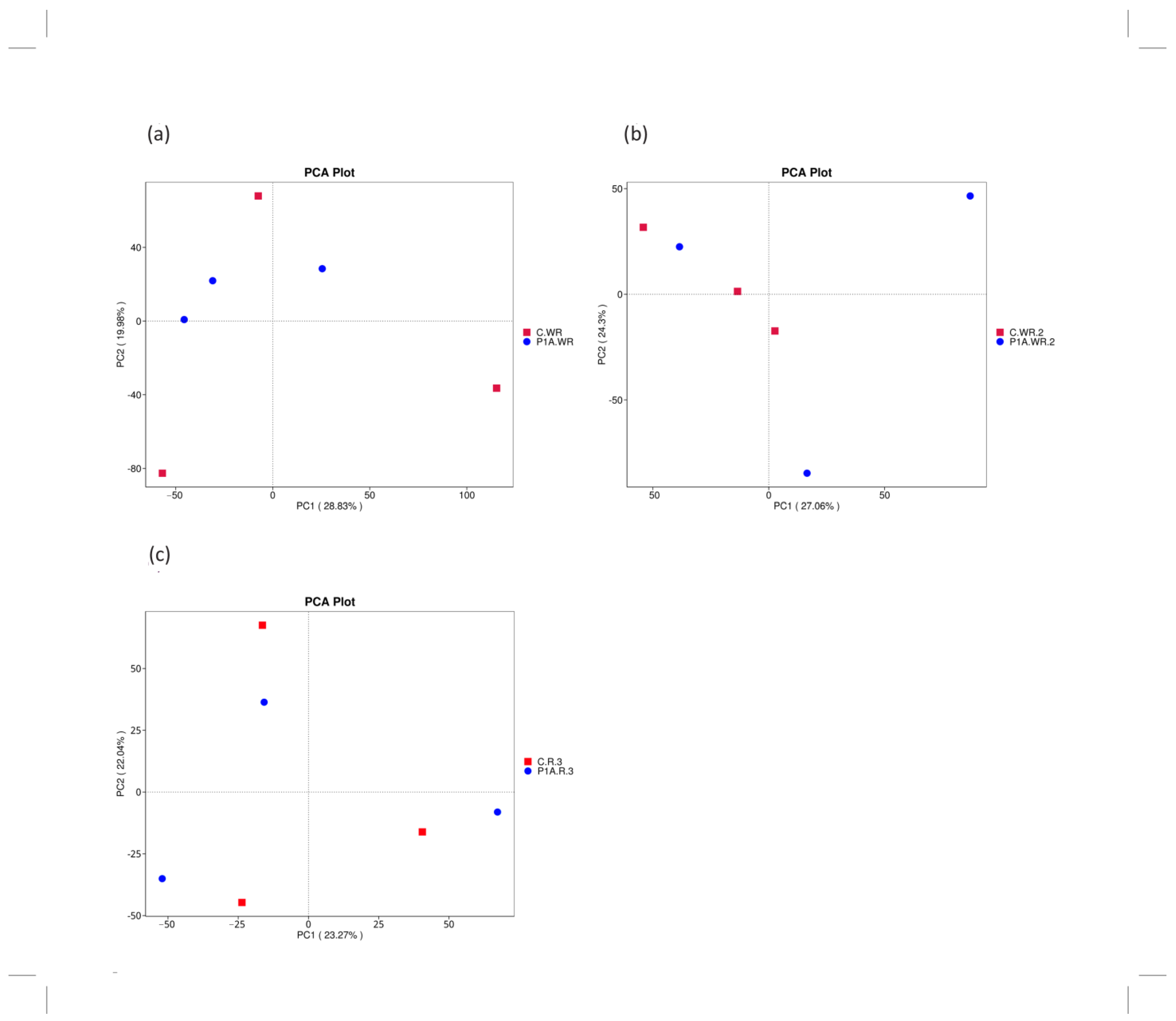
2.3.2. Beta Diversity
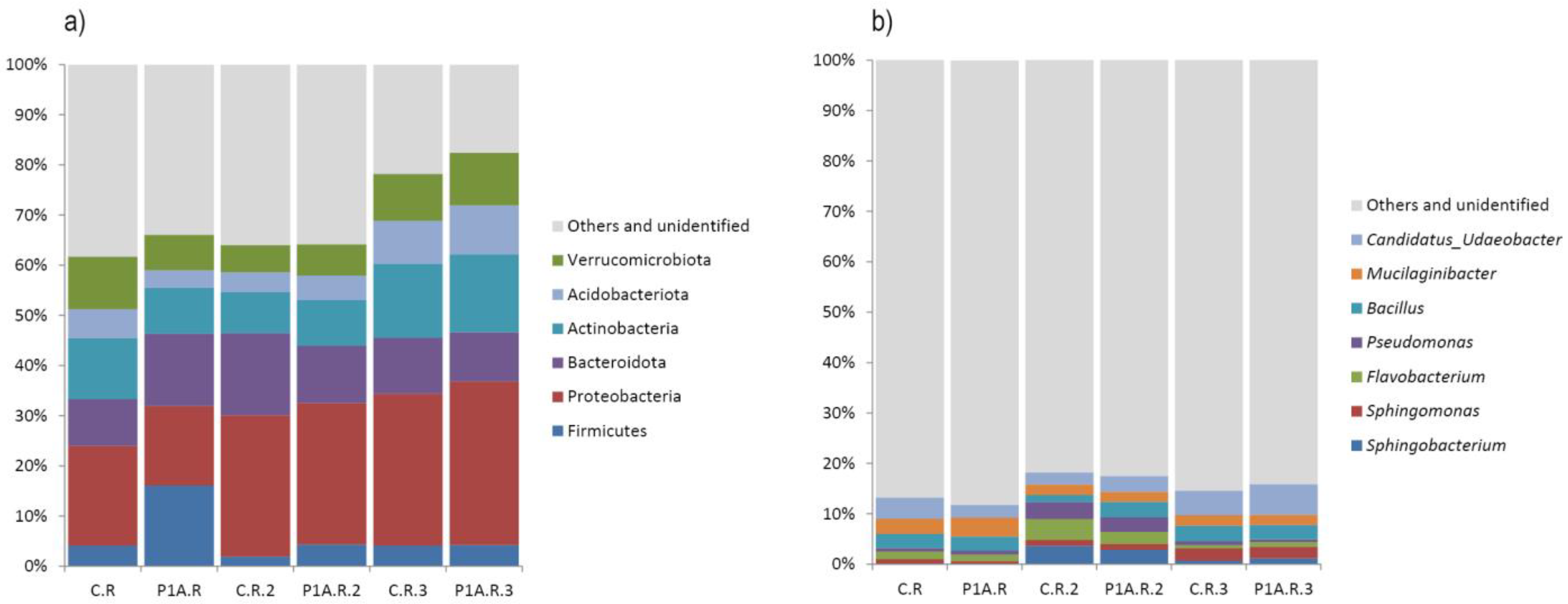
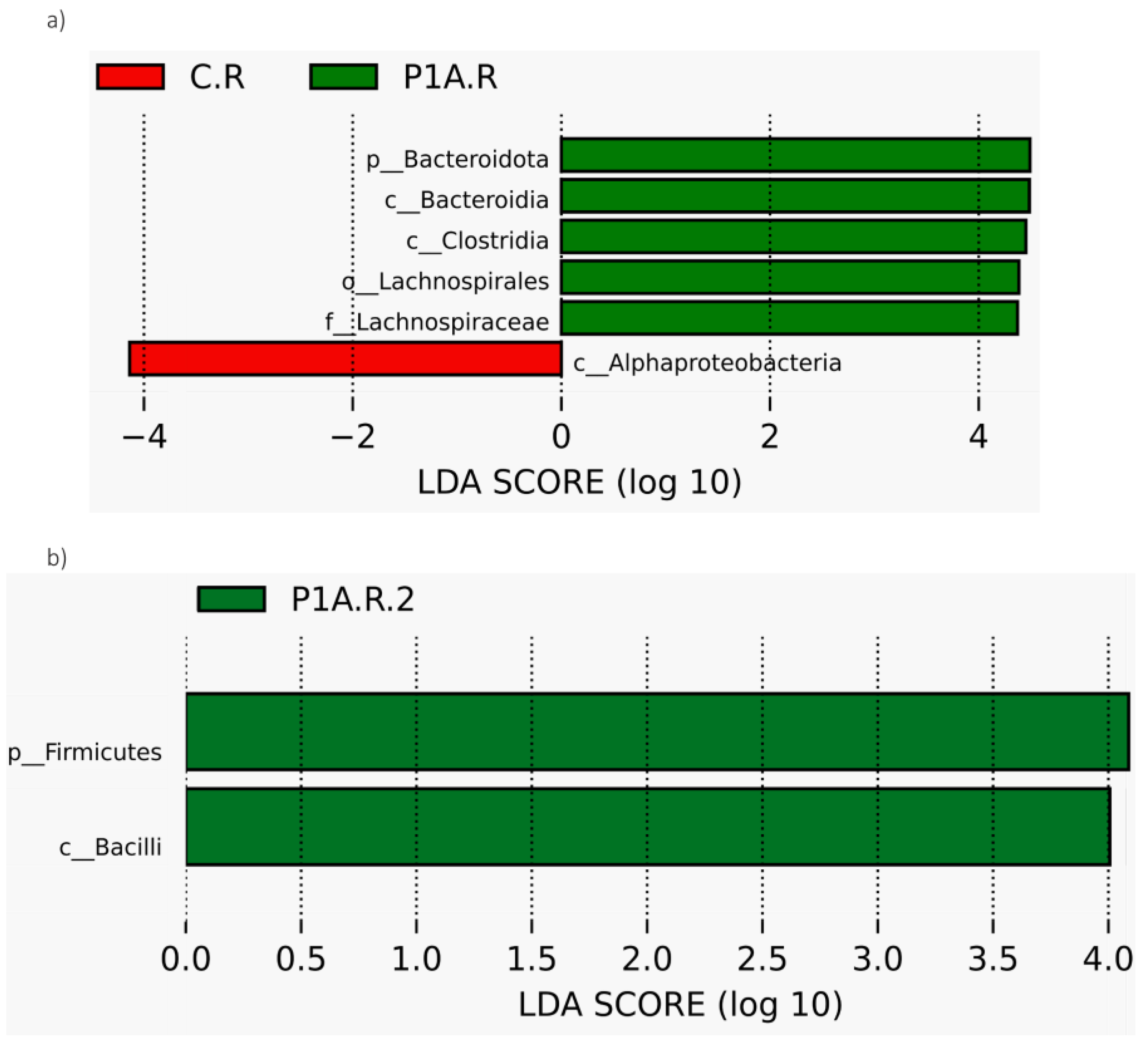
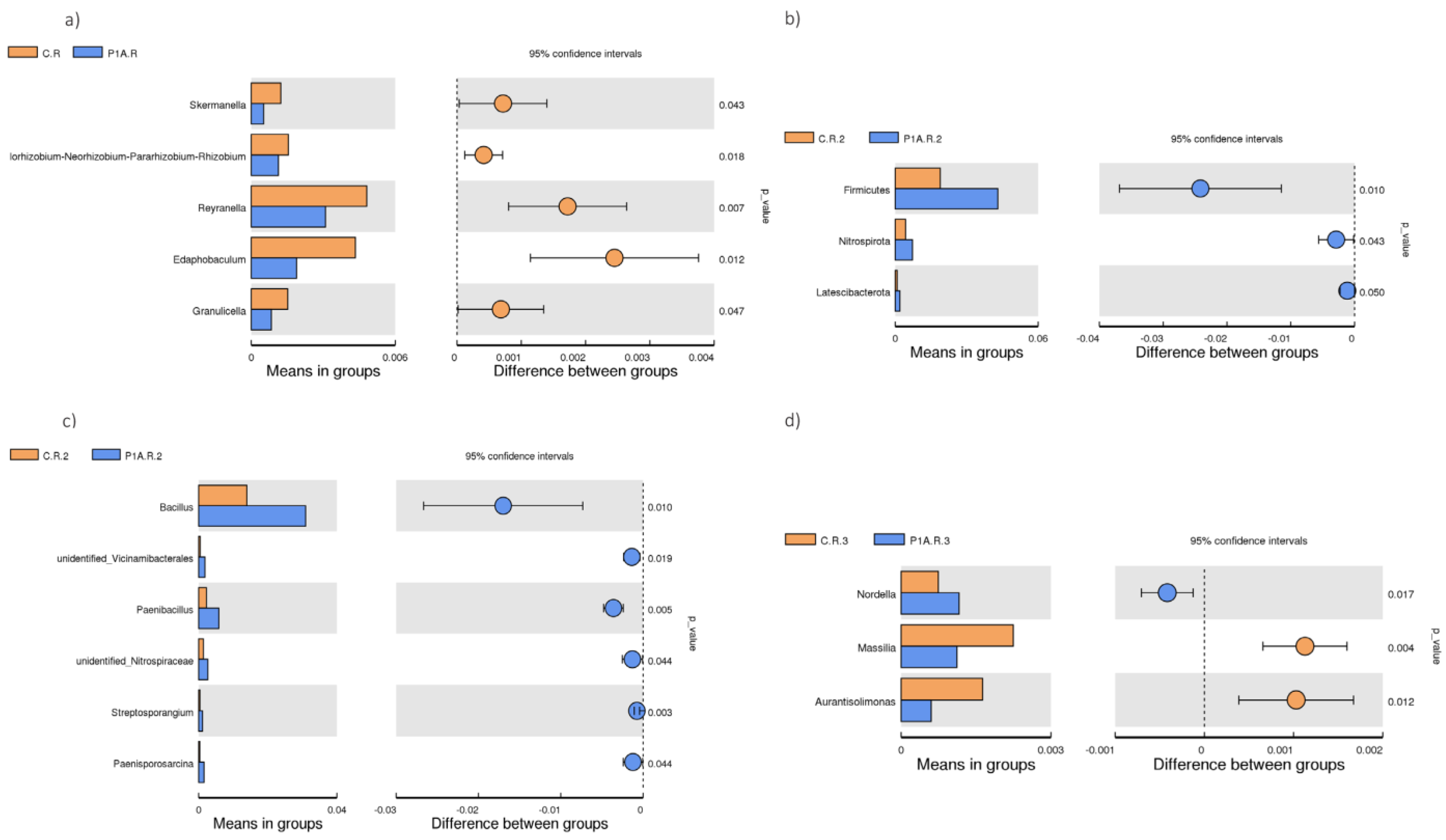
2.3.3. Structure of Bacterial Communities
3. Materials and Methods
3.1. Plant-Growth-Promoting Effects
3.1.1. Isolation, Selection, and Taxonomic Identification of PGPB
3.1.2. IAA Production
3.1.3. Nitrogen Fixation
3.1.4. Phosphate Solubilization
3.1.5. Experimental Treatments and Sampling Procedures
3.2. Physico-Chemical Analysis of Rhizosphere Soil
3.3. Bacterial Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yong, K.J.; Wu, T.Y. Second-Generation Bioenergy from Oilseed Crop Residues: Recent Technologies, Techno-Economic Assessments and Policies. Energy Convers. Manag. 2022, 267, 115869. [Google Scholar] [CrossRef]
- Fang, Y.; Ren, T.; Zhang, S.; Liu, Y.; Liao, S.; Li, X.; Lu, J. Rotation with Oilseed Rape as the Winter Crop Enhances Rice Yield and Improves Soil Indigenous Nutrient Supply. Soil Tillage Res. 2021, 212, 105065. [Google Scholar] [CrossRef]
- Crop Prospects and Food Situation; FAO: Rome, Italy, 2024; ISBN 978-92-5-138629-3.
- Sieling, K.; Kage, H. Efficient N Management Using Winter Oilseed Rape. A Review. Agron. Sustain. Dev. 2010, 30, 271–279. [Google Scholar] [CrossRef]
- Zhan, N.; Xu, K.; Ji, G.; Yan, G.; Chen, B.; Wu, X.; Cai, G. Research Progress in High-Efficiency Utilization of Nitrogen in Rapeseed. Int. J. Mol. Sci. 2023, 24, 7752. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, S.; Wang, H.; Hu, X.; Yang, F.; Tang, M.; Zhang, M.; Zhong, J. Internal Nitrogen and Phosphorus Loading in a Seasonally Stratified Reservoir: Implications for Eutrophication Management of Deep-Water Ecosystems. J. Environ. Manag. 2022, 319, 115681. [Google Scholar] [CrossRef]
- Devlin, M.; Brodie, J. Nutrients and Eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Textbooks in Earth Sciences, Geography and Environment; Springer Nature: Cham, Switzerland, 2023; pp. 75–100. ISBN 978-3-031-10126-7. [Google Scholar]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global Metaanalysis of the Nonlinear Response of Soil Nitrous Oxide (N2O) Emissions to Fertilizer Nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil Acidification from Long-Term Use of Nitrogen Fertilizers on Winter Wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Smagacz, J.; Martyniuk, S. Soil Properties and Crop Yields as Influenced by the Frequency of Straw Incorporation in a Rape-Wheat-Triticale Rotation. J. Water Land Dev. 2023, 56, 1–6. [Google Scholar] [CrossRef]
- Konieczna, A.; Koniuszy, A. Greenhouse gas and ammonia emissions in modeled cereal crop production under Polish agricultural conditions: An example spring barley. J. Water Land Dev. 2024, 61, 39–47. [Google Scholar] [CrossRef]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of Plant Growth-Promoting Bacteria in Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Taxonomy, Ecology, and Cellulolytic Properties of the Genus Bacillus and Related Genera. Agriculture 2023, 13, 1979. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Singh, U.B.; Khan, M.S.; Singh, P.; Kumar, R.; Singh, R.N.; Kumar, A.; Singh, H.V. Bacterial ACC Deaminase: Insights into Enzymology, Biochemistry, Genetics, and Potential Role in Amelioration of Environmental Stress in Crop Plants. Front. Microbiol. 2023, 14, 1132770. [Google Scholar] [CrossRef] [PubMed]
- Górska, E.B.; Stępień, W.; Hewelke, E.; Lata, J.-C.; Gworek, B.; Gozdowski, D.; Sas-Paszt, L.; Bazot, S.; Lisek, A.; Gradowski, M.; et al. Response of Soil Microbiota to Various Soil Management Practices in 100-Year-Old Agriculture Field and Identification of Potential Bacterial Ecological Indicator. Ecol. Indic. 2024, 158, 111545. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Naziębło, A. Paenibacillus as a Biocontrol Agent for Fungal Phytopathogens: Is P. polymyxa the Only One Worth Attention? Microb. Ecol. 2024, 87, 134. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum Lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas Fluorescens Promote Photosynthesis, Carbon Fixation and Cadmium Phytoremediation of Hyperaccumulator Sedum Alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Cellulolytic Properties of a Potentially Lignocellulose-Degrading Bacillus sp. 8E1A Strain Isolated from Bulk Soil. Agronomy 2022, 12, 665. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Kulkova, I.; Wróbel, B.; Dobrzyński, J. Serratia spp. as Plant Growth-Promoting Bacteria Alleviating Salinity, Drought, and Nutrient Imbalance Stresses. Front. Microbiol. 2024, 15, 1342331. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant Root Exudates and Rhizosphere Bacterial Communities Shift with Neighbor Context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Bai, Z.; Wu, S.; Li, X.; Wang, N.; Du, X.; Fan, H.; Zhuang, G.; Bohu, T.; et al. Unraveling Mechanisms and Impact of Microbial Recruitment on Oilseed Rape (Brassica napus L.) and the Rhizosphere Mediated by Plant Growth-Promoting Rhizobacteria. Microorganisms 2021, 9, 161. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, H.; Zhao, H.; Ding, L.; Zhou, J.; Zhang, Y.; Gao, Y.; Ren, Z. Effects of Streptomyces melanosporofaciens X216 on Microbial Diversity in Oilseed Rape Soil. Front. Plant Sci. 2024, 15, 1425798. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Kulkova, I.; Jakubowska, Z.; Wróbel, B. Non-Native PGPB Consortium Altered the Rhizobacterial Community and Slightly Stimulated the Growth of Winter Oilseed Rape (Brassica napus L.) under Field Conditions. Microb. Ecol. 2024. [Google Scholar] [CrossRef]
- Minuț, M.; Diaconu, M.; Roșca, M.; Cozma, P.; Bulgariu, L.; Gavrilescu, M. Screening of Azotobacter, Bacillus and Pseudomonas Species as Plant Growth-Promoting Bacteria. Processes 2022, 11, 80. [Google Scholar] [CrossRef]
- Świątczak, J.; Kalwasińska, A.; Brzezinska, M.S. Plant Growth–Promoting Rhizobacteria: Peribacillus frigoritolerans 2RO30 and Pseudomonas sivasensis 2RO45 for their effect on canola growth under controlled as well as natural conditions. Front. Plant Sci. 2023, 14, 1233237. [Google Scholar] [CrossRef]
- Hassouna, B.A.; Fathi, S.H.; Mostafa, G.A. Testing the Efficiency of Different Isolates of Azotobacter, Azospirillum, and Pseudomonas for Some Traits Related to Plant Growth Promoters on Varieties of Onion. Middle East J. 2020, 9, 1007–1020. [Google Scholar]
- Wani, S.A.; Chand, S.; Wani, M.A.; Ramzan, M.; Hakeem, K.R. Azotobacter chroococcum—A potential biofertilizer in agriculture: An overview. In Soil Science: Agricultural and Environmental Prospectives; Springer: Cham, Switzerland, 2016; pp. 333–348. [Google Scholar] [CrossRef]
- Patil, S.V.; Mohite, B.V.; Patil, C.D.; Koli, S.H.; Borase, H.P.; Patil, V.S. Azotobacter. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 397–426. ISBN 978-0-12-823414-3. [Google Scholar]
- Saribay, G.F. Growth and Nitrogen Fixation Dynamics of Azotobacter chroococcum in Nitrogen-Free and OMW Containing Medium. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2003. [Google Scholar]
- Sharafzadeh, S. Effects of PGPR on growth and nutrients uptake of tomato. Int. J. Adv. Eng. Technol. 2012, 2, 27. [Google Scholar]
- Sultana, U.; Desai, S.; Reddy, G. Successful Colonization of Roots and Plant Growth Promotion of Sorghum (Sorghum bicolor L.) by Seed Treatment with Pseudomonas putida and Azotobacter chroococcum. World J. Microbiol. 2016, 3, 43–49. [Google Scholar]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.X.; Guo, X.; Wang, D.; Chu, H. Bacterial Diversity in Soils Subjected to Long-Term Chemical Fertilization Can Be More Stably Maintained with the Addition of Livestock Manure than Wheat Straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Samain, E.; Duclercq, J.; Ait Barka, E.; Eickermann, M.; Ernenwein, C.; Mazoyon, C.; Sarazin, V.; Dubois, F.; Aussenac, T.; Selim, S. PGPR-Soil Microbial Communities’ Interactions and Their Influence on Wheat Growth Promotion and Resistance Induction against Mycosphaerella Graminicola. Biology 2023, 12, 1416. [Google Scholar] [CrossRef]
- Lee, S.-K.; Chiang, M.-S.; Hseu, Z.-Y.; Kuo, C.-H.; Liu, C.-T. A Photosynthetic Bacterial Inoculant Exerts Beneficial Effects on the Yield and Quality of Tomato and Affects Bacterial Community Structure in an Organic Field. Front. Microbiol. 2022, 13, 959080. [Google Scholar] [CrossRef]
- Tang, L.; Shi, Y.; Zhang, Y.; Yang, D.; Guo, C. Effects of Plant-Growth-Promoting Rhizobacteria on Soil Bacterial Community, Soil Physicochemical Properties, and Soil Enzyme Activities in the Rhizosphere of Alfalfa under Field Conditions. Diversity 2023, 15, 537. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Crecchio, C.; Verbruggen, E. Shifts in the Rhizosphere and Endosphere Colonizing Bacterial Communities Under Drought and Salinity Stress as Affected by a Biofertilizer Consortium. Microb. Ecol. 2022, 84, 483–495. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, S.; Hua, Q.; Qiu, C.; Wu, P.; Liu, X.; Lin, X. The Long-Term Effects of Using Phosphate-Solubilizing Bacteria and Photosynthetic Bacteria as Biofertilizers on Peanut Yield and Soil Bacteria Community. Front. Microbiol. 2021, 12, 693535. [Google Scholar] [CrossRef]
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial Purification and Characterization of Chitinase Produced by Bacillus Licheniformis B307. Heliyon 2020, 6, e03858. [Google Scholar] [CrossRef]
- Huang, Z.; Ni, G.; Zhao, X.; Wang, F.; Qu, M. Characterization of a GH8 β-1,4-Glucanase from Bacillus subtilis B111 and Its Saccharification Potential for Agricultural Straws. J. Microbiol. Biotechnol. 2021, 31, 1446–1454. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, M.S.; Khan, S.S.; Ahmad, W.; Zheng, L.; Shah, S.U.A.; Ullah, M.; Iqbal, A. Identification and Characterization of Thermophilic Amylase Producing Bacterial Isolates from the Brick Kiln Soil. Saudi J. Biol. Sci. 2021, 28, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. BioMed Res. Int. 2020, 2020, 8650957. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, I.Z.; Rahmoune, B.; Auer, S.; Rößler, S.; Lin, T.; Baluska, F.; Dobrev, P.I.; Motyka, V.; Ludwig-Müller, J. Growth and Aluminum Tolerance of Maize Roots Mediated by Auxin- and Cytokinin-Producing Bacillus Toyonensis Requires Polar Auxin Transport. Environ. Exp. Bot. 2020, 176, 104064. [Google Scholar] [CrossRef]
- Do Carmo Dias, B.; Da Mota, F.F.; Jurelevicius, D.; Seldin, L. Genetics and Regulation of Nitrogen Fixation in Paenibacillus brasilensis PB24. Microbiol. Res. 2021, 243, 126647. [Google Scholar] [CrossRef]
- Mažylytė, R.; Kaziūnienė, J.; Orola, L.; Valkovska, V.; Lastauskienė, E.; Gegeckas, A. Phosphate Solubilizing Microorganism Bacillus sp. MVY-004 and Its Significance for Biomineral Fertilizers’ Development in Agrobiotechnology. Biology 2022, 11, 254. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Österman, J.; Wahlberg, N.; Nesme, X.; Lavire, C.; Vial, L.; Paulin, L.; de Lajudie, P.; Lindström, K. Phylogeny of the Rhizobium–Allorhizobium–Agrobacterium clade supports the delineation of Neorhizobium gen. nov. Syst. Appl. Microbiol. 2014, 37, 208–215. [Google Scholar] [CrossRef]
- Maitra, S.; Praharaj, S.; Brestic, M.; Sahoo, R.K.; Sagar, L.; Shankar, T.; Palai, J.B.; Sahoo, U.; Sairam, M.; Pramanick, B.; et al. Rhizobium as biotechnological tools for green solutions: An environment-friendly approach for sustainable crop production in the modern era of climate change. Curr. Microbiol. 2023, 80, 219. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Wang, F.; Yang, W.; Zhou, J.; Muhoza, B.; Mugabowindekwe, M.; Yu, X. Harnessing the power of cellulolytic nitrogen-fixing bacteria for biovalorization of lignocellulosic biomass. Ind. Crop. Prod. 2022, 186, 115235. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, Z.; Zhang, L.; Zhang, C.; Chen, M.; Wang, B.; Lin, M.; Wang, W.; Zhang, W.; Li, X. Skermanella pratensis sp. nov., isolated from meadow soil, and emended description of the genus Skermanella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1605–1609. [Google Scholar] [CrossRef]
- Kröber, M.; Wibberg, D.; Grosch, R.; Eikmeyer, F.; Verwaaijen, B.; Chowdhury, S.P.; Hartmann, A.; Pühler, A.; Schlüter, A. Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Front. Microbiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Yang, R.; Zhao, W.; Zhu, D.; Zhang, X.; Huang, Z. Bacillus amyloliquefaciens FH-1 Significantly Affects Cucumber Seedlings and the Rhizosphere Bacterial Community but Not Soil. Sci. Rep. 2021, 11, 12055. [Google Scholar] [CrossRef] [PubMed]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Mendes, L.W.; Raaijmakers, J.M.; De Hollander, M.; Mendes, R.; Tsai, S.M. Influence of Resistance Breeding in Common Bean on Rhizosphere Microbiome Composition and Function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 10390:2022-09; Soil, Treated Bio-Waste and Sewage Sludge—Determination of pH. Polish Committee for Standardization: Warszawa, Poland, 2022.
- PN-R-04024:1997; Analysis of the Chemical-Agricultural Soil—Determination of the Content of Available Phosphorus, Potassium, Magnesium and Manganese in Organic Soils. Polish Committee for Standardization: Warszawa, Poland, 1997. (In Polish)
- ISO 13878:1998; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). DIN: Berlin, Germany, 1998.
- PN-R-04023; Chemical and Agricultural Analysis of Soil—Determination of the Available Phosphorus Content in Mineral Soils. Polish Committee for Standardization: Warszawa, Poland, 1996.
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
| Treatment | Shoot Length (cm) | Shoot Weight (g) | Root Length (cm) | Root Weight (g) | Seed Yield (g) |
|---|---|---|---|---|---|
| C.R | 157.5 a | 1573.7 a | 18.7 a | 101.2 a | 415.8 a |
| P1A.R | 157 a | 1773.3 a | 18.4 a | 114 a | 528.6 b |
| 1st Time Point | 2nd Time Point | 3rd Time Point | ||||
|---|---|---|---|---|---|---|
| Treatment | C.R | P1A.R | C.R.2 | P1A.R.2 | C.R.3 | P1A.R.3 |
| pH | 4.87 a | 4.83 a | 4.8 a | 4.73 a | 4.83 a | 4.87 a |
| N-NO3 (mg kg−1) | 6.1 a | 3.8 a | 5.3 a | 4.9 a | 13.9 a | 15.1 a |
| N-NH4 (mg kg−1) | 8.77 b | 7.0 a | 11.67 a | 12.27 a | 9.87 a | 10.3 a |
| AP (mg kg−1) | 10.17 a | 10.4 a | 9.27 a | 9.53 a | 10.1 a | 10.27 a |
| TOC (%) | 1.42 a | 1.42 a | 1.45 a | 1.42 a | 1.43 a | 1.43 a |
| TN (%) | 0.127 a | 0.12 a | 0.14 a | 0.127 a | 0.14 a | 0.13 a |
| 1st Time Point | 2nd Time Point | 3rd Time Point | ||||
|---|---|---|---|---|---|---|
| Treatment | C.R | P1A.R | C.R.2 | P1A.R.2 | C.R.3 | P1A.R.3 |
| Chao1 | 2902 a | 2279 a | 1650 a | 1559 a | 2067 a | 2150 a |
| Observed features | 2830 a | 2221 a | 1644 a | 1545 a | 2000 a | 2067 a |
| Shannon | 10.14 a | 9.21 a | 9.28 a | 9.44 a | 9.66 a | 9.86 a |
| Simpson | 0.995 a | 0.990 a | 0.992 a | 0.996 a | 0.993 a | 0.996 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyński, J.; Kulkova, I.; Jakubowska, Z.; Naziębło, A.; Wróbel, B. Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape. Int. J. Mol. Sci. 2024, 25, 13168. https://doi.org/10.3390/ijms252313168
Dobrzyński J, Kulkova I, Jakubowska Z, Naziębło A, Wróbel B. Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape. International Journal of Molecular Sciences. 2024; 25(23):13168. https://doi.org/10.3390/ijms252313168
Chicago/Turabian StyleDobrzyński, Jakub, Iryna Kulkova, Zuzanna Jakubowska, Aleksandra Naziębło, and Barbara Wróbel. 2024. "Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape" International Journal of Molecular Sciences 25, no. 23: 13168. https://doi.org/10.3390/ijms252313168
APA StyleDobrzyński, J., Kulkova, I., Jakubowska, Z., Naziębło, A., & Wróbel, B. (2024). Pseudomonas sp. G31 and Azotobacter sp. PBC2 Changed Structure of Bacterial Community and Modestly Promoted Growth of Oilseed Rape. International Journal of Molecular Sciences, 25(23), 13168. https://doi.org/10.3390/ijms252313168







