Circadian Rhythms of Clock Genes After Transplantation of Mesenchymal Stem Cells with Type 2 Diabetes Mellitus
Abstract
1. Introduction
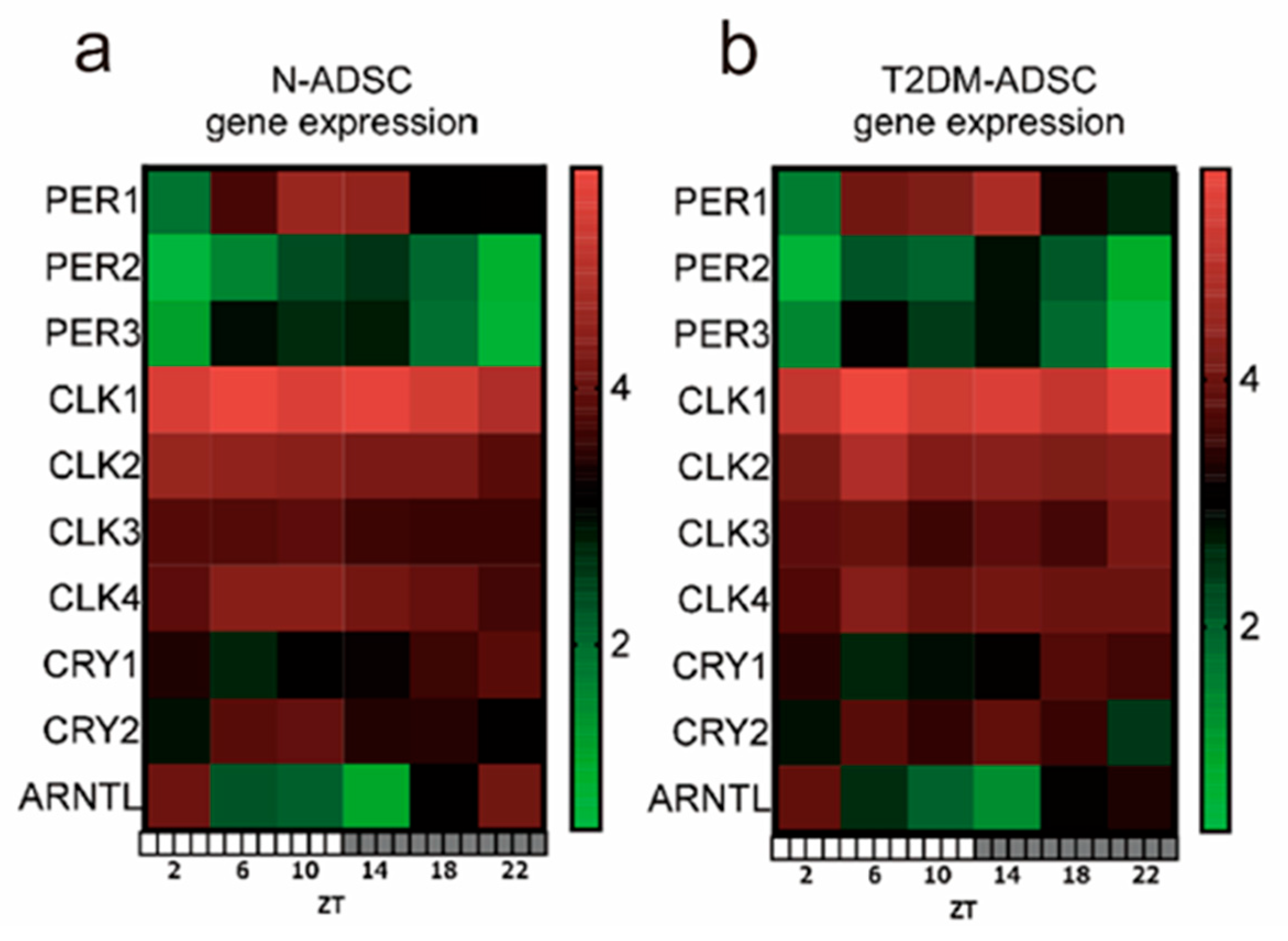
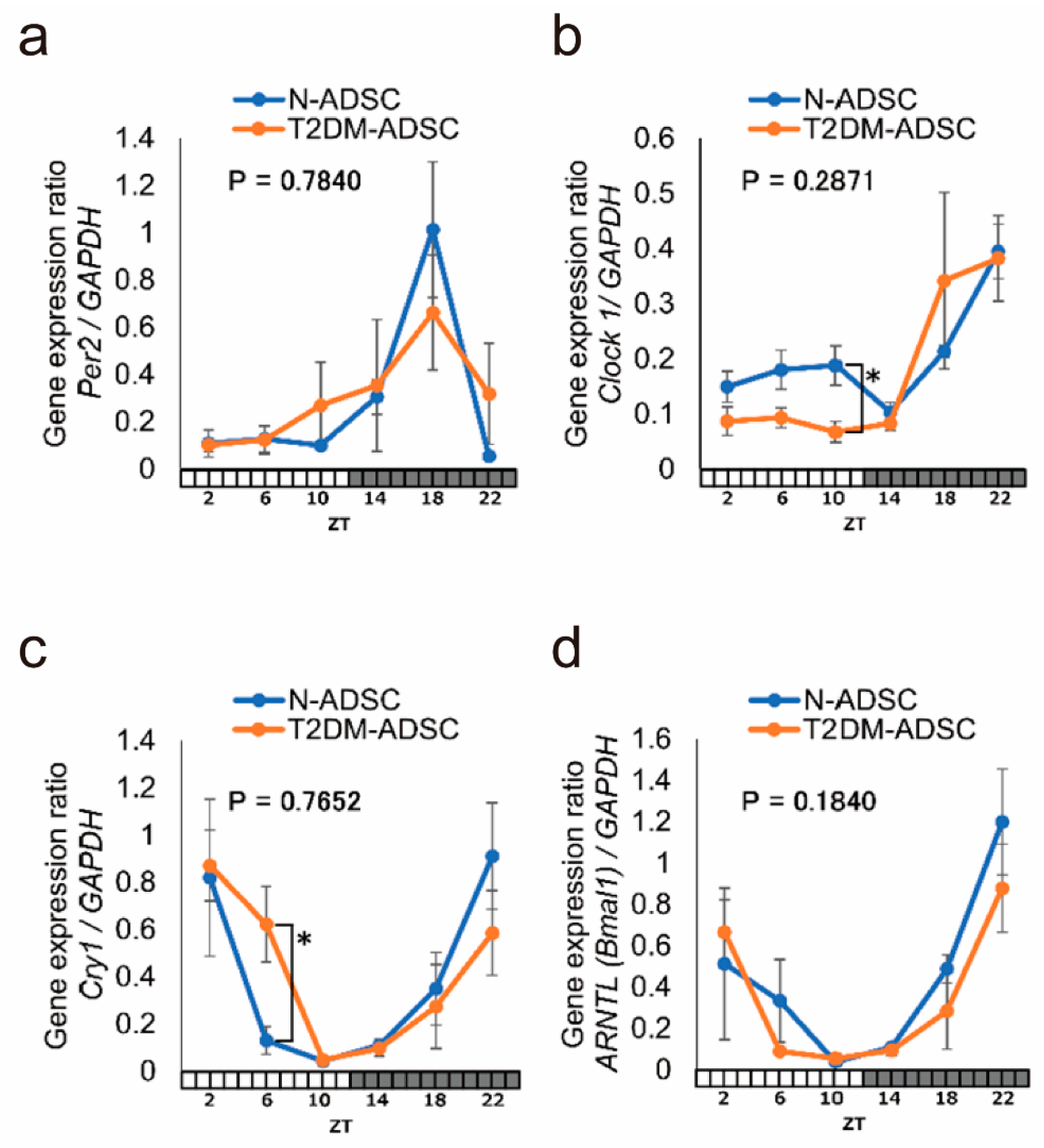
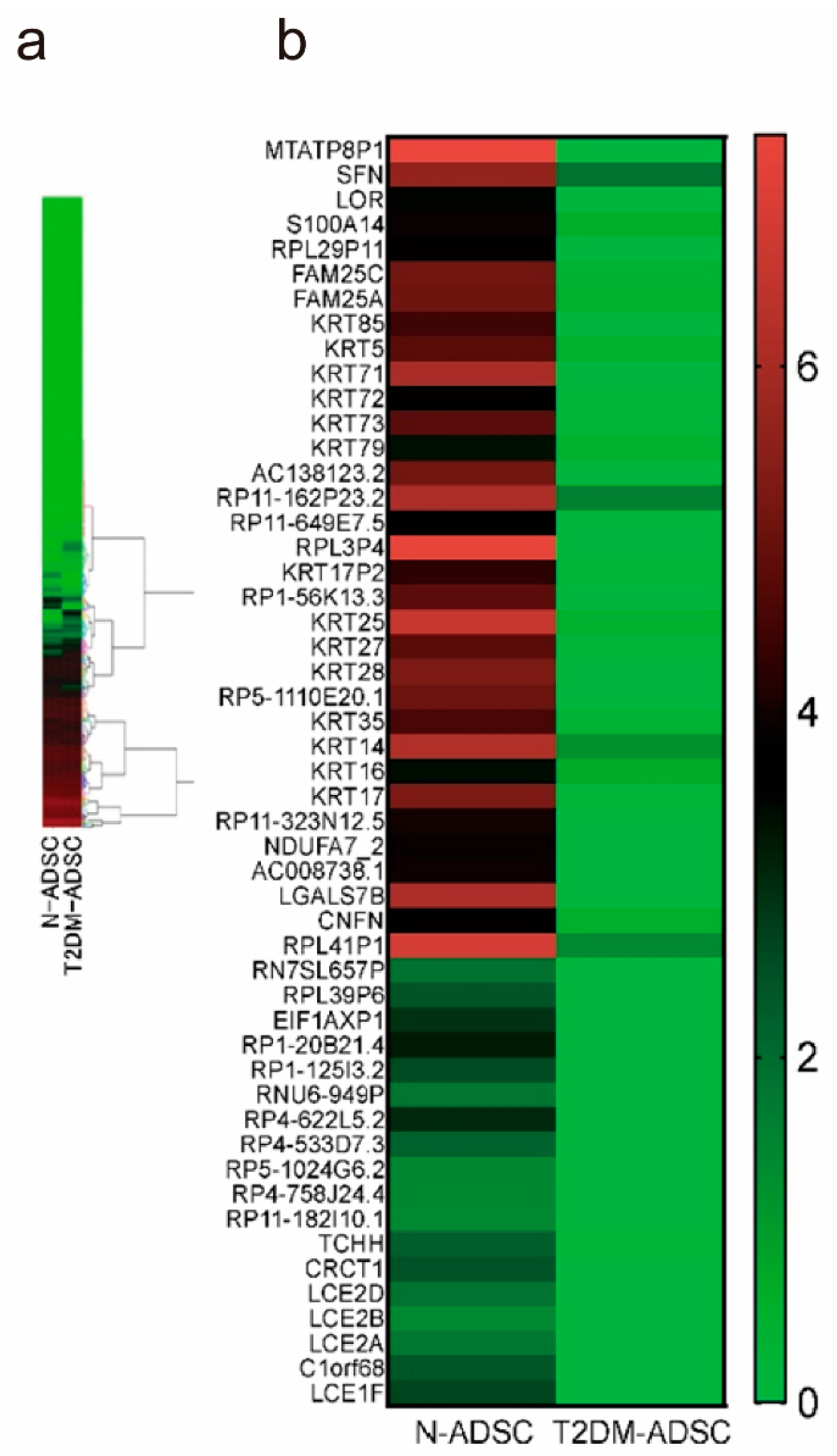
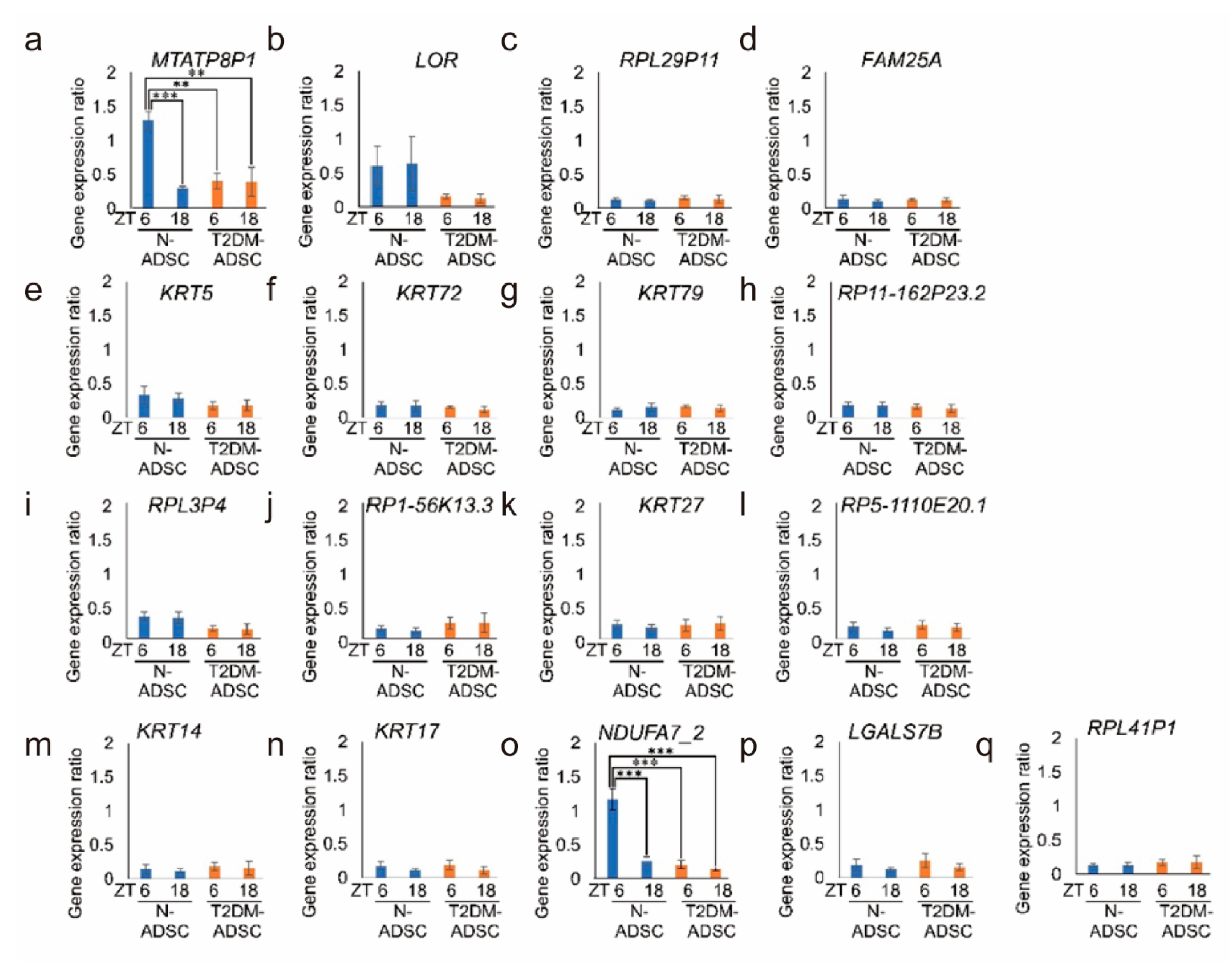
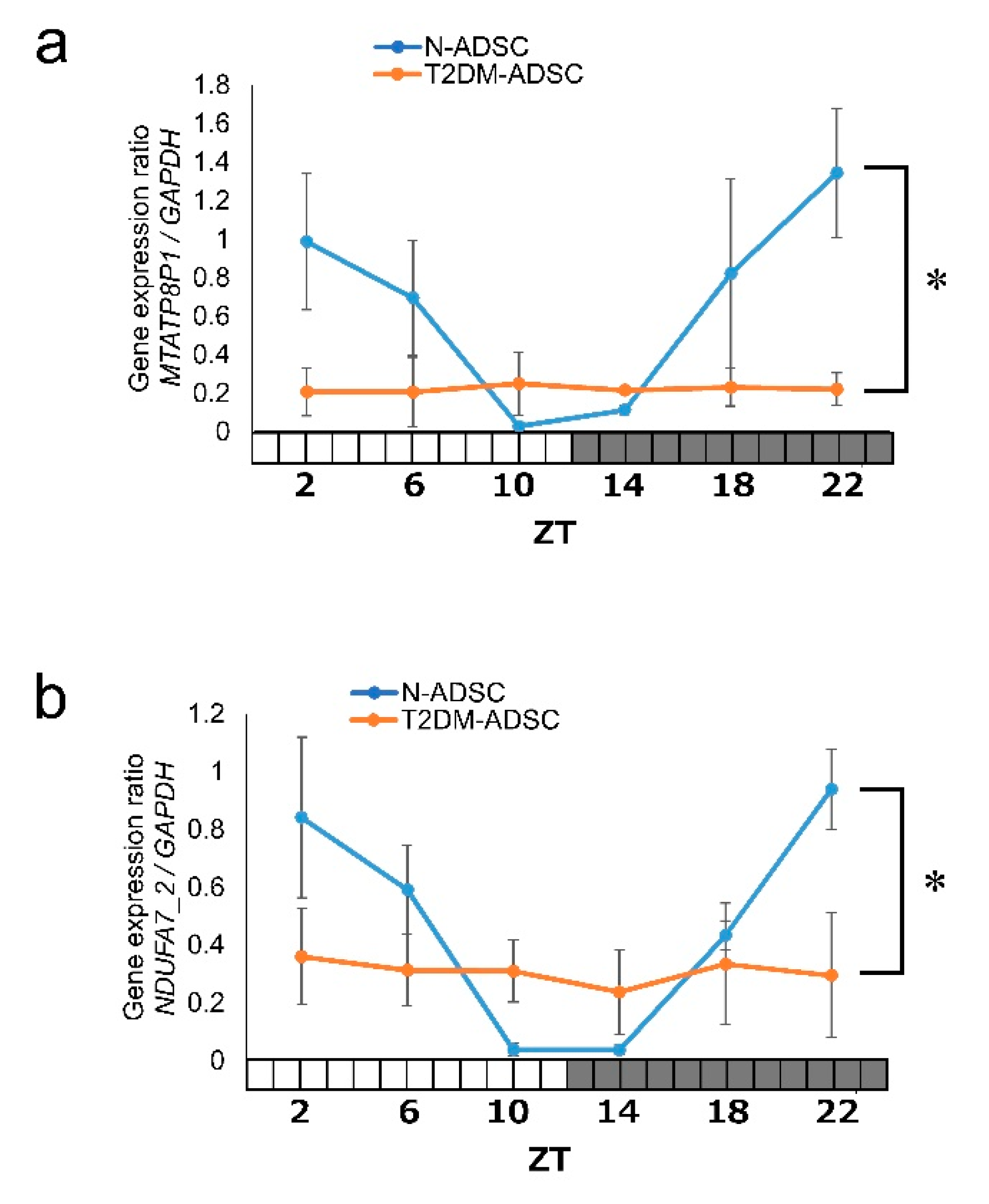
2. Results
3. Discussion
4. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roden, M.; Shulman, G.I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 Diabetes Mellitus as a Disease of the β-Cell (Do Not Blame the Immune System?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Function and Mechanisms of Enteroendocrine Cells and Gut Hormones in Metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Juneja, R.; Beals, J.M.; Antalis, C.J.; Wright, E.E. The Evolution of Insulin and How It Informs Therapy and Treatment Choices. Endocr. Rev. 2020, 41, 733–755. [Google Scholar] [CrossRef]
- Päth, G.; Perakakis, N.; Mantzoros, C.S.; Seufert, J. Stem Cells in the Treatment of Diabetes Mellitus-Focus on Mesenchymal Stem Cells. Metabolism 2019, 90, 1–15. [Google Scholar] [CrossRef]
- Siehler, J.; Blöchinger, A.K.; Meier, M.; Lickert, H. Engineering Islets from Stem Cells for Advanced Therapies of Diabetes. Nat. Rev. Drug Discov. 2021, 20, 920–940. [Google Scholar] [CrossRef]
- de Klerk, E.; Hebrok, M. Stem Cell-Based Clinical Trials for Diabetes Mellitus. Front. Endocrinol. 2021, 12, 631463. [Google Scholar] [CrossRef]
- Sneddon, J.B.; Tang, Q.; Stock, P.; Bluestone, J.A.; Roy, S.; Desai, T.; Hebrok, M. Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges. Cell Stem Cell 2018, 22, 810–823. [Google Scholar] [CrossRef]
- Wu, H.; Mahato, R.I. Mesenchymal Stem Cell-Based Therapy for Type 1 Diabetes. Discov. Med. 2014, 17, 139–143. [Google Scholar]
- Aguayo-Mazzucato, C.; Bonner-Weir, S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018, 27, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Brusko, T.M.; Russ, H.A.; Stabler, C.L. Strategies for Durable β Cell Replacement in Type 1 Diabetes. Science 2021, 373, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Saka, Y.; Furuhashi, K.; Katsuno, T.; Kim, H.; Ozaki, T.; Iwasaki, K.; Haneda, M.; Sato, W.; Tsuboi, N.; Ito, Y.; et al. Adipose-Derived Stromal Cells Cultured in a Low-Serum Medium, but Not Bone Marrow-Derived Stromal Cells, Impede Xenoantibody Production. Xenotransplantation 2011, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, S.; Ozaki, T.; Maruyama, S.; Saka, Y.; Kobori, M.; Omae, K.; Yamaguchi, H.; Niimi, T.; Toriyama, K.; Kamei, Y.; et al. Novel Culture System of Mesenchymal Stromal Cells from Human Subcutaneous Adipose Tissue. Stem Cells Dev. 2009, 18, 533–543. [Google Scholar] [CrossRef]
- Ebrahim, N.; Dessouky, A.A.; Mostafa, O.; Hassouna, A.; Yousef, M.M.; Seleem, Y.; El Gebaly, E.A.E.A.M.; Allam, M.M.; Farid, A.S.; Saffaf, B.A.; et al. Adipose Mesenchymal Stem Cells Combined with Platelet-Rich Plasma Accelerate Diabetic Wound Healing by Modulating the Notch Pathway. Stem Cell Res. Ther. 2021, 12, 392. [Google Scholar] [CrossRef]
- Horiguchi, M.; Hata, S.; Tsurudome, Y.; Ushijima, K. Characterizing the Degeneration of Nuclear Membrane and Mitochondria of Adipose-Derived Mesenchymal Stem Cells from Patients with Type II Diabetes. J. Cell Mol. Med. 2021, 25, 4298–4306. [Google Scholar] [CrossRef]
- Horiguchi, M.; Okada, Y.; Turudome, Y.; Ushijima, K. Exosome Degeneration in Mesenchymal Stem Cells Derived from Patients with Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 10906. [Google Scholar] [CrossRef]
- Horiguchi, M.; Tsurudome, Y.; Ushijima, K. AMFR and DCTN2 Genes Cause Transplantation Resistance of Adipose-Derived Mesenchymal Stem Cells in Type 1 Diabetes Mellitus. Front. Pharmacol. 2022, 13, 1005293. [Google Scholar] [CrossRef]
- Horiguchi, M.; Turudome, Y.; Ushijima, K. The Transplantation Resistance of Type II Diabetes Mellitus Adipose-Derived Stem Cells Is Due to G6PC and IGF1 Genes Related to the FoxO Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 6595. [Google Scholar] [CrossRef]
- Zhao, X.; Cho, H.; Yu, R.T.; Atkins, A.R.; Downes, M.; Evans, R.M. Nuclear Receptors Rock around the Clock. EMBO Rep. 2014, 15, 518–528. [Google Scholar] [CrossRef]
- Hastings, M.H.; Brancaccio, M.; Maywood, E.S. Circadian Pacemaking in Cells and Circuits of the Suprachiasmatic Nucleus. J. Neuroendocrinol. 2014, 26, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Tsurudome, Y.; Akamine, T.; Horiguchi, M.; Wada, Y.; Fujimura, A.; Ushijima, K. Potential Mechanism of Hepatic Lipid Accumulation During a Long-Term Rest Phase Restricted Feeding in Mice. Chronobiol. Int. 2022, 39, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Tsurudome, Y.; Morita, N.; Horiguchi, M.; Ushijima, K. Decreased ZO1 Expression Causes Loss of Time-Dependent Tight Junction Function in the Liver of Ob/Ob Mice. Mol. Biol. Rep. 2022, 49, 11881–11890. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, R.; Ye, M.; Zhang, L.; Zheng, J.; Yang, Y.; Wei, X.; Zhao, Q. The Circadian Clock Has Roles in Mesenchymal Stem Cell Fate Decision. Stem Cell Res. Ther. 2022, 13, 200. [Google Scholar] [CrossRef]
- Benitah, S.A.; Welz, P.-S. Circadian Regulation of Adult Stem Cell Homeostasis and Aging. Cell Stem Cell 2020, 26, 817–831. [Google Scholar] [CrossRef]
- Dierickx, P.; Van Laake, L.W.; Geijsen, N. Circadian Clocks: From Stem Cells to Tissue Homeostasis and Regeneration. EMBO Rep. 2018, 19, 18–28. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Hogeveen, M.; Nijtmans, L.G.J.; van den Brand, M.A.M.; Janssen, A.J.M.; Diepstra, J.H.S.; van den Brandt, F.C.A.; van den Heuvel, L.P.; Hol, F.A.; Hofste, T.G.J.; et al. A Novel Mitochondrial ATP8 Gene Mutation in a Patient with Apical Hypertrophic Cardiomyopathy and Neuropathy. J. Med. Genet. 2008, 45, 129–133. [Google Scholar] [CrossRef]
- Ware, S.M.; El-Hassan, N.; Kahler, S.G.; Zhang, Q.; Ma, Y.-W.; Miller, E.; Wong, B.; Spicer, R.L.; Craigen, W.J.; Kozel, B.A.; et al. Infantile Cardiomyopathy Caused by a Mutation in the Overlapping Region of Mitochondrial ATPase 6 and 8 Genes. J. Med. Genet. 2009, 46, 308–314. [Google Scholar] [CrossRef]
- Fernandez-Vizarra, E.; Zeviani, M. Mitochondrial Disorders of the OXPHOS System. FEBS Lett. 2021, 595, 1062–1106. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Chen, S.; Wu, Y.; Lin, S.; Duan, Y.; Zheng, K.; Zhang, L.; Gu, X.; Hong, W.; et al. A Novel Potentially Causative Variant of NDUFAF7 Revealed by Mutation Screening in a Chinese Family with Pathologic Myopia. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4182–4192. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Rasouli, M.; Reitz, C.J.; Posen, I.; Margulets, V.; Dhingra, R.; Khatua, T.N.; Thliveris, J.A.; Martino, T.A.; Kirshenbaum, L.A. Mitochondrial Autophagy and Cell Survival Is Regulated by the Circadian Clock Gene in Cardiac Myocytes During Ischemic Stress. Autophagy 2021, 17, 3794–3812. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Metabolism and Cancer: The Circadian Clock Connection. Nat. Rev. Cancer 2009, 9, 886–896. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]

| Category | p-Value | Molecules |
|---|---|---|
| Cell Morphology | 2.88 × 10−49–2.98 × 10−2 | EPCAM, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LORICRIN, mir-199, MSX2, PKP1, SERPINB5, SFN, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TCHH, TYRP1 |
| Embryonic Development | 2.3 × 10−43–3.75 × 10−2 | COL10A1, DCT, EPCAM, HOXC13, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LHX2, LORICRIN, mir-27, MSX2, PKP1, SERPINB5, SFN, SOX21, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TFAP2A, TFAP2B, TYRP1 |
| Hair and Skin Development and Function | 2.3 × 10−43–3.71 × 10−2 | DCT, EPCAM, HOXC13, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LGALS7/LGALS7B, LHX2, LORICRIN, mir-27, MSX2, PKP1, SFN, SOX21, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TFAP2A, TYRP1 |
| Organ Development | 2.3 × 10−43–3.62 × 10−2 | COL10A1, DCT, HOXC13, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LGALS7/LGALS7B, LHX2, LORICRIN, mir-199, mir-27, MSX2, PKP1, SFN, SOX21, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TFAP2A, TFAP2B, TYRP1 |
| Organismal Development | 2.3 × 10−43–4.01 × 10−2 | COL10A1, DCT, EPCAM, ESRP1, GPR87, HOXC13, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LHX2, LORICRIN, mir-199, mir-27, MSX2, PKP1, RAB25, SERPINB5, SFN, SOX21, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TFAP2A, TFAP2B, TYRP1 |
| Tissue Development | 2.3 × 10−43–4.01 × 10−2 | COL10A1, DCT, EPCAM, ESRP1, GPR87, HOXC13, KRT1, KRT12, KRT14, KRT15, KRT16, KRT17, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33A, KRT35, KRT5, KRT6A, KRT6B, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT79, KRT85, KRT86, KRTAP2-1, LGALS7/LGALS7B, LHX2, LORICRIN, mir-27, MSX2, PKP1, RAB25, SCT, SERPINB5, SFN, SOX21, SP6, SPINK5, SPRR1A, SPRR1B, ST14, TFAP2A, TFAP2B, TYRP1 |
| Forward primer | |||||
| Gene Symbol | Acc. No. | Position | Forward | GC% | Tm |
| GAPDH | NM_002046.4 | 415 | GCACCGTCAAGGCTGAGAAC | 60 | 63.3 |
| Per2 | NM_022817.2 | 4929 | CGTTGGAACCACCCAGACATC | 57.1 | 64.9 |
| CLOCK | NM_004898.2 | 5092 | CCCACATTCCCATGAATATCAAGA | 41.7 | 64 |
| Cry1 | NM_004075.3 | 2458 | AATATCCCAGGTTGTAGCAGCAGTG | 48 | 64.6 |
| Bmal1 | NM_001178.4 | 2134 | GCCTACTATCAGGCCAGGCTCA | 59.1 | 65 |
| Reverse primer | |||||
| Gene Symbol | Acc. No. | Reverse | GC% | Tm | |
| GAPDH | NM_002046.4 | 415 | TGGTGAAGACGCCAGTGGA | 57.9 | 64 |
| Per2 | NM_022817.2 | 4929 | ATGCAGTCGCAAGCTGTCAGA | 52.4 | 64.5 |
| CLOCK | NM_004898.2 | 5092 | GCTATTGCCATATTCCACAACATCC | 44 | 64.6 |
| Cry1 | NM_004075.3 | 2458 | GTTTGCTGACTGTCGCCATGA | 52.4 | 64.6 |
| Bmal1 | NM_001178.4 | 2134 | AGCCATTGCTGCCTCATCATTAC | 47.8 | 64.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiguchi, M.; Yoshihara, K.; Watanabe, K.; Tsurudome, Y.; Mizukami, Y.; Ushijima, K. Circadian Rhythms of Clock Genes After Transplantation of Mesenchymal Stem Cells with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 13145. https://doi.org/10.3390/ijms252313145
Horiguchi M, Yoshihara K, Watanabe K, Tsurudome Y, Mizukami Y, Ushijima K. Circadian Rhythms of Clock Genes After Transplantation of Mesenchymal Stem Cells with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(23):13145. https://doi.org/10.3390/ijms252313145
Chicago/Turabian StyleHoriguchi, Michiko, Kenichi Yoshihara, Kenji Watanabe, Yuya Tsurudome, Yoichi Mizukami, and Kentaro Ushijima. 2024. "Circadian Rhythms of Clock Genes After Transplantation of Mesenchymal Stem Cells with Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 23: 13145. https://doi.org/10.3390/ijms252313145
APA StyleHoriguchi, M., Yoshihara, K., Watanabe, K., Tsurudome, Y., Mizukami, Y., & Ushijima, K. (2024). Circadian Rhythms of Clock Genes After Transplantation of Mesenchymal Stem Cells with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 25(23), 13145. https://doi.org/10.3390/ijms252313145






