Is There a Relationship Between Prenatal Dexamethasone and Postnatal Fructose Overexposure and Testicular Development, Function, and Oxidative Stress Parameters in Rats?
Abstract
1. Introduction
2. Results
2.1. Body Weight, Testicular Weight, and Gonadosomatic Index
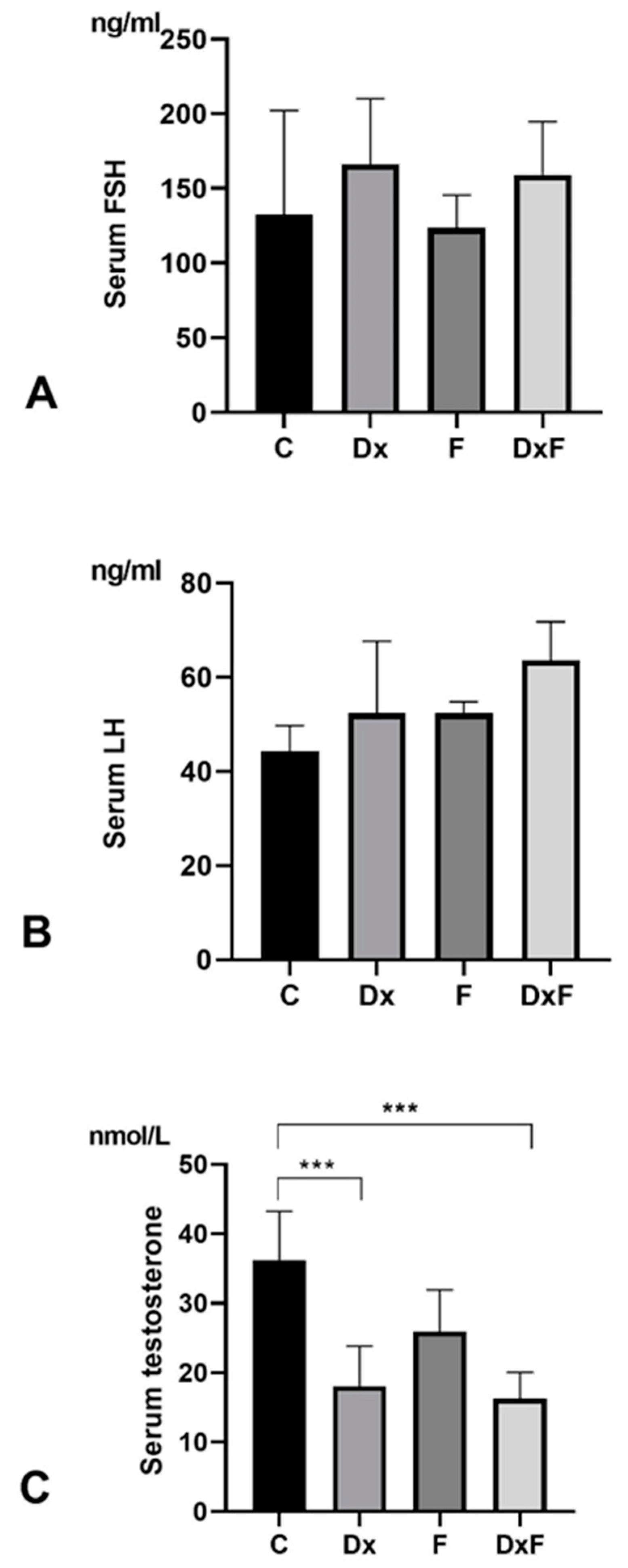
2.2. Histomorphometric, Stereological, and Biochemical Analyses
2.3. Oxidative Stress Parameters
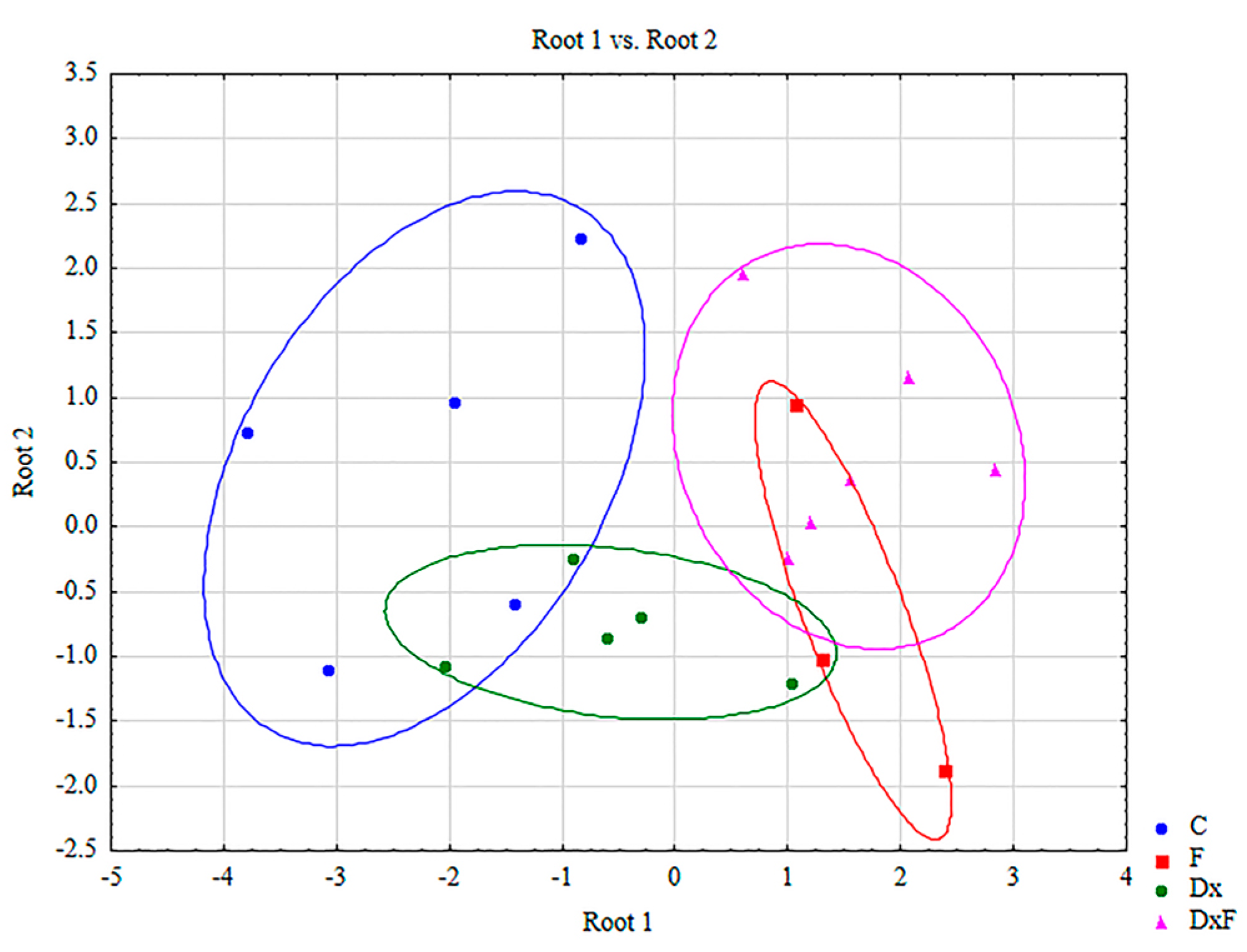
2.4. Discriminant Function Analysis
2.5. Spearman Rank Order Correlations
3. Discussion
4. Materials and Methods
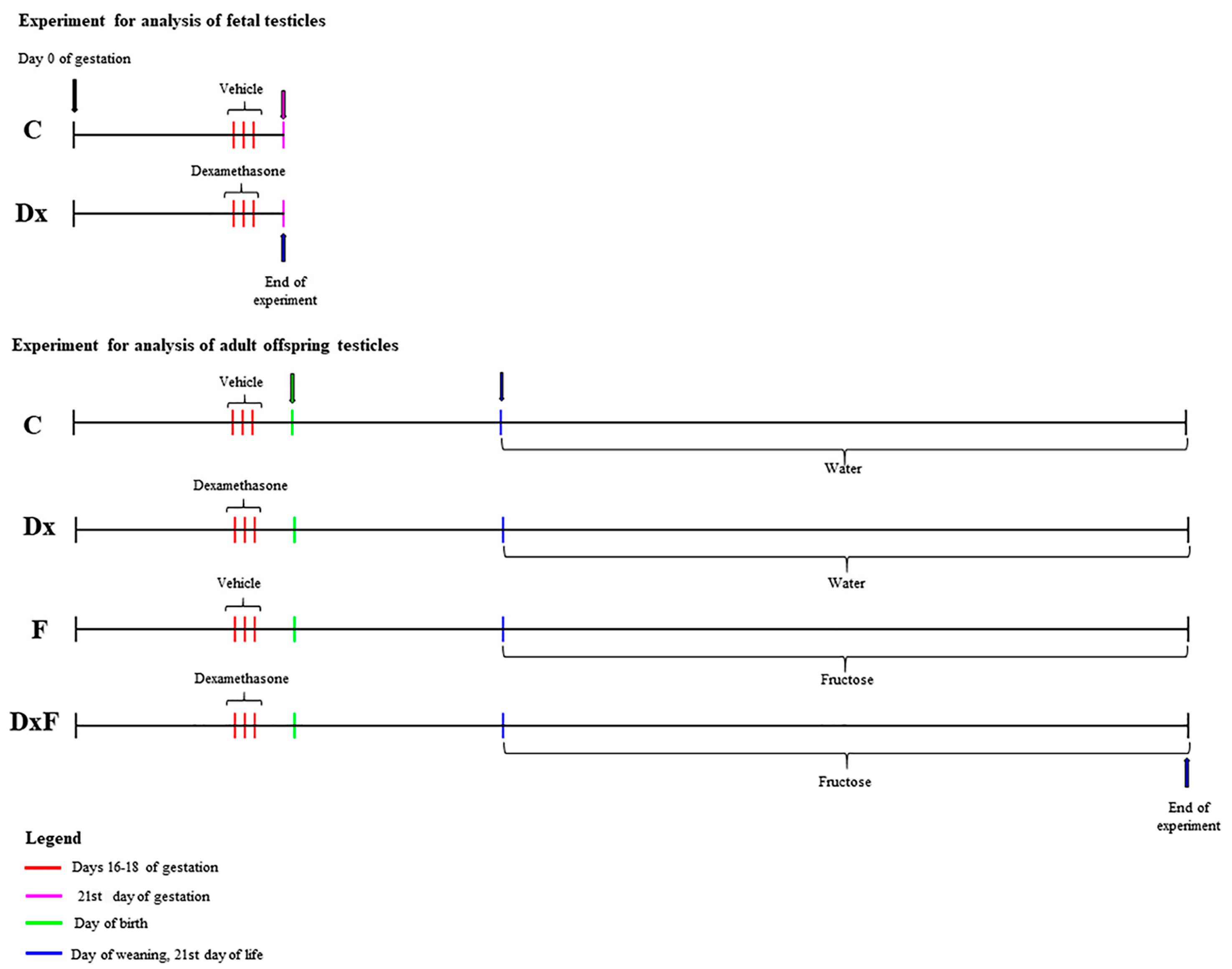
4.1. Animals and Experimental Design
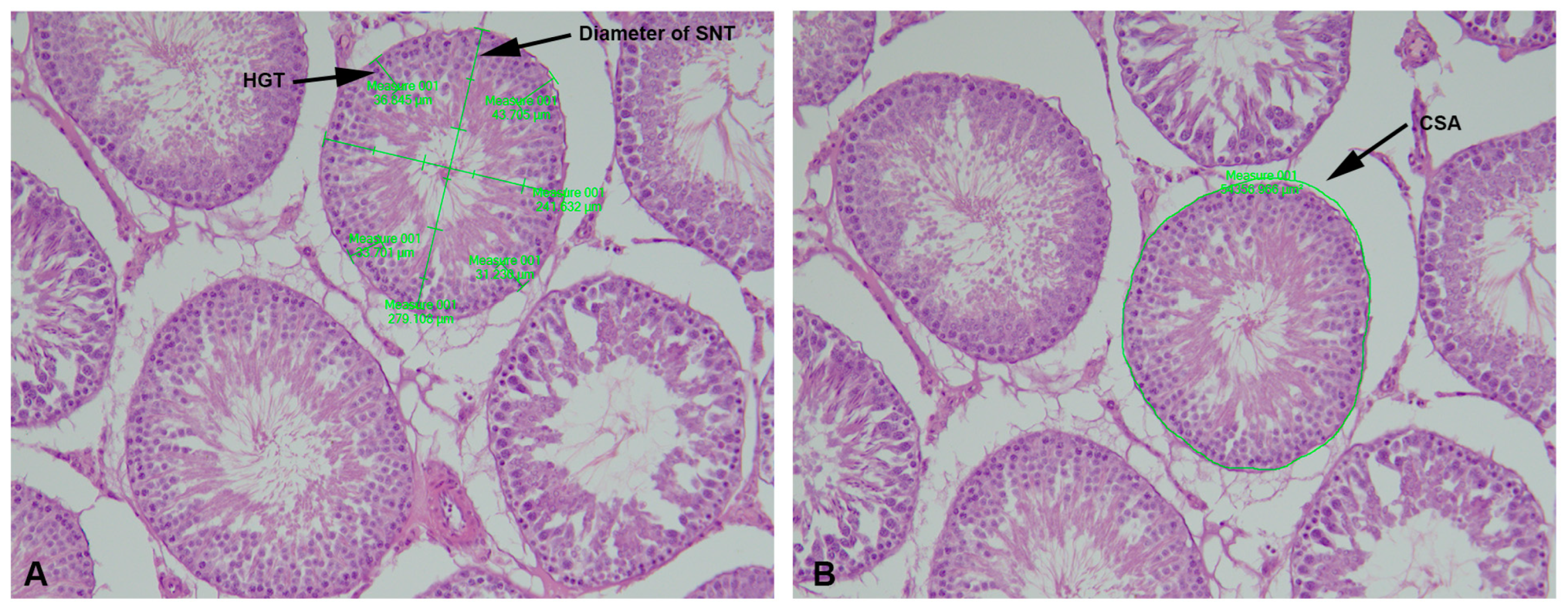
4.2. Stereological and Morphometric Analyses
4.3. Biochemical Analyses
4.4. Determination of Oxidative Stress Biomarkers in the Testicles
4.5. Determination of Nonenzymatic Antioxidants
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, N.P.; Bellingham, M.; Robinson, J.E. Prenatal programming of neuroendocrine reproductive function. Theriogenology 2016, 86, 340–348. [Google Scholar] [CrossRef]
- Rabadán-Diehl, C.; Nathanielsz, P.J. From Mice to Men: Research models of developmental programming. Dev. Orig. Health Dis. 2013, 4, 3–9. [Google Scholar] [CrossRef]
- Hacking, D.; Watkins, A.; Fraser, S.; Wolfe, R.; Nolan, T. Respiratory distress syndrome and birth order in premature twins. Arch. Dis. Child. Fetal Neonatal. Ed. 2001, 84, 117–121. [Google Scholar] [CrossRef]
- Benediktsson, R.; Lindsay, R.S.; Noble, J.; Seckl, J.R.; Edwards, C.R.V. Glucocorticoid exposure in utero: New model for adult hypertension. Lancet 1993, 341, 339–341. [Google Scholar] [CrossRef]
- Green, B.B.; Armstrong, D.A.; Lesseur, C.; Paquette, A.G.; Guerin, D.J.; Kwan, L.E.; Marsit, C.J. The Role of Placental 11-Beta Hydroxysteroid Dehydrogenase Type 1 and Type 2 Methylation on Gene Expression and Infant Birth Weight. Biol. Reprod. 2015, 92, 149. [Google Scholar] [CrossRef]
- Arias, A.; Schander, J.A.; Bariani, M.V.; Correa, F.; Domínguez Rubio, A.P.; Cella, M.; Cymeryng, C.B.; Wolfson, M.L.; Franchi, A.M.; Aisemberg, J. Dexamethasone-induced intrauterine growth restriction modulates expression of placental vascular growth factors and fetal and placental growth. Mol. Hum. Reprod. 2021, 27, 27. [Google Scholar] [CrossRef]
- Dagklis, T.; Sen, C.; Tsakiridis, I.; Villalaín, C.; Allegaert, K.; Wellmann, S.; Kusuda, S.; Serra, B.; Sanchez Luna, M.; Huertas, E.; et al. The use of antenatal corticosteroids for fetal maturation: Clinical practice guideline by the WAPM-World Association of Perinatal Medicine and the PMF-Perinatal Medicine foundation. J. Perinat. Med. 2022, 50, 375–385. [Google Scholar] [CrossRef]
- Murphy, V.E.; Fittock, R.J.; Zarzycki, P.K.; Delahunty, M.M.; Smith, R.; Clifton, V.L. Metabolism of synthetic steroids by the human placenta. Placenta 2007, 28, 39–46. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Zhu, S.; Li, X.; Shi, W.; Dai, Z.; Hao, L.; Wang, X.J. Adverse effects of prenatal dexamethasone exposure on fetal development. Reprod. Immunol. 2022, 151, 103619. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. 2024. Available online: https://www.fondazionemisi.it/images/Covid19_Treatment_Guidelines_NIH.pdf (accessed on 1 December 2020).
- Jeje, S.O.; Raji, Y. Maternal treatment with dexamethasone during gestation alters sexual development markers in the F1 and F2 male offspring of Wistar rats. J. Dev. Orig. Health Dis. 2017, 8, 101–112. [Google Scholar] [CrossRef]
- Li, H.; Spade, D.J. Reproductive toxicology: Environmental exposures, fetal testis development and function: Phthalates and beyond. Reproduction 2021, 162, 147–167. [Google Scholar] [CrossRef]
- Medaglia, D.S.A.; Vieira, H.R.; Silveira, S.D.S.; Siervo, G.E.M.L.; Marcon, M.S.D.S.; Mathias, P.C.F.; Fernandes, G.S.A. High-fructose diet during puberty alters the sperm parameters, testosterone concentration, and histopathology of testes and epididymis in adult Wistar rats. J. Dev. Orig. Health Dis. 2022, 13, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 6846. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Bhat, S.F.; Pinney, S.E.; Kennedy, K.M.; McCourt, C.R.; Mundy, M.A.; Surette, M.G.; Sloboda, D.M.; Simmons, R.A. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J. Physiol. 2021, 599, 1487–1511. [Google Scholar] [CrossRef]
- Ko, E.A.; Kim, H.R.; Kim, Y.B.; Kim, H.S.; Lee, S.H. Effect of high fructose corn syrup (HFCS) intake on the female reproductive organs and lipid accumulation in adult rats. Dev. Reprod. 2017, 21, 151–156. [Google Scholar] [CrossRef]
- Yildirim, O.G.; Sumlu, E.; Aslan, E.; Koca, H.B.; Pektas, M.B.; Sadi, G.; Akar, F. High-fructose in drinking water initiates activation of inflammatory cytokines and testicular degeneration in rat. Toxicol. Mech. Methods 2019, 29, 224–232. [Google Scholar] [CrossRef]
- Yildirim, O.G.; Guney, C.; Alcigir, M.E.; Akar, F. High-fructose consumption suppresses insulin signaling pathway accompanied by activation of macrophage and apoptotic markers in rat testis. Reprod. Biol. 2023, 23, 100815. [Google Scholar] [CrossRef]
- Akar, F.; Yildirim, O.G.; Yucel Tenekeci, G.; Tunc, A.S.; Demirel, M.A.; Sadi, G. Dietary high-fructose reduces barrier proteins and activates mitogenic signalling in the testis of a rat model: Regulatory effects of kefir supplementation. Andrologia 2022, 54, 14342. [Google Scholar] [CrossRef]
- Aslankoc, R.; Ozmen, O. The effects of high-fructose corn syrup consumption on testis physiopathology-The ameliorative role of melatonin. Andrologia 2019, 51, e13327. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- De Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2011, 686137. [Google Scholar] [CrossRef]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef]
- Zambrano, E.; Nathanielsz, P.W.; Rodríguez-González, G.L. Developmental programming and ageing of male reproductive function. Eur. J. Clin. Investig. 2021, 51, e13637. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Munkhzaya, M.; Tungalagsuvd, A.; Kawami, T.; Murakami, M.; Yamasaki, M.; Kato, T.; Kuwahara, A.; Yasui, T.; et al. Prenatal exposure to glucocorticoids affects body weight, serum leptin levels, and hypothalamic neuropeptide-Y expression in pre-pubertal female rat offspring. Int. J. Dev. Neurosci. 2014, 36, 1–4. [Google Scholar] [CrossRef]
- Guo, J.; Fang, M.; Zhuang, S.; Qiao, Y.; Huang, W.; Gong, Q.; Xu, D.; Zhang, Y.; Wang, H. Prenatal dexamethasone exposure exerts sex-specific effect on placental oxygen and nutrient transport ascribed to the differential expression of IGF2. Ann. Transl. Med. 2020, 8, 233. [Google Scholar] [CrossRef]
- Ristić, N.; Severs, W.; Nestorović, N.; Jarić, I.; Manojlović-Stojanoski, M.; Trifunović, S.; Pendovski, L.; Milošević, V. Effects of prenatal dexamethasone on the rat pituitary gland and gonadotropic cells in female offspring. Cells Tissues Organs 2016, 201, 148–158. [Google Scholar] [CrossRef]
- Pampanini, V.; Germani, D.; Puglianiello, A.; Stukenborg, J.-B.; Reda, A.; Savchuk, I.; Rós Kjartansdóttir, K.; Cianfarani, S.; Söder, O. Impact of uteroplacental insufficiency on postnatal rat male gonad. J. Endocrinol. 2016, 232, 247–257. [Google Scholar] [CrossRef]
- Lim, K.; Armitage, J.A.; Stefanidis, A.; Oldfield, B.J.; Black, M.J. IUGR in the absence of postnatal “catch-up” growth leads to improved whole body insulin sensitivity in rat offspring. Pediatr. Res. 2011, 70, 339–344. [Google Scholar] [CrossRef]
- Gruver-Yates, A.; Cidlowski, J. Tissue-specific actions of glucocorticoids on apoptosis: A double-edged sword. Cells 2013, 2, 202–223. [Google Scholar] [CrossRef]
- Gao, H.B.; Tong, M.H.; Hu, Y.Q.; Guo, Q.S.; Ge, R.; Hardy, M.P. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology 2002, 143, 130–138. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, J.Y.; Kim, M.H. Prenatal exposure to dexamethasone disturbs sex-determining gene expression and fetal testosterone production in male embryos. Biochem. Biophys. Res. Commun. 2016, 471, 149–155. [Google Scholar] [CrossRef]
- Jeje, S.O.; Ola-Mudathir, F.K.; Raji, Y. Experimental maternal treatment with dexamethasone during lactation induces neonatal testicular and epididymal oxidative stress; Implications for early postnatal exposure. Pathophysiology 2017, 24, 261–265. [Google Scholar] [CrossRef]
- Ferrari, F.; de Paiva Foletto, M.; Franzói de Moraes, S.M.; Barnabé Peres, S.; Segatelli, T.M.; Mareze da Costa, C.E. Testis morphophysiology of rats treated with nandrolone decanoate and submitted to physical training. Acta. Sci. Health Sci. 2013, 35, 161–167. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Gaby, A.R. Adverse effects of dietary fructose. Altern. Med. Rev. 2005, 10, 294–302. [Google Scholar]
- Lê, K.A.; Tappy, L. Metabolic effects of fructose. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Massa, M.L.; Arbeláez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 15, 1–6. [Google Scholar] [CrossRef]
- Reddy, S.S.; Ramatholisamma, P.; Karuna, R.; Saralakumari, D. Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male wistar rats. Food Chem. Toxico. 2009, 47, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. 2008. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Dalziel, S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2006, 19, CD004454. [Google Scholar]
- Carbone, D.L.; Zuloaga, D.G.; Hiroi, R.; Foradori, C.D.; Legare, M.E.; Handa, R.J. Prenatal dexamethasone exposure potentiates dietinduced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology 2012, 153, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef]
- Ristić, N.; Nestorović, N.; Manojlović-Stojanoski, M.; Filipović, B.; Šošić-Jurjević, B.; Milošević, V.; Sekulić, M. Maternal dexamethasone treatment reduces ovarian follicle number in neonatal rat offspring. J. Microsc. 2008, 232, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, M.E.; Adrover, E.; Baier, C.J.; Bourguignon, N.S.; Monteleone, M.C.; Brocco, M.A.; González-Calvar, S.I.; Antonelli, M.C. Prenatal maternal restraint stress exposure alters the reproductive hormone profile and testis development of the rat male offspring. Stress 2013, 16, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Abiaka, C.; Al-Awadi, F.; Olusi, S. Effect of Prolonged Storage on the Activities of Superoxide Dismutase, Glutathione Reductase, and Glutathione Peroxidase. Clin. Chem. 2000, 46, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.L.; Farr, A.L.; Randall, R.I. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Tamura, M.; Oschino, N.; Chance, B. Some characteristics of hydrogen and alkyl-hydroperoxides metabolizing systems in cardiac tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef]
- Glatzle, D.; Vulliemuier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef]
- Habig, W.H.; Pubst, M.J.; Jakoby, W.B. Glutathione S-transferase. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Motulsky, H.M.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression–a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Darlington, R.B.; Weinsberg, S.; Walberg, H. Canonical variate analysis and related techniques. Rev. Edu. Res. 1973, 43, 433–454. [Google Scholar] [CrossRef]
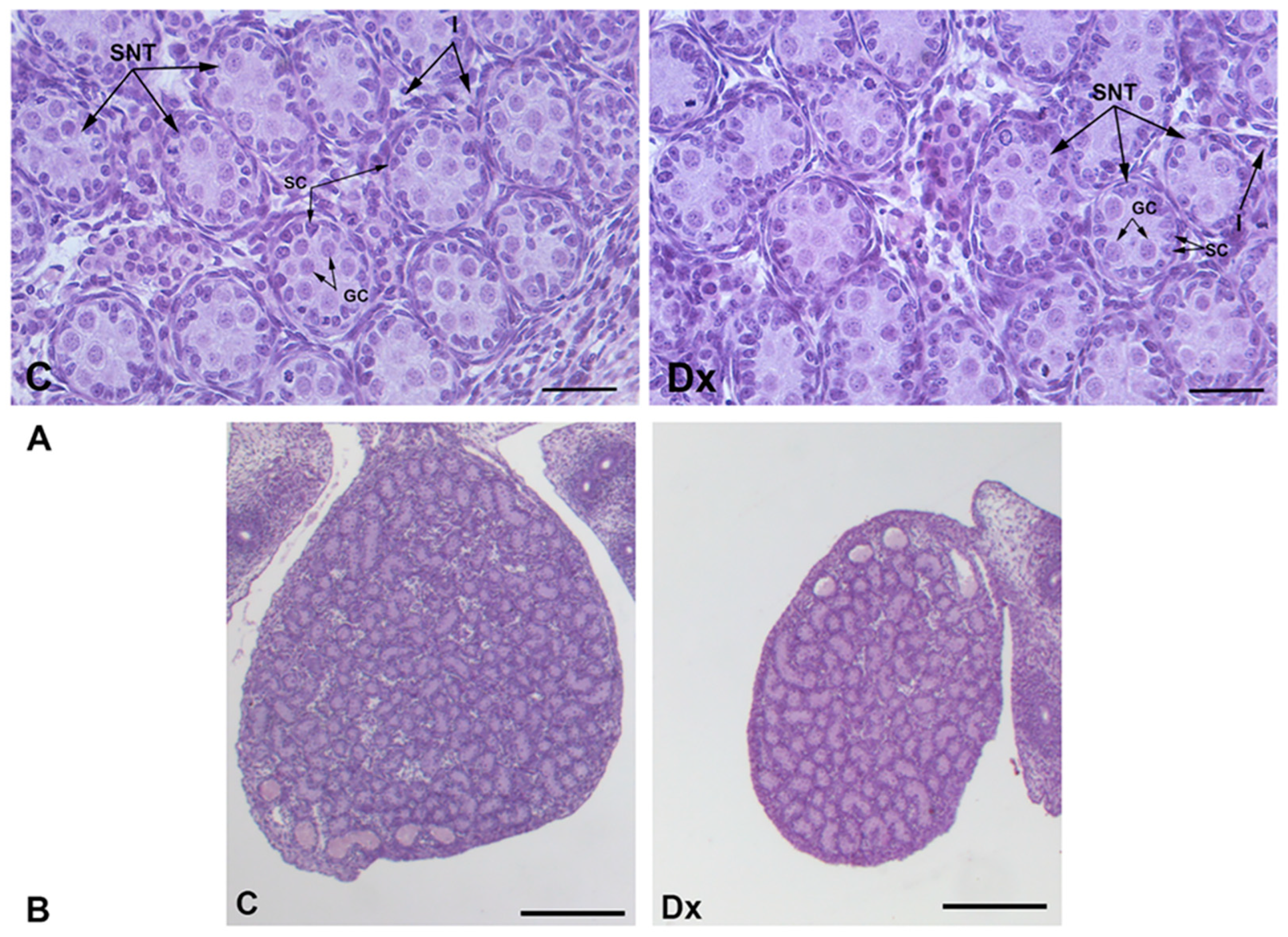
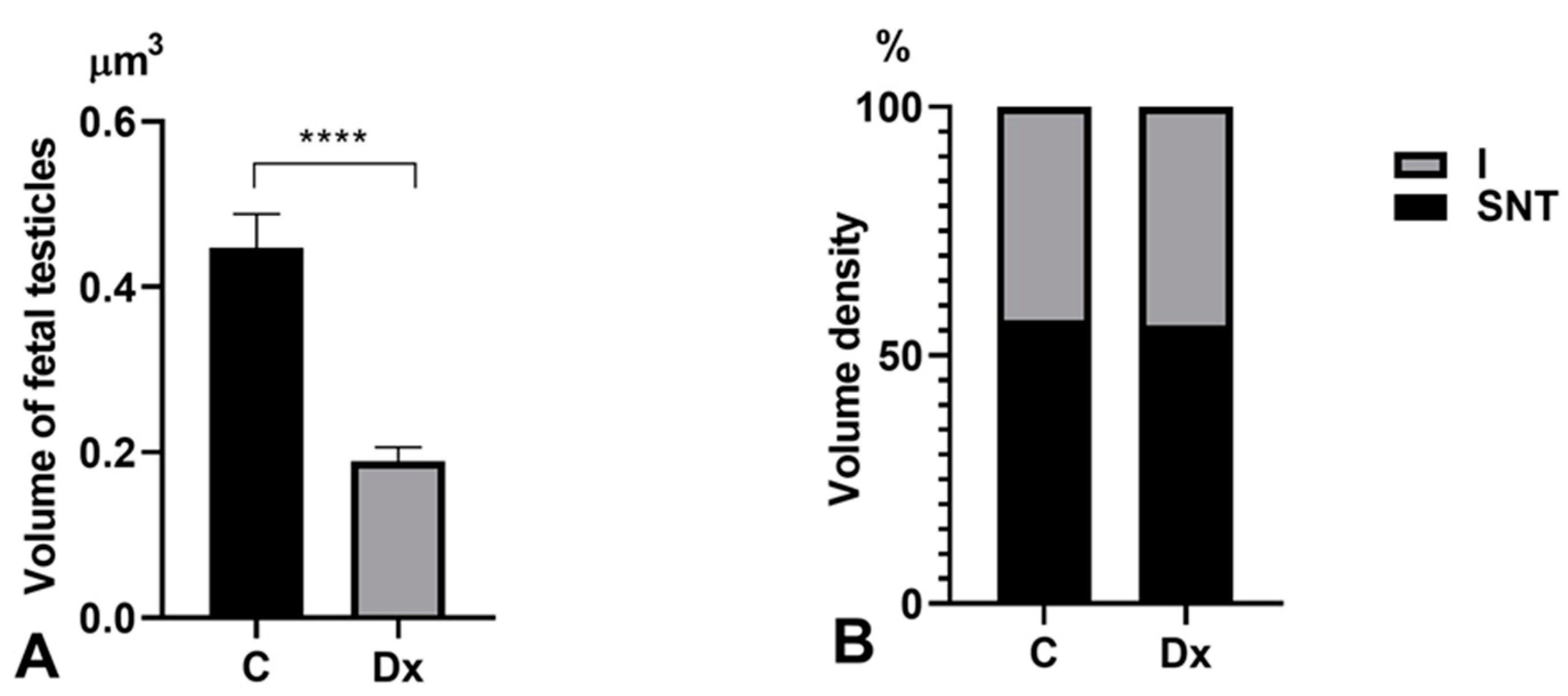
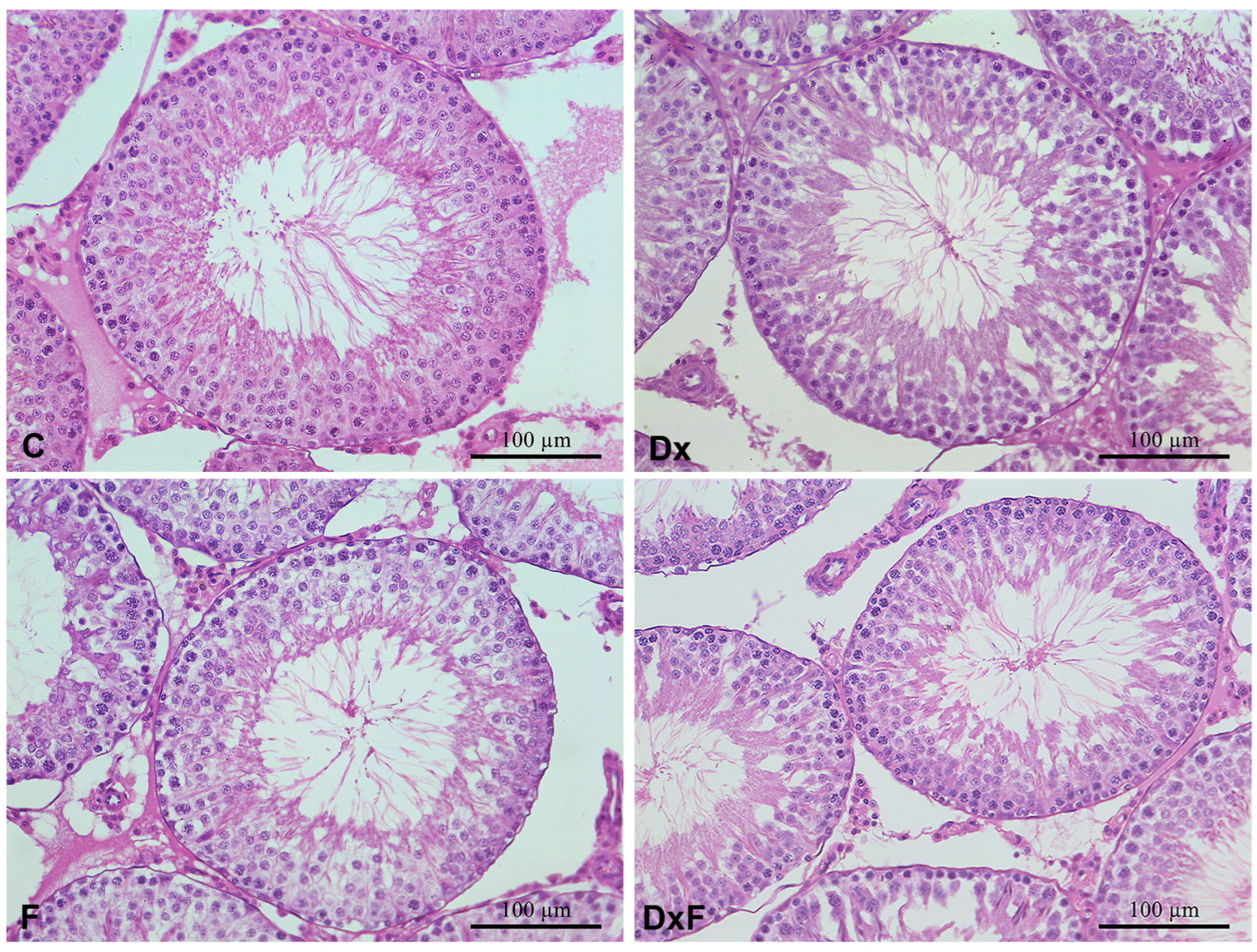
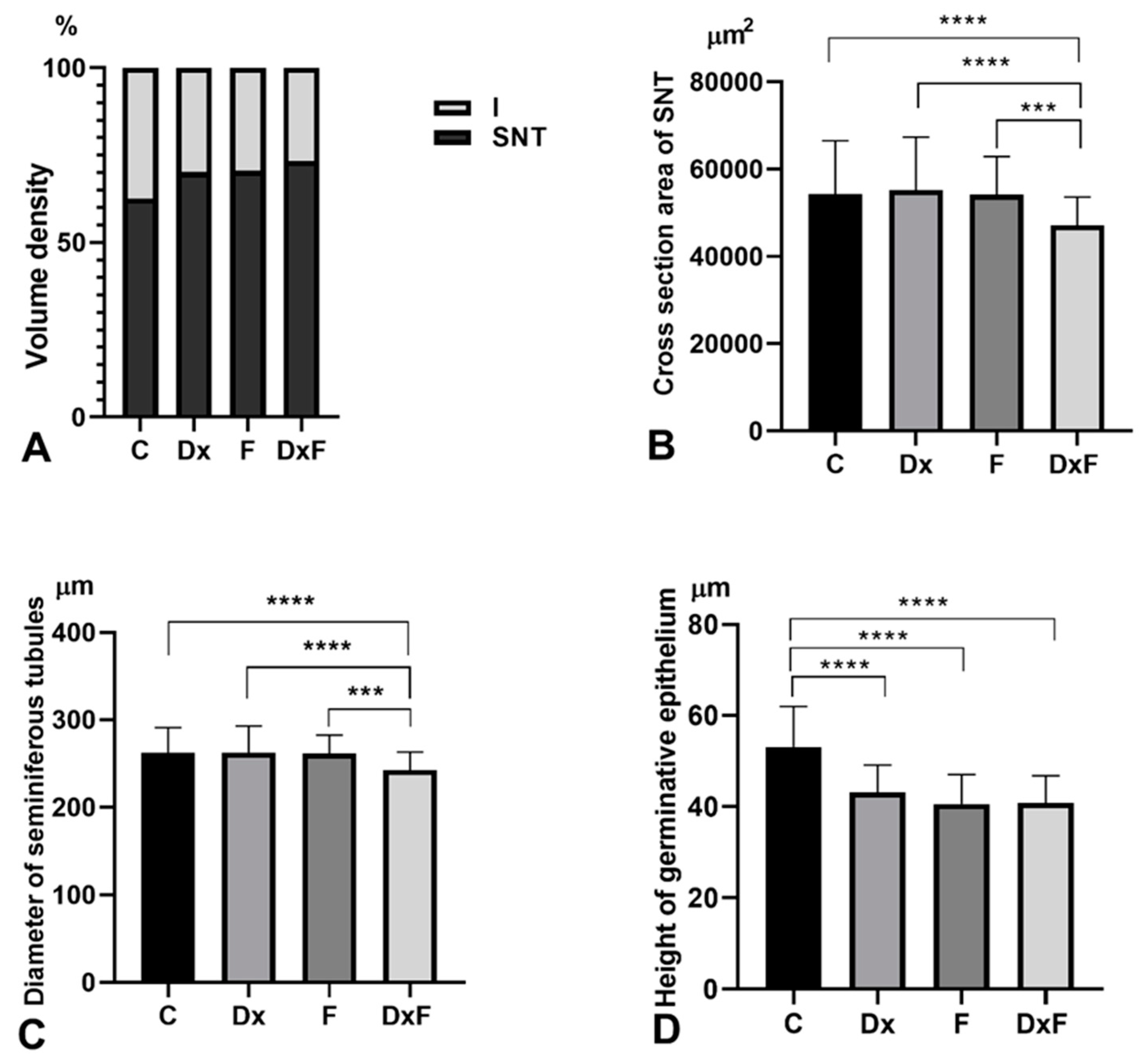
| Gestational Length (days) | Litter Size | Female–Male Ratio | Fetal bw (g) | Adult bw (g) | Testicular Weight (g) | GSI (gw/bw ∗ 100) | Vft/bw | |
|---|---|---|---|---|---|---|---|---|
| C | 22 ± 0 | 11 ± 2 | 1:1 ± 0.1 | 5.5 ± 0.4 | 322 ± 54 | 1.49 ± 0.13 | 0.46 ± 0.05 | 0.09 ± 0.007 |
| Dx | 22 ± 0 | 11 ± 3 | 1:1 ± 0.3 | 4.3 ± 0.4 A | 314 ± 30 | 1.46 ± 0.12 | 0.46 ± 0.03 | 0.05 ± 0.003 A |
| F | 370 ± 63 | 1.63 ± 0.14 | 0.44 ± 0.03 | |||||
| DxF | 383 ± 42 | 1.46 ± 0.17 | 0.38 ± 0.03 B,C,D |
| C | Dx | F | DxF | |
|---|---|---|---|---|
| SOD (U/g wet mass) | 12,578.9 ± 857.9 | 13,076.53 ± 1834.35 | 15,474.56 ± 13,076.64 | 9744.31 ± 3282.78 E,F |
| CAT (μmol H2O2/min/g wet mass) | 857.9 ± 145.29 | 754.47 ± 52.37 | 1158.03 ± 708.59 | 911.01 ± 420.92 |
| GSH-Px (μmol NADPH/min/g wet mass) | 134,797.4 ± 34,722.1 | 91,157 ± 41738.28 A | 66,945.32 ± 23,556.9 B | 78,472.65 ± 24,074.25 C |
| GR (nmol NADPH/min/g wet mass) | 1783.8 ± 1090.36 | 1186.49 ± 630.84 A | 428.30 ± 132.61 B,D | 325.08 ± 174.99 C,E |
| GST (nmol GSH/min/g wet mass) | 90,630.0 ± 15,689.36 | 99,731.25 ± 6062.29 | 95,837.50 ± 27,172.89 | 88,337.50 ± 9785.61 |
| GSH (µmol/g wet mass) | 3253.6 ± 1237.35 | 3319.92 ± 499.33 | 2536.06 ± 317.59 | 2123.17 ± 480.77 C,E |
| SH (nmol/g wet mass) | 719.6 ± 120.83 | 828.42 ± 118.18 | 883.60 ± 310.24 | 707.92 ± 104.80 |
| Group | Root 1 | Root 2 |
|---|---|---|
| C | −2.22189 | 0.451185 |
| F | 1.57741 | −0.644622 |
| Dx | −0.57059 | −0.811620 |
| DxF | 1.53837 | 0.622674 |
| Variable | Wilks’ Lambda | Partial Lambda | F-Remove (3.9) | p-Value | Toler. | 1-Toler. (R-Sqr.) |
|---|---|---|---|---|---|---|
| SOD | 0.146698 | 0.878118 | 0.416399 | 0.745528 | 0.622707 | 0.377293 |
| CAT | 0.140828 | 0.914720 | 0.279694 | 0.838790 | 0.771553 | 0.228447 |
| GSH-Px | 0.164153 | 0.784747 | 0.822889 | 0.513479 | 0.792215 | 0.207785 |
| GR | 0.216311 | 0.595523 | 2.037586 | 0.179149 | 0.868542 | 0.131458 |
| GST | 0.134973 | 0.954403 | 0.143325 | 0.931384 | 0.538255 | 0.461745 |
| GSH | 0.197505 | 0.652228 | 1.599618 | 0.256856 | 0.568103 | 0.431897 |
| SH | 0.167259 | 0.770174 | 0.895226 | 0.480321 | 0.560317 | 0.439683 |
| Variable | Root 1 | Root 2 |
|---|---|---|
| SOD | −0.309496 | −0.143654 |
| CAT | 0.097974 | 0.265807 |
| GSH-Px | −0.400054 | 0.651859 |
| GR | −0.766891 | −0.226217 |
| GST | 0.282339 | 0.097549 |
| GSH | −0.848148 | −0.429790 |
| SH | 0.536050 | −0.741471 |
| Eigenval. | 3.198399 | 0.525628 |
| Cum. prop. | 0.812603 | 0.946147 |
| Pair of Variables | Valid N | Spearman R | t(N-2) | p-Value |
|---|---|---|---|---|
| Treatments and SOD | 18 | −0.431228 | −1.91180 | 0.043980 * |
| Treatments and CAT | 18 | −0.148844 | −0.60208 | 0.555556 |
| Treatments and GSH-Px | 19 | −0.420989 | −1.91362 | 0.042660 * |
| Treatments and GR | 19 | −0.586733 | −2.98743 | 0.008273 * |
| Treatments and GST | 18 | 0.003212 | 0.01285 | 0.989906 |
| Treatments and GSH | 19 | −0.403713 | −1.81941 | 0.086506 |
| Treatments and SH | 19 | −0.057284 | −0.23657 | 0.815814 |
| SOD | CAT | GSH-Px | GR | GST | GSH | SH | |
|---|---|---|---|---|---|---|---|
| −0.431228 | −0.148844 | −0.420989 | −0.586733 * | 0.003212 | −0.403713 | −0.057284 | |
| SOD | 0.080882 | 0.010325 | 0.416322 | 0.484366 * | 0.408880 | 0.415075 | |
| CAT | 0.080882 | 0.029928 | −0.214765 | 0.036765 | −0.122807 | −0.021672 | |
| GSH-Px | 0.010325 | 0.029928 | 0.314173 | −0.106295 | 0.428070 | 0.192982 | |
| GR | 0.416322 | −0.214765 | 0.314173 | 0.162106 | 0.475647 * | 0.103554 | |
| GST | 0.484366 * | 0.036765 | −0.106295 | 0.162106 | 0.364293 | 0.525284 * | |
| GSH | 0.408880 | −0.122807 | 0.428070 | 0.475647 * | 0.364293 | 0.522807 * | |
| SH | 0.415075 | −0.021672 | 0.192982 | 0.103554 | 0.525284 * | 0.522807 * |
| Offspring from Control Dams | Offspring from Dx-Treated Dams | |
|---|---|---|
| Standard commercial feed/drinking water ad libitum | C (n = 6) | Dx (n = 6) |
| Standard commercial feed/10% (w/v) fructose solution | F (n = 6) | DxF (n = 6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristić, N.; Borković-Mitić, S.; Manojlović-Stojanoski, M.; Nestorović, N.; Filipović, B.; Šošić-Jurjević, B.; Trifunović, S.; Mitić, B.; Čukuranović-Kokoris, J.; Pavlović, S. Is There a Relationship Between Prenatal Dexamethasone and Postnatal Fructose Overexposure and Testicular Development, Function, and Oxidative Stress Parameters in Rats? Int. J. Mol. Sci. 2024, 25, 13112. https://doi.org/10.3390/ijms252313112
Ristić N, Borković-Mitić S, Manojlović-Stojanoski M, Nestorović N, Filipović B, Šošić-Jurjević B, Trifunović S, Mitić B, Čukuranović-Kokoris J, Pavlović S. Is There a Relationship Between Prenatal Dexamethasone and Postnatal Fructose Overexposure and Testicular Development, Function, and Oxidative Stress Parameters in Rats? International Journal of Molecular Sciences. 2024; 25(23):13112. https://doi.org/10.3390/ijms252313112
Chicago/Turabian StyleRistić, Nataša, Slavica Borković-Mitić, Milica Manojlović-Stojanoski, Nataša Nestorović, Branko Filipović, Branka Šošić-Jurjević, Svetlana Trifunović, Bojan Mitić, Jovana Čukuranović-Kokoris, and Slađan Pavlović. 2024. "Is There a Relationship Between Prenatal Dexamethasone and Postnatal Fructose Overexposure and Testicular Development, Function, and Oxidative Stress Parameters in Rats?" International Journal of Molecular Sciences 25, no. 23: 13112. https://doi.org/10.3390/ijms252313112
APA StyleRistić, N., Borković-Mitić, S., Manojlović-Stojanoski, M., Nestorović, N., Filipović, B., Šošić-Jurjević, B., Trifunović, S., Mitić, B., Čukuranović-Kokoris, J., & Pavlović, S. (2024). Is There a Relationship Between Prenatal Dexamethasone and Postnatal Fructose Overexposure and Testicular Development, Function, and Oxidative Stress Parameters in Rats? International Journal of Molecular Sciences, 25(23), 13112. https://doi.org/10.3390/ijms252313112









