Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline
Abstract
1. Introduction
2. Results
2.1. Confirmation and Antibiotic Treatments of the RCMs
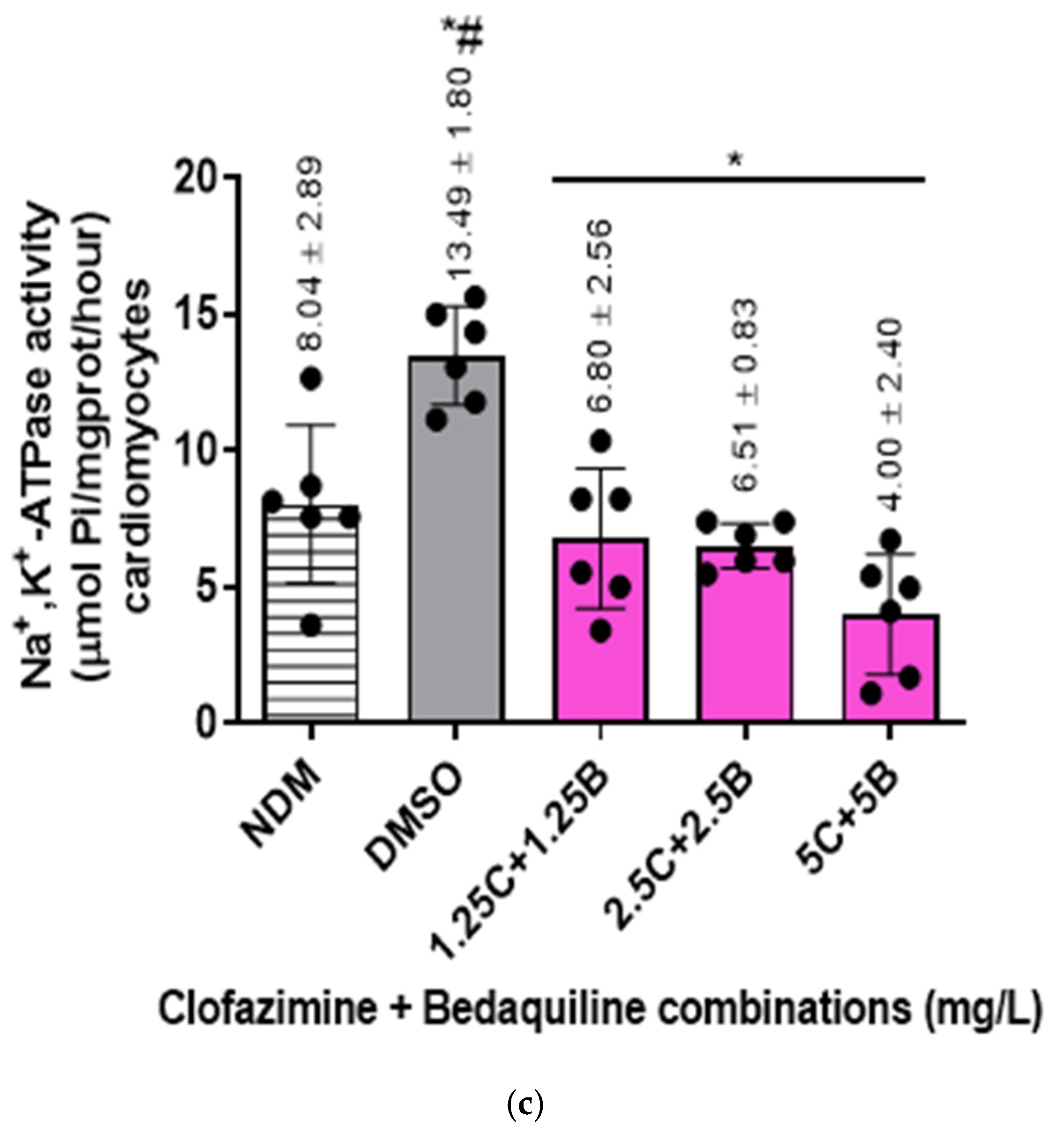
2.2. Na+,K+-ATPase Activity of the RCMs
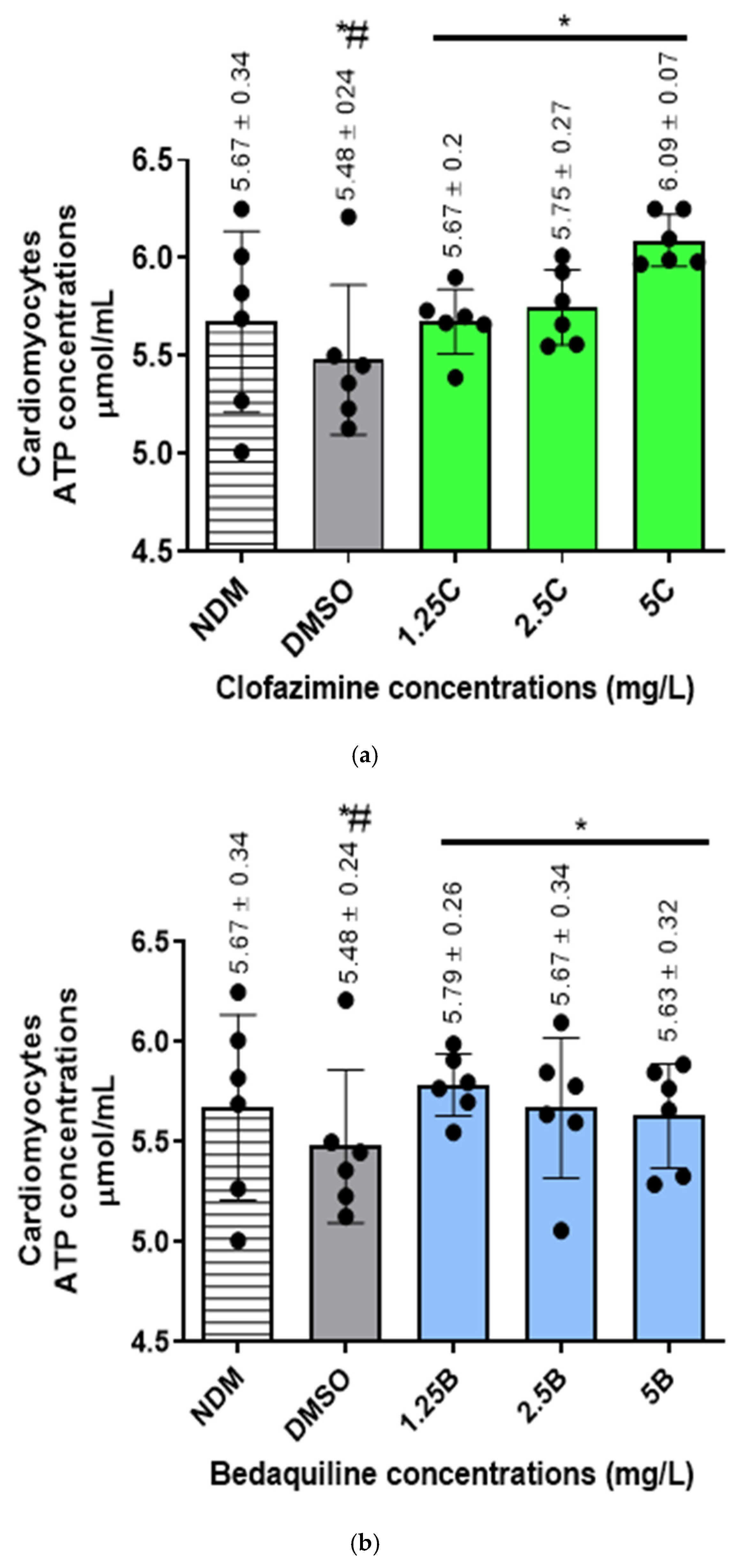
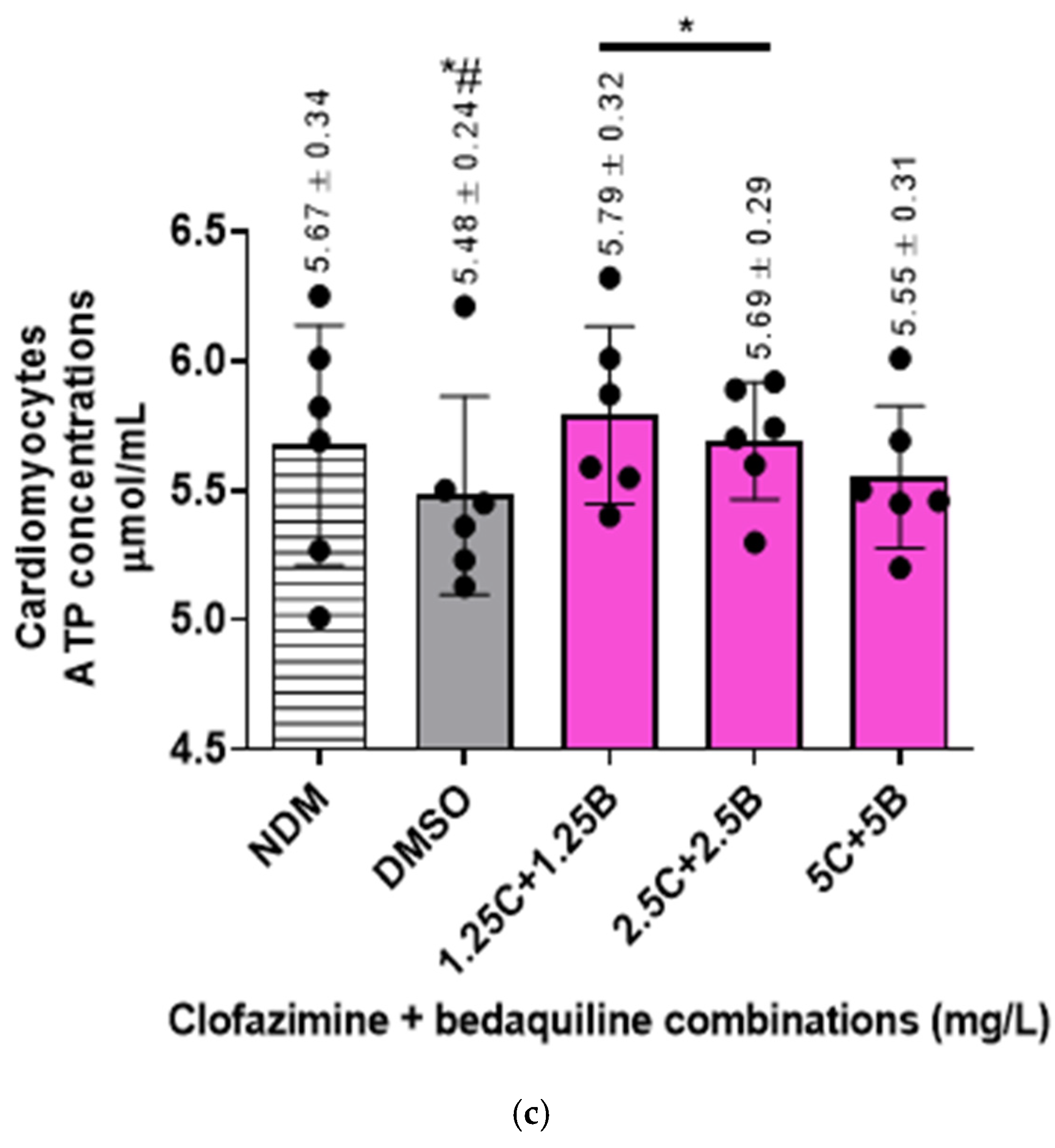
2.3. The Intracellular ATP Concentrations of the RCMs
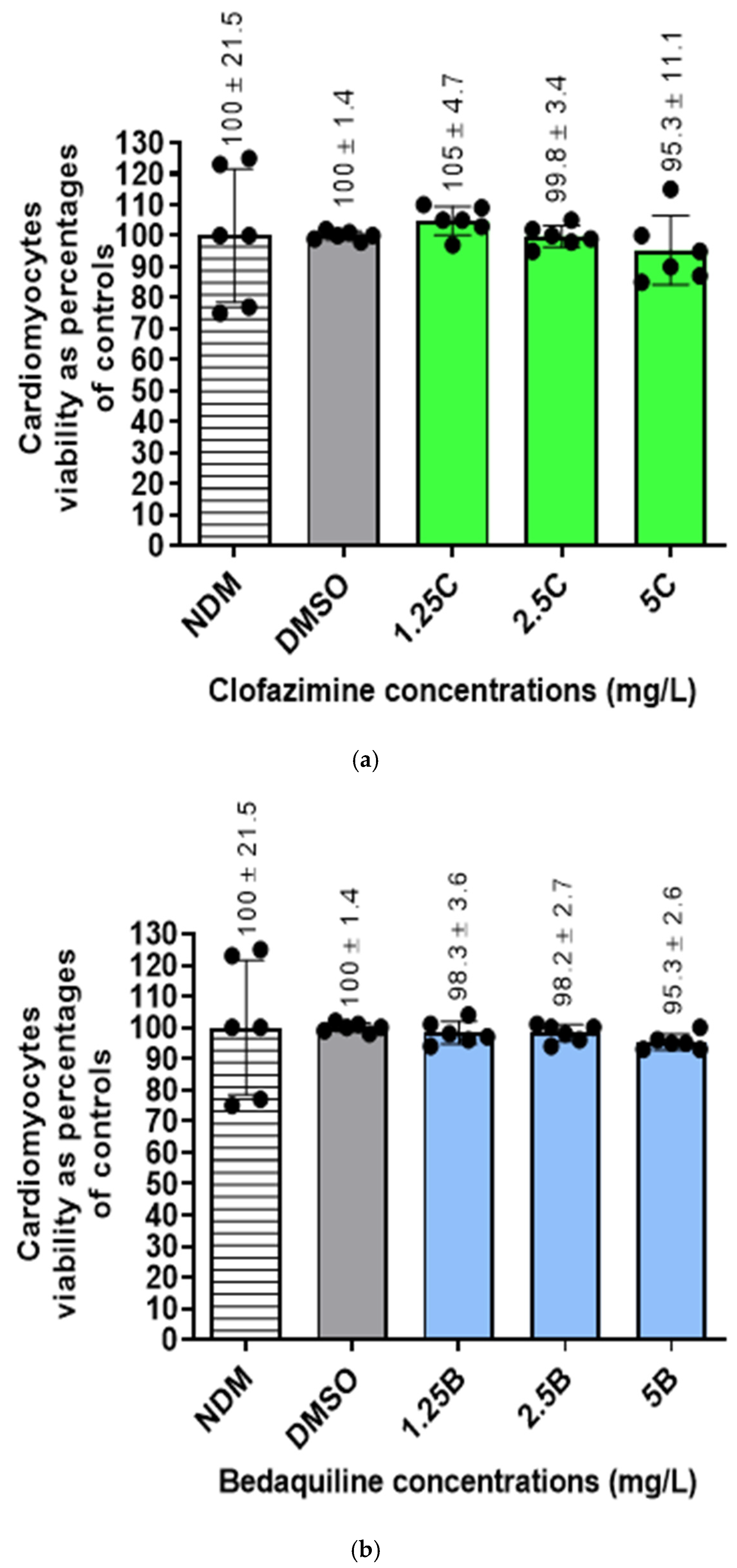
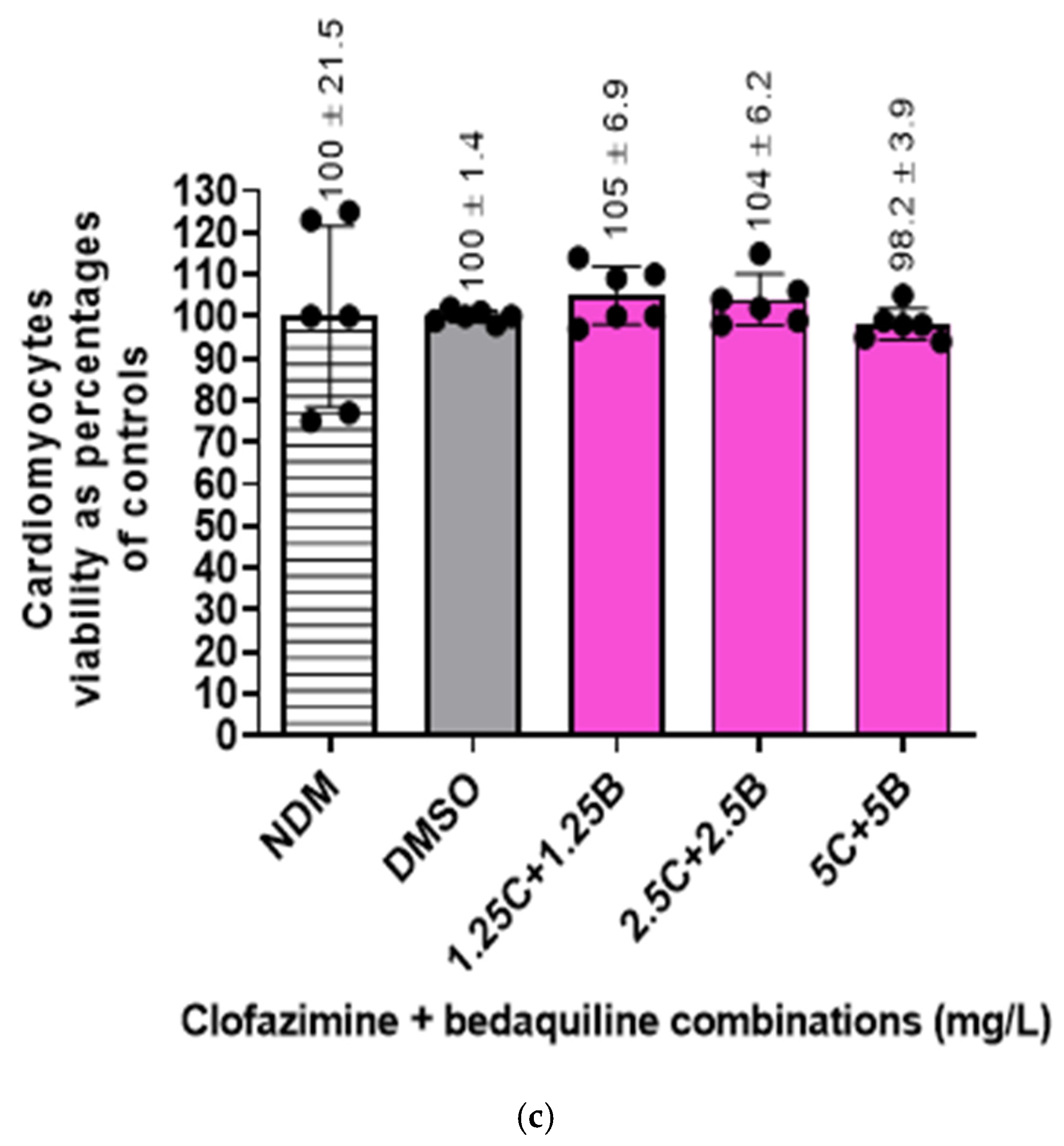
2.4. Viability of the RCMs
2.5. Molecular Docking Analysis
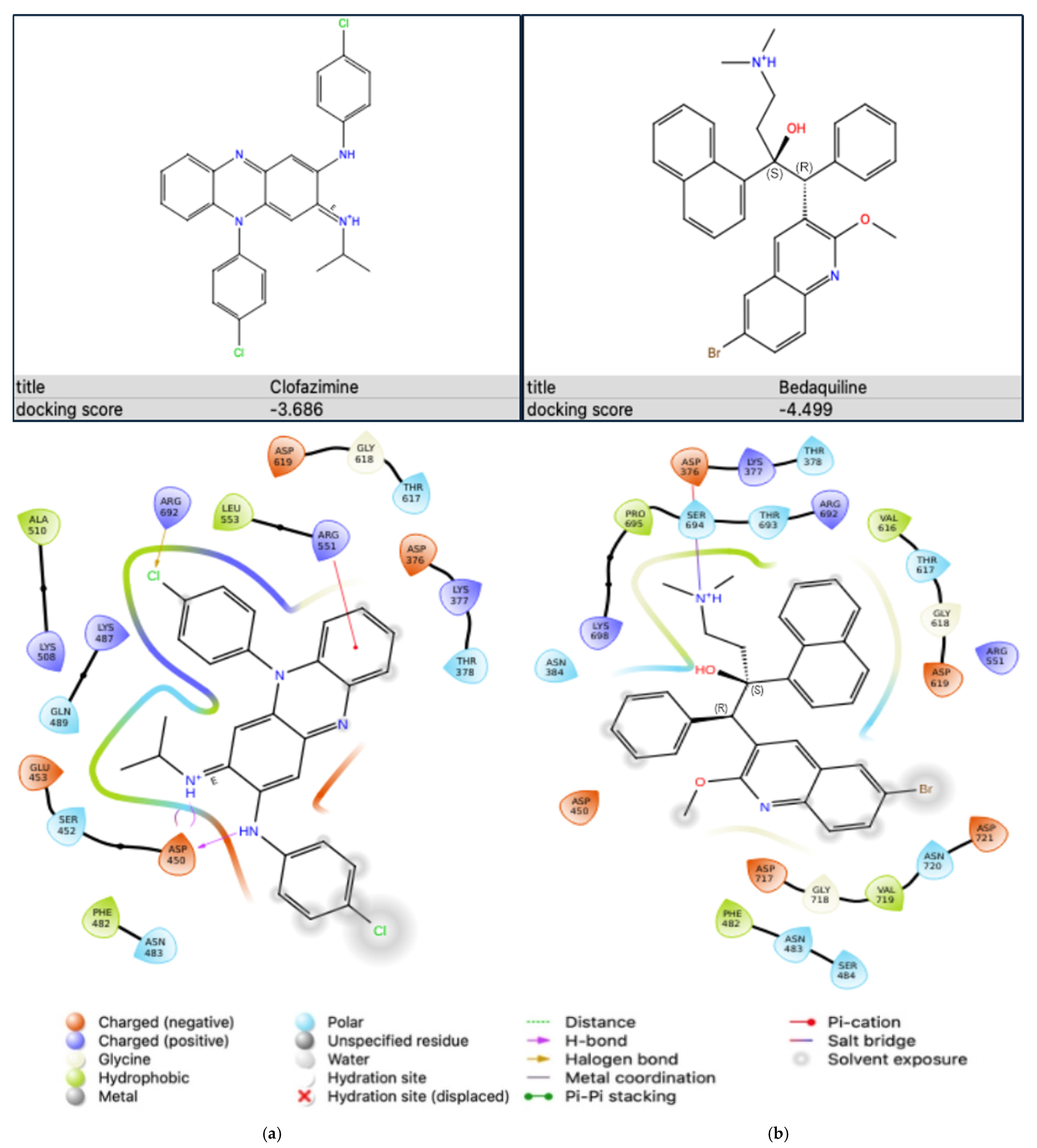
2.5.1. Antibiotic-Na+,K+-ATPase Interaction
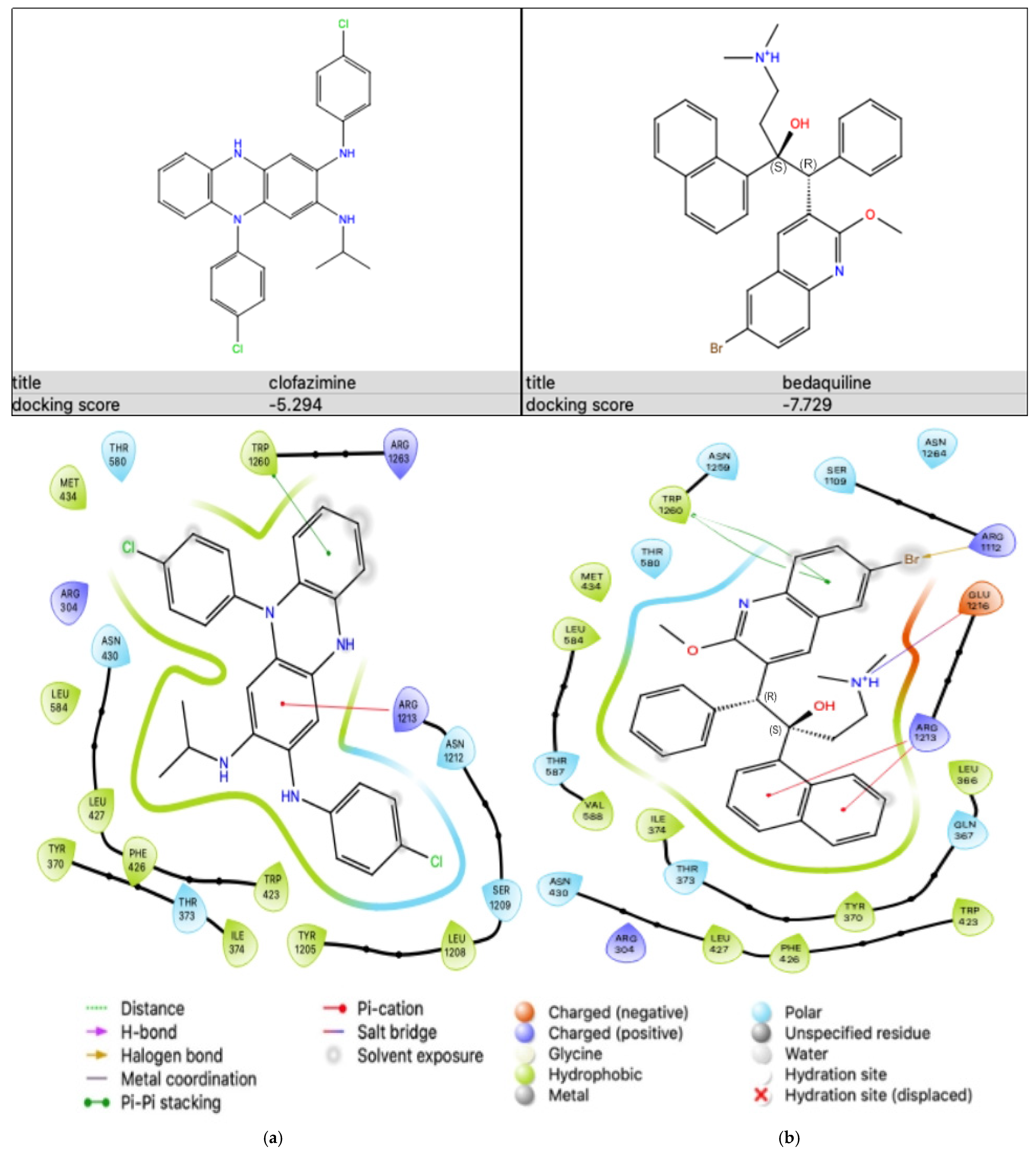
2.5.2. Antibiotic-KATP Channel Interaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Antibiotics and Other Chemicals
4.1.2. Assay Kits
4.1.3. RCMs and Growth Media
4.2. Methods
4.2.1. Preparation of the RCMs
4.2.2. Antibiotic Reaction Assays
4.2.3. Na+,K+-ATPase Activity
Protein Extraction
Na+,K+-ATPase Enzymatic Activity Assay
- y: ODstandard − ODblank (ODblank is the OD value when the standard concentration is 0);
- x: the concentration of the standard;
- ΔA660: ODsample − ODcontrol;
- b: the y intercept of the standard curve
- a: the slope of the standard curve;
- V1: the total volume of the reaction system (0.25 mL);
- Cpr: the concentration of protein in sample (mg protein/mL);
- V2: the volume of added sample (0.1 mL);
- t: the time of the enzymatic reaction (1/6 h);
- f: the dilution factor of the sample before testing.
4.2.4. ATP Concentration Determination
ATP Extraction
ATP Quantification
- B = ATP concentration in the reaction well from standard curve (µmol);
- V = the sample volume added into sample wells (mL);
- D = the dilution factor.
4.2.5. Cellular Viability Determination
4.2.6. Computational Molecular Docking Analysis
Ligand Preparation
Protein Preparation
- Protein molecules
- 2.
- Protein preparation
Active Site Determination and Receptor Grid Generation
Molecular Docking
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Deun, A.; Maug, A.K.; Salim, M.A.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Cholo, M.C.; Mothiba, M.T.; Fourie, B.; Anderson, R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J. Antimicrob. Chemother. 2017, 72, 338–353. [Google Scholar] [CrossRef]
- Ndjeka, N.; Ismail, N.A. Bedaquiline and clofazimine: Successes and challenges. Lancet Microbe 2020, 1, 139–140. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2021; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 25 November 2024).
- Dooley, K.E.; Rosenkranz, S.L.; Conradie, F.; Moran, L.; Hafner, R.; Von Groote-Bidlingmaier, F.; Lama, J.R.; Shenje, J.; De Los Rios, J.; Comins, K.; et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: A phase 2, open-label, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pereiro, J.; Sánchez-Montalvá, A.; Aznar, M.L.; Espiau, M. MDR tuberculosis treatment. Medicina 2022, 58, 188. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Bern, H.; Chiang, C.Y.; Goodall, R.L.; Nunn, A.J.; Rusen, I.D.; Meredith, S.K. QT prolongation in the STREAM. stage 1 Trial. Int. J. Tuberc. Lung Dis. 2022, 26, 334–340. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Hill, A.P.; Perrin, M.J.; Heide, J.; Campbell, T.J.; Mann, S.A.; Vandenberg, J.I. Kinetics of drug interaction with the Kv11.1 potassium channel. Mol. Pharmacol. 2014, 85, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Borin, D.; Pecorari, I.; Pena, B.; Sbaizero, O. Novel insights into cardiomyocytes provided by atomic force microscopy. Semin. Cell Dev. Biol. 2018, 73, 4–12. [Google Scholar] [CrossRef]
- Liu, X.; Pu, W.; He, L.; Li, Y.; Zhao, H.; Li, Y.; Liu, K.; Huang, X.; Weng, W.; Wang, Q.D.; et al. Cell proliferation fate mapping reveals regional cardiomyocyte cell-cycle activity in subendocardial muscle of left ventricle. Nat. Commun. 2021, 12, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Jadhav, H.; Shinde, Y.; Jagtap, V.; Girase, R.; Patel, H. Optimizing bedaquiline for cardiotoxicity by structure based virtual screening, DFT analysis and molecular dynamic simulation studies to identify selective MDR-TB inhibitors. Silico Pharmacol. 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Hadova, K.; Kralova, E.; Doka, G.; Bies Pivackova, L.; Kmecova, Z.; Krenek, P.; Klimas, J. Isolated downregulation of HCN2 in ventricles of rats with streptozotocin-induced diabetic cardiomyopathy. BMC Cardiovasc. Disord. 2021, 21, 118. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, N.; Sun, J.; Lu, J.X.; Abbasi, S.; Wu, G.; Lee, A.S.; Sawamura, T.; Cheng, J.; Chen, C.H.; et al. Atherogenic L5 LDL induces cardiomyocyte apoptosis and inhibits KATP channels through CaMKII activation. Lipids Health Dis. 2020, 19, 189. [Google Scholar] [CrossRef]
- Patton, B.L.; Zhu, P.; ElSheikh, A.; Driggers, C.M.; Shyng, S.L. Dynamic duo: Kir6 and SUR in KATP channel structure and function. Channels 2024, 18, 2327708. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R. Inward rectifier potassium currents as a target for atrial fibrillation therapy. J. Cardiovasc. Pharmacol. 2008, 52, 129–135. [Google Scholar] [CrossRef]
- Kang, J.A.; Park, S.H.; Jeong, S.P.; Han, M.H.; Lee, C.R.; Lee, K.M.; Kim, N.; Song, M.R.; Choi, M.; Ye, M.; et al. Epigenetic regulation of KCNA3-encoding Kv1.3 potassium channel by cereblon contributes to regulation of CD4+ T-cell activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8771–8776. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shapiro, J.I. The physiological and clinical importance of sodium potassium ATPase in cardiovascular diseases. Curr. Opin. Pharmacol. 2016, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.R.; Pan, F.; Parvez, S.; Fleig, A.; Chong, C.R.; Xu, J.; Dang, Y.; Zhang, J.; Jiang, H.; Penner, R.; et al. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS ONE 2008, 3, 4009. [Google Scholar] [CrossRef]
- Leanza, L.; Trentin, L.; Becker, K.A.; Frezzato, F.; Zoratti, M.; Semenzato, G.; Gulbins, E.; Szabo, I. Clofazimine, Psora-4 and PAP-1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia. Leukemia 2013, 27, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Leanza, L.; O’reilly, P.; Doyle, A.; Venturini, E.; Zoratti, M.; Szegezdi, E.; Szabo, I. Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines. Curr. Pharm. Des. 2014, 20, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Faouzi, M.; Starkus, J.; Penner, R. State-dependent blocking mechanism of Kv1.3 channels by the antimycobacterial drug clofazimine. Br. J. Pharmacol. 2015, 172, 5161–5173. [Google Scholar] [CrossRef]
- Anderson, R.; Smit, M.J. Clofazimine and B669 inhibit the proliferative responses and Na+,K+-adenosine triphosphatase activity of human lymphocytes by a lysophospholipid-dependent mechanism. Biochem. Pharmacol. 1993, 46, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Yu, Z.L.; Lou, J.C.; Ma, X.C.; Zhang, B. Update on the effects of the sodium pump α1 subunit on human glioblastoma: From the laboratory to the clinic. Expert Opin. Investig. Drugs 2018, 27, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Felippe Gonçalves-De-Albuquerque, C.; Ribeiro Silva, A.; Ignácio Da Silva, C.; Caire Castro-Faria-Neto, H.; Burth, P. Na+/K+ pump and beyond: Na+,K+-ATPase as a modulator of apoptosis and autophagy. Molecules 2017, 22, 578. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.J.; Bijlani, S.; De Sautu, M.; Spontarelli, K.; Young, V.C.; Gatto, C.; Artigas, P. FXYD protein isoforms differentially modulate human Na+/K+ pump function. J. Gen. Physiol. 2020, 152, e202012660. [Google Scholar] [CrossRef]
- Rindler, T.N.; Lasko, V.M.; Nieman, M.L.; Okada, M.; Lorenz, J.N.; Lingrel, J.B. Knockout of the Na+,K+-ATPase α2-isoform in cardiac myocytes delays pressure overload-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Sepp, M.; Sokolova, N.; Jugai, S.; Mandel, M.; Peterson, P.; Vendelin, M. Tight coupling of Na+,K+-ATPase with glycolysis demonstrated in permeabilized rat cardiomyocytes. PLoS ONE 2014, 9, 994–1013. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Ge, N.; Huang, T.; Zhang, M.; Wang, H. Sodium pump alpha-2 subunit (ATP1α2) alleviates cardiomyocyte anoxia-reoxygenation injury via inhibition of endoplasmic reticulum stress-related apoptosis. Can J. Physiol. Pharmacol. 2018, 96, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase Revisited: On its mechanism of action, role in cancer, and activity modulation. Molecules 2021, 28, 1905. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Webb, S.T. Cations: Potassium, calcium, and magnesium. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 195–198. [Google Scholar] [CrossRef]
- McDonough, A.A.; Youn, J.H. Potassium homeostasis: The knowns, the unknowns, and the health benefits. Physiology 2017, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, R.; Tung, L. Contribution of potassium channels to action potential repolarization of human embryonic stem cell-derived cardiomyocytes. Br. J. Pharmacol. 2019, 176, 2780–2794. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Li, Z.; Pang, Y.; Jing, W.; Liu, P.; Wu, T.; Cai, C.; Shi, J.; Qin, Z.; et al. Clofazimine improves clinical outcomes in multidrug-resistant tuberculosis: A randomized controlled trial. Clin. Microbiol. Infect. 2019, 25, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, W.; Schurig-Briccio, L.A.; Lindert, S.; Shoen, C.; Hitchings, R.; Li, J.; Wang, Y.; Baig, N.; Zhou, T.; et al. Antiinfectives targeting enzymes and the proton motive force. Proc. Natl. Acad. Sci. USA 2015, 112, 7073–7082. [Google Scholar] [CrossRef] [PubMed]
- Pontali, E.; Sotgiu, G.; Tiberi, S.; Ambrosio, L.; Centis, R.; Migliori, G.B. Cardiac safety of bedaquiline: A systematic and critical analysis of the evidence. Eur. Respir. J. 2017, 50, 1701462. [Google Scholar] [CrossRef] [PubMed]
- Cumberland, M.J.; Riebel, L.L.; Roy, A.; O’shea, C.; Holmes, A.P.; Denning, C.; Kirchhof, P.; Rodriguez, B.; Gehmlich, K. Basic research approaches to evaluate cardiac arrhythmia in heart failure and beyond. Front. Physiol. 2022, 13, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.S.; Hajouli, S. Arrhythmias. Last Update: June 5, 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Guglielmetti, L.; Jaspard, M.; Le Dû, D.; Lachâtre, M.; Marigot-Outtandy, D.; Bernard, C.; Veziris, N.; Robert, J.; Yazdanpanah, Y.; Caumes, E.; et al. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur. Respir. J. 2017, 49, 1601–1679. [Google Scholar] [CrossRef]
- Ndjeka, N.; Schnippel, K.; Master, I.; Meintjes, G.; Maartens, G.; Romero, R.; Padanilam, X.; Enwerem, M.; Chotoo, S.; Singh, N.; et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur. Respir. J. 2018, 52, 1801528. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.; Danila, E.; Maryandyshev, A.; Dalcolmo, M.; Miliauskas, S.; Kuksa, L.; Manga, S.; Skrahina, A.; Diktanas, S.; Codecasa, L.R.; et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: First global report. Eur. Respir. J. 2019, 54, 1901522. [Google Scholar] [CrossRef] [PubMed]
- Kashongwe, I.M.; Mawete, F.; Mbulula, L.; Nsuela, D.J.; Losenga, L.; Anshambi, N.; Aloni, M.; Kaswa, M.; Kayembe, J.M.N.; Umba, P.; et al. Outcomes and adverse events of pre- and extensively drug-resistant tuberculosis patients in Kinshasa, Democratique Republic of the Congo: A retrospective cohort study. PLoS ONE 2020, 15, 236–264. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.Y.; Prieto, A.; Bogati, H.; Sannino, L.; Akai, N.; Marquardt, T. Adverse events using shorter MDR-TB regimens: Outcomes from Port Moresby, Papua New Guinea. Public Health Action. 2021, 11, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nakamura, I.; Takebayashi, Y.; Watanabe, H. A fatal case of repeated ventricular fibrillation due to torsade de pointes following repeated administration of metoclopramide. Clin. Case Rep. 2022, 10, 6213. [Google Scholar] [CrossRef] [PubMed]
- Olona, A.; Hateley, C.; Guerrero, A.; Ko, J.H.; Johnson, M.R.; Anand, P.K.; Thomas, D.; Gil, J.; Behmoaras, J. Cardiac glycosides cause cytotoxicity in human macrophages and ameliorate white adipose tissue homeostasis. Br. J. Pharmacol. 2022, 179, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Yan, R.; Huang, B.; Ye, F.; Wu, L.; Chi, X.; Shi, Y.; Zhou, Q. Cryo-EM structures of recombinant human sodium-potassium pump determined in three different states. Nat. Commun. 2022, 13, 3957. [Google Scholar] [CrossRef]
- Ding, D.; Hou, T.; Wei, M.; Wu, J.X.; Chen, L. The inhibition mechanism of the SUR2A-containing KATP channel by a regulatory helix. Nat. Commun. 2023, 14, 3608. [Google Scholar] [CrossRef] [PubMed]
- Driggers, C.M.; Shyng, S.L. Mechanistic insights on KATP channel regulation from cryo-EM structures. J. Gen. Physiol. 2023, 155, e202113046. [Google Scholar] [CrossRef] [PubMed]
- Haruna, T.; Horie, M.; Kouchi, I.; Nawada, R.; Tsuchiya, K.; Akao, M.; Otani, H.; Murakami, T.; Sasayama, S. Coordinate interaction between ATP-sensitive K+ channel and Na+, K+-ATPase modulates ischemic preconditioning. Circulation 1998, 98, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.; Cox, D.J.; O’Connell, F.; Basdeo, S.A.; Gogan, K.M.; Ó’Maoldomhnaigh, C.; O’Sullivan, J.; Keane, J.; Phelan, J.J. The effect of tuberculosis antimicrobials on the immunometabolic profiles of primary human macrophages stimulated with Mycobacterium tuberculosis. Int. J. Mol. Sci. 2021, 22, 12189. [Google Scholar] [CrossRef]
- Killilea, D.W.; Killilea, A.N. Mineral requirements for mitochondrial function: A connection to redox balance and cellular differentiation. Free Radic. Biol. Med. 2022, 182, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Pott, A.; Bock, S.; Berger, I.M.; Frese, K.; Dahme, T.; Keßler, M.; Rinné, S.; Decher, N.; Just, S.; Rottbauer, W. Mutation of the Na+,K+-ATPase ATP1a1a.1 causes QT interval prolongation and bradycardia in zebrafish. J. Mol. Cell Cardiol. 2018, 20, 42–52. [Google Scholar] [CrossRef]
- Shattock, M.J.; Ottolia, M.; Bers, D.M.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ exchange and Na+,K+-ATPase in the heart. Physiol. J. 2015, 593, 1361–1382. [Google Scholar] [CrossRef]
- Liess, C.; Radda, G.K.; Clarke, K. Measurement of cardiomyocyte diameter in the isolated rat heart using diffusion-weighted H-MRS: Changes with ischaemia. Proc. Intl. Sot. Mag. Reson. Med. 2000, 8, 468. Available online: https://cds.ismrm.org/ismrm-2000/PDF2/0468.pdf (accessed on 25 November 2024).
- Kadota, S.; Pabon, L.; Reinecke, H.; Murry, C.E. In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Rep. 2017, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Yang, M.; Xin, G.; Cui, W.; Xie, Z.; Silverstein, R.L. Cardiotonic steroids stimulate macrophage inflammatory responses through a pathway involving CD36, TLR4 and Na+,K+-ATPase. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1462–1469. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mmakola, K.; Balmith, M.; Steel, H.; Said, M.; Potjo, M.; van der Mescht, M.; Hlatshwayo, N.; Meyer, P.; Tintinger, G.; Anderson, R.; et al. Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline. Int. J. Mol. Sci. 2024, 25, 13022. https://doi.org/10.3390/ijms252313022
Mmakola K, Balmith M, Steel H, Said M, Potjo M, van der Mescht M, Hlatshwayo N, Meyer P, Tintinger G, Anderson R, et al. Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline. International Journal of Molecular Sciences. 2024; 25(23):13022. https://doi.org/10.3390/ijms252313022
Chicago/Turabian StyleMmakola, Khomotso, Marissa Balmith, Helen Steel, Mohamed Said, Moliehi Potjo, Mieke van der Mescht, Nomsa Hlatshwayo, Pieter Meyer, Gregory Tintinger, Ronald Anderson, and et al. 2024. "Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline" International Journal of Molecular Sciences 25, no. 23: 13022. https://doi.org/10.3390/ijms252313022
APA StyleMmakola, K., Balmith, M., Steel, H., Said, M., Potjo, M., van der Mescht, M., Hlatshwayo, N., Meyer, P., Tintinger, G., Anderson, R., & Cholo, M. (2024). Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline. International Journal of Molecular Sciences, 25(23), 13022. https://doi.org/10.3390/ijms252313022






