Gut-Microbiota-Derived Butyric Acid Overload Contributes to Ileal Mucosal Barrier Damage in Late Phase of Chronic Unpredictable Mild Stress Mice
Abstract
1. Introduction
2. Results
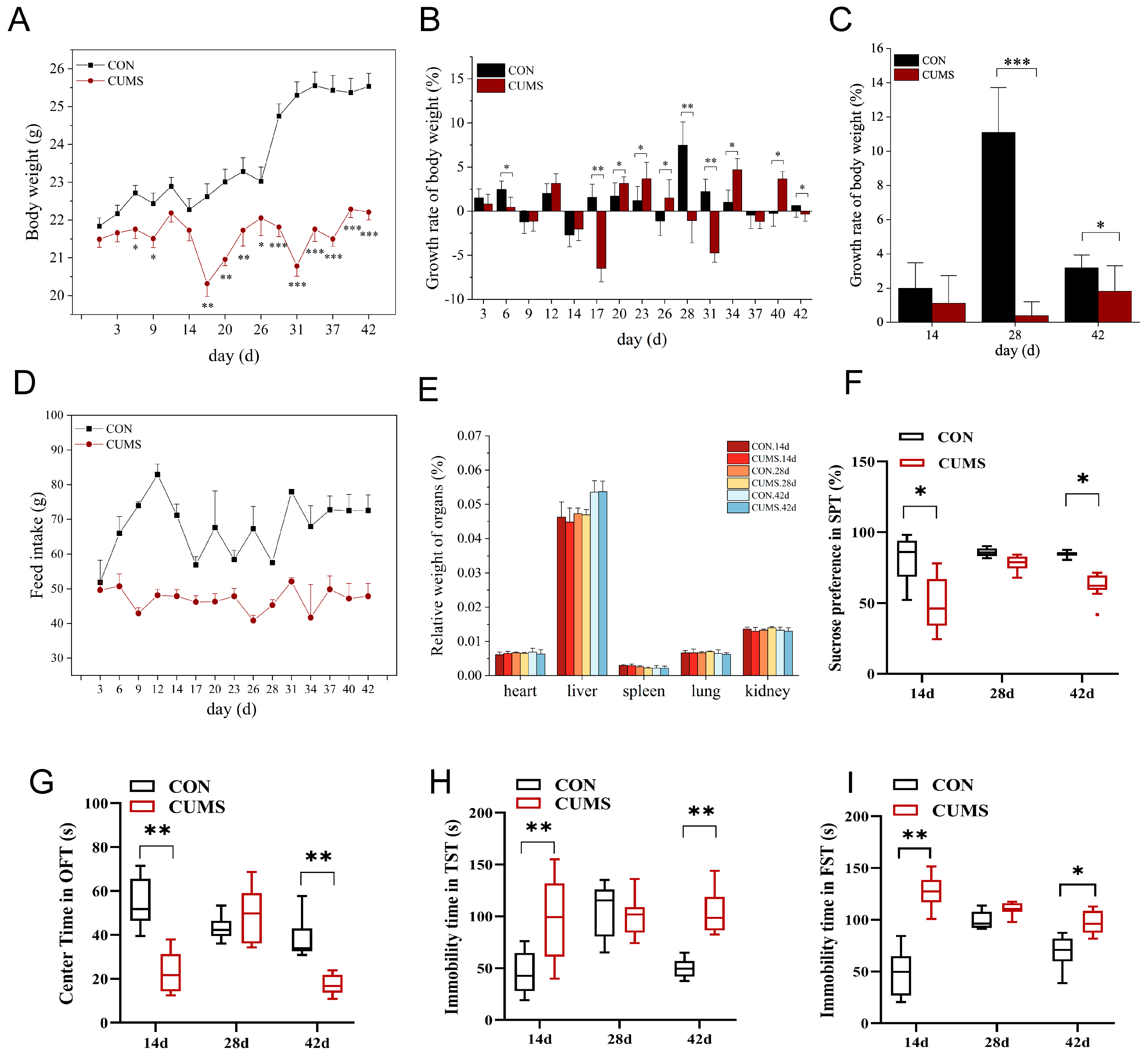
2.1. Effects of CUMS on Mice Body Weight, Food Intake, and Viscera Coefficient
2.2. Effect of CUMS on Depression Behavior of Mice
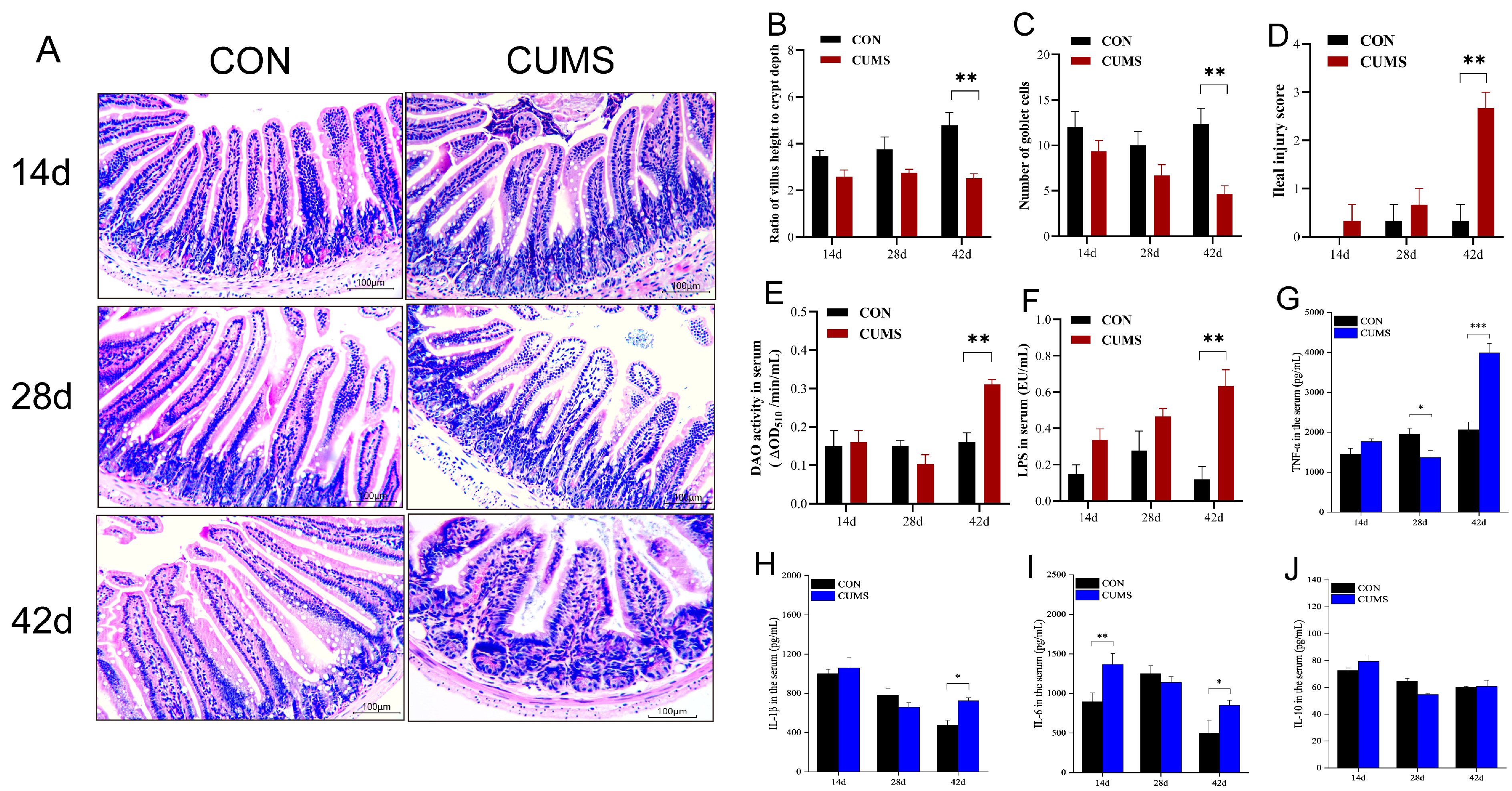
2.3. Effect of CUMS on the Mice IMBD
2.4. Effect of CUMS on Inflammatory Markers Levels in the Serum of Mice
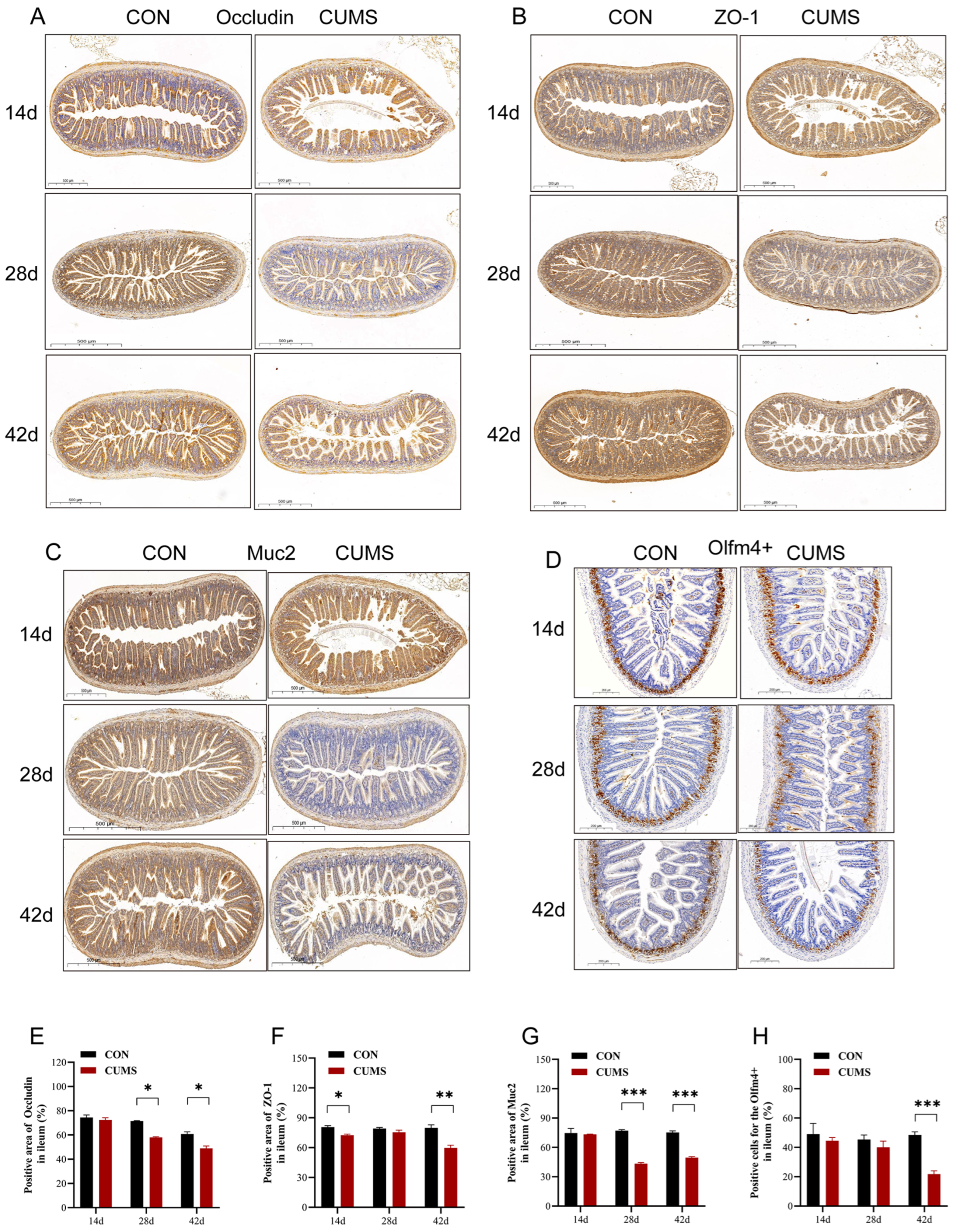
2.5. Effect of CUMS on Ileal Occludin, Zonula Occludens-1 (ZO-1), Mucoprotein 2 (Muc2), and Olfactomedin 4 (Olfm4+) Protein Expressions in Mice
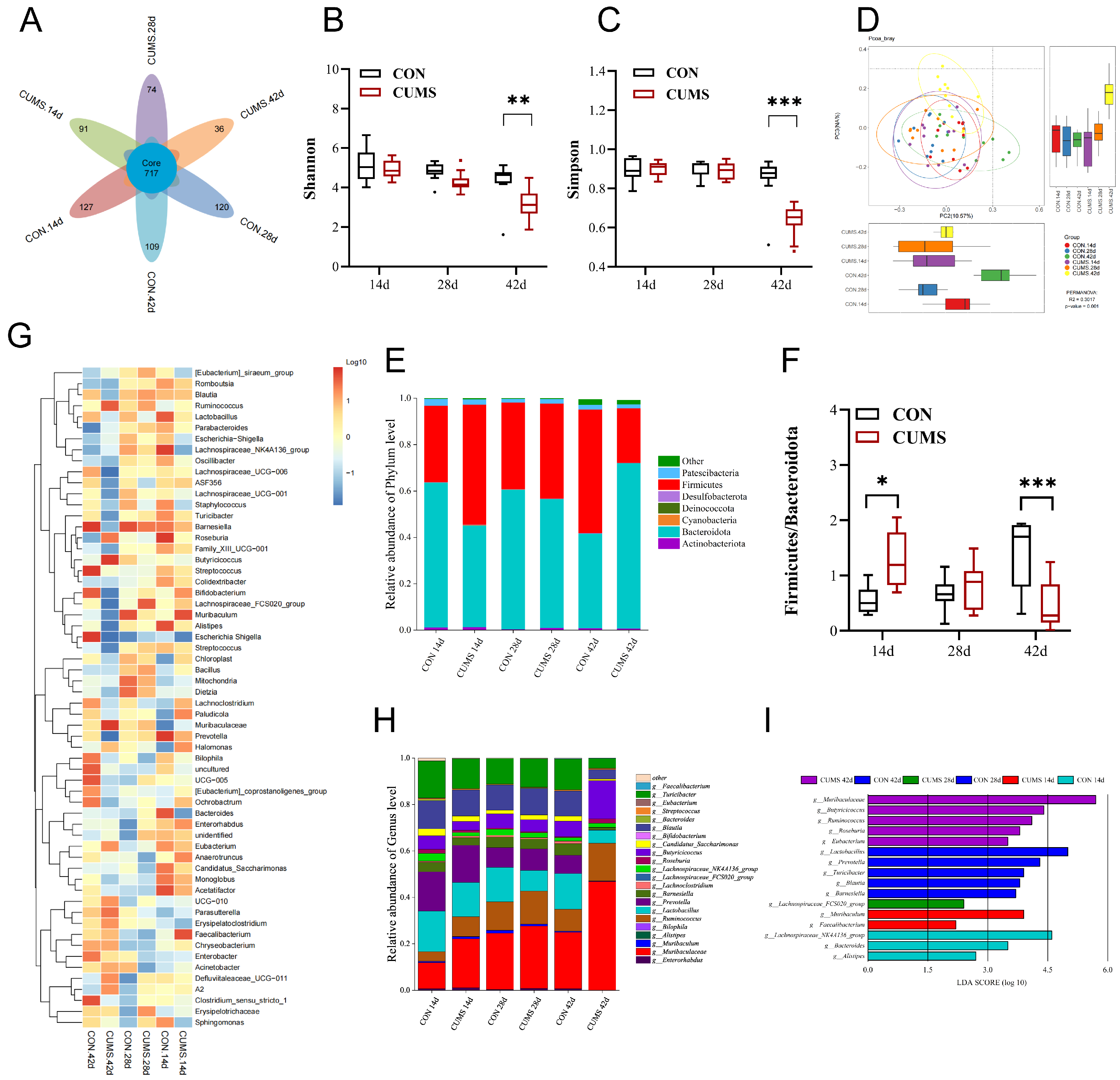
2.6. Effect of CUMS on the Composition and Function of Ileal Gut Microbiota in Mice
2.6.1. Diversity of the Ileal Gut Microbiota
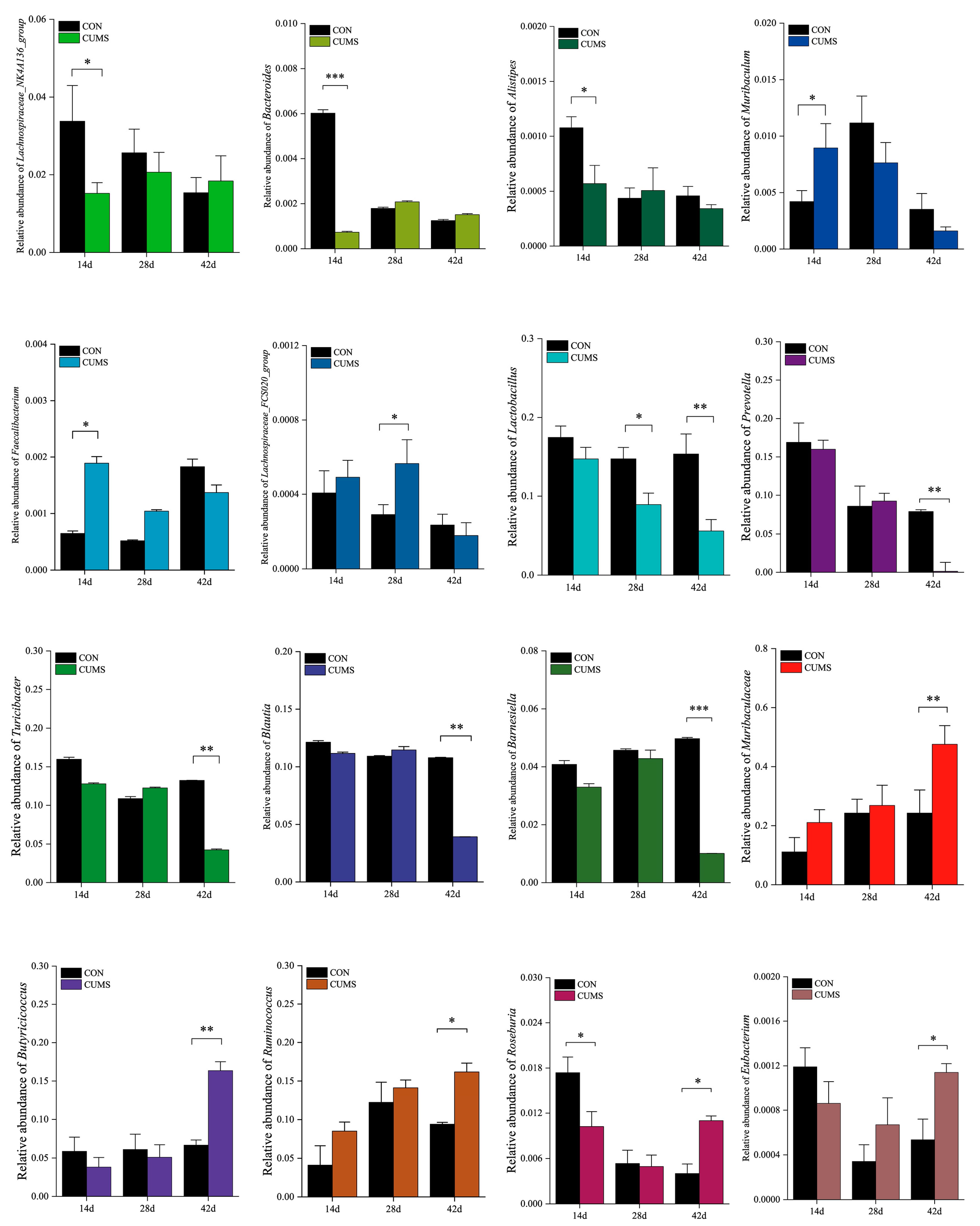
2.6.2. Composition of the Ileal Gut Microbiota in Mice
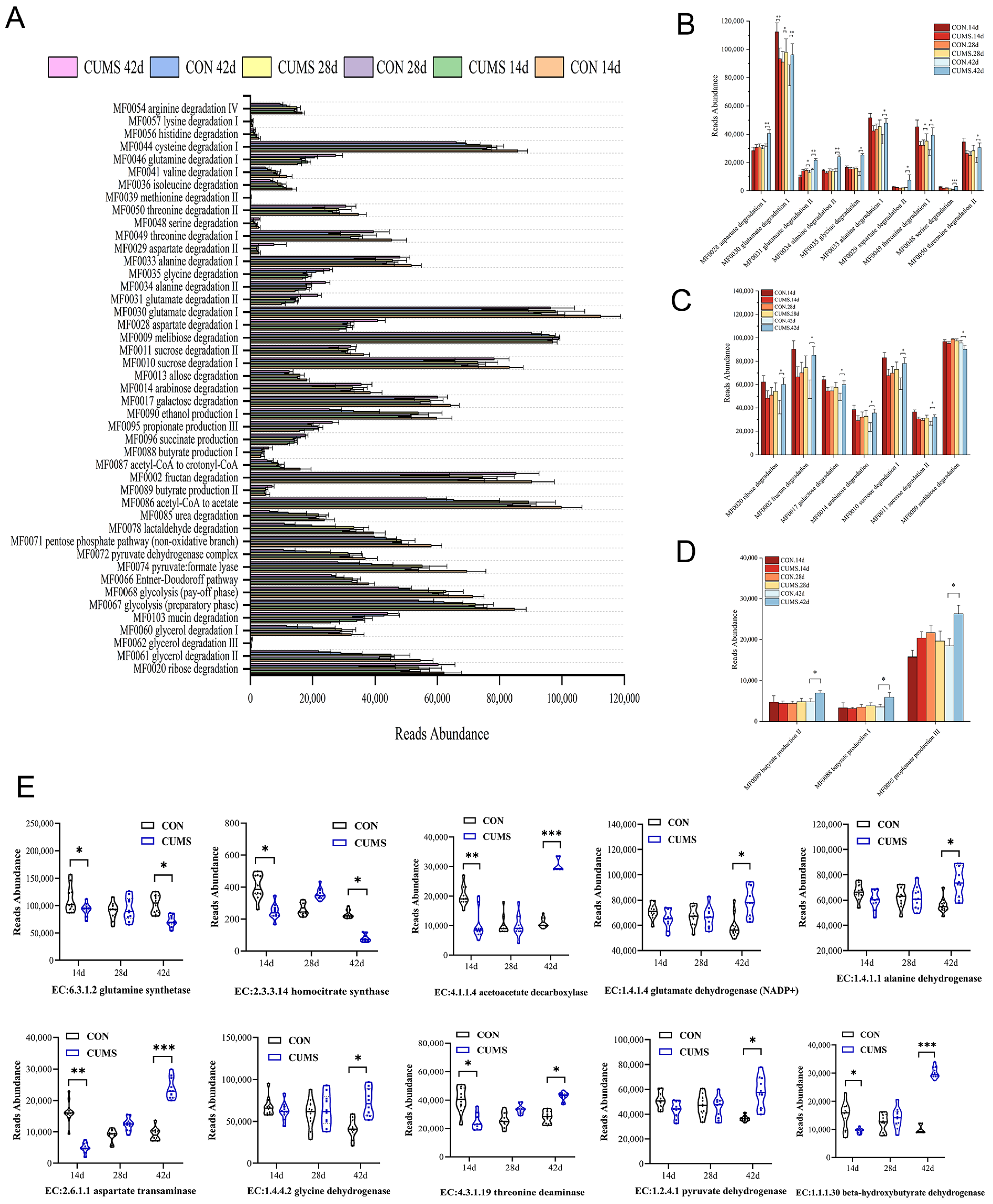
2.6.3. Predictive Analysis of Ileal Microbiota Metabolism
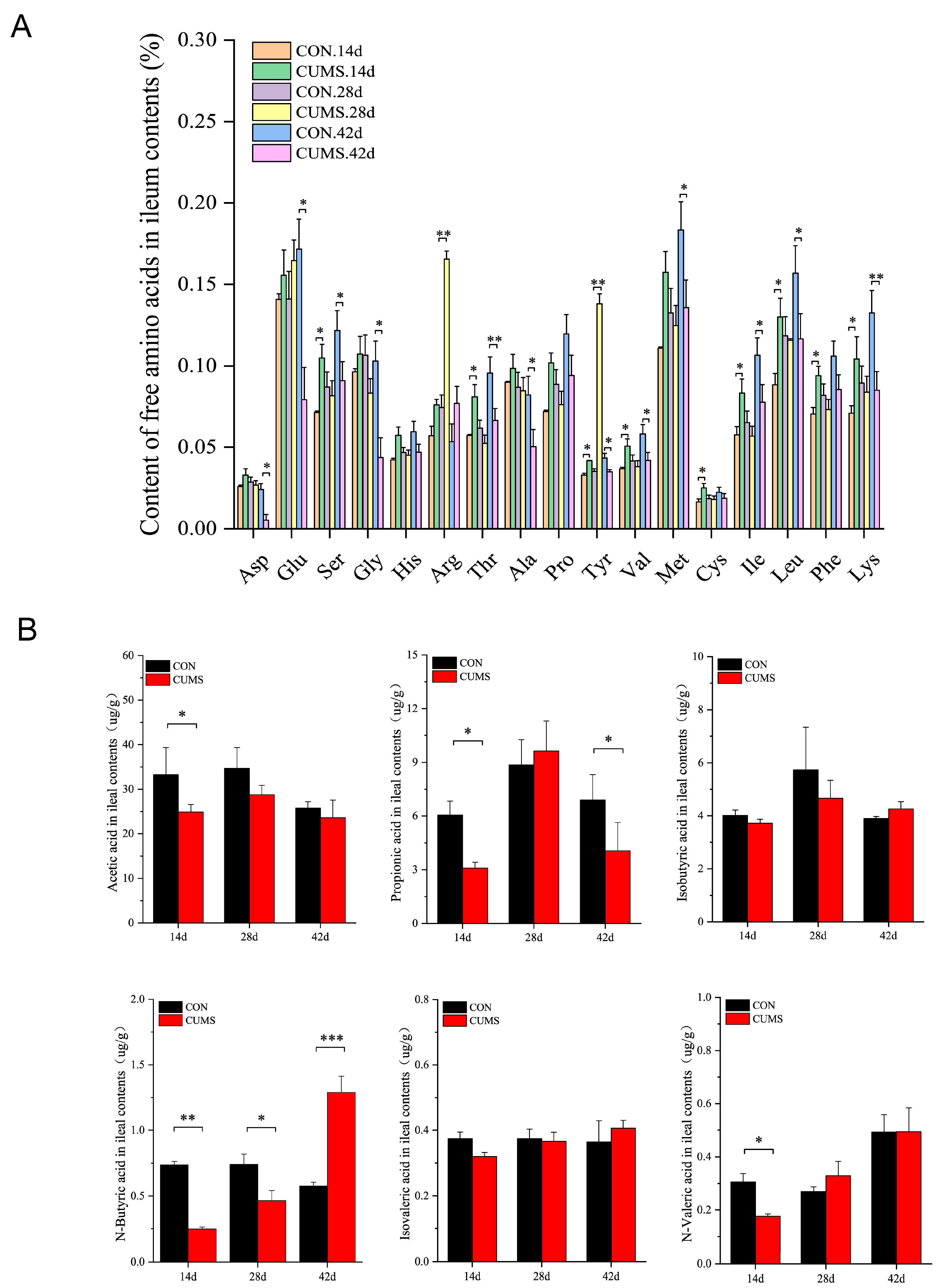
2.7. Effects of CUMS on FAAs and SCFAs Levels in the Ileal Contents of Mice
2.8. Correlation Analysis
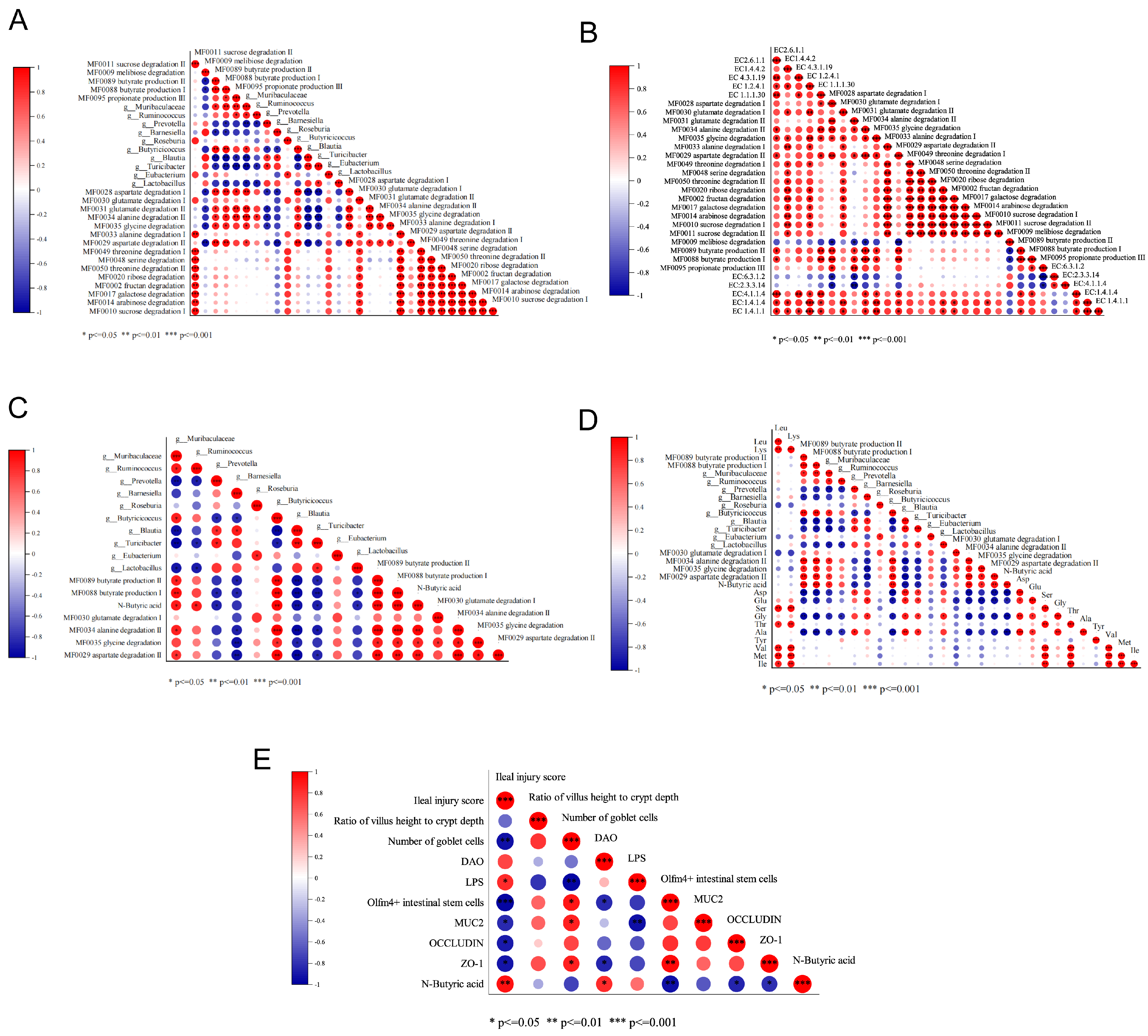
2.8.1. Correlation Analysis of FAAs, BA, Gut Key Marker Bacteria, and Gene Abundance of Predicted Metabolic Pathways and Associated Enzymes
2.8.2. Correlation Analysis of BA and IMBD Indicators
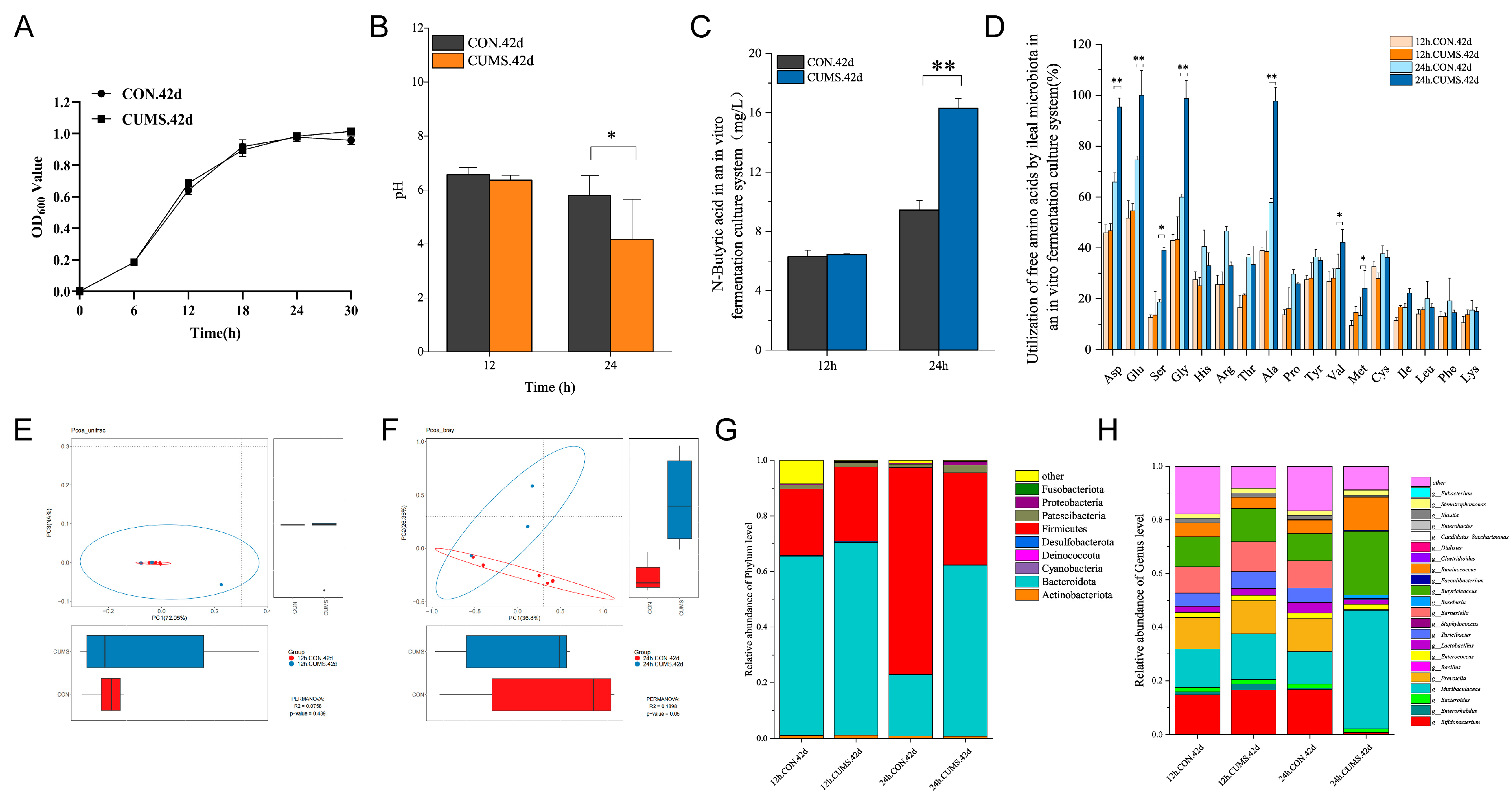
2.9. 42 d Ileal Flora In Vitro Culture
2.9.1. Growth Curves, pH and BA Levels, and FAA Consumption Rates in Ileal Flora In Vitro Culture
2.9.2. Analysis of the In Vitro Culture Ileal Flora Composition
3. Discussion
4. Materials and Methods
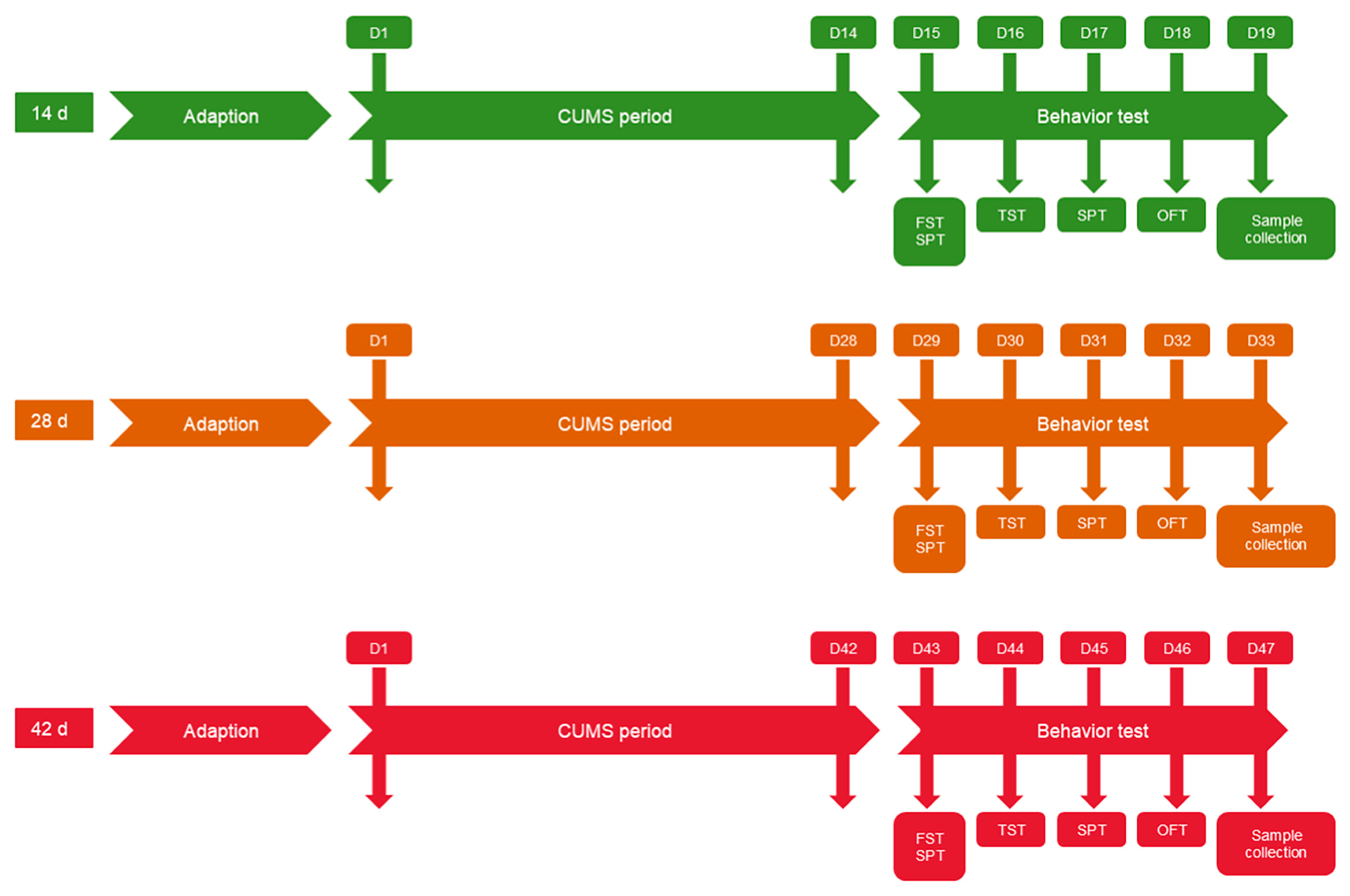
4.1. Experimental Animals and Study Design
4.2. CUMS Procedure
4.3. Mice Behavioral Experiments
4.4. Physiological Variables and Sample Collection
4.5. Measurement of Inflammatory Markers, Diamine Oxidase (DAO), and Lipopolysaccharide (LPS)
4.6. H&E Staining and Analysis of Ileum Tissue
4.7. Immunohistochemical (IHC) Staining and Analysis of Ileum Tissue
4.8. Gut Microbiota Analysis
4.9. Free Amino Acid (FAA) Analysis
4.10. Short-Chain Fatty Acid (SCFA) Analysis
4.11. Ileal Flora In Vitro Culture
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathour, D.; Shah, S.; Khan, S.; Singh, P.K.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Role of gut microbiota in depression: Understanding molecular pathways, recent research, and future direction. Behav. Brain Res. 2023, 436, 114081. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, J.; Zhu, P.; Xie, H.; Lu, L.; Bai, W.; Pan, W.; Shi, R.; Ye, J.; Xia, B.; et al. Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: The potential involvement of gut-brain axis. Food Res. Int. 2022, 157, 111289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Gui, S.; Zeng, B.; Pu, J.; Zheng, P.; Zeng, L.; Luo, Y.; Wu, Y.; Zhou, C. Proteomics analysis of the gut–brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, G.; Song, X.; Qian, Y.; Liao, Z.; Sui, L.; Ai, L.; Xia, Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J. Nutr. 2023, 153, 924–939. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, C.; Liu, Y.; Zhao, H.; Long, L.; Gai, X.; Zhao, H. Soluble dietary fiber protects intestinal mucosal barrier by improving intestinal flora in a murine model of sepsis. Biomed. Pharmacother 2020, 129, 110343. [Google Scholar] [CrossRef]
- Nakao, M.; Shirotsuki, K.; Sugaya, N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc. Med. 2021, 15, 16. [Google Scholar] [CrossRef]
- Lu, X.; Ce, Q.; Jin, L.; Zheng, J.; Sun, M.; Tang, X.; Li, D.; Sun, J. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021, 12, 573–586. [Google Scholar] [CrossRef]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef]
- Russo, S.; Chan, K.; Li, L.; Parise, L.; Cathomas, F.; LeClair, K.; Shimo, Y.; Lin, H.Y.; Durand-de Cuttoli, R.; Aubry, A.; et al. Stress-activated brain-gut circuits disrupt intestinal barrier integrity and social behaviour. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Geng, S.; Yang, L.; Cheng, F.; Zhang, Z.; Li, J.; Liu, W.; Li, Y.; Chen, Y.; Bao, Y.; Chen, L. Gut microbiota are associated with psychological stress-induced defections in intestinal and blood–brain barriers. Front. Microbiol. 2020, 10, 3067. [Google Scholar] [CrossRef]
- De Sousa, E.M.F.; De Sauvage, F.J. Cellular Plasticity in Intestinal Homeostasis and Disease. Cell Stem Cell 2019, 24, 54–64. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Kelly, J.; Kennedy, P.; Cryan, J.; Dinan, T.; Clarke, G.; Hyland, N. Breaking Down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Gong, X.; Huang, C.; Mao, Q.; Zeng, L.; Zheng, P.; Qin, Y.; Ye, F.; Lian, B.; et al. Effects of chronic stress on intestinal amino acid pathways. Physiol. Behav. 2019, 204, 199–209. [Google Scholar] [CrossRef]
- Li, Y.; Wan, H.; Ma, R.; Liu, T.; Chen, Y.; Dong, Y. Chronic Stress That Changed Intestinal Permeability and Induced Inflammation Was Restored by Estrogen. Int. J. Mol. Sci. 2023, 24, 12822. [Google Scholar] [CrossRef]
- Swaid, F.; Sukhotnik, I.; Matter, I.; Berkowitz, D.; Hadjittofi, C.; Pollak, Y.; Lavy, A. Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following acetic acid induced intestinal injury in rats. Nutr. Metab. 2013, 10, 53. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Han, W.; Zheng, Y.; Wang, L.; An, C. Disordered gut microbiota and changes in short-chain fatty acids and inflammatory processes in stress-vulnerable mice. J. Neuroimmunol. 2023, 383, 578172. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, R.; Yu, Y.; Liu, X.; Tian, Z. Protective effect of sodium butyrate on intestinal barrier damage and uric acid reduction in hyperuricemia mice. Biomed. Pharmacother. 2023, 161, 114568. [Google Scholar] [CrossRef]
- Karim, M.R.; Iqbal, S.; Mohammad, S.; Morshed, M.N.; Haque, M.A.; Mathiyalagan, R.; Yang, D.C.; Kim, Y.J.; Song, J.H.; Yang, D.U. Butyrate’s (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer. Arch. Microbiol. 2024, 206, 137. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Zhong, Q.; Bai, S.J.; Zhou, C.J.; Tian, T.; Chen, J.J. Associations Between Disordered Microbial Metabolites and Changes of Neurotransmitters in Depressed Mice. Front. Cell. Infect. Microbiol. 2022, 12, 906303. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatr. 2024, 37, e101374. [Google Scholar] [CrossRef]
- Nan, N.; Wang, Q.; Li, M.J.; Xu, Y.S.; Guo, X.M.; Xu, R.; Ma, Z.; Wang, S.H.; Li, J.; Zhao, H.; et al. The Microbiota-Dependent Treatment of Wuzhuyu Decoction for Chronic Migraine Model Rat Associated with Anxiety-Depression Like Behavior. Oxid. Med. Cell. Longev. 2023, 2023, 2302653. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota–gut–brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl. Psychiatry 2018, 8, 187. [Google Scholar] [CrossRef]
- Zhao, Y.; Coulson, E.J.; Su, X.; Zhang, J.; Sha, B.; Xu, H.; Deng, Y.; Chen, Y.; Cao, J.; Wang, Y. Identification of 14-3-3 epsilon as a regulator of the neural apoptotic pathway for chronic-stress-induced depression. Iscience 2021, 24, 102043. [Google Scholar] [CrossRef]
- Sakai, Y.; Li, H.; Inaba, H.; Funayama, Y.; Ishimori, E.; Kawatake-Kuno, A.; Yamagata, H.; Seki, T.; Hobara, T.; Nakagawa, S. Gene-environment interactions mediate stress susceptibility and resilience through the CaMKIIβ/TARPγ-8/AMPAR pathway. Iscience 2021, 24, 102504. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, L.; Deng, Q.; Sun, L.; Zhao, Y.; Fang, Z.; Wang, C.; Zhao, J. Carboxymethyl pachymaran attenuates short-term stress induced depressive behaviours and over-expression of occludin and claudin-2 in the blood–brain-barrier by regulating inflammatory cytokines-JNK/ERK/p38 pathway. J. Funct. Foods 2023, 103, 105490. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: Roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Tang, W.; Sun, Q.; Chen, L.; Wang, X.; Liu, Q.; Yu, S.; Yu, S.; Liu, C.; et al. Chronic Unpredictable Mild Stress in Rats Induces Colonic Inflammation. Front. Physiol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Huang, L.; Ma, Z.; Ze, X.; Zhao, X.; Zhang, M.; Lv, X.; Zheng, Y.; Liu, H. Gut microbiota decreased inflammation induced by chronic unpredictable mild stress through affecting NLRP3 inflammasome. Front. Cell. Infect. Microbiol. 2023, 13, 1189008. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Pawluski, J.; Steinbusch, H.W.; Kunikullaya, U.K.; Song, C. The effect of chronic stress on behaviors, in-flammation and lymphocyte subtypes in male and female rats. Behav. Brain Res. 2023, 439, 114220. [Google Scholar] [CrossRef]
- Teng, T.; Shively, C.A.; Li, X.; Jiang, X.; Neigh, G.N.; Yin, B.; Zhang, Y.; Fan, L.; Xiang, Y.; Wang, M. Chronic unpredictable mild stress produces depressive-like behavior, hypercortisolemia, and metabolic dysfunction in adolescent cynomolgus monkeys. Transl. Psychiatry 2021, 11, 9. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Wang, L.; Cai, X.; Xie, H.; Yi, F.; Huang, R.; Fang, C.; Xie, P.; Zhou, J. Chronic mild stress-induced protein dysregulations correlated with susceptibility and resiliency to depression or anxiety revealed by quantitative proteomics of the rat prefrontal cortex. Transl. Psychiatry 2021, 11, 143. [Google Scholar] [CrossRef]
- Cameron, H.A.; Schoenfeld, T.J. Behavioral and structural adaptations to stress. Front. Neuroendocrinol. 2018, 49, 106–113. [Google Scholar] [CrossRef]
- Radley, J.J.; Herman, J.P. Preclinical models of chronic stress: Adaptation or pathology? Biol. Psychiatry 2023, 94, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20; quiz 21–22. [Google Scholar] [CrossRef]
- Saunders, P.; Kosecka, U.; McKay, D.; Perdue, M. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 1994, 267, G794–G799. [Google Scholar] [CrossRef]
- Santos, A.J.M.; Lo, Y.H.; Mah, A.T.; Kuo, C.J. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary macronutrients and the gut microbiome: A precision nutrition approach to improve cardiometabolic health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480. e1412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflammation 2021, 18, 254. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, W.; Ma, Q.; Yang, Z.; Fan, Y.; Ju, Y.; Guo, R.; Wang, Q.; Mu, X.; Zhao, B. Role of short-chain fatty acids in the gut-brain axis in schizophrenia: Contribution to immune activation and pathophysiology in humans and mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sun, D.; Luo, J.; Ye, W.; Wang, C.; Deng, Q.; Fang, Z.; Sun, L.; Gooneratne, R. Ziziphus Jujube Polysaccharides inhibit over-abundance of fecal butyric acid in mildly stressed growing mice to ameliorate depression-like behavior. Food Biosci. 2024, 62, 104875. [Google Scholar] [CrossRef]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2022, 13, 1103836. [Google Scholar] [CrossRef]
- Dicks, L.M.; Hurn, D.; Hermanus, D. Gut bacteria and neuropsychiatric disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ju, P.; Xue, T.; Ali, U.; Cui, D.; Chen, J. Alteration of faecal microbiota balance related to long-term deep meditation. Gen. Psychiatry 2023, 36, e100893. [Google Scholar] [CrossRef] [PubMed]
- Frolova, M.S.; Suvorova, I.A.; Iablokov, S.N.; Petrov, S.N.; Rodionov, D.A. Genomic reconstruction of short-chain fatty acid production by the human gut microbiota. Front. Mol. Biosci. 2022, 9, 949563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, P. Characteristics of Oral-Gut Microbiota in Model Rats with CUMS-Induced Depression. Neuropsychiatr. Dis. Treat. 2024, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, Z.; Zhou, L.; Sun, L.; Xiao, L.; Wang, G. Swimming Exercise Modulates Gut Microbiota in CUMS-Induced Depressed Mice. Neuropsychiatr. Dis. Treat. 2022, 18, 749–760. [Google Scholar] [CrossRef]
- Song, H.; Jeon, D.; Unno, T. Evaluation of Prebiotics through an In Vitro Gastrointestinal Digestion and Fecal Fermentation Experiment: Further Idea on the Implementation of Machine Learning Technique. Foods 2022, 11, 2490. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Rui, L.; Wentian, L.; Meiyu, P.; Hong, Z. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Abdallah, A.; Elemba, E.; Zhong, Q.; Sun, Z. Gastrointestinal Interaction between Dietary Amino Acids and Gut Microbiota: With Special Emphasis on Host Nutrition. Curr. Protein Pept. Sci. 2020, 21, 785–798. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, R.; Grandjean, T.; Stabler, S.; Bauduin, M.; Gosset, P.; Kipnis, E.; Dessein, R. Gut colonisation with multidrug-resistant Klebsiella pneumoniae worsens Pseudomonas aeruginosa lung infection. Nat. Commun. 2023, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liang, X.; Lu, H.; Li, X.; Zhang, Z.; Lu, X.; Wang, H.; Meng, Y.; Roy, A.; Luo, W. Adaptation of gut microbiome and host metabolic systems to lignocellulosic degradation in bamboo rats. ISME J. 2022, 16, 1980–1992. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Guo, Z.; Zhong, Y.; Zhou, L.; Xu, P.; Gao, N.; Lu, J.; Yan, X.; Cao, H. Unveiling the microbiota-metabolite-myocardium axis: A novel perspective on cardiovascular health. Front. Microbiol. 2024, 15, 1389311. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Amiar, M.R.; Ocaña-Wilhelmi, L.; Soler-Humanes, R.; Arranz-Salas, I.; Garrido-Sánchez, L.; Gutiérrez-Repiso, C.; Tinahones, F.J. Non-alcoholic fatty liver disease in patients with morbid obesity: The gut microbiota axis as a potential pathophysiology mechanism. J. Gastroenterol. 2024, 59, 329–341. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Y.; Qin, J.; Ma, C.; Jin, Y.; Wang, X.; Chen, K.; Ouyang, P. Increasing lysine level improved methanol assimilation toward butyric acid production in Butyribacterium methylotrophicum. Biotechnol. Biofuels Bioprod. 2023, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.; Nouwen, J.L.; Thio, H.B. Butyrate properties in immune-related diseases: Friend or foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Qureshi, N.; Liu, S.; Saha, B.C. Butyric Acid Production by Fermentation: Employing Potential of the Novel Clostridium tyrobutyricum Strain NRRL 67062. Fermentation 2022, 8, 491. [Google Scholar] [CrossRef]
- Botta, C.; Acquadro, A.; Greppi, A.; Barchi, L.; Bertolino, M.; Cocolin, L.; Rantsiou, K. Genomic assessment in Lactobacillus plantarum links the butyrogenic pathway with glutamine metabolism. Sci. Rep. 2017, 7, 15975. [Google Scholar] [CrossRef] [PubMed]
- Stø, K.; Valeur, J.; Ueland, T.; Malmstrøm, G.H.; Bjerkeli, V.; Trøseid, M.; Hov, J.R.; Holm, K.; Vestad, B.; Halvorsen, B. Fecal level of butyric acid, a microbiome-derived metabolite, is increased in patients with severe carotid atherosclerosis. Sci. Rep. 2022, 12, 22378. [Google Scholar] [CrossRef]
- Wang, J.; Lang, T.; Shen, J.; Dai, J.; Tian, L.; Wang, X. Core gut bacteria analysis of healthy mice. Front. Microbiol. 2019, 10, 887. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lee, E.M.; Wang, Y.; Zhou, C.; Pruss, K.M.; Henrissat, S.; Chen, R.Y.; Kao, C.; Hibberd, M.C.; Lynn, H.M. Prevotella copri and microbiota members mediate the beneficial effects of a therapeutic food for malnutrition. Nat. Microbiol. 2024, 9, 922–937. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lan, T.; Han, Y.; Zhao, H.; Wang, C.; Xu, Z.; Chen, Z.; Tao, M.; Li, H.; Song, Y.; et al. Tributyrin alleviates gut microbiota dysbiosis to repair intestinal damage in antibiotic-treated mice. PLoS ONE 2023, 18, e0289364. [Google Scholar] [CrossRef]
- Weng, J.; Zhu, Y.; Wen, A.; Qin, L. Butyric acid generation from kidney beans mediated by gut microbiota based on the butyryl-CoA:acetate CoA-transferase pathway. Food Biosci. 2024, 57, 103520. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20-76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Xu, L.; Zhang, X. Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness. Foods 2021, 10, 2312. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.; Miao, Y.; Ng, S.C. Prevotella species in the human gut is primarily comprised of Prevotella copri, Prevotella stercorea and related lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, D.; Deng, Q.; Sun, L.; Hu, L.; Fang, Z.; Zhao, J.; Gooneratne, R. Elephantopus scaber L. Polysaccharides Alleviate Heat Stress-Induced Systemic Inflammation in Mice via Modulation of Characteristic Gut Microbiota and Metabolites. Nutrients 2024, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, L.; Zhang, Y.; Yang, Z.; Zhang, C.; Li, Y.; Kalueff, A.V.; Li, W.; Song, C. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J. Ethnopharmacol. 2020, 250, 112487. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Ip, S.P.; Ko, K.M.; Tsai, S.H.; Che, C.T. Peony glycosides produce antidepressant-like action in mice exposed to chronic unpredictable mild stress: Effects on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1211–1216. [Google Scholar] [CrossRef]
- Song, N.; Du, J.; Gao, Y.; Yang, S. Epitranscriptome of the ventral tegmental area in a deep brain-stimulated chronic unpredictable mild stress mouse model. Transl. Neurosci. 2020, 11, 402–418. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; You, B.; Tang, W.; Gan, T.; Feng, C.; Li, C.; Yang, R. Serum Metabonomic Study on the Antidepressant-like Effects of Ellagic Acid in a Chronic Unpredictable Mild Stress-Induced Mouse Model. J. Agric. Food Chem. 2020, 68, 9546–9556. [Google Scholar] [CrossRef]
- Katz, R.J.; Roth, K.A.; Carroll, B.J. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci. Biobehav. Rev. 1981, 5, 247–251. [Google Scholar] [CrossRef]
- Yu-Lu, W.; Meng, L.U.; Yue-Yue, W.; Ru, J.; Yan, C.; Shu-Ling, Z.; Yu, L. Effect of Puyu Capsules on behavior, HPA axis and CREB-BDNF pathway expression in hippocampus of depressed mice. Zhongguo Zhong Yao Za Zhi 2020, 45, 4971–4977. [Google Scholar] [CrossRef]
- Sun, D.; Wang, C.; Sun, L.; Hu, L.; Fang, Z.; Deng, Q.; Zhao, J.; Gooneratne, R. Preliminary Report on Intestinal Flora Disorder, Faecal Short-Chain Fatty Acid Level Decline and Intestinal Mucosal Tissue Weakening Caused by Litchi Extract to Induce Systemic Inflammation in HFA Mice. Nutrients 2022, 14, 776–794. [Google Scholar] [CrossRef]
- Generoso, S.V.; Viana, M.L.; Santos, R.G.; Arantes, R.M.; Martins, F.S.; Nicoli, J.R.; Machado, J.A.; Correia, M.I.; Cardoso, V.N. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur. J. Nutr. 2011, 50, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.T.; Chiu, K.; Zheng, E.; Martinez, A.; Chiu, J.; Raj, K.; Stasiak, S.; Lai, N.Z.E.; Arcanjo, R.B.; Flaws, J.A.; et al. Subchronic exposure to environmentally relevant concentrations of di-(2-ethylhexyl) phthalate differentially affects the colon and ileum in adult female mice. Chemosphere 2022, 309, 136680. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, R.; Wang, Y.; Sun, L.; Tao, S.; Li, X.; Gooneratne, R.; Zhao, J. Diversity and succession of microbial communities and chemical analysis in dried Lutianus erythropterus during storage. Int. J. Food Microbiol. 2020, 314, 108416. [Google Scholar] [CrossRef]
- Dong, Y.S.; Sui, L.P.; Yang, F.; Ren, X.X.; Xing, Y.; Xiu, Z.L. Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: An in vitro study. Food Chem. 2022, 394, 133561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Qiu, M.; Wang, S.; Luo, J.; Huang, L.; Deng, Q.; Fang, Z.; Sun, L.; Gooneratne, R. Gut-Microbiota-Derived Butyric Acid Overload Contributes to Ileal Mucosal Barrier Damage in Late Phase of Chronic Unpredictable Mild Stress Mice. Int. J. Mol. Sci. 2024, 25, 12998. https://doi.org/10.3390/ijms252312998
Wang C, Qiu M, Wang S, Luo J, Huang L, Deng Q, Fang Z, Sun L, Gooneratne R. Gut-Microbiota-Derived Butyric Acid Overload Contributes to Ileal Mucosal Barrier Damage in Late Phase of Chronic Unpredictable Mild Stress Mice. International Journal of Molecular Sciences. 2024; 25(23):12998. https://doi.org/10.3390/ijms252312998
Chicago/Turabian StyleWang, Chen, Mei Qiu, Shuo Wang, Jinjin Luo, Ling Huang, Qi Deng, Zhijia Fang, Lijun Sun, and Ravi Gooneratne. 2024. "Gut-Microbiota-Derived Butyric Acid Overload Contributes to Ileal Mucosal Barrier Damage in Late Phase of Chronic Unpredictable Mild Stress Mice" International Journal of Molecular Sciences 25, no. 23: 12998. https://doi.org/10.3390/ijms252312998
APA StyleWang, C., Qiu, M., Wang, S., Luo, J., Huang, L., Deng, Q., Fang, Z., Sun, L., & Gooneratne, R. (2024). Gut-Microbiota-Derived Butyric Acid Overload Contributes to Ileal Mucosal Barrier Damage in Late Phase of Chronic Unpredictable Mild Stress Mice. International Journal of Molecular Sciences, 25(23), 12998. https://doi.org/10.3390/ijms252312998




