Allergic Disposition of IVF-Conceived Mice
Abstract
1. Introduction
2. Results
2.1. Characteristics of IVF and Outcomes
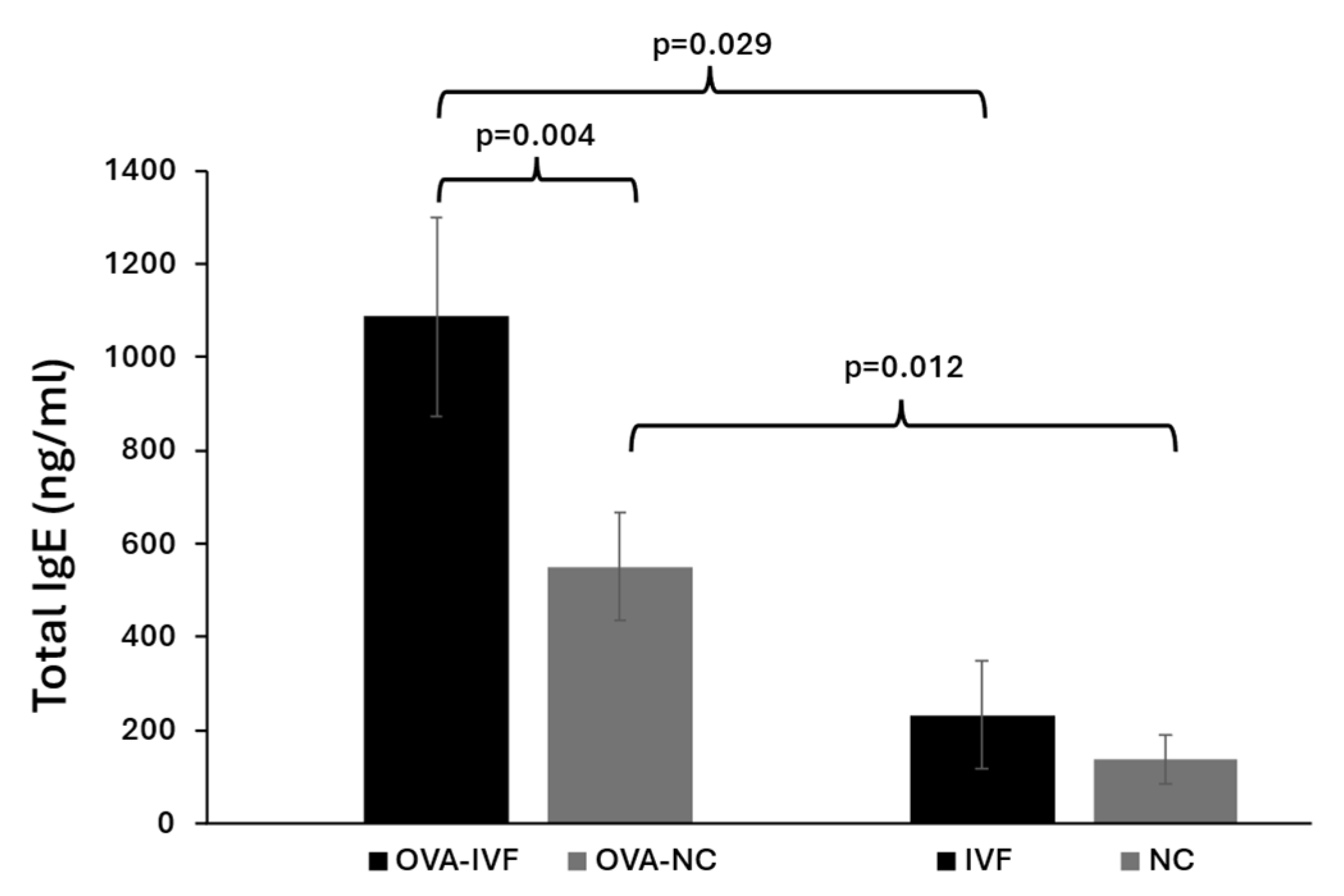
2.2. Serum Levels of Total IgE in Naturally and IVF-Conceived Offspring
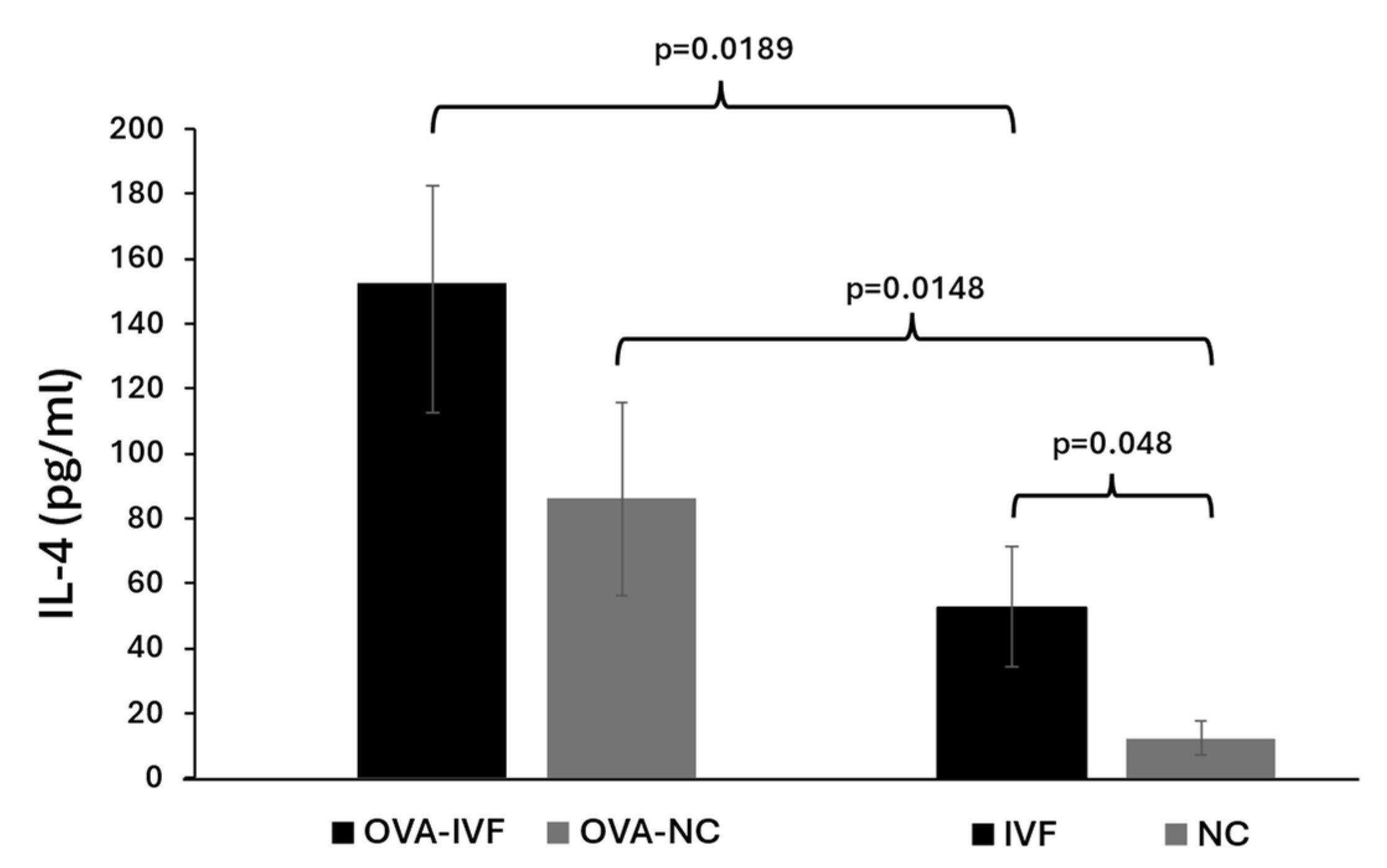
2.3. Serum Levels of IL-4 in Naturally and IVF-Conceived Offspring
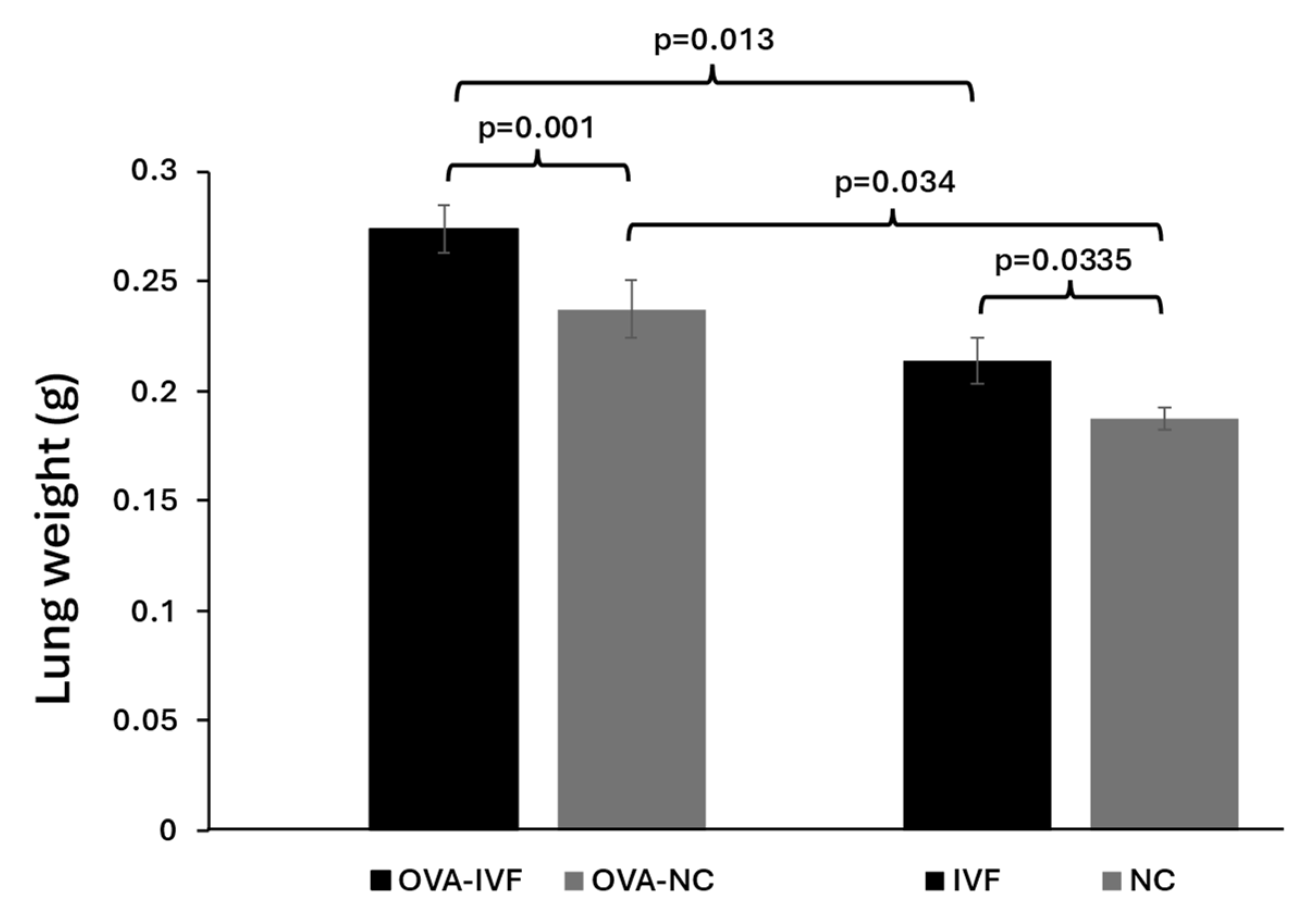
2.4. Lung Weights in Naturally and IVF-Conceived Offspring
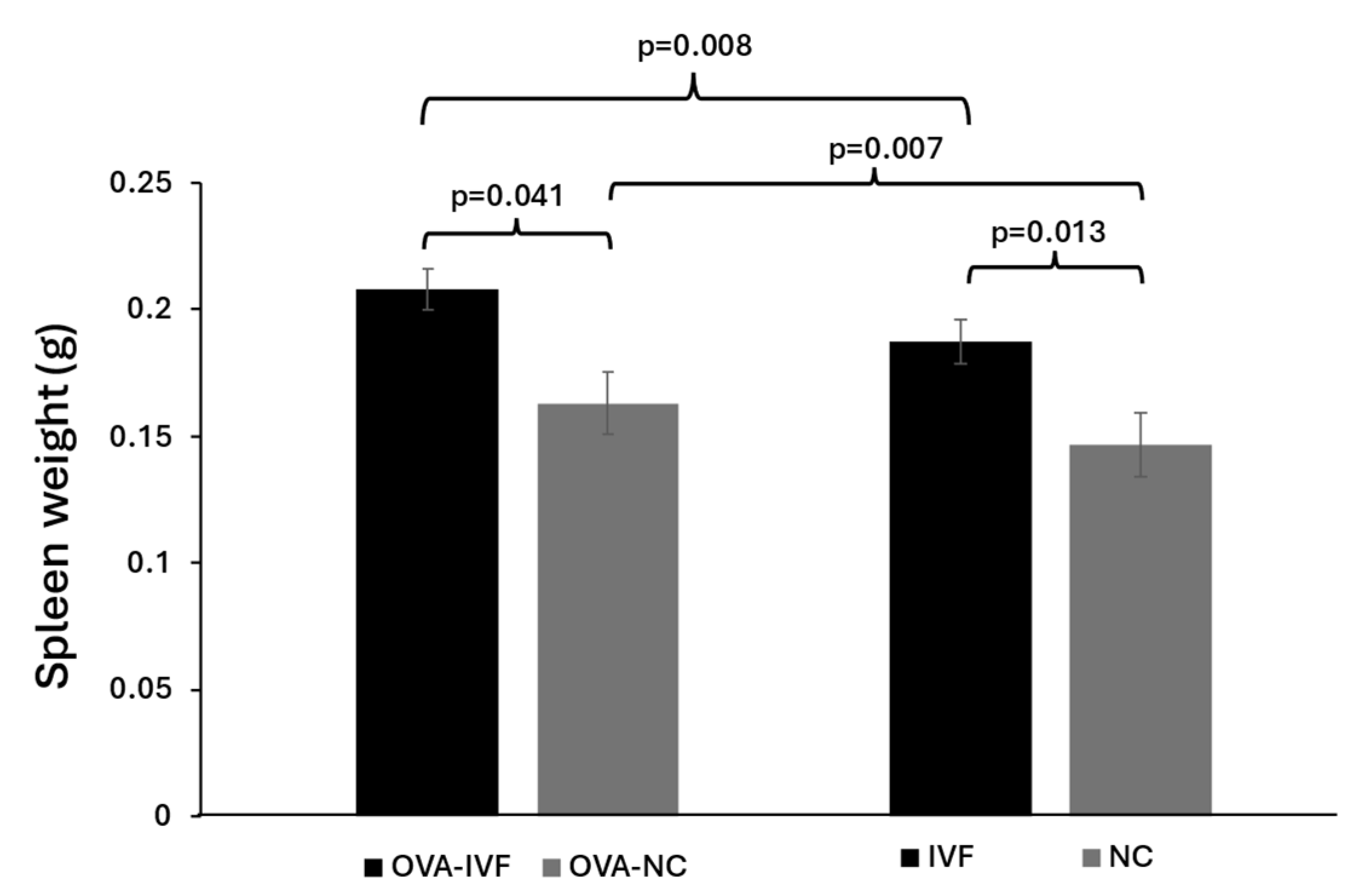
2.5. Spleen Weights in Naturally and IVF-Conceived Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Gametes
4.3. In Vitro Fertilization (IVF) and Natural Conception
4.4. Pseudo-Pregnant Mice and Embryo Transfer
4.5. Experimental Groups
4.6. Measurement of Organ Weights
4.7. Evaluation of Total IgE and IL-4 Concentrations
4.8. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, H.; Aghebati-Maleki, L.; Rashidiani, S.; Csabai, T.; Nnaemeka, O.B.; Szekeres-Bartho, J. Long-Term Effects of ART on the Health of the Offspring. Int. J. Mol. Sci. 2023, 24, 13564. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gonzalez, R.; Moreira, P.; Bilbao, A.; Jiménez, A.; Pérez-Crespo, M.; Ramírez, M.A.; De Fonseca, F.R.; Pintado, B.; Gutiérrez-Adán, A. Long-Term Effect of in Vitro Culture of Mouse Embryos with Serum on mRNA Expression of Imprinting Genes, Development, and Behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 5880–5885. [Google Scholar] [CrossRef] [PubMed]
- Heber, M.F.; Ptak, G.E. The Effects of Assisted Reproduction Technologies on Metabolic Health and Disease. Biol. Reprod. 2021, 104, 734–744. [Google Scholar] [CrossRef]
- Laprise, S.L. Implications of Epigenetics and Genomic Imprinting in Assisted Reproductive Technologies. Mol. Reprod. Dev. 2009, 76, 1006–1018. [Google Scholar] [CrossRef]
- Hargreave, M.; Jensen, A.; Toender, A.; Andersen, K.K.; Kjaer, S.K. Fertility Treatment and Childhood Cancer Risk: A Systematic Meta-Analysis. Fertil. Steril. 2013, 100, 150–161. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, N.; Le, F.; Li, L.; Lou, H.-Y.; Liu, X.-Z.; Zheng, Y.-M.; Qian, Y.-Q.; Chen, Y.-L.; Jiang, X.-H.; et al. Superovulation Induced Changes of Lipid Metabolism in Ovaries and Embryos and Its Probable Mechanism. PLoS ONE 2015, 10, e0132638. [Google Scholar] [CrossRef]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice Generated by In Vitro Fertilization Exhibit Vascular Dysfunction and Shortened Life Span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Sacker, A.; Kelly, Y.; Redshaw, M.; Kurinczuk, J.J.; Quigley, M.A. Asthma in Children Born after Infertility Treatment: Findings from the UK Millennium Cohort Study. Hum. Reprod. 2013, 28, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Fathi, F.; Karimi, H.; Amidi, F.; Mehdinejadiani, S.; Moeini, A.; Bahram Rezai, M.; Hoseini, S.; Sobhani, A. Altered TH1, TH2, TH17 Balance in Assisted Reproductive Technology Conceived Mice. J. Reprod. Immunol. 2020, 139, 103117. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, P.; Fakhari, S.; Faryabi, M.R.; Esmaeili, P.; Banafshi, O.; Mohammadi, E.; Fathi, F.; Mokarizadeh, A. Altered Helper T Cell-Mediated Immune Responses in Male Mice Conceived through in Vitro Fertilization. Reprod. Toxicol. 2017, 69, 196–203. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, W.; Xi, B.; Ma, J.; Hu, J.; Fang, M.; Hu, K.; Qin, Y.; You, L.; Cao, Y.; et al. Increased Risk of Metabolic Dysfunction in Children Conceived by Assisted Reproductive Technology. Diabetologia 2020, 63, 2150–2157. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic Diseases and Asthma: A Global Public Health Concern and a Call to Action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.E.; Xu, C.-J.; Den Dekker, H.T.; Lee, M.K.; Sikdar, S.; Ruiz-Arenas, C.; Merid, S.K.; Rezwan, F.I.; Page, C.M.; Ullemar, V.; et al. Epigenome-Wide Meta-Analysis of DNA Methylation and Childhood Asthma. J. Allergy Clin. Immunol. 2019, 143, 2062–2074. [Google Scholar] [CrossRef]
- Tsabouri, S.; Lavasidis, G.; Efstathiadou, A.; Papasavva, M.; Bellou, V.; Bergantini, H.; Priftis, K.; Ntzani, E.E. Association between Childhood Asthma and History of Assisted Reproduction Techniques: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2021, 180, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.T.; Bieneman, A.P.; Chichester, K.L.; Hamilton, R.G.; Xiao, H.; Saini, S.S.; Liu, M.C. Decreases in Human Dendritic Cell–Dependent TH2-like Responses after Acute In Vivo IgE Neutralization. J. Allergy Clin. Immunol. 2010, 125, 896–901.e6. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting Key Proximal Drivers of Type 2 Inflammation in Disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. The Role of Lymphocytes in Allergic Disease. J. Allergy Clin. Immunol. 2000, 105, 399–408. [Google Scholar] [CrossRef]
- Ericson, A.; Nygren, K.; Olausson, P.; Källén, B. Hospital Care Utilization of Infants Born after IVF. Hum. Reprod. 2002, 17, 929–932. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Zhou, Z.; Sha, J.; Li, Y.; Liu, J. Altered Global Gene Expressions of Human Placentae Subjected to Assisted Reproductive Technology Treatments. Placenta 2010, 31, 251–258. [Google Scholar] [CrossRef]
- Sun, L.-Z.; Elsayed, S.; Aasen, T.B.; Van Do, T.; Aardal, N.P.; Florvaag, E.; Vaali, K. Comparison between Ovalbumin and Ovalbumin Peptide 323-339 Responses in Allergic Mice: Humoral and Cellular Aspects. Scand. J. Immunol. 2010, 71, 329–335. [Google Scholar] [CrossRef]
- Warren, K.J.; Dickinson, J.D.; Nelson, A.J.; Wyatt, T.A.; Romberger, D.J.; Poole, J.A. Ovalbumin-Sensitized Mice Have Altered Airway Inflammation to Agriculture Organic Dust. Respir. Res. 2019, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Macpherson, A.J. Immune Adaptations That Maintain Homeostasis with the Intestinal Microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.E. Immunoglobulin E: Role in Asthma and Allergic Disease: Lessons from the Clinic. Pharmacol. Ther. 2007, 113, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Miyake, K.; Iki, M.; Tsutsui, H.; Karasuyama, H. Recent Advances in Understanding Basophil-mediated Th2 Immune Responses. Immunol. Rev. 2017, 278, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Wali, S.; Nurieva, R. T Helper 2 and T Follicular Helper Cells: Regulation and Function of Interleukin-4. Cytokine Growth Factor Rev. 2016, 30, 29–37. [Google Scholar] [CrossRef]
- Mitter, V.R.; Håberg, S.E.; Magnus, M.C. Early Childhood Respiratory Tract Infections According to Parental Subfertility and Conception by Assisted Reproductive Technologies. Hum. Reprod. 2022, 37, 2113–2125. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and Function of the Spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Toyoshima, S.; Wakamatsu, E.; Ishida, Y.; Obata, Y.; Kurashima, Y.; Kiyono, H.; Abe, R. The Spleen Is the Site Where Mast Cells Are Induced in the Development of Food Allergy. Int. Immunol. 2017, 29, 31–45. [Google Scholar] [CrossRef]
- Aljahdali, A.; Airina, R.K.R.I.; Velazquez, M.A.; Sheth, B.; Wallen, K.; Osmond, C.; Watkins, A.J.; Eckert, J.J.; Smyth, N.R.; Fleming, T.P. The Duration of Embryo Culture after Mouse IVF Differentially Affects Cardiovascular and Metabolic Health in Male Offspring. Hum. Reprod. 2020, 35, 2497–2514. [Google Scholar] [CrossRef]
- Papi, A.; Blasi, F.; Canonica, G.W.; Morandi, L.; Richeldi, L.; Rossi, A. Treatment Strategies for Asthma: Reshaping the Concept of Asthma Management. Allergy Asthma Clin. Immunol. 2020, 16, 75. [Google Scholar] [CrossRef]
- Glover, V. Prenatal Stress and Its Effects on the Fetus and the Child: Possible Underlying Biological Mechanisms. In Perinatal Programming of Neurodevelopment; Antonelli, M.C., Ed.; Advances in Neurobiology; Springer: New York, NY, USA, 2015; Volume 10, pp. 269–283. [Google Scholar] [CrossRef]
- Zijlmans, M.A.C.; Korpela, K.; Riksen-Walraven, J.M.; De Vos, W.M.; De Weerth, C. Maternal Prenatal Stress Is Associated with the Infant Intestinal Microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef] [PubMed]
| Cycle | Ovulated Oocytes | Two-Cell Stage | IVF Rate (%) | Four-Cell Stage | Blastocysts | Transferred Blastocysts | No. of Recipients | Offspring | Birth Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 15 | 42.85 | 12 | 8 | 8 | 1 | 4 | 50 |
| 2 | 37 | 26 | 70.27 | 26 | 24 | 22 | 2 | 4 | 18.8 |
| 3 | 40 | 30 | 75 | 26 | 25 | 12 | 1 | 3 | 25 |
| 4 | 24 | 18 | 75 | 18 | 18 | 15 | 1 | 3 | 20 |
| 5 | 20 | 16 | 80 | 14 | 14 | 14 | 1 | 3 | 21.42 |
| 6 | 50 | 32 | 64 | 30 | 24 | 24 | 2 | 6 | 25 |
| 7 | 37 | 26 | 70 | 26 | 24 | 10 | 1 | 2 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, H.; Bognar, Z.; Csabai-Tanics, T.; Obodo, B.N.; Szekeres-Bartho, J. Allergic Disposition of IVF-Conceived Mice. Int. J. Mol. Sci. 2024, 25, 12993. https://doi.org/10.3390/ijms252312993
Ahmadi H, Bognar Z, Csabai-Tanics T, Obodo BN, Szekeres-Bartho J. Allergic Disposition of IVF-Conceived Mice. International Journal of Molecular Sciences. 2024; 25(23):12993. https://doi.org/10.3390/ijms252312993
Chicago/Turabian StyleAhmadi, Hamid, Zoltan Bognar, Timea Csabai-Tanics, Basil Nnaemeka Obodo, and Julia Szekeres-Bartho. 2024. "Allergic Disposition of IVF-Conceived Mice" International Journal of Molecular Sciences 25, no. 23: 12993. https://doi.org/10.3390/ijms252312993
APA StyleAhmadi, H., Bognar, Z., Csabai-Tanics, T., Obodo, B. N., & Szekeres-Bartho, J. (2024). Allergic Disposition of IVF-Conceived Mice. International Journal of Molecular Sciences, 25(23), 12993. https://doi.org/10.3390/ijms252312993






