Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review
Abstract
1. Introduction
2. Methods
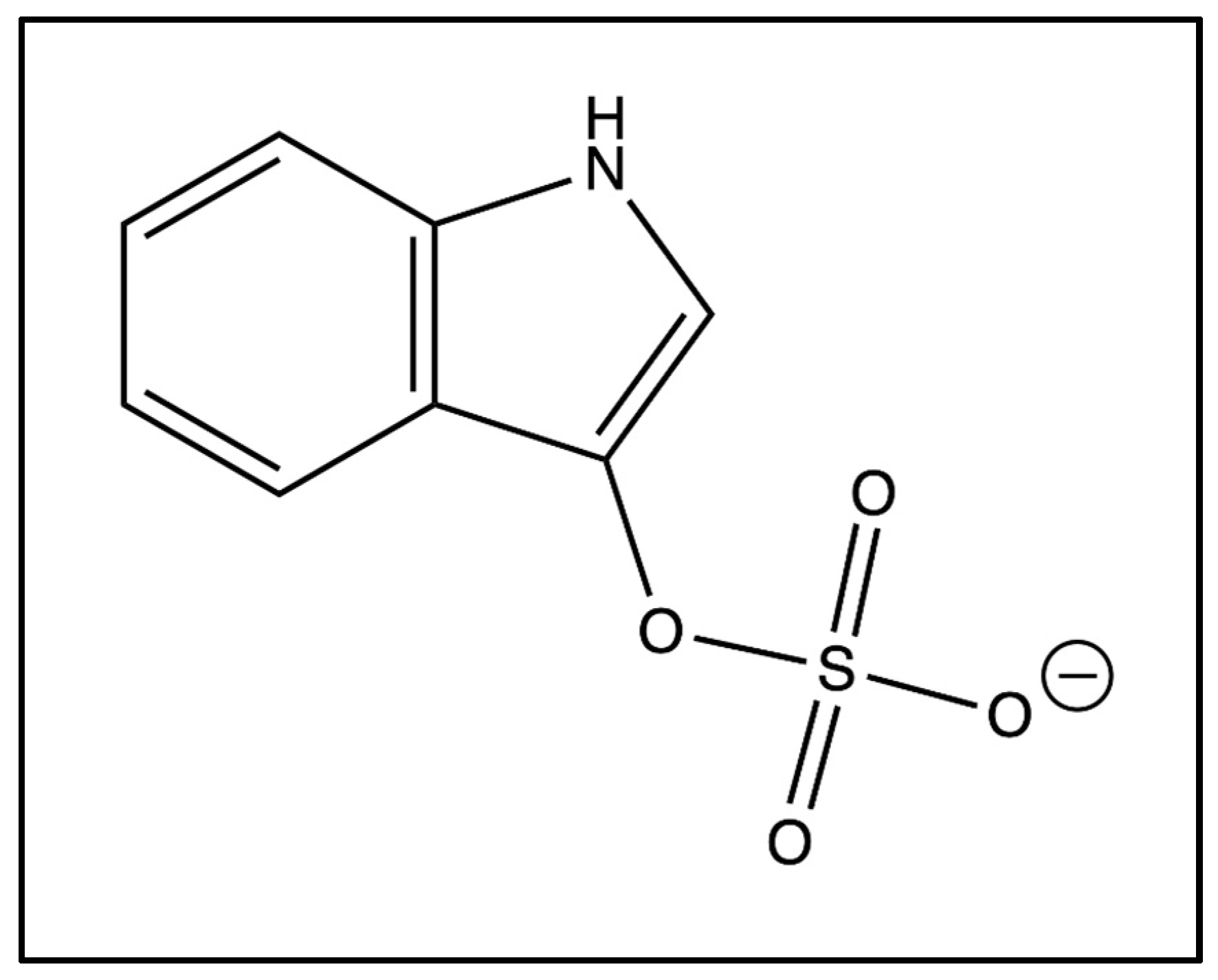
3. Metabolism of Indoxyl Sulfate
4. Toxin Classification
4.1. Neurotoxin
4.2. Uremic Toxin
4.3. Nephrotoxin
4.4. Cardiotoxin
4.5. Osteotoxin
4.6. Myotoxin
5. Other Disorders with Elevated Levels of Indoxyl Sulfate
5.1. Chronic Kidney Disease & Acute Kidney Injury
5.2. Parkinson’s Disease
5.3. Mood Disorders
6. Elevated Levels of Indoxyl Sulfate in Autism
7. Treatments
8. Discussion
9. Limitations
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olesova, D.; Galba, J.; Piestansky, J.; Celusakova, H.; Repiska, G.; Babinska, K.; Ostatnikova, D.; Katina, S.; Kovac, A. A novel UHPLC-MS method targeting urinary metabolomic markers for autism spectrum disorder. Metabolites 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Baškovič, B.; Finderle, P.; Bobrowska-Korczak, B.; Gątarek, P.; Rosiak, A.; Giebułtowicz, J.; Vrhovšek, M.J.; Kałużna-Czaplińska, J. Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. Int. J. Mol. Sci. 2023, 24, 7078. [Google Scholar] [CrossRef]
- Mrochek, J.E.; Dinsmore, S.R.; Ohrt, D.W. Monitoring Phenylalanine—Tyrosine Metabolism by High-Resolution Liquid Chromatography of Urine. Clin. Chem. 1973, 19, 927–936. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- The Metabolomic Innovation Centre. Human Metabolome Database: Showing Metabocard for Indoxyl Sulfate (HMDB0000682). Available online: https://hmdb.ca/metabolites/HMDB0000682 (accessed on 1 January 2023).
- Zhao, Y.-Y.; Cheng, X.-L.; Wei, F.; Bai, X.; Tan, X.-J.; Lin, R.-C.; Mei, Q. Intrarenal metabolomic investigation of chronic kidney disease and its TGF-β1 mechanism in induced-adenine rats using UPLC Q-TOF/HSMS/MSE. J. Proteome Res. 2013, 12, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Barichella, M.; Cancello, R.; Cavanna, F.; Iorio, L.; Cereda, E.; Bolliri, C.; Maria, P.Z.; Bianchi, F.; Cestaro, B.; et al. Increased urinary indoxyl sulfate (indican): New insights into gut dysbiosis in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 389–393. [Google Scholar] [CrossRef]
- Karbowska, M.; Hermanowicz, J.M.; Tankiewicz-Kwedlo, A.; Kalaska, B.; Kaminski, T.W.; Nosek, K.; Wisniewska, R.J.; Pawlak, D. Neurobehavioral effects of uremic toxin–indoxyl sulfate in the rat model. Sci. Rep. 2020, 10, 9483. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gomez-Fernández, A.; de la Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Gil-Campos, M. Metabolic profiling in children with autism spectrum disorder with and without mental regression: Preliminary results from a cross-sectional case–control study. Metabolomics 2019, 15, 99. [Google Scholar] [CrossRef]
- Brydges, C.R.; Fiehn, O.; Mayberg, H.S.; Schreiber, H.; Dehkordi, S.M.; Bhattacharyya, S.; Cha, J.; Choi, K.S.; Craighead, W.E.; Krishnan, R.R.; et al. Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Sci. Rep. 2021, 11, 21011. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Huang, M.-F.; Liang, S.-S.; Hwang, S.-J.; Tsai, J.-C.; Liu, T.-L.; Wu, P.-H.; Yang, Y.-H.; Kuo, K.-C.; Kuo, M.-C.; et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. NeuroToxicology 2016, 53, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Chen, Y.-J.; Fukuji, Y.; Kondoh, H.; Yanagida, M. Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc. Natl. Acad. Sci. USA 2021, 118, e2022857118. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; de Edelenyi, F.S.; Naudon, L.; Druesne-Pecollo, N.; Hercberg, S.; Kesse-Guyot, E.; Latino-Martel, P.; Galan, P.; Rabot, S. Relation between mood and the host-microbiome co-metabolite 3-indoxylsulfate: Results from the observational prospective nutrinet-santé study. Microorganisms 2021, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-S.; Chen, J.; Zou, J.-Z.; Zhong, Y.-H.; Teng, J.; Ji, J.; Chen, Z.-W.; Liu, Z.-H.; Shen, B.; Nie, Y.-X.; et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 111–119. [Google Scholar] [CrossRef]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: Interaction between astrocytes and microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef]
- Griffin, A.; Berry, B.; Spencer, S.-K.; Bowles, T.; Wallace, K. Indoxyl Sulfate Administration during Pregnancy Contributes to Renal Injury and Increased Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2023, 24, 11968. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl sulfate induces intestinal barrier injury through IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, C.-W.; Yang, Y.; Hsiao, Y.-C.; Ru, H.; Lu, K. High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat. Commun. 2021, 12, 6000. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Taguchi, N.; Ozawa, K.; Taguchi, K.; Kobayashi, T. Indoxyl sulfate decreases uridine adenosine tetraphosphate–induced contraction in rat renal artery. Pflügers Arch. Eur. J. Physiol. 2022, 474, 1285–1294. [Google Scholar] [CrossRef]
- Ntranos, A.; Park, H.-J.; Wentling, M.; Tolstikov, V.; Amatruda, M.; Inbar, B.; Kim-Schulze, S.; Frazier, C.; Button, J.; Kiebish, M.A.; et al. Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain 2022, 145, 569–583. [Google Scholar] [CrossRef]
- Ribeiro, A.; Liu, F.; Srebrzynski, M.; Rother, S.; Adamowicz, K.; Wadowska, M.; Steiger, S.; Anders, H.-J.; Schmaderer, C.; Koziel, J.; et al. Uremic Toxin Indoxyl Sulfate Promotes Macrophage-Associated Low-Grade Inflammation and Epithelial Cell Senescence. Int. J. Mol. Sci. 2023, 24, 8031. [Google Scholar] [CrossRef]
- Schakenraad, L.; Van Es, M.; Meerman, J.; Broek, P.V.D.; Van Hove, H.; Van Drongelen, J.; Eliesen, G.; Russel, F.; Greupink, R. Transfer of uremic solutes across the human term placenta: An ex vivo study in the dual-side perfused cotyledon. Placenta 2021, 104, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Li, J.-R.; Wang, Y.-Y.; Lin, S.-Y.; Ou, Y.-C.; Lin, C.-J.; Wang, J.-D.; Liao, S.-L.; Chen, C.-J. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging 2021, 13, 6681–6701. [Google Scholar] [CrossRef]
- Won, A.J.; Kim, S.; Kim, Y.G.; Kim, K.-B.; Choi, W.S.; Kacew, S.; Kim, K.S.; Jung, J.H.; Lee, B.M.; Kim, S.; et al. Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol. Biosyst. 2016, 12, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef]
- Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of Indole and Indole-Related Compounds by the Intestinal Microbiota and Consequences for the Host: The Good, the Bad, and the Ugly. Microorganisms 2022, 10, 930. [Google Scholar] [CrossRef]
- Montgomery, T.L.; Eckstrom, K.; Lile, K.H.; Caldwell, S.; Heney, E.R.; Lahue, K.G.; D’alessandro, A.; Wargo, M.J.; Krementsov, D.N. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome 2022, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Banoglu, E.; Jha, G.G.; King, R.S. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. PROGRESS IN UREMIC TOXIN RESEARCH: Protein-bound toxins—Update 2009. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- Yang, K.; Du, G.; Liu, J.; Zhao, S.; Dong, W. Gut microbiota and neonatal acute kidney injury biomarkers. Pediatr. Nephrol. 2023, 38, 3529–3547. [Google Scholar] [CrossRef]
- Liu, W.-C.; Wu, C.-C.; Lim, P.-S.; Chien, S.-W.; Hou, Y.-C.; Zheng, C.-M.; Shyu, J.-F.; Lin, Y.-F.; Lu, K.-C. Effect of uremic toxin-indoxyl sulfate on the skeletal system. Clin. Chim. Acta 2018, 484, 197–206. [Google Scholar] [CrossRef]
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007, 71, 738–743. [Google Scholar] [CrossRef]
- Arinze, N.V.; Yin, W.; Lotfollahzadeh, S.; Napoleon, M.A.; Richards, S.; Walker, J.A.; Belghasem, M.; Ravid, J.D.; Kamel, M.H.; Whelan, S.A.; et al. Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease. J. Clin. Investig. 2022, 132, e142260. [Google Scholar] [CrossRef]
- Sato, E.; Mori, T.; Mishima, E.; Suzuki, A.; Sugawara, S.; Kurasawa, N.; Saigusa, D.; Miura, D.; Morikawa-Ichinose, T.; Saito, R.; et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef]
- Madella, A.M.; Van Bergenhenegouwen, J.; Garssen, J.; Masereeuw, R.; Overbeek, S.A. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins 2022, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- Lis, A.W.; McLaughlin, D.I.; McLaughlin, R.K.; Lis, E.W.; Stubbs, E.G. Profiles of Ultraviolet-absorbing Components of Urine from Autistic Children, as Obtained by High-Resolution Ion-Exchange Chromatography. Clin. Chem. 1976, 22, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Miot, S.; Akbaraly, T.; Michelon, C.; Couderc, S.; Crepiat, S.; Loubersac, J.; Picot, M.-C.; Pernon, É.; Gonnier, V.; Jeandel, C.; et al. Comorbidity Burden in Adults With Autism Spectrum Disorders and Intellectual Disabilities—A Report From the EFAAR (Frailty Assessment in Ageing Adults With Autism Spectrum and Intellectual Disabilities) Study. Front. Psychiatry 2019, 10, 617. [Google Scholar] [CrossRef]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation deficit in “low-functioning” autistic children: A pilot study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]

| Author | Condition | Type of Sample | Patient Levels | Control Levels | Patient/Control Ratio | Findings |
|---|---|---|---|---|---|---|
| Dieme 2015 [5] | Autism | Urine | Visual Data | Visual Data | Not applicable | IS urinary concentrations higher in ASD patients compared to controls (p = 0.01) |
| Gevi 2016 [4] | Autism | Urine | Not applicable | Not applicable | Not applicable | IS urinary concentrations higher in ASD patients compared to controls (p < 0.001) |
| Mrochek 1973 [3] | Autism | Urine | Mild: 4 mg/24 h, Severe: 6 mg/24 h, Neurological Seizures: 7 mg/24 h | 3.4 mg/24 h | Mild: 1.18, Severe: 1.76, Seizures: 2.06 | IS urinary concentrations higher in ASD patients compared to controls; concentrations increase with severity |
| Olesova 2020 [1] | Autism | Urine | 32.63 ± 10.38 μmol/mmol Creatinine (Cr) | 18.95 ± 7.11 μmol/mmol Cr | 1.72 | IS urinary concentrations higher in ASD patients compared to controls (p = 0.00004) |
| Osredkar 2023 [2] | Autism | Urine | 55.64 μg/mg | 52.62 μg/mg | 1.06 | IS urinary concentrations higher in ASD patients compared to controls (p = 0.0182) |
| Rangel-Huerta 2019 [10] | Autism | Plasma | Visual Data | Visual Data | Not applicable | IS urinary concentrations higher in ASD patients with no regression compared to controls (p < 0.001) |
| Brydges 2021 [11] | Anxiety | Serum | Visual Data | Visual Data | Not applicable | IS significantly correlated with Hamilton Psychic Anxiety (p = 0.0001) and Hamilton Total Anxiety (p = 0.002) |
| Yeh 2016 [12] | Cognitive Impairment | Serum | 22.0 ± 25.8 mg/mL | 4.8 ± 5.1 mg/mL | 4.58 | IS independently associated with poor executive function; correlation varried depending on stage of Chronic Kidney Disease (CKD) |
| Teruya 2021 [13] | Dementia | Plasma | Not applicable | Not applicable | 1.93 | IS higher in dementia patients’ plasma than in healthy elderly (p = 0.015) |
| Philippe 2021 [14] | Depression | Urine | 234.2 μmol/mg Cr | 197.3 μmol/mg Cr | 1.19 | IS urinary concentrations higher in ASD patients compared to controls (p = 0.0098) |
| Cao 2015 [15] | Heart Failure | Plasma | Low IS Plasma Group: £32.35 mg/mL High IS Plasma Group: >32.35 mg/mL | Not applicable | Not applicable | Incidence of first heart failure event significantly higher in high IS group compared to low IS group (p < 0.001) |
| Cassani 2015 [8] | Parkinson’s | Urine | 48.3 ± 4.9 mg/L | 24.9 ± 4.6 mg/L | 1.94 | Parkinson’s patients had urinary IS concentrations twice as high as controls (p = 0.001) |
| Sankowski 2020 [16] | Parkinson’s | Cerebrospinal fluid (CSF)/Plasma | CSF/Plasma: 1.6 ± 0.9% | CSF/Plasma: 0.42 ± 0.21% | 3.81 | IS concentrations significantly higher in Parkinson’s cerebrospinal fluid (CSF) with motor fluctuation compared to Parkinson’s without motor fluctuation (p < 0.05); CSF to plasma ratio of IS in Parkinson’s group four times greater than control (p < 0.05) |
| Author | Indoxyl Sulfate Administered | Groups | R-Value & p-Value |
|---|---|---|---|
| Adesso 2017 [17] | Cells: 15–60 mM for 24 h; Injected mice: 800 mg/kg IS | C6 cells group; Primary astrocytes and mixed glial cells group; Injected mice group; Control mice group | Enhanced reactive oxygen species (ROS) in C6 cells: p < 0.001; Reduced nuclear factor erythroid 2-related factor 2 (Nrf2) Nuclear Translocation in C6 cells: p < 0.01; Reduced heme oxygenase 1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone dehydrogenase 1 (NQO1), and superoxide dismutase (SOD) expression in C6 cells: p < 0.01; Induced aryl hydrocarbon receptor (AhR) activation in C6 cells: p < 0.001; Induced p65 nuclear factor kappa-B (NF-kB) nuclear translocation in C6 cells: p < 0.05; Influenced oxidative stress and pro-inflammatory parameters in primary astrocytes and mixed glial cell cultures: p < 0.001 (greater effect on mixed culture than astrocytes alone: p < 0.05); Increased cellular death in neuronal cultures: p < 0.05; IS serum concentration compared to control: p < 0.001 |
| Bobot 2020 [18] | 1 g/L of water for 14 days | Rat Group 1: Induced Chronic Kidney Disease (CKD) (adenine-rich diet or 5/6 nephrectomy) Rat Group 2: Control | Blood brain barrier (BBB) permeability & Cognitive Impairment: R = −0.90, p < 0.001; BBB permeability & IS levels: R = 0.68, p = 0.006 |
| Griffin 2023 [19] | 100 mg/kg body weight, 200 mg/kg body weight | Pregnancy-related acute kidney injury (AKI) group of rats; Normal pregnant 100 mg/kg group; Normal pregnant 200 mg/kg; Normal pregnant group | Severity of decrease in weight associated with amount of IS administered: p = 0.002; IS administration significantly increased BBB permeability: p < 0.0001 |
| Huang 2020 [20] | IS-injected mice (intraperitoneal injection, 100 mg/kg daily for 8 weeks) | Transepithelial electrical resistance, permeability assay and transmission electron microscopy carried out to evaluate the damaging effect of IS on intestinal barrier in intestinal epithelial cells (in vitro study); IS-injected mice (intraperitoneal injection, 100 mg/kg daily for 8 weeks), and CKD mice (in vivo studies); CKD mice then treated with Kremezin (AST-120) (oral absorbent for IS) and gene knockout mice used to verify mechanism and explore possible interventions for IS-induced intestinal barrier injury | Not applicable |
| Jaglin 2018 [21] | Injected 500 mg/kg in rat cecum, also colonized germ-free rats with indole producing bacteria E. coli | Injection experiment: 12 injected, 12 control (injected with corn oil), Colonization experiment: 12 I+, 12 I− (control) | Injection experiment: distance traveled (p < 0.001), rearing (p < 0.001), eye blinking frequency (p < 0.001), Colonization experiment: Eye blinking frequency (p < 0.01) |
| Karbowska 2020 [9] | 100–200 mg/kg body weight/day for 4 weeks | Rat Group 1: 100 mg/kg body weight/day; Rat Group 2: 200 mg/kg body weight/day; Rat Group 3: Control | IS in Brainstem: control vs. 100 IS: p = 0.0093; control vs. 200 IS: p < 0.0001; Groomings: p = 0.0371; Time to exit: p = 0.0133; Latency: p = 0.0002; Correct Response: p = 0.0116 |
| Lai 2022 [22] | Profile of fecal, blood sera, and cerebral cortical brain tissues | Germ-free C57BL/6 mice and their age-matched conventionally raised specific-pathogen-free counterparts | Not applicable |
| Lekawanvijit 2010 [23] | Isolated rat cardiac myocytes and fibroblasts and human leukaemia monocytic cell line cells incubated with 0.1 nM to 300 mM | Control group; IS incubation groups | Neonatal cardiac fibroblasts (NCF) collagen synthesis: p < 0.05; Neonatal cardiac myocytes (NCM) hypertrophy: p < 0.001 |
| Matsumoto 2022 [24] | Isolated rat renal arteries incubated in 1.0 mM indoxyl sulfate for 1 h | Control group; IS incubation group | p < 0.05 |
| Ntranos 2022 [25] | Cultured rat neurons exposed to cerebrospinal fluid (CSF) samples collected from multiple sclerosis patients | 20 healthy controls; 16 relapsing remitting multiple sclerosis patients (RRMS); 17 patients with secondary progressive multiple sclerosis (SPMS) | Levels of IS in RRMS patients compared to control: p = 0.0537; Levels of IS in SPMS patients compared to control: p = 0.03795; Correlation of IS levels to cortical volume: r = −0.45, p = 0.037; Correlation of IS levels to levels of neurofilament light chain: r = 0.58, p = 0.011 |
| Ribeiro 2023 [26] | 250 mM (low concentrations), 60 mg/mL media (high concentrations) checked at 24 and 48 h | Macrophages and tubular epithelial (UT7/EPO) cells, incubated with IS at low concentrations and concentrations found in uremic patients, alone and with lipopolysaccharide challenge; control group | p < 0.05 |
| Schakenraad 2021 [27] | Not applicable | Placental transfer studied in healthy term placentas via ex vivo dual-side human cotyledon perfusion technique | Not applicable |
| Sun 2021 [28] | 100 mg/kg body weight | Rat Group 1: Control; Rat Group 2: Unilateral nephrectomy & 100 mg/kg | p < 0.05 |
| Won 2016 [29] | Not applicable | Cisplatin-induced acute kidney injury (AKI) rats; Control group | p < 0.01 |
| Zhao 2012 [7] | Not applicable | Rat Group 1: Adenine-induced chronic renal failure; Rat Group 2: Control | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, Z.R.; Flynn, C.K.; Adams, J.B. Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review. Int. J. Mol. Sci. 2024, 25, 12973. https://doi.org/10.3390/ijms252312973
Hill ZR, Flynn CK, Adams JB. Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review. International Journal of Molecular Sciences. 2024; 25(23):12973. https://doi.org/10.3390/ijms252312973
Chicago/Turabian StyleHill, Zoë R., Christina K. Flynn, and James B. Adams. 2024. "Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review" International Journal of Molecular Sciences 25, no. 23: 12973. https://doi.org/10.3390/ijms252312973
APA StyleHill, Z. R., Flynn, C. K., & Adams, J. B. (2024). Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review. International Journal of Molecular Sciences, 25(23), 12973. https://doi.org/10.3390/ijms252312973





