Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus—Clinical Practice Position Statement of the Polish Society of Nephrology
Abstract
1. Introduction
1.1. Epidemiology of T2D and CKD Worldwide
1.2. Pathophysiology of Diabetic Renal Complications and Their Clinical Consequences
1.3. The Main Goals of CKD in T2D Treatment
2. Antihyperglycemic Drugs
2.1. Statement 2.1
2.1.1. Statement 2.1.1
- Comment to Statement 2.1.1
- SGLT2i in the cardiovascular outcome trials (CVOTs)
- SGLT2i in the renal outcome trials
- SGLT2i in the heart failure trials and meta-analysis
- GLP1RA in the CVOT and renal outcome trials
2.1.2. Statement 2.1.2
- Comment to Statement 2.1.2
2.1.3. Statement 2.1.3
- Comment to Statement 2.1.3
2.1.4. Statement 2.1.4
- Comment to Statement 2.1.4
2.2. Statement 2.2
- Comment to Statement 2.2
- HbA1c measurement in CKD
2.3. Statement 2.3
- Comment to Statement 2.3
- GLP1RAs in CVOTs
- “Twincretin” agent (tirzepatide) in the renal outcome trial
- Aspects of co-antihyperglycemic treatment and metabolic control in CVOTs with SGLT2i and GLP1RA
2.4. Statement 2.4
- Comment to Statement 2.4
- Metformin
- Metformin can be used if an eGFR is not lower than 30 mL/min/1.73 m2. The dosage should be reduced when the eGFR falls below 45 mL/min/1.73 m2 (to a maximum of 1000 mg per day) [108].
- There are two reasons to avoid metformin in patients with eGFR < 30 mL/min/1.73 m2:
- ○
- ○
- lack of high-quality evidence for the benefits of continuing metformin in patients with eGFR lower than 30 mL/min/1.73 m2. Many studies demonstrated that metformin use in patients with T2D and eGFR > 30 mL/min/1.73 m2 confers a survival benefit (22% reduction of mortality; HR 0.78; 95% CI 0.63–0.96) and a 30%–40% reduction in cardiovascular and diabetes-related events [111].
- Metformin should be withheld during acute illnesses with a risk of tissue hypoxia or sudden deterioration in renal function, particularly in AKI and sepsis [112].
- It is not necessary to discontinue metformin before intravenous contrast media administration [2].
- During metformin therapy, attention should be paid to vitamin B12 deficiency [29].
- Sulfonylureas
- Patients with T2D and CKD < 60 mL/min/1.73 m2 who are on sulfonylureas treatment (with or without concomitant insulin therapy) are at increased risk of hypoglycemia.
- Sulfonylureas with the lowest risk of hypoglycemia and hepatic metabolism (glipizide, glimepiride, gliquidone, and gliclazide) are reasonable agents for patients with an eGFR > 30 mL/min/1.73 m2.
- Most sulfonylureas should be avoided in advanced renal impairment (eGFR < 30 mL/min/1.73 m2). The only sulfonylurea agent that might be safely used in patients with eGFR < 30 mL/min/1.73 m2 is metabolized mainly in the liver to largely inactive metabolites, gliquidone.
- Given the lack of excess cardiovascular events and a lower risk of doubling Scr in patients with eGFR ≥ 60 mL/min/1.73 m2 (HR: 0.21, 95% CI: 0.04–0.99), gliclazide should be a drug of choice in these patients, i.e., patients with normal kidney function [113].
- Meglitinides
- Repaglinide, due to its hepatic metabolism (via cytochrome P450), can be considered for T2D therapy in CKD patients as monotherapy or in addition to metformin.
- Nateglinide is hepatically metabolized with renal excretion of active metabolites that can accumulate and cause hypoglycemia. Therefore, it should be avoided in patients with advanced CKD or ESRD.
- Repaglinide dose reduction is advised in patients with eGFR < 30 mL/min/1.73 m2 [29].
- Thiazolidinediones: Pioglitazone
- Patients with T2D and CKD of all stages can be considered for treatment with pioglitazone.
- Pioglitazone should be avoided in patients with advanced CKD and fluid overload, especially those with preexisting heart failure, given the risk of edema and worsening heart failure [29].
- We suggest discontinuing pioglitazone in CKD patients with T2D, experiencing hip fracture during treatment, or with painless hematuria until bladder cancer is excluded [29].
- Dipeptidyl Peptidase-4 (DPP4) Inhibitors
- Patients with T2D and CKD of all stages, including those requiring dialysis, can be considered for treatment with DPP4 inhibitors without the risk of hypoglycemia [114].
- DPP4 inhibitors appear to have a neutral effect on the risk of diabetes-related kidney disease and kidney outcomes [29].
- Doses of DPP4 inhibitors (sitagliptin, saxagliptin, and vildagliptin) should be appropriately reduced by the degree of renal impairment [29].
- Sodium–Glucose Co-Transporter-2 Inhibitors (SGLT-2 Inhibitors)
- Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs)
- Alpha-Glucosidase Inhibitors A
- Acarbose, or miglitol, can be safely used in patients with all CKD stages [117].
- They are minimally effective in lowering blood HbA1c concentration (mean 0.5 to 0.7% reduction) and are associated with limiting gastrointestinal side effects [117].
- Insulin
- Co-Formulation
3. Antihypertensive Therapy
3.1. Statement 3.1
3.1.1. Statement 3.1.1
- Comment to Statement 3.1.1
3.1.2. Statement 3.1.2
- Comment to Statement 3.1.2
3.2. Statement 3.2
Statement 3.2.1
- Comment to Statement 3.2.1
3.3. Statement 3.3
Statement 3.3.1
- Comment to Statement 3.3.1
3.4. Statement 3.4
3.4.1. Statement 3.4.1
- Comment to Statement 3.4.1
3.4.2. Statement 3.4.2
- Comment to Statement 3.4.2
3.5. Statement 3.5
- Comment to Statement 3.5
3.6. Statement 3.6
- Comment to Statement 3.6
3.7. Statement 3.7
- Comment to Statement 3.7
4. Inhibition of Renin–Angiotensin–Aldosterone Axis
4.1. Statement 4.1
- Comment to Statement 4.1
4.1.1. Statement 4.1.1
- Comment to Statement 4.1.1
4.1.2. Statement 4.1.2
- Comment to Statement 4.1.2
4.1.3. Statement 4.1.3
- Comment to Statement 4.1.3
4.1.4. Statement 4.1.4
- Comment to Statement 4.1.4
4.1.5. Statement 4.1.5
- Comment to Statement 4.1.5
4.2. Statement 4.2
- Comment to Statement 4.2
4.3. Statement 4.3
4.3.1. Statement 4.3.1
- Comment to Statement 4.3.1
4.3.2. Statement 4.3.2
- Comment to Statement 4.3.2
5. Metabolic Acidosis Treatment
5.1. Statement 5.1
- Comment to Statement 5.1
5.2. Statement 5.2
- Comment to Statement 5.2
6. Limitations of Current Evidence Concerning Pharmacological Nephroprotection
7. Cost-Effectiveness of Pharmacological Nephroprotection
8. Conclusive Remarks
9. Brief Summary
- Effective Antihyperglycemic Treatment: Optimized glucose control is crucial, with sodium–glucose cotransporter-2 inhibitors (SGLT2i) or glucagon-like peptide-1 receptor agonists (GLP1RA) preferred for their nephroprotective or lowering albuminuria effects.
- SGLT2 inhibitors and semaglutide: Independently from diabetic metabolic control, the use of SGLT2 inhibitors like empagliflozin, dapagliflozin, and canagliflozin or GLP1RA semaglutide is strongly recommended for their dual role in kidney protection and cardiovascular benefit, with a well-documented safety profile across CKD stages in T2D patients.
- Antihypertensive Therapy: Optimal blood pressure management is essential, typically incorporating renin–angiotensin system inhibitors (RASi), specifically angiotensin receptor blockers (ARB) or, in the case of ARB intolerance, ACE inhibitors (ACEi), at the maximally tolerated dose.
- RASi and Mineralocorticoid Receptor Antagonists: For T2D patients with persistent albuminuria, despite optimal use of ARB or ACEi and SGLT2i or semaglutide, finerenone, a non-steroidal mineralocorticoid receptor antagonist, is recommended to reduce kidney disease progression further.
- Sodium Bicarbonate for Metabolic Acidosis: In patients with metabolic acidosis, sodium bicarbonate should be used with a target serum bicarbonate level of 24–28 mmol/L.
- Monitoring: Regularly monitor renal function (eGFR, serum creatinine) and electrolytes (serum potassium and sodium) to manage the safety and efficacy of therapy.
- Lifestyle Modifications: Encourage dietary adjustments, physical activity, smoking cessation, and weight management.
- Individualization: Tailor treatment goals based on comorbidities, patient age, and risk factors.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stompór, T.; Adamczak, M.; Kurnatowska, I.; Naumnik, B.; Nowicki, M.; Tylicki, L.; Winiarska, A.; Krajewska, M. Pharmacological Nephroprotection in Non-Diabetic Chronic Kidney Disease-Clinical Practice Position Statement of the Polish Society of Nephrology. J. Clin. Med. 2023, 12, 5184. [Google Scholar] [CrossRef]
- Chmielewski, M.; Serafin, Z.; Kamińska, D.; Skrobisz, K.; Kozak, O.; Olczyk, P.; Rutkowski, P.; Adamczak, M.; Szurowska, E.; Krajewska, M. The use of intravascular contrast media in patients with impaired kidney function-joint clinical practice position statement of the Polish Society of Nephrology and the Polish Medical Society of Radiology. Pol. J. Radiol. 2024, 89, e161–e171. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Cukrzyca. Available online: https://archiwum.mz.gov.pl/zdrowie-i-profilaktyka/choroby-cywilizacyjne/cukrzyca/ (accessed on 15 September 2024).
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Renal Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef]
- Woodhams, L.; Sim, T.F.; Chalmers, L.; Yeap, B.; Green, D.; Schlaich, M.; Schultz, C.; Hillis, G. Diabetic kidney disease in type 2 diabetes: A review of pathogenic mechanisms, patient-related factors and therapeutic options. PeerJ 2021, 9, e11070. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Targeting Redox Imbalance as an Approach for Diabetic Kidney Disease. Biomedicines 2020, 8, 40. [Google Scholar] [CrossRef]
- Magee, G.M.; Bilous, R.W.; Cardwell, C.R.; Hunter, S.J.; Kee, F.; Fogarty, D.G. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 2009, 52, 691–697. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef]
- Vallon, V.; Komers, R. Pathophysiology of the diabetic kidney. Compr. Physiol. 2011, 1, 1175–1232. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.S.; Torquato, B.G.S.; da Silva, C.A.; Dos Reis Monteiro, M.L.G.; Dos Santos Martins, A.L.M.; da Silva, M.V.; Dos Reis, M.A.; Machado, J.R. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Salti, T.; Khazim, K.; Haddad, R.; Campisi-Pinto, S.; Bar-Sela, G.; Cohen, I. Glucose Induces IL-1α-Dependent Inflammation and Extracellular Matrix Proteins Expression and Deposition in Renal Tubular Epithelial Cells in Diabetic Kidney Disease. Front. Immunol. 2020, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Milas, O.; Gadalean, F.; Vlad, A.; Dumitrascu, V.; Velciov, S.; Gluhovschi, C.; Bob, F.; Popescu, R.; Ursoniu, S.; Jianu, D.C.; et al. Pro-inflammatory cytokines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J. Diabetes Complicat. 2020, 34, 107479. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 2017, 13, 311–318. [Google Scholar] [CrossRef]
- Gu, H.F. Genetic and Epigenetic Studies in Diabetic Kidney Disease. Front. Genet. 2019, 10, 507. [Google Scholar] [CrossRef]
- Kourtidou, C.; Tziomalos, K. The Role of Histone Modifications in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 6007. [Google Scholar] [CrossRef]
- Kuo, F.C.; Chao, C.T.; Lin, S.H. The Dynamics and Plasticity of Epigenetics in Diabetic Kidney Disease: Therapeutic Applications Vis-à-Vis. Int. J. Mol. Sci. 2022, 23, 843. [Google Scholar] [CrossRef]
- Schelling, J.R. The Contribution of Lipotoxicity to Diabetic Kidney Disease. Cells 2022, 11, 3236. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Wyld, M.L.R.; Morton, R.L.; Aouad, L.; Magliano, D.; Polkinghorne, K.R.; Chadban, S. The impact of comorbid chronic kidney disease and diabetes on health-related quality-of-life: A 12-year community cohort study. Nephrol. Dial. Transplant. 2021, 36, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Ustyugova, A.; Déruaz-Luyet, A.; O’Keeffe-Rosetti, M.; Brodovicz, K.G. Health Care Costs by Type of Expenditure across eGFR Stages among Patients with and without Diabetes, Cardiovascular Disease, and Heart Failure. J. Am. Soc. Nephrol. 2020, 31, 1594–1601. [Google Scholar] [CrossRef]
- Adamczak, M.; Zeier, M.; Dikow, R.; Ritz, E. Kidney and hypertension. Kidney Int. Suppl. 2002, 80, 62–67. [Google Scholar] [CrossRef][Green Version]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Amod, A.; Buse, J.B.; McGuire, D.K.; Pieber, T.R.; Pop-Busui, R.; Pratley, R.E.; Zinman, B.; Hansen, M.B.; Jia, T.; Mark, T.; et al. Glomerular Filtration Rate and Associated Risks of Cardiovascular Events, Mortality, and Severe Hypoglycemia in Patients with Type 2 Diabetes: Secondary Analysis (DEVOTE 11). Diabetes Ther. 2020, 11, 53–70. [Google Scholar] [CrossRef]
- Karalliedde, J.; Winocour, P.; Chowdhury, T.A.; De, P.; Frankel, A.H.; Montero, R.M.; Pokrajac, A.; Banerjee, D.; Dasgupta, I.; Fogarty, D.; et al. Clinical practice guidelines for management of hyperglycaemia in adults with diabetic kidney disease. Diabet. Med. 2022, 39, e14769. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, L.; Jia, X.; Pang, X.; Xiang, Q.; Zhao, X.; Ma, L.; Liu, Z.; Hu, K.; Wang, Z.; et al. Comparative Lipid-Lowering/Increasing Efficacy of 7 Statins in Patients with Dyslipidemia, Cardiovascular Diseases, or Diabetes Mellitus: Systematic Review and Network Meta-Analyses of 50 Randomized Controlled Trials. Cardiovasc. Ther. 2020, 23, 3987065. [Google Scholar] [CrossRef]
- Burke, M.; Faruque, L.I.; Lloyd, A.; Ahmad, N.; Liu, Y.; Tiv, S.; Millard, T.; Gagliardi, L.; Kolanu, N.; Barmanray, R.D.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573, Erratum in BMJ 2022, 376, o109. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Mosenzon, O.; Wiviott, S.D.; Cahn, A.; Rozenberg, A.; Yanuv, I.; Goodrich, E.L.; Murphy, S.A.; Heerspink, H.J.L.; Zelniker, T.A.; Dwyer, J.P.; et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Clinical Benefit of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 435–447. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group. SGLT2 Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Cesaro, A.; Gallinoro, E.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in diabetic patients with acute myocardial infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT registry. Diabetes Res. Clin. Pract. 2023, 202, 110766. [Google Scholar] [CrossRef] [PubMed]
- Özkan, U.; Gürdoğan, M. The Effect of SGLT2 Inhibitors on the Development of Contrast-Induced Nephropathy in Diabetic Patients with Non-ST Segment Elevation Myocardial Infarction. Medicina 2023, 59, 505. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Oshima, M.; Perkovic, V.; Agarwal, R.; Arnott, C.; Bakris, G.; Cannon, C.P.; Charytan, D.M.; Edwards, R.; Górriz, J.L.; et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: The CREDENCE trial. Eur. Heart J. 2021, 42, 4891–4901. [Google Scholar] [CrossRef]
- Neuen, B.L.; Oshima, M.; Agarwal, R.; Arnott, C.; Cherney, D.Z.; Edwards, R.; Langkilde, A.M.; Mahaffey, K.W.; McGuire, D.K.; Neal, B.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People With Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation 2022, 145, 1460–1470. [Google Scholar] [CrossRef]
- Neuen, B.L.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019, 11, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Ferrannini, E.; Umpierrez, G.E.; Peters, A.L.; Rosenstock, J.; Carroll, A.K.; Lapuerta, P.; Banks, P.; Agarwal, R. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and severe renal impairment. Diabetes Obes. Metab. 2021, 23, 2632–2642. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Gorriz, J.L.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 2352–2361. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Stompór, T.; Winiarska, A. Kidneys in heart failure: Impact of flozins. Kardiol. Pol. 2023, 81, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B.; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Fonseca, V.; Mosenzon, O.; Raz, I.; Goldman, B.; Idorn, T.; von Scholten, B.J.; Poulter, N.R. Effects of Liraglutide Versus Placebo on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Circulation 2018, 138, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.E.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 2022, 145, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bosch-Traberg, H.; Cherney, D.Z.I.; Hadjadj, S.; Lawson, J.; Mosenzon, O.; Rasmussen, S.; Bain, S.C. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 2023, 103, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Botros, F.T.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019, 394, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mosenzon, O.; Schechter, M.; Leibowitz, G. Kidney Outcomes With Glucagon-Like Peptide-1 Receptor Agonists in Patients With Type 2 Diabetes. Adv. Chronic Kidney Dis. 2021, 28, 347–360. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Barreto, J.; Borges, C.; Rodrigues, T.B.; Jesus, D.C.; Campos-Staffico, A.M.; Nadruz, W.; Luiz da Costa, J.; Bueno de Oliveira, R.; Sposito, A.C. Pharmacokinetic Properties of Dapagliflozin in Hemodialysis and Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2023, 18, 1051–1058. [Google Scholar] [CrossRef]
- De La Flor, J.C.; Villa, D.; Cruzado, L.; Apaza, J.; Valga, F.; Zamora, R.; Marschall, A.; Cieza, M.; Deira, J.; Rodeles, M. Efficacy and Safety of the Use of SGLT2 Inhibitors in Patients on Incremental Hemodialysis: Maximizing Residual Renal Function, Is There a Role for SGLT2 Inhibitors? Biomedicines 2023, 11, 1908. [Google Scholar] [CrossRef]
- Lim, J.H.; Kwon, S.; Jeon, Y.; Kim, Y.H.; Kwon, H.; Kim, Y.S.; Lee, H.; Kim, Y.L.; Kim, C.D.; Park, S.H.; et al. The Efficacy and Safety of SGLT2 Inhibitor in Diabetic Kidney Transplant Recipients. Transplantation 2022, 106, e404–e412. [Google Scholar] [CrossRef]
- Demir, M.E.; Özler, T.E.; Merhametsiz, Ö.; Sözener, U.; Uyar, M.; Ercan, Z.; Demir, S.B.; Sezer, S.; Sarıyıldız, G.T. The results of SGLT-2 inhibitors use in kidney transplantation: 1-year experiences from two centers. Int. Urol. Nephrol. 2023, 55, 2989–2999. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Garg, N.; Pabich, S.; Mandelbrot, D.A.; Swanson, K.J. Sodium-glucose cotransporter-2 inhibitor use in kidney transplant recipients. World J. Transplant. 2023, 13, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, L.; Montero, N.; Cruzado, J.M. Searching in the maze: Sodium-glucose cotransporter-2 inhibitors in kidney transplant recipients to improve survival. Clin. Kidney J. 2023, 16, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-glucose cotransporter-2 inhibitor therapy in kidney transplant patients with type 2 or post-transplant diabetes: An observational multicentre study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Mahalwar, G.; Mathew, R.O.; Rangaswami, J. Sodium-glucose cotransporter 2 inhibitors and cardiorenal outcomes in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2024, 33, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Miyagi, K. Empagliflozin in kidney transplant recipients with chronic kidney disease G3a-4 and metabolic syndrome: Five Japanese cases. BMC Nephrol. 2022, 23, 168. [Google Scholar] [CrossRef]
- De la Flor, J.C.; Lorenzo, J.D.; Marschall, A.; Valga, F.; Vázquez TMCícero, E.R. Efficacy and Safety of Semaglutide, a Glucagon-Like Peptide-1 Receptor Agonist in Real-Life: A Case Series of Patients in Maintenance Incremental Hemodialysis. Case Rep. Nephrol. Dial. 2022, 12, 238–247. [Google Scholar] [CrossRef]
- Lawrence, S.E.; Chandran, M.M.; Park, J.M.; Sweiss, H.; Jensen, T.; Choksi, P.; Crowther, B. Sweet and simple as syrup: A review and guidance for use of novel antihyperglycemic agents for post-transplant diabetes mellitus and type 2 diabetes mellitus after kidney transplantation. Clin. Transplant. 2023, 37, e14922. [Google Scholar] [CrossRef]
- Dotan, I.; Rudman, Y.; Turjeman, A.; Akirov, A.; Steinmetz, T.; Calvarysky, B.; Diker Cohen, T. Glucagon-like Peptide 1 Receptor Agonists and Cardiovascular Outcomes in Solid Organ Transplant Recipients With Diabetes Mellitus. Transplantation 2024, 108, e121–e128. [Google Scholar] [CrossRef]
- Sato, T.; Azuma, Y.; Ozone, C.; Okazaki, M.; Takeda, A.; Okada, M.; Futamura, K.; Hiramitsu, T.; Goto, N.; Narumi, S.; et al. Possible Advantage of Glucagon-Like Peptide 1 Receptor Agonists for Kidney Transplant Recipients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 2597–2603. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Cherney, D.Z.I. Clinical Implications of an Acute Dip in eGFR after SGLT2 Inhibitor Initiation. Clin. J. Am. Soc. Nephrol. 2021, 16, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Jongs, N.; Chertow, G.M.; Greene, T.; McMurray, J.J.V.; Langkilde, A.M.; Correa-Rotter, R.; Kashihara, N.; Rossing, P.; Sjöström, C.D.; Stefánsson, B.V.; et al. Correlates and Consequences of an Acute Change in eGFR in Response to the SGLT2 Inhibitor Dapagliflozin in Patients with CKD. J. Am. Soc. Nephrol. 2022, 33, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Leibensperger, M.; Zhang, J.; Huan, Y. A Meta-Analysis of the Effects of SGLT-2 Inhibitors on Serum Electrolytes. 2021. Phase 1. Paper 41. Available online: https://jdc.jefferson.edu/si_ctr_2023_phase1/41 (accessed on 15 September 2024).
- Luo, X.; Xu, J.; Zhou, S.; Xue, C.; Chen, Z.; Mao, Z. Influence of SGLT2i and RAASi and Their Combination on Risk of Hyperkalemia in DKD: A Network Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2023, 18, 1019–1030. [Google Scholar] [CrossRef]
- Berns, J.S.; Glickman, J.D.; DeSantis, A. Management of Hyperglycemia in Patients with Type 2 Diabetes and Advanced Chronic Kidney Disease or End-Stage Kidney Disease. Available online: https://www.uptodate.com/contents/management-of-hyperglycemia-in-patients-with-type-2-diabetes-and-advanced-chronic-kidney-disease-or-end-stage-kidney-disease#:~:text=This%20topic%20reviews%20monitoring%20glycemic%20control (accessed on 15 September 2024).
- Shurraw, S.; Hemmelgarn, B.; Lin, M.; Majumdar, S.R.; Klarenbach, S.; Manns, B.; Bello, A.; James, M.; Turin, T.C.; Tonelli, M.; et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: A population-based cohort study. Arch. Intern. Med. 2011, 171, 1920–1927. [Google Scholar] [CrossRef]
- Coca, S.G.; Ismail-Beigi, F.; Haq, N.; Krumholz, H.M.; Parikh, C.R. Role of Intensive glucose control in development of renal end points in type 2 diabetes mellitus: Systematic review and meta-analysis. Arch. Intern. Med. 2012, 172, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gutiérrez, R.; Montori, V.M. Glycemic control for patients with type 2 diabetes mellitus: Our evolving faith in the face of evidence. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 504–512. [Google Scholar] [CrossRef]
- Zoungas, S.; Arima, H.; Gerstein, H.C.; Holman, R.R.; Woodward, M.; Reaven, P.; Hayward, R.A.; Craven, T.; Coleman, R.L.; Chalmers, J.; et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: A meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017, 5, 431–437. [Google Scholar] [CrossRef]
- Lo, C.; Lui, M.; Ranasinha, S.; Teede, H.J.; Kerr, P.G.; Polkinghorne, K.R.; Nathan, D.M.; Zheng, H.; Zoungas, S. Defining the relationship between average glucose and HbA1c in patients with type 2 diabetes and chronic kidney disease. Diabetes Res. Clin. Pract. 2014, 104, 84–91. [Google Scholar] [CrossRef]
- Freedman, B.I.; Shihabi, Z.K.; Andries, L.; Cardona, C.Y.; Peacock, T.P.; Byers, J.R.; Russell, G.B.; Stratta, R.J.; Bleyer, A.J. Relationship between assays of glycemia in diabetic subjects with advanced chronic kidney disease. Am. J. Nephrol. 2010, 31, 375–379. [Google Scholar] [CrossRef]
- Konya, J.; Ng, J.M.; Cox, H.; Cooke, M.; Lewis, N.; Bhandari, S.; Atkin, S.L.; Kilpatrick, E.S. Use of complementary markers in assessing glycaemic control in people with diabetic kidney disease undergoing iron or erythropoietin treatment. Diabet. Med. 2013, 30, 1250–1254. [Google Scholar] [CrossRef]
- Nathan, D.M.; McGee, P.; Steffes, M.W.; Lachin, J.M.; DCCT/EDIC Research Group. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014, 63, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Tsapas, A.; Avgerinos, I.; Karagiannis, T.; Malandris, K.; Manolopoulos, A.; Andreadis, P.; Liakos, A.; Matthews, D.R.; Bekiari, E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2020, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- von Scholten, B.J.; Kreiner, F.F.; Rasmussen, S.; Rossing, P.; Idorn, T. The potential of GLP-1 receptor agonists in type 2 diabetes and chronic kidney disease: From randomised trials to clinical practice. Ther. Adv. Endocrinol. Metab. 2022, 19, 20420188221112490. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Vistisen, D.; Kivimäki, M.; Perreault, L.; Hulman, A.; Witte, D.R.; Brunner, E.J.; Tabák, A.; Jørgensen, M.E.; Færch, K. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: The Whitehall II cohort study. Diabetologia 2019, 62, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Mima, A.; Nomura, A.; Fujii, T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: A narrative review. Biomed. Pharmacother. 2023, 165, 115032. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C., 3rd; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2023, 381, e074068. [Google Scholar] [CrossRef]
- Mima, A.; Gotoda, H.; Lee, R.; Murakami, A.; Akai, R.; Lee, S. Effects of incretin-based therapeutic agents including tirzepatide on renal outcomes in patients with type 2 diabetes: A systemic review and meta-analysis. Metabol. Open 2023, 17, 100236. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Gibson, A.K.; McGill, J.B.; Yan, Y.; Maddukuri, G.; Al-Aly, Z. Comparative Effectiveness of the Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin Versus Other Antihyperglycemics on Risk of Major Adverse Kidney Events. Diabetes Care 2020, 43, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Broncel, M.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Cyranka, K.; Czupryniak, L.; Dzida, G.; et al. Standards of Care in Diabetes. The position of Diabetes Poland–2024. Curr. Top. Diabetes 2023, 3, 1–348. [Google Scholar] [CrossRef]
- Stompór, T.; Adamczak, M.; Masajtis-Zagajewska, A.; Mazanowska, O.; Maziarska, K.; Witkowska, A.; Więcek, A. Diagnosis and treatment of type 2 diabetes mellitus in patients with chronic kidney disease and eGFR < 60 mL/min—A position statement of the Polish Society of Nephrology Working Group on Metabolic and Endocrine Disorders in Kidney Diseases. Endokrynol. Pol. 2020, 70, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, B.; Wu, A.; Shin, J.I.; Sang, Y.; Alexander, G.C.; Secora, A.; Inker, L.A.; Coresh, J.; Chang, A.R.; Grams, M.E. Association of Metformin Use With Risk of Lactic Acidosis Across the Range of Kidney Function: A Community-Based Cohort Study. JAMA Intern. Med. 2018, 178, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: Systematic review. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, C.B.; Stanifer, J.W.; Mock, C.K.; Wang, X.; Tang, S.; Nagi, A.; Kosinski, A.S.; et al. Clinical Outcomes of Metformin Use in Populations With Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann. Intern. Med. 2017, 166, 191–200. [Google Scholar] [CrossRef]
- Home, P.; Mant, J.; Diaz, J.; Turner, C.; Guideline Development Group. Management of type 2 diabetes: Summary of updated NICE guidance. BMJ 2008, 336, 1306–1308. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, C.J.; Lee, H.S.; Choe, E.Y.; Lee, B.W.; Ahn, C.W.; Cha, B.S.; Lee, H.C.; Balkau, B.; Kang, E.S. Comparing kidney outcomes in type 2 diabetes treated with different sulphonylureas in real-life clinical practice. Diabetes Metab. 2015, 41, 208–215. [Google Scholar] [CrossRef]
- Snyder, R.W.; Berns, J.S. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin. Dial. 2004, 17, 365–370. [Google Scholar] [CrossRef]
- Monami, M.; Dicembrini, I.; Martelli, D.; Mannucci, E. Safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of randomized clinical trials. Curr. Med. Res. Opin. 2011, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther. Adv. Endocrinol. Metab. 2013, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.B.; Sloan, L.; Newman, J.; Patel, S.; Sauce, C.; von Eynatten, M.; Woerle, H.J. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: A 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 2013, 36, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Yang, C.Y.; Kuo, S.; Kuo, T.H.; Roan, J.N.; Li, C.Y.; Wang, M.C.; Ou, H.T. Hepatic and cardiovascular safety of acarbose among type 2 diabetes patients with end-stage renal disease: A nationwide population-based longitudinal study. Diabetes Res. Clin. Pract. 2021, 172, 108489. [Google Scholar] [CrossRef] [PubMed]
- Biesenbach, G.; Rami, A.; Schmekal, B.; Eichbauer-Sturm, G. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet. Med. 2003, 20, 642–645. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, T.; Heianza, Y.; Li, X.; Fan, M.; Fonseca, V.A.; Qi, L. Type 2 Diabetes and Hypertension. Circ. Res. 2019, 124, 930–937. [Google Scholar] [CrossRef]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core curriculum 2019. Am J Kidney Dis 2019, 74, 120–131. [Google Scholar] [CrossRef]
- Judd, E.; Calhoun, D.A. Management of hypertension in CKD: Beyond the guidelines. Adv. Chronic Kidney Dis. 2015, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Yang, J.; Tan, T.C.; Cabrera, C.S.; Stefansson, B.V.; Greasley, P.J.; Ordonez, J.D. Kaiser Permanente Northern California CKD Outcomes Study. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018, 19, 146. [Google Scholar] [CrossRef]
- Obi, Y.; Kalantar-Zadeh, K.; Shintani, A.; Kovesdy, C.P.; Hamano, T. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: A post hoc analysis of a randomized clinical trial. J. Intern. Med. 2018, 283, 314–327. [Google Scholar] [CrossRef]
- Schrier, R.W.; Estacio, R.O.; Mehler, P.S.; Hiatt, W.R. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: A summary of the ABCD trial. Nat. Clin. Pract. Nephrol. 2007, 3, 428–438. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998, 317, 703–713. [Google Scholar] [CrossRef]
- Ismail-Beigi, F.; Craven, T.E.; O’Connor, P.J.; Karl, D.; Calles-Escandon, J.; Hramiak, I.; Genuth, S.; Cushman, W.C.; Gerstein, H.C.; Probstfield, J.L. Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int. 2012, 81, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Greene, T.; Boucher, R.; Cushman, W.C.; Wei, G.; Stoddard, G.; Ix, J.H.; Chonchol, M.; Kramer, H.; Cheung, A.K.; et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: Secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol. 2018, 6, 555–563. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef]
- Pohl, M.A.; Blumenthal, S.; Cordonnier, D.J.; De Alvaro, F.; Deferrari, G.; Eisner, G.; Esmatjes, E.; Gilbert, R.E.; Hunsicker, L.G.; de Faria, J.B.; et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J. Am. Soc. Nephrol. 2005, 16, 3027–3037. [Google Scholar] [CrossRef]
- de Galan, B.E.; Perkovic, V.; Ninomiya, T.; Pillai, A.; Patel, A.; Cass, A.; Neal, B.; Poulter, N.; Harrap, S.; Mogensen, C.E.; et al. Lowering blood pressure reduces renal events in type 2 diabetes. J. Am. Soc. Nephrol. 2009, 20, 883–892. [Google Scholar] [CrossRef]
- Cooper-DeHoff, R.M.; Gong, Y.; Handberg, E.M.; Bavry, A.A.; Denardo, S.J.; Bakris, G.L.; Pepine, C.J. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010, 304, 61–68. [Google Scholar] [CrossRef]
- Thomopoulos, C.; Parati, G.; Zanchetti, A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10—Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J. Hypertens. 2017, 35, 922–944. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Xu, W.; Lu, N.; Cao, J.; Yu, S. Effects of intensive blood pressure lowering on mortality and cardiovascular and renal outcomes in type 2 diabetic patients: A meta-analysis. PLoS ONE 2019, 14, e0215362. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Kruzliak, P.; Komornikova, A.; Celecova, Z.; Krahulec, B.; Balaz, D.; Sabaka, P.; Caprnda, M.; Kucera, M.; Rodrigo, L.; et al. Orthostatic hypotension in diabetic patients-10-year follow-up study. J. Diabetes Complicat. 2016, 30, 67–71. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Redon, J.; Mancia, G.; Sleight, P.; Schumacher, H.; Gao, P.; Pogue, J.; Fagard, R.; Verdecchia, P.; Weber, M.; Böhm, M.; et al. Safety and efficacy of low blood pressures among patients with diabetes: Subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). J. Am. Coll. Cardiol. 2012, 59, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Ito, S.; Izzo, J.L., Jr.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2011, 364, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.; Muirhead, N.; Rene de Cotret, P.; Chiu, A.; Pichette, V.; Tobe, S.; SMART (Supra Maximal Atacand Renal Trial) Investigators. Supramaximal dose of candesartan in proteinuric renal disease. J. Am. Soc. Nephrol. 2009, 20, 893–900. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Fassi, A.; Ilieva, A.P.; Bruno, S.; Iliev, I.P.; Brusegan, V.; Rubis, N.; Gherardi, G.; Arnoldi, F.; Ganeva, M.; et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2004, 351, 1941–1951. [Google Scholar] [CrossRef]
- Barnett, A.H.; Bain, S.C.; Bouter, P.; Karlberg, B.; Madsbad, S.; Jervell, J.; Mustonen, J.; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N. Engl. J. Med. 2004, 351, 1952–1961. [Google Scholar] [CrossRef]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar] [CrossRef]
- Hou, F.F.; Zhang, X.; Zhang, G.H.; Xie, D.; Chen, P.Y.; Zhang, W.R.; Jiang, J.P.; Liang, M.; Wang, G.B.; Liu, Z.R.; et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 2006, 354, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Schumacher, H.; Redon, J.; Verdecchia, P.; Schmieder, R.; Jennings, G.; Yusoff, K.; Ryden, L.; Liu, G.L.; Teo, K.; et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). Circulation 2011, 124, 1727–1736. [Google Scholar] [CrossRef]
- Tatti, P.; Pahor, M.; Byington, R.P.; Di Mauro, P.; Guarisco, R.; Strollo, G.; Strollo, F. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care 1998, 21, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Jamerson, K.; Weber, M.A.; Bakris, G.L.; Dahlöf, B.; Pitt, B.; Shi, V.; Hester, A.; Gupte, J.; Gatlin, M.; Velazquez, E.J.; et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N. Engl. J. Med. 2008, 359, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, C.; Weidmann, P.; De Chatel, R.; Reubi, F. Hypertension associated with early stage kidney disease. Complementary roles of circulating renin, the body sodium/volume state and duration of hypertension. Am. J. Med. 1976, 61, 739–747. [Google Scholar] [CrossRef]
- Surma, S.; Więcek, A.; Adamczak, M. Diuretics—A review of the current state of knowledge. Renal Dis. Transplant. Forum 2023, 16, 81–92. [Google Scholar]
- Lièvre, M.; Gueyffier, F.; Ekbom, T.; Fagard, R.; Cutler, J.; Schron, E.; Marre, M.; Boissel, J.P. Efficacy of diuretics and beta-blockers in diabetic hypertensive patients. Results from a meta-analysis. The INDANA Steering Committee. Diabetes Care 2000, 23, B65–B71. [Google Scholar]
- Curb, J.D.; Pressel, S.L.; Cutler, J.A.; Savage, P.J.; Applegate, W.B.; Black, H.; Camel, G.; Davis, B.R.; Frost, P.H.; Gonzalez, N.; et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA 1996, 276, 1886–1892. [Google Scholar] [CrossRef]
- Kostis, J.B.; Wilson, A.C.; Freudenberger, R.S.; Cosgrove, N.M.; Pressel, S.L.; Davis, B.R.; SHEP Collaborative Research Group. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am. J. Cardiol. 2005, 95, 29–35. [Google Scholar] [CrossRef]
- Patel, A.; ADVANCE Collaborative Group; MacMahon, S.; Chalmers, J.; Neal, B.; Woodward, M.; Billot, L.; Harrap, S.; Poulter, N.; Marre, M.; et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 2007, 370, 829–840. [Google Scholar] [CrossRef]
- Scheen, A.J. Type 2 Diabetes and Thiazide Diuretics. Curr. Diab Rep. 2018, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Dussol, B.; Moussi-Frances, J.; Morange, S.; Somma-Delpero, C.; Mundler, O.; Berland, Y. A pilot study comparing furosemide and hydrochlorothiazide in patients with hypertension and stage 4 or 5 chronic kidney disease. J. Clin. Hypertens. 2012, 14, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.D.; Agarwal, R. Thiazides are useful agents in CKD. J. Am. Soc. Hypertens. 2016, 10, 288–289. [Google Scholar] [CrossRef]
- Lin, J.J.; Chang, H.C.; Ku, C.T.; Chen, H.Y. Hydrochlorothiazide hypertension treatment induced metabolic effects in type 2 diabetes: A meta-analysis of parallel-design RCTs. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2926–2946. [Google Scholar]
- Agarwal, R.; Sinha, A.D.; Cramer, A.E.; Balmes-Fenwick, M.; Dickinson, J.H.; Ouyang, F.; Tu, W. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2021, 385, 2507–2519. [Google Scholar] [CrossRef]
- Teles, F.; Peçanha de Miranda Coelho, J.A.; Albino, R.M.; Verçosa Pacheco, F.C.; Rodrigues de Oliveira, E.; Silveira, M.A.D.; Diógenes, M.; Feitosa, A.; Bezerra, R. Effectiveness of thiazide and thiazide-like diuretics in advanced chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2023, 45, 2163903. [Google Scholar] [CrossRef] [PubMed]
- Brater, D.C. Diuretic therapy. N. Engl. J. Med. 1998, 339, 387–395. [Google Scholar] [CrossRef]
- Ravioli, S.; Bahmad, S.; Funk, G.C.; Schwarz, C.; Exadaktylos, A.; Lindner, G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: Results of a cross-sectional study. Am. J. Med. 2021, 134, 1148–1154. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S.; Więcek, A. Hyponatremia in patients with arterial hypertension: Pathophysiology and management. Arch. Med. Sci. 2023, 19, 1630–1645. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Altered Prostaglandin Signaling as a Cause of Thiazide-Induced Hyponatremia. Am. J. Kidney Dis. 2018, 71, 769–771. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Rossing, K.; Schjoedt, K.J.; Smidt, U.M.; Boomsma, F.; Parving, H.H. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care 2005, 28, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; MacDonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Hypertension in chronic kidney disease-treatment standard 2023. Nephrol. Dial. Transplant. 2023, 38, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Ruospo, M.; Natale, P.; Bolignano, D.; Navaneethan, S.D.; Palmer, S.C.; Strippoli, G.F. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD007004. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Nowack, C.; Kolkhof, P.; Ferreira, A.C.; Schloemer, P.; Filippatos, G.; et al. Design and Baseline Characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease Trial. Am. J. Nephrol. 2019, 50, 333–344. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Lehnhardt, A.; Kemper, M.J. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr. Nephrol. 2011, 26, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, J.G. Treatment of Severe Hyperkalemia: Confronting 4 Fallacies. Kidney Int. Rep. 2017, 3, 47–55. [Google Scholar] [CrossRef]
- Adamczak, M.; Masajtis-Zagajewska, A.; Mazanowska, O.; Madziarska, K.; Stompór, T.; Więcek, A. Diagnosis and Treatment of Metabolic Acidosis in Patients with Chronic Kidney Disease—Position Statement of the Working Group of the Polish Society of Nephrology. Kidney Blood Press. Res. 2018, 43, 959–969. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Pitt, B.; Weir, M.R.; Freeman, M.W.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Zawadzki, R.; Berman, L.; Bushinsky, D.A. AMETHYST-DN Investigators. Effect of Patiromer on Serum Potassium Level in Patients with Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 2015, 314, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.D.; Spinowitz, B.S.; Lerma, E.V.; Singh, B.; Packham, D.K.; Al-Shurbaji, A.; Kosiborod, M. Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: An 11-month open-label extension of HARMONIZE. Am. J. Nephrol. 2019, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, G.; Hasegawa, K.; Kuji, T.; Ogawa, N.; Shimura, G.; Umemura, S.; Tochikubo, O. Effects of doxazosin on ambulatory blood pressure and sympathetic nervous activity in hypertensive Type 2 diabetic patients with overt nephropathy. Diabet. Med. 2005, 22, 1394–1400. [Google Scholar] [CrossRef]
- Mori, Y.; Matsubara, H.; Nose, A.; Shibasaki, Y.; Masaki, H.; Kosaki, A.; Okigaki, M.; Fujiyama, S.; Tanaka-Uchiyama, Y.; Hasegawa, T.; et al. Safety and availability of doxazosin in treating hypertensive patients with chronic renal failure. Hypertens. Res. 2001, 24, 359–563. [Google Scholar] [CrossRef]
- Yildiz, A.; Hursit, M.; Celik, A.V.; Kayacan, S.M.; Yazici, H.; Akkaya, V.; Gürol, A.O.; Karsidag, K. Doxazosin, but not amlodipine decreases insulin resistance in patients with chronic renal failure: A prospective, randomized-controlled study. Clin. Nephrol. 2002, 58, 405–410. [Google Scholar] [CrossRef]
- Damianaki, A.; Polychronopoulou, E.; Wuerzner, G.; Burnier, M. New Aspects in the Management of Hypertension in Patients with Chronic Kidney Disease not on Renal Replacement Therapy. High. Blood Press. Cardiovasc. Prev. 2022, 29, 125–135. [Google Scholar] [CrossRef]
- Ringholm, L.; Damm, J.A.; Vestgaard, M.; Damm, P.; Mathiesen, E.R. Diabetic Nephropathy in Women with Preexisting Diabetes: From Pregnancy Planning to Breastfeeding. Curr. Diab Rep. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.M.; Drager, L.F.; Giorgi, D.M.A.; Pereira, A.C.; Barreto-Filho, J.A.S.; Nogueira, A.R.; Mill, J.G.; Lotufo, P.A.; Amodeo, C.; Batista, M.C.; et al. Spironolactone Versus Clonidine as a Fourth-Drug Therapy for Resistant Hypertension: The ReHOT Randomized Study (Resistant Hypertension Optimal Treatment). Hypertension 2018, 71, 681–690. [Google Scholar] [CrossRef]
- Lambert, A.E.; Mpoy, M.; Vandeleene, B.; Ketelslegers, J.M. Treatment of hypertension in diabetic patients. Am. J. Med. 1989, 87, 30S–33S. [Google Scholar] [CrossRef]
- Paolillo, S.; Dell’Aversana, S.; Esposito, I.; Poccia, A.; Perrone Filardi, P. The use of β-blockers in patients with heart failure and comorbidities: Doubts, certainties and unsolved issues. Eur. J. Intern. Med. 2021, 88, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Acampora, R.; Marfella, R.; De Rosa, N.; Ziccardi, P.; Ragone, R.; De Angelis, L.; D’Onofrio, F. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann. Intern. Med. 1997, 126, 955–999. [Google Scholar] [CrossRef]
- Rashid, A.M.; Khan, M.S.; Fudim, M.; DeWald, T.A.; DeVore, A.; Butler, J. Management of heart failure with reduced ejection fraction. Curr. Probl. Cardiol. 2023, 48, 101596. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Fonseca, V.; Katholi, R.E.; McGill, J.B.; Messerli, F.H.; Phillips, R.A.; Raskin, P.; Wright, J.T., Jr.; Oakes, R.; Lukas, M.A.; et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: A randomized controlled trial. JAMA 2004, 292, 2227–2236. [Google Scholar] [CrossRef]
- Vasavada, N.; Saha, C.; Agarwal, R. A double-blind randomized crossover trial of two loop diuretics in chronic kidney disease. Kidney Int. 2003, 64, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Parving, H.H.; Lehnert, H.; Bröchner-Mortensen, J.; Gomis, R.; Andersen, S.; Arner, P.; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 2001, 345, 870–878. [Google Scholar] [CrossRef]
- Makino, H.; Haneda, M.; Babazono, T.; Moriya, T.; Ito, S.; Iwamoto, Y.; Kawamori, R.; Takeuchi, M.; Katayama, S.; INNOVATION Study Group. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007, 30, 1577–1578. [Google Scholar] [CrossRef]
- Dunkler, D.; Gao, P.; Lee, S.F.; Heinze, G.; Clase, C.M.; Tobe, S.; Teo, K.K.; Gerstein, H.; Mann, J.F.; Oberbauer, R.; et al. Risk Prediction for Early CKD in Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2015, 10, 1371–1379. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Remuzzi, G.; Parving, H.H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E.; Mitch, W.E.; Brenner, B.M. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004, 65, 2309–2320. [Google Scholar] [CrossRef]
- Persson, F.; Lindhardt, M.; Rossing, P.; Parving, H.H. Prevention of microalbuminuria using early intervention with renin-angiotensin system inhibitors in patients with type 2 diabetes: A systematic review. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316652047. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.; Craig, M.; Schena, F.P.; Craig, J.C. Antihypertensive agents for primary prevention of diabetic nephropathy. J. Am. Soc. Nephrol. 2005, 16, 3081–3091. [Google Scholar] [CrossRef]
- Wolf, G.; Butzmann, U.; Wenzel, U. The renin-angiotensin system and progression of renal disease: From hemodynamics to cell biology. Nephron Physiol. 2003, 93, p3–p13. [Google Scholar] [CrossRef]
- Tylicki, L.; Biedunkiewicz, B.; Chamienia, A.; Wojnarowski, K.; Zdrojewski, Z.; Rutkowski, B. Randomized placebo-controlled study on the effects of losartan and carvedilol on albuminuria in renal transplant recipients. Transplantation 2006, 81, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Rutkowski, B. Low-dose angiotensin II receptor antagonists and angiotensin II-converting enzyme inhibitors alone or in combination for treatment of primary glomerulonephritis. Scand. J. Urol. Nephrol. 2004, 38, 427–433. [Google Scholar] [CrossRef]
- Coresh, J.; Heerspink, H.J.L.; Sang, Y.; Matsushita, K.; Arnlov, J.; Astor, B.C.; Black, C.; Brunskill, N.J.; Carrero, J.J.; Feldman, H.I.; et al. Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019, 7, 115–127. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Greene, T.; Tighiouart, H.; Gansevoort, R.T.; Coresh, J.; Simon, A.L.; Chan, T.M.; Hou, F.F.; Lewis, J.B.; Locatelli, F.; et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019, 7, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, L.; Lizakowski, S.; Rutkowski, P.; Renke, M.; Sulikowska, B.; Heleniak, Z.; Donderski, R.; Bednarski, R.; Przybylska, M.; Manitius, J.; et al. The enhanced renin-angiotensin-aldosteron system pharmacological blockade-which is the best? Kidney Blood Press. Res. 2012, 36, 335–343. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Kamath, N.S.; El Kossi, M.; El Nahas, A.M. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 3977–3982. [Google Scholar] [CrossRef]
- Fu, E.L.; Evans, M.; Clase, C.M.; Tomlinson, L.A.; van Diepen, M.; Dekker, F.W.; Carrero, J.J. Stopping Renin-Angiotensin System Inhibitors in Patients with Advanced CKD and Risk of Adverse Outcomes: A Nationwide Study. J. Am. Soc. Nephrol. 2021, 32, 424–435. [Google Scholar] [CrossRef]
- Nistor, I.; De Sutter, J.; Drechsler, C.; Goldsmith, D.; Soler, M.J.; Tomson, C.; Wiecek, A.; Donciu, M.D.; Bolignano, D.; Van Biesen, W.; et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2018, 33, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Ruggenenti, P.; Perna, A.; Dimitrov, B.D.; de Zeeuw, D.; Hille, D.A.; Shahinfar, S.; Carides, G.W.; Brenner, B.M.; RENAAL Study Group. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: A post hoc analysis of the RENAAL trial results. J. Am. Soc. Nephrol. 2004, 15, 3117–3125. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P.; STOP ACEi Trial Investigators. Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef]
- D’Elia, L.; Rossi, G.; Schiano di Cola, M.; Savino, I.; Galletti, F.; Strazzullo, P. Meta-Analysis of the Effect of Dietary Sodium Restriction with or without Concomitant Renin-Angiotensin-Aldosterone System-Inhibiting Treatment on Albuminuria. Clin. J. Am. Soc. Nephrol. 2015, 10, 1542–1552. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Holtkamp, F.A.; Parving, H.H.; Navis, G.J.; Lewis, J.B.; Ritz, E.; de Graeff, P.A.; de Zeeuw, D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012, 82, 330–337. [Google Scholar] [CrossRef]
- Bakris, G.L.; Weir, M.R. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch. Intern. Med. 2000, 160, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, F.A.; de Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; de Graeff, P.A.; Hillege, H.J.; Parving, H.H.; Brenner, B.M.; Shahinfar, S.; Lambers Heerspink, H.J. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Barzilay, J.; Gao, P.; Smyth, A.; Schmieder, R.E.; Tobe, S.; Teo, K.K.; Yusuf, S.; Mann, J.F. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017, 91, 683–690. [Google Scholar] [CrossRef]
- Bandak, G.; Sang, Y.; Gasparini, A.; Chang, A.R.; Ballew, S.H.; Evans, M.; Arnlov, J.; Lund, L.H.; Inker, L.A.; Coresh, J.; et al. Hyperkalemia After Initiating Renin-Angiotensin System Blockade: The Stockholm Creatinine Measurements (SCREAM) Project. J. Am. Heart Assoc. 2017, 6, e005428. [Google Scholar] [CrossRef]
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, B.; Tylicki, L. Nephroprotective action of renin-angiotensin-aldosterone system blockade in chronic kidney disease patients: The landscape after ALTITUDE and VA NEPHRON-D trails. J. Ren. Nutr. 2015, 25, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef]
- Lizakowski, S.; Tylicki, L.; Renke, M.; Rutkowski, P.; Heleniak, Z.; Sławińska-Morawska, M.; Aleksandrowicz, E.; Łysiak-Szydłowska, W.; Rutkowski, B. Effect of aliskiren on proteinuria in non-diabetic chronic kidney disease: A double-blind, crossover, randomised, controlled trial. Int. Urol. Nephrol. 2012, 44, 1763–1770. [Google Scholar] [CrossRef][Green Version]
- Parving, H.H.; Brenner, B.M.; McMurray, J.J.; de Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; Nicolaides, M.; et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef]
- Epstein, M.; Kovesdy, C.P.; Clase, C.M.; Sood, M.M.; Pecoits-Filho, R. Aldosterone, Mineralocorticoid Receptor Activation, and CKD: A Review of Evolving Treatment Paradigms. Am. J. Kidney Dis. 2022, 80, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, U.F.; Adams-Huet, B.; Raskin, P.; Vega, G.L.; Toto, R.D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2641–2650. [Google Scholar] [CrossRef]
- Currie, G.; Taylor, A.H.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.J.; Townend, J.N.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef]
- Epstein, M.; Williams, G.H.; Weinberger, M.; Lewin, A.; Krause, S.; Mukherjee, R.; Patni, R.; Beckerman, B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2006, 1, 940–951. [Google Scholar] [CrossRef]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’Marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L.; Heerspink, H.J.L.; ROTATE-3 study group; et al. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Chan, J.C.N.; Kooy, A.; McCafferty, K.; Schernthaner, G.; et al. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes With or Without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int. Rep. 2021, 7, 36–45. [Google Scholar] [CrossRef]
- Neuen, B.L.; Heerspink, H.J.L.; Vart, P.; Claggett, B.L.; Fletcher, R.A.; Arnott, C.; de Oliveira Costa, J.; Falster, M.O.; Pearson, S.-A.; Mahaffey, K.W.; et al. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment with SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared with Conventional Care in Patients with Type 2 Diabetes and Albuminuria. Circulation 2024, 149, 450–462. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis. 2021, 7, 452–467. [Google Scholar] [CrossRef]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Skiba, K.; Gojowy, D.; Szotowska, M.; Bartmańska, M.; Kolonko, A.; Cierpka, L.; Więcek, A.; Adamczak, M. Metabolic acidosis in kidney transplant recipients. Pol. Arch. Intern. Med. 2018, 128, 587–593. [Google Scholar] [CrossRef]
- Kuczera, P.; Ciaston-Mogilska, D.; Oslizlo, B.; Hycki, A.; Wiecek, A.; Adamczak, M. The Prevalence of Metabolic Acidosis in Patients with Different Stages of Chronic Kidney Disease: Single-Centre Study. Kidney Blood Press. Res. 2020, 45, 863–872. [Google Scholar] [CrossRef]
- Tangri, N.; Mathur, V.; Reaven, N.; Funk, S.E.; Wesson, D.E. Relationship between Metabolic Acidosis and Chronic Kidney Disease Progression across Racial and Ethnic Groups: An Observational, Retrospective Cohort Study. Am. J. Nephrol. 2022, 53, 603–613. [Google Scholar] [CrossRef]
- Dobre, M.; Yang, W.; Chen, J.; Drawz, P.; Hamm, L.L.; Horwitz, E.; Hostetter, T.; Jaar, B.; Lora, C.M.; Nessel, L.; et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 2013, 62, 670–678. [Google Scholar] [CrossRef]
- Raphael, K.L.; Zhang, Y.; Wei, G.; Greene, T.; Cheung, A.K.; Beddhu, S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol. Dial. Transplant. 2013, 28, 1207–1213. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Bellasi, A.; Raphael, K.L.; Santoro, D.; Aucella, F.; Garofano, L.; Ceccarelli, M.; Di Lullo, L.; Capolongo, G.; Di Iorio, M.; et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI Study. J. Nephrol. 2019, 32, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Hultin, S.; Hood, C.; Campbell, K.L.; Toussaint, N.D.; Johnson, D.W.; Badve, S.V. A Systematic Review and Meta-Analysis on Effects of Bicarbonate Therapy on Kidney Outcomes. Kidney Int. Rep. 2020, 6, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Merriam, R.L.; Morris, R.C., Jr. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am. J. Clin. Nutr. 2002, 76, 1308–1316. [Google Scholar] [CrossRef]
- Scialla, J.J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef]
- Bellos, I.; Lagiou, P.; Benetou, V.; Marinaki, S. Safety and Efficacy of Sodium-Glucose Transport Protein 2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Diabetic Kidney Transplant Recipients: Synthesis of Evidence. J. Clin. Med. 2024, 13, 6181. [Google Scholar] [CrossRef]
- Clemens, K.K.; Ernst, J.; Khan, T.; Reichert, S.; Khan, Q.; LaPier, H.; Chiu, M.; Stranges, S.; Sahi, G.; Castrillon-Ramirez, F.; et al. Glucagon-like peptide 1 receptor agonists in end-staged kidney disease and kidney transplantation: A narrative review. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1111–1120. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E.; Caliskan, Y.; Lentine, K.L. Mineralocorticoid receptor blockage in kidney transplantation: Too much of a good thing or not? Int. Urol. Nephrol. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Toan, A.T.N.; Phung, T.L.; Dang, T.T.; Alcusky, M.J.; Amante, D.J.; Nguyen, H.L.; Goldberg, R.J. Cost-effectiveness of sodium-glucose cotransporter 2 inhibitors in the treatment of chronic kidney disease: A systematic review. Expert. Rev. Pharmacoecon. Outcomes Res. 2024, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maruyama-Sakurai, K.; Tachimori, H.; Saito, E.; Kohsaka, S.; Segawa, Y.; Miyata, H.; Igarashi, A. Cost-effectiveness of sodium-glucose cotransporter-2 inhibitors in the treatment of diabetic nephropathy in Japan. Diabetes Obes. Metab. 2024, 26, 5546–5555. [Google Scholar] [CrossRef] [PubMed]
- Kongmalai, T.; Prawjaeng, J.; Hadnorntun, P.; Leelahavarong, P.; Chaikledkaew, U.; Thakkinstian, A.; Srinonprasert, V. Cost-Utility and Budget Impact Analysis of Adding SGLT-2 Inhibitors to Standard Treatment in Type 2 Diabetes Patients with Heart Failure: Utilizing National Database Insights from Thailand. Pharmacoecon. Open 2024. [Google Scholar] [CrossRef] [PubMed]
- McEwan, P.; Davis, J.A.; Gabb, P.D.; Wheeler, D.C.; Rossing, P.; Chertow, G.M.; Correa-Rotter, R.; Tamura, K.; Barone, S.; Sanchez, J.J.G. Dapagliflozin in chronic kidney disease: Cost-effectiveness beyond the DAPA-CKD trial. Clin. Kidney J. 2024, 17, sfae025. [Google Scholar] [CrossRef]
- Li, X.; Hoogenveen, R.; El Alili, M.; Knies, S.; Wang, J.; Beulens, J.W.J.; Elders, P.J.M.; Nijpels, G.; Barone, S.; Garcia Sanchez, J.J.; et al. Cost-Effectiveness of SGLT2 Inhibitors in a Real-World Population: A MICADO Model-Based Analysis Using Routine Data from a GP Registry. Pharmacoeconomics 2023, 41, 1249–1262. [Google Scholar] [CrossRef]
- McEwan, P.; Foos, V.; Martin, B.; Chen, J.; Evans, M. Estimating the value of sodium-glucose cotransporter-2 inhibitors within the context of contemporary guidelines and the totality of evidence. Diabetes Obes. Metab. 2023, 25, 1830–1838. [Google Scholar] [CrossRef]
- Choi, J.G.; Winn, A.N.; Skandari, M.R.; Franco, M.I.; Staab, E.M.; Alexander, J.; Wan, W.; Zhu, M.; Huang, E.S.; Philipson, L.; et al. First-Line Therapy for Type 2 Diabetes with Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Cost-Effectiveness Study. Ann. Intern. Med. 2022, 175, 1392–1400. [Google Scholar] [CrossRef]
- Yang, C.T.; Yao, W.Y.; Ou, H.T.; Kuo, S. Value of GLP-1 receptor agonists versus long-acting insulins for type 2 diabetes patients with and without established cardiovascular or chronic kidney diseases: A model-based cost-effectiveness analysis using real-world data. Diabetes Res. Clin Pract. 2023, 198, 110625. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, Y.R.; Ou, H.T.; Kuo, S. Cost-effectiveness of GLP-1 receptor agonists versus insulin for the treatment of type 2 diabetes: A real-world study and systematic review. Cardiovasc. Diabetol. 2021, 20, 21. [Google Scholar] [CrossRef]
- Franchi, M.; Pellegrini, G.; Avogaro, A.; Buzzetti, G.; Candido, R.; Cavaliere, A.; Consoli, A.; Marzona, I. Comparing the effectiveness and cost-effectiveness of sulfonylureas and newer diabetes drugs as second-line therapy for patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2024, 12, e003991. [Google Scholar] [CrossRef] [PubMed]
- Laursen, H.V.B.; Jørgensen, E.P.; Vestergaard, P.; Ehlers, L.H. A Systematic Review of Cost-Effectiveness Studies of Newer Non-Insulin Antidiabetic Drugs: Trends in Decision-Analytical Models for Modelling of Type 2 Diabetes Mellitus. Pharmacoeconomics 2023, 41, 1469–1514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, Y.; Li, Q.; Wang, G. Cost-Effectiveness of Newer Antidiabetic Drugs as Second-Line Treatment for Type 2 Diabetes: A Systematic Review. Adv. Ther. 2023, 40, 4216–4235. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Hong, G.; Xu, Y.; Mernagh, P.; Pochopień, M.; Li, H. Cost-effectiveness of Finerenone in Addition to Standard of Care for Patients with Chronic Kidney Disease and Type 2 Diabetes in China. Adv. Ther. 2024, 41, 3138–3158. [Google Scholar] [CrossRef]
- Quist, S.W.; van Schoonhoven, A.V.; Bakker, S.J.L.; Pochopień, M.; Postma, M.J.; van Loon, J.M.T.; Paulissen, J.H.J. Cost-effectiveness of finerenone in chronic kidney disease associated with type 2 diabetes in The Netherlands. Cardiovasc. Diabetol. 2023, 22, 328. [Google Scholar] [CrossRef]

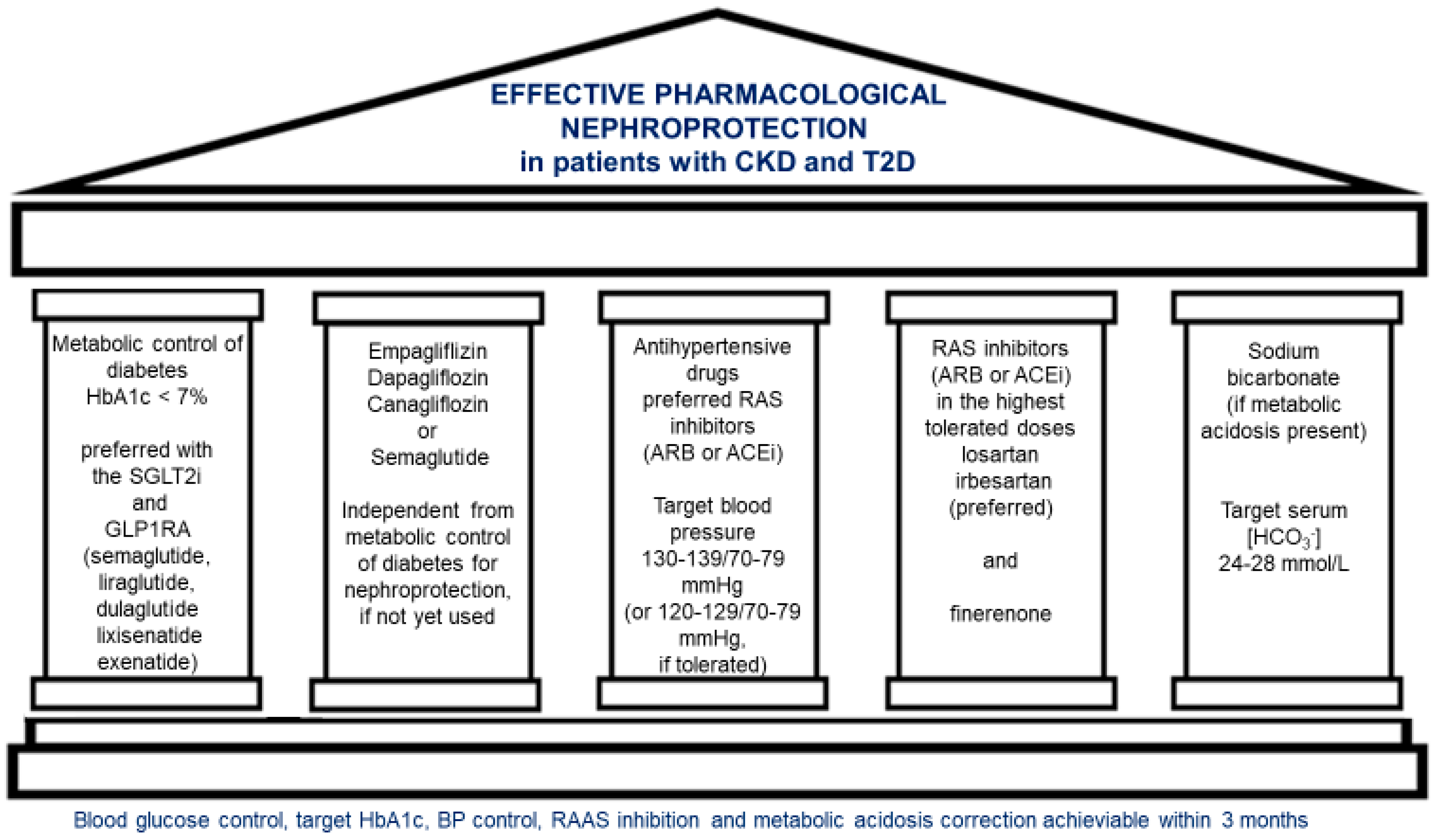
| Trial Acronym | HbA1c Range at Baseline (Inclusion Criterion) | Antihyperglycemic Treatment | |||
|---|---|---|---|---|---|
| Active Arm | Placebo Arm | ||||
| CANVAS | 7–10.5% | Insulin: 49.9% Metformin: 76.7% SU: 43.6% DPP4i: 12.0% GLP1RA: 3.8% | Insulin: 50.7% Metformin: 77.7% SU: 42.2% DPP4i: 13.0% GLP1RA: 4.3% | ||
| EMPAREG-Outcome | 7.0–9.0% | Insulin: 11.5% Metformin: 4.8% SU: 7.0% DPP4i: 8.3% GLP1RA: 2.4% TZD: 2.9% | Insulin: 5.8% Metformin: 3.7% SU: 3.8% DPP4i: 5.6% GLP1RA: 1.4% TZD: 1.2% | ||
| DECLARE-TIMI 58 | 6.5–12.0% | Insulin: 41.6% Metformin: 81.8% SU: 42.1% DPP4i: 16.5% GLP1RA: 4.6% | Insulin: 40.2% Metformin: 82.2% SU: 43.2% DPP4i: 17.1% GLP1RA: 4.1% | ||
| CREDENCE | 6.5–12.0% (6.5–10.5% in Germany) | Insulin: 66.0% Metformin: 58.0% SU: 27.8% DPP4i: 17.2% GLP1RA: 4.0% TZD: 3.2% Alpha-glucosidase inhibitors: 3.0% | Insulin: 65.1% Metformin: 57.7% SU: 29.9% DPP4i: 17.0% GLP1RA: 4.3% TZD: 3.0% Alpha-glucosidase inhibitors: 3.3% | ||
| LEADER | ≥7.0% | Insulin: 43.7% Metformin: 75.8% SU: 50.8% DPP4i: <0.1% GLP1RA: 0 TZD: 6.3% Alpha-glucosidase inhibitors: 3.0% Glinides: 3.8 | Insulin: 45.6% Metformin: 77.1% SU: 50.6% DPP4i: <0.1% GLP1RA: 0 TZD: 6.0% Alpha-glucosidase inhibitors: 2.6% Glinides: 3.7 | ||
| REWIND | ≤9.5% | Insulin: 24.0% Metformin: 81.3% SU: 45.9% DPP4i: 5.4% TZD: 2.0% Other: 0.3% | Insulin: 23.7% Metformin: 81.1% SU: 46.1% DPP4i: 6.0% TZD: 1.4% Other: 0.4% | ||
| PIONEER-6 | No HbA1c criterion | Insulin: 60.8% Metformin: 76.7% SU: 32.5% DPP4i: 0.1% GLP1RA: 0.1% TZD: 4.1% Alpha-glucosidase inhibitors: 2.3% SGLT2i: 10.4% | Insulin: 60.4% Metformin: 78.0% SU: 32.0% DPP4i: 0 GLP1RA: 0 TZD: 3.3% Alpha-glucosidase inhibitors: 2.7% SGLT2i: 8.8% | ||
| SUSTAIN-6 | ≥7.0% | Semaglutide 0.5 mg | Semaglutide 1.0 mg | Placebo 0.5 mg | Placebo 1.0 |
| Insulin: 58.0% Metformin: 74.7% SU: 42.3% DPP4i: 0.1% GLP1RA: 0.1% TZD: 1.7% Glinides: 3.0 SGLT2i: 0 Alpha-glucosidase inhibitors: 1.1% | Insulin: 58.0% Metformin: 72.3% SU: 42.5% DPP4i: 0.2% TZD: 2.6% Glinides: 2.8% SGLT2i: 0.1% Alpha-glucosidase inhibitors: 0.9 | Insulin: 58.1% Metformin: 71.1% SU: 44.1% DPP4i: 0.2% TZD: 2.2% Glinides: 2.9% Alpha-glucosidase inhibitors: 1.9% SGLT2i: 0.2% | Insulin: 58.0% Metformin: 74.8% SU: 42.3% DPP4i: 0 TZD: 2.8% Alpha-glucosidase inhibitors: 2.8% SGLT2i: 0.2% | ||
| Renal Impairment-CKD Stage and eGFR (mL/min/1.73 m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
G1–2 | G3a | G3b | G4 | G5 | |||||
Drug | Class of drug | eGFR > 60 | eGFR 45–59 | eGFR 30–44 | eGFR 15–30 | eGFR < 15 | |||
| Metformin | Biguanide | Reduce dose to 2 × 500 mg | |||||||
| Gliquidone | Sulfonylurea | ||||||||
| Glimepiride | Sulfonylurea | Dose adjusted to glycemia, monitor CBG | |||||||
| Glipizide | Sulfonylurea | Dose adjuste to glycemia, monitor CBG | |||||||
| Gliclazide | Sulfonylurea | Dose adjusted to glycemia, monitor CBG | |||||||
| Repaglinide | Meglitinide | Dose reduction advised, monitor CBG | |||||||
| Sitagliptin | DPP4i | Reduce dose to 50 mg | Reduce dose to 25 mg | ||||||
| Saxagliptin | DPP4i | Reduce dose to 2.5 mg, avoid treatment in dialyzed patients | |||||||
| Vildagliptin | DPP4i | Reduce dose to 50 mg if eGFR < 50 | |||||||
| Linagliptin | DDP4i | ||||||||
| Pioglitazone | Thiazolidinediones | Avoid treatment in dialyzed patients | |||||||
| Dulaglutide | GLP1RA | ||||||||
| Exenatide (twice daily) | GLP1RA | Gradually increase dose if eGFR 30–50 | |||||||
| Exenatide (once daily) | GLP1RA | ||||||||
| Liraglutide | GLP1RA | ||||||||
| Lixisenatide | GLP1RA | ||||||||
| Semaglutide (oral/injectable) | GLP1RA | ||||||||
| Tirzepatide | GLP-1 and GIP agonist | Caution advised | |||||||
| Dapagliflozin | SGLT2i | If eGFR < 25 do not start, continuation only, discontinuation in dialyzed patients | |||||||
| Empagliflozin | SGLT2i | If eGFR < 20 do not start, continuation only, discontinuation in dialyzed patients | |||||||
| Canagliflozin | SGLT2i | Initiate at 100 mg and gradually increase to 300 mg if needed | Initiate at 100 mg or continuation | Do not start, continuation 100 mg, discontinuation in dialyzed patients | |||||
| Acarbose | Alpha-glucosidase inhibitor | ||||||||
 no need to adjust the dose to eGFR.
no need to adjust the dose to eGFR.  recommended dose adjustment to eGFR.
recommended dose adjustment to eGFR.  not recommended the use of the drug. Abbreviations: CBG, capillary blood glucose; CKD, chronic kidney disease; eGFR, an estimate of glomerular filtration rate, usually calculated using Cockcroft–Gault equation; DPP4i, dipeptidyl peptidase-4 inhibitors; GLP1RA = glucagon-like peptide 1 ago-nists; GIP, glucose-dependent insulinotropic polypeptide; SGLT2i, sodium–glucose co-transporter-2 inhibitor.
not recommended the use of the drug. Abbreviations: CBG, capillary blood glucose; CKD, chronic kidney disease; eGFR, an estimate of glomerular filtration rate, usually calculated using Cockcroft–Gault equation; DPP4i, dipeptidyl peptidase-4 inhibitors; GLP1RA = glucagon-like peptide 1 ago-nists; GIP, glucose-dependent insulinotropic polypeptide; SGLT2i, sodium–glucose co-transporter-2 inhibitor.| Pillar/ Level of Recommendation | Recommendations | Key Drugs |
|---|---|---|
| 1. Effective Antihyperglycemic Treatment (1A) | Maintain HbA1c <7% to prevent CKD progression. Adjust targets (7–8%) in patients at high hypoglycemia risk. Caution glucose monitoring in advanced CKD. Antihyperglycemic drugs needed to be chosen based on eGFR with dose adjustment and treatment cessation according to eGFR. Use SGLT2 inhibitors. For advanced CKD, consider GLP-1 receptor agonists. | - SGLT2i: empagliflozin, dapagliflozin, canagliflozin - GLP1RA: semaglutide, dulaglutide, liraglutide |
| 2. Sodium–Glucose Co-Transporter 2 Inhibitors (SGLT2i) and Semaglutide (1A) | Initiate SGLT2i or semaglutide for T2D and CKD, except when eGFR is below drug-specific thresholds. Continue even in advanced CKD stages if tolerated, until dialysis or transplant. | - Empagliflozin - Dapagliflozin - Canagliflozin - Semaglutide |
| 3. Blood Pressure Control (1B) | Tailor antihypertensive treatment to reach individualized blood pressure targets: 130–139/70–79 mmHg or 120–129/70–79 mmHg if tolerated. Use RASi (ARB/ACEi) in the first line at the maximally tolerated doses. | - ARBs: e.g., losartan, irbesartan |
| 4. Renin–Angiotensin System Inhibition (RASi) (1A) | Use angiotensin II receptor blockers (ARBs) or, in the case of ARBs, intolerance ACE inhibitors (ACEi) as first-line nephroprotective agents. Combine with SGLT2i or GLP1RA when possible. Add finerenone in cases of persistent albuminuria (>30 mg/day) despite RAS blockade and SGLT2i use if hyperkalemia risk is low. | - ARBs: e.g., losartan, irbesartan - Finerenone |
| 5. Management of Metabolic Acidosis (2B) | Treat with sodium bicarbonate to maintain serum bicarbonate levels within the range 24–28 mmol/L. | - Sodium bicarbonate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczak, M.; Kurnatowska, I.; Naumnik, B.; Stompór, T.; Tylicki, L.; Krajewska, M. Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus—Clinical Practice Position Statement of the Polish Society of Nephrology. Int. J. Mol. Sci. 2024, 25, 12941. https://doi.org/10.3390/ijms252312941
Adamczak M, Kurnatowska I, Naumnik B, Stompór T, Tylicki L, Krajewska M. Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus—Clinical Practice Position Statement of the Polish Society of Nephrology. International Journal of Molecular Sciences. 2024; 25(23):12941. https://doi.org/10.3390/ijms252312941
Chicago/Turabian StyleAdamczak, Marcin, Ilona Kurnatowska, Beata Naumnik, Tomasz Stompór, Leszek Tylicki, and Magdalena Krajewska. 2024. "Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus—Clinical Practice Position Statement of the Polish Society of Nephrology" International Journal of Molecular Sciences 25, no. 23: 12941. https://doi.org/10.3390/ijms252312941
APA StyleAdamczak, M., Kurnatowska, I., Naumnik, B., Stompór, T., Tylicki, L., & Krajewska, M. (2024). Pharmacological Nephroprotection in Chronic Kidney Disease Patients with Type 2 Diabetes Mellitus—Clinical Practice Position Statement of the Polish Society of Nephrology. International Journal of Molecular Sciences, 25(23), 12941. https://doi.org/10.3390/ijms252312941







