Genes, Gene Loci, and Their Impacts on the Immune System in the Development of Multiple Sclerosis: A Systematic Review
Abstract
1. Introduction
1.1. Background
1.2. Knowledge Gap
1.3. Aims and Objectives
2. Materials and Methods
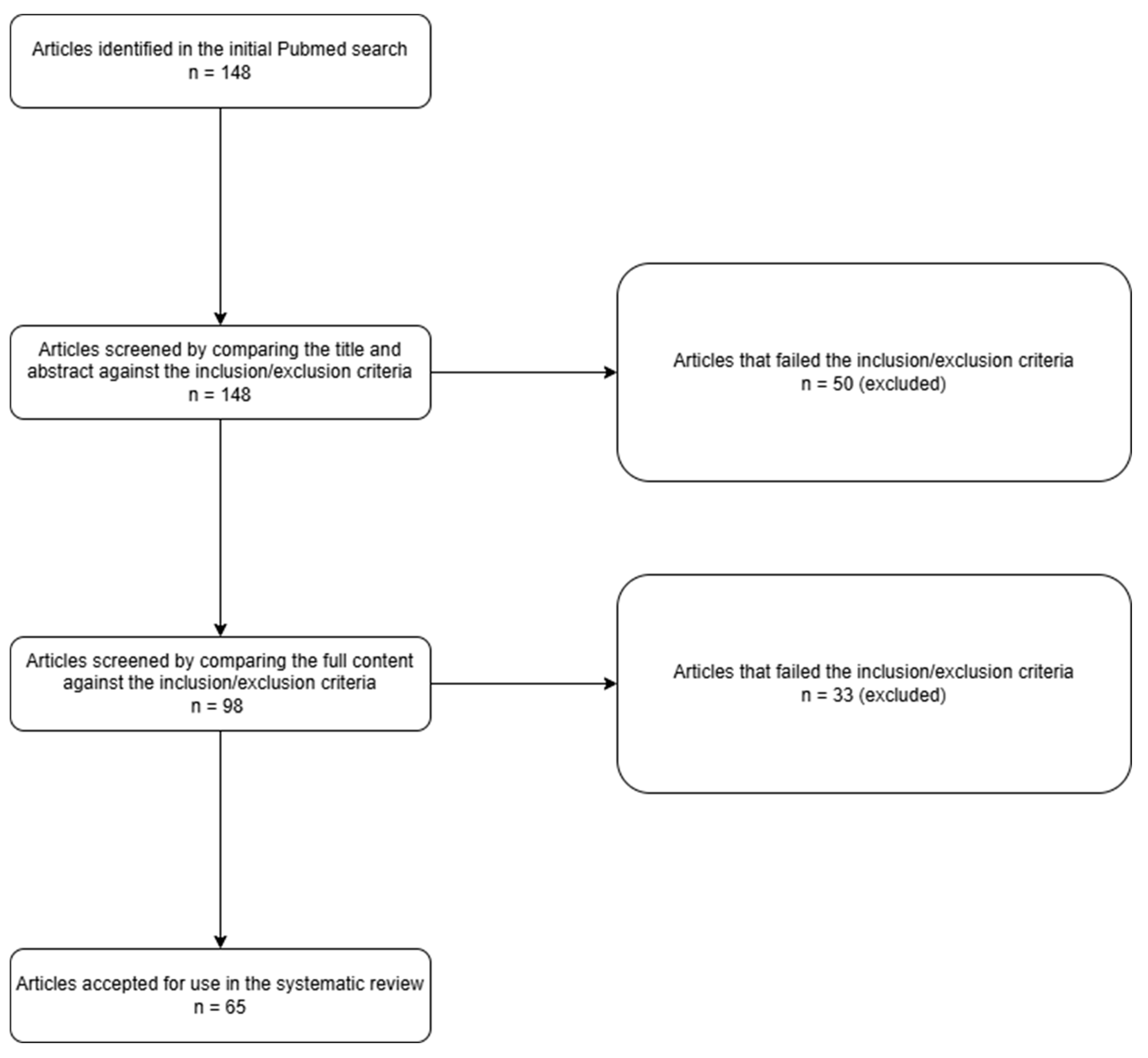
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Data Synthesis
2.5. Quality Assessment
3. Results
3.1. Overview of Selected Studies
3.2. Key Findings
| Main Author | Paper Type | Article Title | Reason for Relevance | Gene Loci Information |
|---|---|---|---|---|
| International Multiple Sclerosis Genetics Consortium (2019) [8] | Research Study | Multiple Sclerosis Genomic Map Implicates Peripheral Immune Cells and Microglia in Susceptibility [8] | The article provides key insights into the involvement of genetic factors and immune cells in MS susceptibility. | rs10191329 (DYSF-ZNF638 locus) is associated with MS susceptibility. |
| International Multiple Sclerosis Genetics Consortium (2023) [9] | Research Study | Locus for Severity Implicates CNS Resilience in Progression of Multiple Sclerosis [9] | This article discusses the genetic loci related to MS severity and CNS resilience, which are vital in understanding the progression of MS. | - |
| Chan V (2020) [10] | Narrative Review | Epigenetics in Multiple Sclerosis [10] | This reviews the role of epigenetic modifications in MS. | rs123456 influences DNA methylation patterns. |
| Li X (2017) [11] | Review | DNA Methylation: A New Player in Multiple Sclerosis [11] | This highlights the role of DNA methylation in MS diagnoses and treatment. | - |
| Baranzini S (2017) [12] | Review | The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years [12] | This provides a comprehensive overview of genetic research in MS and how it has evolved. | - |
| Kucukali C (2015) [13] | Review | Epigenetics of Multiple Sclerosis: An Updated Review [13] | This provides an updated review of epigenetic research and its latest developments. | - |
| Maglione A (2021) [14] | Perspective Paper | Host Genetics and Gut Microbiome: Perspectives for Multiple Sclerosis [14] | This explores the interactions between genetics and the gut microbiome in MS to provide a wider perspective on the disease’s dynamics. | rs2853035 impacts the gut microbiome, which in turn affects MS progression. |
| Lu H (2021) [15] | Mendelian Randomization Study | Circulating Interleukins and Risk of Multiple Sclerosis: A Mendelian Randomization Study [15] | This investigates the role of interleukins in the development of MS, focusing on genetic methods. | - |
| Senent J (2023) [16] | Metanalysis | A Deep Transcriptome Meta-Analysis Reveals Sex Differences in Multiple Sclerosis [16] | This examines sex-based differences in an MS transcriptome analysis, which plays an important role in developing personalized treatments. | - |
| Bashinskava V (2015) [17] | Review | A Review of Genome-Wide Association Studies for Multiple Sclerosis: A Classical and Hypothesis-Driven Approach [17] | This provides a comprehensive review of GWAS in MS. | - |
| Gerdes L (2020) [18] | Research Study | Immune Signatures of Prodromal Multiple Sclerosis in Monozygotic Twins [18] | This investigates early immune system signatures in MS, providing crucial insight into the disease’s development. | - |
| Menascu S (2021) [19] | Research Study | Clinical and Transcriptional Recovery Profiles in Pediatric and Adult Multiple Sclerosis Patients [19] | This assesses the recovery profiles of MS patients to determine the effects of factors such as age and genetics. | - |
| Ostkamp P (2021) [20] | Observational Study | Sunlight Exposure Exerts Immunomodulatory Effects to Reduce Multiple Sclerosis Severity [20] | This discusses the effects of sunlight on the severity of MS, which may influence prevention and treatment strategies. This information also helps shed light on the environmental dynamics of the disease. | - |
| Faber H (2020) [21] | Research Study | Gene Expression in Spontaneous Experimental Autoimmune Encephalomyelitis is Linked to Human Multiple Sclerosis Risk Genes [21] | This establishes a connection between experimental models and risk genes associated with MS. | - |
| Stojkovic L (2024) [22] | Research Study | Targeted RNAseq Revealed the Gene Expression Signature of Ferroptosis-related Processes Associated with Disease Severity in Patients with Multiple Sclerosis [22] | This identifies gene expression patterns related to ferroptosis and disease severity in MS patients. | - |
| Key Finding | Additional Details | Gene Loci Information |
|---|---|---|
| Major genetic risk factors involved in multiple sclerosis | According to the latest GWAS, MS susceptibility genes exist in all major immune cell types, as shown in Figure 1 [23]. Genes and gene loci, such as rs10191329 in the DYSF-ZNF638 locus, increase susceptibility to MS among young adults [8]. | The gene loci increase susceptibility to MS, especially among young adults. |
| Minor genetic risk factors involved in multiple sclerosis | Rare and low-frequency genetic variants, such as mutations in KIF5A and REEP1 genes, are significant MS risk factors [24,25]. | - |
| Epigenetic modifications and their impact on multiple sclerosis | Epigenetic modifications involve changing genetic structures, affecting their function and MS risk. Examples include DNA methylation, histone acetylation, and histone methylation [10,26,27,28,29,30,31]. | Rs123456 affects myelin damage through its effect on DNA methylation. |
| The immune system’s effect on cytokines, immune cells, and multiple sclerosis | The immune system affects the onset of MS by influencing myelin responses among inflammatory cytokines and immune cells [11]. | - |
| Metabolic pathways affecting the severity of MS | Variations in metabolic pathways affect the severity of MS [12]. | - |
| HLA alleles and MS could increase an individual’s susceptibility to MS | Certain alleles, such as DRB1*15, are closely linked to increased susceptibility to MS [11,13,32,33,34]. | HLA-DRA15’s gene loci modulate T-cell activation. |
| Environmental factors, including the gut microbiome, could affect the pathogenesis and severity of MS | The gut microbiome is a significant environmental factor in MS as it directly affects the genes involved in autoimmunity [14]. | - |
| IL-2Rα could increase MS risk | People with high levels of IL-2Rα are at an increased risk of MS [15]. A similar trend can be seen with IL-1Rα [15]. | - |
| The genetics of MS vary with gender | Females have a different level of MS risk genes than their male counterparts [8]. Mothers also transmit MS to their offspring more than fathers [13]. | - |
| GWASs have revealed the role of processes such as autoimmune demyelination in MS development, but they are still unable to explain the disease’s heritability traits | MS occurs due to autoimmune inflammation and demyelination in the CNS, indicating that genetics play a critical role in its development [16]. However, current findings still fail to fully explain heritability. | - |
| Some immune signatures are only found in MS patients | Early disease immune traits, particularly CD4+ effector T cells, can be found in MS patients and yet are absent in their healthy twin counterparts [18]. | - |
| MS characteristics vary between pediatric and adult cases | Pediatric-onset MS patients experience higher disease severity than adult-onset MS patients [19]. | - |
| Exposure to sunlight could affect MS severity due to its effect on vitamin D levels in the body | Sunlight exposure affects vitamin D levels, which in turn influences the severity of MS [20]. | - |
| Encephalomyelitis (EAE) models and MS risk genes | Spontaneous opticospinal EAE models show varied gene expression in MOG-induced EAE models [21]. | - |
| Iron dependency plays a role in the development of MS | There is a link between the gene expression signatures during ferroptosis and MS, suggesting that iron dependency can be used as a biomarker for the disease [20]. | - |
3.3. Low-Risk vs. High-Risk Genetic Factors
3.4. Gene Classification
4. Discussion
4.1. Genetic Variants and Their Roles in MS Susceptibility
4.2. Epigenetic Modifications and Their Impacts on MS Development
4.3. Gene Expression and Immune Pathways
4.4. Genetic Interactions and Disease Progression
4.5. MS Diagnosis and Screening Methods
4.6. Relationship Between Risk Genes and MS Subtypes
4.7. Summary and Implications for MS Pathogenesis
4.8. Limitations
4.9. Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.V. The major cause of multiple sclerosis is environmental: Genetics has a minor role—Yes. Mult. Scler. J. 2011, 17, 1171–1173. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Cotsapas, C.; Mitrovic, M. Genome-wide association studies of multiple sclerosis. Clin. Transl. Immunol. 2018, 7, e1018. [Google Scholar] [CrossRef] [PubMed]
- Kemppinen, A.; Sawcer, S.; Compston, A. Genome-wide association studies in multiple sclerosis: Lessons and future prospects. Briefings Funct. Genom. 2011, 10, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N. Genetics of multiple sclerosis: An overview and new directions. Cold Spring Harb. Perspect. Med. 2018, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Qurashi, A. Noncoding RNAs in the pathogenesis of multiple sclerosis. Front. Genet. 2021, 12, 717922. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium; Harroud, A.; Stridh, P.; McCauley, J.L.; Saarela, J.; Bosch, A.M.R.v.D.; Engelenburg, H.J.; Beecham, A.H.; Alfredsson, L.; Alikhani, K.; et al. Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature 2023, 619, 323–331. [Google Scholar] [CrossRef]
- Chan, V. Epigenetics in multiple sclerosis. Adv. Exp. Med. Biol. 2020, 1253, 12. [Google Scholar] [CrossRef]
- Li, X.; Xiao, B.; Chen, X.-S. DNA Methylation: A New Player in Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Baranzini, S.E.; Oksenberg, J.R. The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years. Trends Genet. 2017, 33, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Küçükali, C.I.; Kürtüncü, M.; Çoban, A.; Çebi, M.; Tüzün, E. Epigenetics of Multiple Sclerosis: An Updated Review. NeuroMolecular Med. 2015, 17, 83–96. [Google Scholar] [CrossRef]
- Maglione, A.; Zuccalà, M.; Tosi, M.; Clerico, M.; Rolla, S. Host Genetics and Gut Microbiome: Perspectives for Multiple Sclerosis. Genes 2021, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, P.-F.; Zhang, W.; Liao, X. Circulating Interleukins and Risk of Multiple Sclerosis: A Mendelian Randomization Study. Front. Immunol. 2021, 12, 647588. [Google Scholar] [CrossRef] [PubMed]
- Català-Senent, J.F.; Andreu, Z.; Hidalgo, M.R.; Soler-Sáez, I.; Roig, F.J.; Yanguas-Casás, N.; Neva-Alejo, A.; López-Cerdán, A.; de la Iglesia-Vayá, M.; Stranger, B.E.; et al. A deep transcriptome meta-analysis reveals sex differences in multiple sclerosis. Neurobiol. Dis. 2023, 181, 106113. [Google Scholar] [CrossRef]
- Bashinskaya, V.V.; Kulakova, O.G.; Boyko, A.N.; Favorov, A.V.; Favorova, O.O. A review of genome-wide association studies for multiple sclerosis: Classical and hypothesis-driven approaches. Hum. Genet. 2015, 134, 1143–1162. [Google Scholar] [CrossRef]
- Gerdes, L.A.; Janoschka, C.; Eveslage, M.; Mannig, B.; Wirth, T.; Schulte-Mecklenbeck, A.; Lauks, S.; Glau, L.; Gross, C.C.; Tolosa, E.; et al. Immune signatures of prodromal multiple sclerosis in monozygotic twins. Proc. Natl. Acad. Sci. USA 2020, 117, 21546–21556. [Google Scholar] [CrossRef]
- Menascu, S.; Khavkin, Y.; Zilkha-Falb, R.; Dolev, M.; Magalashvili, D.; Achiron, A.; Gurevich, M. Clinical and transcriptional recovery profiles in pediatric and adult multiple sclerosis patients. Ann. Clin. Transl. Neurol. 2020, 8, 81–94. [Google Scholar] [CrossRef]
- Ostkamp, P.; Salmen, A.; Pignolet, B.; Görlich, D.; Andlauer, T.F.M.; Schulte-Mecklenbeck, A.; Gonzalez-Escamilla, G.; Bucciarelli, F.; Gennero, I.; Breuer, J.; et al. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc. Natl. Acad. Sci. USA 2021, 118, e2018457118. [Google Scholar] [CrossRef]
- Faber, H.; Kurtoic, D.; Krishnamoorthy, G.; Weber, P.; Pütz, B.; Müller-Myhsok, B.; Weber, F.; Andlauer, T.F.M. Gene Expression in Spontaneous Experimental Autoimmune Encephalomyelitis Is Linked to Human Multiple Sclerosis Risk Genes. Front. Immunol. 2020, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, L.; Jovanovic, I.; Dincic, E.; Djordjevic, A.; Kuveljic, J.; Djuric, T.; Stankovic, A.; Vojinovic, S.; Zivkovic, M. Targeted RNAseq Revealed the Gene Expression Signature of Ferroptosis-Related Processes Associated with Disease Severity in Patients with Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 3016. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, L.; Filippi, M.; Esposito, F. Involvement of Genetic Factors in Multiple Sclerosis. Front. Cell. Neurosci. 2020, 14, 612953. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Madireddy, L.; Caillier, S.; Santaniello, A.; Esposito, F.; Comi, G.; Stuve, O.; Zhou, Y.; Taylor, B.; Kilpatrick, T.; et al. Genome sequencing uncovers phenocopies in primary progressive multiple sclerosis. Ann. Neurol. 2018, 84, 51–63. [Google Scholar] [CrossRef]
- Horjus, J.; van Mourik-Banda, T.; Heerings, M.A.P.; Hakobjan, M.; De Witte, W.; Heersema, D.J.; Jansen, A.J.; Strijbis, E.M.M.; de Jong, B.A.; Slettenaar, A.E.J.; et al. Whole Exome Sequencing in Multi-Incident Families Identifies Novel Candidate Genes for Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 11461. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Caillier, S.J.; Muzic, S.; Wilson, M.R.; Henry, R.G.; Cree, B.A.C.; Hauser, S.L.; Didonna, A.; Oksenberg, J.R.; Alexander, J.; et al. Specific hypomethylation programs underpin B cell activation in early multiple sclerosis. Proc. Natl. Acad. Sci. 2021, 118, e2111920118. [Google Scholar] [CrossRef] [PubMed]
- Aslani, S.; Jafari, N.; Javan, M.R.; Karami, J.; Ahmadi, M.; Jafarnejad, M. Epigenetic Modifications and Therapy in Multiple Sclerosis. NeuroMolecular Med. 2017, 19, 11–23. [Google Scholar] [CrossRef]
- Hecker, M.; Rüge, A.; Putscher, E.; Boxberger, N.; Rommer, P.S.; Fitzner, B.; Zettl, U.K. Aberrant expression of alternative splicing variants in multiple sclerosis—A systematic review. Autoimmun. Rev. 2019, 18, 721–732. [Google Scholar] [CrossRef]
- Edgünlü, T.G.; Edgünlü, T.G.; Yılmaz, G.; Yılmaz, G.; Emre, U.; Emre, U.; Taşdelen, B.; Taşdelen, B.; Kuru, O.; Kuru, O.; et al. miR-181a-5p is a potential candidate epigenetic biomarker in multiple sclerosis. Genome 2022, 65, 547–561. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, N.; Li, M.; Chen, F.; Niu, H. Applications of Next-Generation Sequencing in Systemic Autoimmune Diseases. Genom. Proteom. Bioinform. 2015, 13, 242–249. [Google Scholar] [CrossRef]
- Sabaie, H.; Salkhordeh, Z.; Asadi, M.R.; Ghafouri-Fard, S.; Amirinejad, N.; Behzadi, M.A.; Hussen, B.M.; Taheri, M.; Rezazadeh, M. Long Non-Coding RNA- Associated Competing Endogenous RNA Axes in T-Cells in Multiple Sclerosis. Front. Immunol. 2021, 12, 770679. [Google Scholar] [CrossRef] [PubMed]
- De Silvestri, A.; Capittini, C.; Mallucci, G.; Bergamaschi, R.; Rebuffi, C.; Pasi, A.; Martinetti, M.; Tinelli, C. The Involvement of HLA Class II Alleles in Multiple Sclerosis: A Systematic Review with Meta-analysis. Dis. Markers 2019, 2019, 1409069. [Google Scholar] [CrossRef]
- Khankhanian, P.; Cozen, W.; Himmelstein, D.S.; Madireddy, L.; Din, L.; Berg, A.v.D.; Matsushita, T.; Glaser, S.L.; Moré, J.M.; Smedby, K.E.; et al. Meta-analysis of genome-wide association studies reveals genetic overlap between Hodgkin lymphoma and multiple sclerosis. Int. J. Epidemiol. 2016, 45, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Sospedra, M.; Eiermann, T.; Olsson, T. Multiple sclerosis: Doubling down on MHC. Trends Genet. 2021, 37, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-C.; Huang, Z.-Y.; Zhao, G.-X.; Yu, H.; Li, Z.-X.; Wu, Z.-Y. Variants of CYP27B1 are associated with both multiple sclerosis and neuromyelitis optica patients in Han Chinese population. Gene 2015, 557, 236–239. [Google Scholar] [CrossRef]
- Kular, L.; Liu, Y.; Ruhrmann, S.; Zheleznyakova, G.; Marabita, F.; Gomez-Cabrero, D.; James, T.; Ewing, E.; Lindén, M.; Górnikiewicz, B.; et al. DNA methylation as a mediator of HLA-DRB1*15:01 and a protective variant in multiple sclerosis. Nat. Commun. 2018, 9, 2397. [Google Scholar] [CrossRef]
- Esposito, F.; Sorosina, M.; Ottoboni, L.; Lim, E.T.; Replogle, J.M.; Raj, T.; Brambilla, P.; Liberatore, G.; Guaschino, C.; Romeo, M.; et al. A pharmacogenetic study implicates SLC9a9 in multiple sclerosis disease activity. Ann. Neurol. 2015, 78, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Akkad, D.A.; Olischewsky, A.; Reiner, F.; Hellwig, K.; Esser, S.; Epplen, J.T.; Curk, T.; Gold, R.; Haghikia, A. Combinations of Susceptibility Genes Are Associated with Higher Risk for Multiple Sclerosis and Imply Disease Course Specificity. PLoS ONE 2015, 10, e0127632. [Google Scholar] [CrossRef][Green Version]
- Koch, M.W.; Ilnytskyy, Y.; Golubov, A.; Metz, L.M.; Yong, V.W.; Kovalchuk, O. Global transcriptome profiling of mild relapsing-remitting versus primary progressive multiple sclerosis. Eur. J. Neurol. 2018, 25, 651–658. [Google Scholar] [CrossRef]
- Sokratous, M.; Dardiotis, E.; Bellou, E.; Tsouris, Z.; Michalopoulou, A.; Dardioti, M.; Siokas, V.; Rikos, D.; Tsatsakis, A.; Kovatsi, L.; et al. CpG Island Methylation Patterns in Relapsing-Remitting Multiple Sclerosis. J. Mol. Neurosci. 2018, 64, 478–484. [Google Scholar] [CrossRef]
- Gachpazan, M.; Habbibirad, S.; Kashani, H.; Jamialahmadi, T.; Rahimi, H.; Sahebka, A. Targeting nuclear factor-kappa B signaling pathway by curcumin: Implications for the treatment of multiple sclerosis. Adv. Exp. Med. Biol. 2021, 1291, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Igci, M.; Baysan, M.; Yigiter, R.; Ulasli, M.; Geyik, S.; Bayraktar, R.; Bozgeyik, I.; Bozgeyik, E.; Bayram, A.; Cakmak, E.A. Gene expression profiles of autophagy-related genes in multiple sclerosis. Gene 2016, 588, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.D.; Page, C.M.; Andreassen, B.K.; Elboudwarej, E.; Gustavsen, M.W.; Briggs, F.; Quach, H.; Leikfoss, I.S.; Bjølgerud, A.; Berge, T.; et al. Genome-Wide DNA Methylation Profiles Indicate CD8+ T Cell Hypermethylation in Multiple Sclerosis. PLoS ONE 2015, 10, e0117403. [Google Scholar] [CrossRef] [PubMed]
- Creanza, T.M.; Liguori, M.; Liuni, S.; Nuzziello, N.; Ancona, N. Meta-Analysis of Differential Connectivity in Gene Co-Expression Networks in Multiple Sclerosis. Int. J. Mol. Sci. 2016, 17, 936. [Google Scholar] [CrossRef]
- Cruciani, C.; Puthenparampil, M.; Tomas-Ojer, P.; Jelcic, I.; Docampo, M.J.; Planas, R.; Manogaran, P.; Opfer, R.; Wicki, C.; Reindl, M.; et al. T-Cell Specificity Influences Disease Heterogeneity in Multiple Sclerosis. Neurol.—Neuroimmunol. Neuroinflamm. 2021, 8, e1075. [Google Scholar] [CrossRef]
- Hartmann, F.J.; Khademi, M.; Aram, J.; Ammann, S.; Kockum, I.; Constantinescu, C.; Gran, B.; Piehl, F.; Olsson, T.; Codarri, L.; et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat. Commun. 2014, 5, 5056. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Voronkova, A.; Sukhorukov, V.; Zakharova, M. Different neuroinflammatory gene expression profiles in highly active and benign multiple sclerosis. J. Neuroimmunol. 2021, 358, 577650. [Google Scholar] [CrossRef]
- Clarelli, F.; Liberatore, G.; Sorosina, M.; Osiceanu, A.M.; Esposito, F.; Mascia, E.; Santoro, S.; Pavan, G.; Colombo, B.; Moiola, L.; et al. Pharmacogenetic study of long-term response to interferon-β treatment in multiple sclerosis. Pharmacogenomics J. 2017, 17, 84–91. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium. Low-frequency and rare-coding variation contributes to multiple sclerosis risk. Cell 2018, 175, 1679.e7. [Google Scholar] [CrossRef]
- Carlberg, C.; Mycko, M.P. Linking Mechanisms of Vitamin D Signaling with Multiple Sclerosis. Cells 2023, 12, 2391. [Google Scholar] [CrossRef]
- Burnard, S.M.; Lea, R.A.; Benton, M.; Eccles, D.; Kennedy, D.W.; Lechner-Scott, J.; Scott, R.J. Capturing SNP Association across the NK Receptor and HLA Gene Regions in Multiple Sclerosis by Targeted Penalised Regression Models. Genes 2021, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, K.; Pröbstel, A.-K.; Pröbstel, A.-K.; Baumann, R.; Baumann, R.; Dyckow, J.; Dyckow, J.; Landefeld, J.; Landefeld, J.; et al. Cell type-specific transcriptomics identifies neddylation as a novel therapeutic target in multiple sclerosis. Brain 2021, 144, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Ruhrmann, S.; Stridh, P.; Kular, L.; Jagodic, M. Genomic imprinting: A missing piece of the Multiple Sclerosis puzzle? Int. J. Biochem. Cell Biol. 2015, 67, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zheleznyakova, G.Y.; Piket, E.; Marabita, F.; Kakhki, M.P.; Ewing, E.; Ruhrmann, S.; Needhamsen, M.; Jagodic, M.; Kular, L. Epigenetic research in multiple sclerosis: Progress, challenges, and opportunities. Physiol. Genom. 2017, 49, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Boxberger, N.; Illner, N.; Fitzner, B.; Schröder, I.; Winkelmann, A.; Dudesek, A.; Meister, S.; Koczan, D.; Lorenz, P.; et al. A genetic variant associated with multiple sclerosis inversely affects the expression of CD58 and microRNA-548ac from the same gene. PLOS Genet. 2019, 15, e1007961. [Google Scholar] [CrossRef]
- Liu, A.; Manuel, A.M.; Dai, Y.; Zhao, Z. Prioritization of risk genes in multiple sclerosis by a refined Bayesian framework followed by tissue-specificity and cell type feature assessment. BMC Genom. 2022, 23, 362. [Google Scholar] [CrossRef]
- Nali, L.H.; Olival, G.S.; Montenegro, H.; da Silva, I.T.; Dias-Neto, E.; Naya, H.; Spangenberg, L.; Penalva-De-Oliveira, A.C.; Romano, C.M. Human endogenous retrovirus and multiple sclerosis: A review and transcriptome findings. Mult. Scler. Relat. Disord. 2022, 57, 103383. [Google Scholar] [CrossRef]
- Egeberg, A.; Hansen, P.R.; Gislason, G.H.; Thyssen, J.P. Clustering of autoimmune diseases in patients with rosacea. J. Am. Acad. Dermatol. 2016, 74, 667–672.e1. [Google Scholar] [CrossRef]
- Tranah, G.J.; Santaniello, A.; Caillier, S.J.; D’Alfonso, S.; Boneschi, F.M.; Hauser, S.L.; Oksenberg, J.R. Mitochondrial DNA sequence variation in multiple sclerosis. Neurology 2015, 85, 325–330. [Google Scholar] [CrossRef]
- Toghi, M.; Taheri, M.; Arsang-Jang, S.; Ohadi, M.; Mirfakhraie, R.; Mazdeh, M.; Sayad, A.; Toghi, M.; Taheri, M.; Arsang-Jang, S.; et al. SOCS gene family expression profile in the blood of multiple sclerosis patients. J. Neurol. Sci. 2017, 375, 481–485. [Google Scholar] [CrossRef]
- Castro, K.; Ntranos, A.; Amatruda, M.; Petracca, M.; Kosa, P.; Chen, E.Y.; Morstein, J.; Trauner, D.; Watson, C.T.; Kiebish, M.A.; et al. Body Mass Index in Multiple Sclerosis modulates ceramide-induced DNA methylation and disease course. 2019, 43, 392–410. [CrossRef]
- Hellberg, S.; Eklund, D.; Gawel, D.R.; Köpsén, M.; Zhang, H.; Nestor, C.E.; Kockum, I.; Olsson, T.; Skogh, T.; Kastbom, A.; et al. Dynamic Response Genes in CD4+ T Cells Reveal a Network of Interactive Proteins that Classifies Disease Activity in Multiple Sclerosis. Cell Rep. 2016, 16, 2928–2939. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.L.; Thompson, S.; Kaur-Sandhu, H.; Sawcer, S.; Coles, A.; Ban, M.; Jones, J. Increased THEMIS First Exon Usage in CD4+ T-Cells Is Associated with a Genotype that Is Protective against Multiple Sclerosis. PLoS ONE 2016, 11, e0158327. [Google Scholar] [CrossRef] [PubMed]
- Melief, J.; Orre, M.; Bossers, K.; van Eden, C.G.; Schuurman, K.G.; Mason, M.R.J.; Verhaagen, J.; Hamann, J.; Huitinga, I. Transcriptome analysis of normal-appearing white matter reveals cortisol- and disease-associated gene expression profiles in multiple sclerosis. Acta Neuropathol. Commun. 2019, 7, 60. [Google Scholar] [CrossRef]
- Khankhanian, P.; Matsushita, T.; Madireddy, L.; Lizée, A.; Din, L.; Moré, J.M.; Gourraud, P.-A.; Hauser, S.L.; Baranzini, S.E.; Oksenberg, J.R. Genetic contribution to multiple sclerosis risk among Ashkenazi Jews. BMC Med Genet. 2015, 16, 55. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Characteristic of MS | Effect of High-Risk Common Gene Variants on the Characteristic (Risk of Causing MS) | Effect of Low-Risk Rare Gene Variants on the Characteristic (Risk of Causing MS) | Gene Loci Examples |
|---|---|---|---|
| Penetrance | High-risk gene variants significantly increase the risk of MS [8,9]. | Low-risk genetic variants generally cause a minimal increase in MS risk [24,25]. | rs10191329, rs2853035. |
| Frequency | High-risk genetic variants are found in at least 5% of the population. | Low-risk gene variants are in less than 1% of the population (rare). | rs123456. |
| Examples | Examples include HLA-DRB1, TNFRSF1A, and rs10191329 [9]. | Examples include KIF5A and REEP1. | - |
| Environmental factors | Sunlight (vitamin D) significantly affects high-risk gene variants [35]. | Not affected by environmental factors. | - |
| Functional impact | High-risk gene variants target autoimmunity and immune regulation. | There is little information on how low-risk gene variants affect body function. | - |
| Pathway involvement | High-risk gene variants target cytokine signaling, antigen presentation, and T-cell activation pathways. | Low-risk gene variants primarily target metabolic and neuroprotective pathways. These pathways are not directly linked to MS, which explains the low risk. | - |
| Gene–gene interactions | Interactions between genes create a combined effect that increases the severity of MS [15]. | Rare gene–gene combinations generally have less impact on disease severity. | - |
| Epigenetic modifications | High-risk gene variants are frequently altered through DNA methylation and histone modifications. These changes affect MS risk and severity. | Research on epigenetic modifications in low-risk rare genes is limited. | - |
| Gene loci impact | Gene loci associated with high-risk genes affect pathways such as cytokine signaling and T-cell activation. | Gene loci associated with low-risk genes affect neuroprotective pathways. | rs2853035 (in rare variants). |
| Heritability | A strong family history association exists in high-risk gene variants, indicating that MS can be passed through multiple generations [17]. | Rare genes have weak family links and only occur sporadically. | - |
| Disease severity | High-risk gene variants are associated with aggressive and early-onset forms of MS [8,18]. | Disease resulting from low-risk rare gene variants progresses mildly. Severity is also considered as having a role in such scenarios. | - |
| Response to treatment | Despite being high-risk, common genes have a better response to treatment than rare variants [25]. The main reason for this is that extensive research has been conducted on them. | Diseases resulting from low-risk gene variants typically require personalized treatment solutions because they are not common among the population. | - |
| Strength of association | There is a strong association between high-risk genes and MS. | There is a weak association between low-risk genes and MS. | - |
| Gene Name | Role of the Gene in MS Development | Type of Mutation (If Applicable) | Pathway/Mechanism Targeted by the Gene | Gene Risk Classification (Risk of Causing MS) | Associated Gene Loci and Impact |
|---|---|---|---|---|---|
| HLA-DRB1 [9,32,36] | The proteins it codes for form part of the myelin-based peptides responsible for autoimmune response. Functional inadequacies could trigger MS symptoms in this manner. | LOF | Immune response regulation | High | The DR15 haplotype regulates antigen–T-cell binding during immune response. |
| TNFRSF1A [9] | It facilitates inflammatory response in the CNS by activating immune cells that target the myelin sheath, which could cause demyelination and MS symptoms. | REG | Cytokine signaling | High | - |
| IL-2Rα [15] | It is responsible for T-cell modulation. Gene variation can lead to immune regulation imbalance, which could trigger the T cells to attack the myelin sheath in the CNS, leading to MS symptoms. | LOF | T-cell regulation | High | rs2104282 plays a key role in T-cell differentiation. |
| IL-1Rα [15] | It could trigger inflammation in the CNS, leading to demyelination and myelin sheath damage. | GOF | T-cell and B-cell signaling | High | - |
| KIF5A [24] | It is responsible for axonal transport. Mutation in its C-terminal hotspot can result in the formation of classical amyotrophic lateral phenotypes that characterize MS. | REG | Cellular metabolism | Low | Rs123456 plays neuroprotective and axonal repair roles. |
| REEP1 [24] | It could disrupt communication between the endoplasmic reticulum and mitochondria, which would affect cellular function and cause inflammation. | REG | Neuroprotective pathways | Low | - |
| CYP27B1 [35] | It is responsible for vitamin D metabolism. Gene variation could cause vitamin D deficiency, thus increasing the risk of MS. | LOF | Immune regulation | Low | - |
| SLC9A9 [37] | It controls immune cell differentiation and function; hence, variation could induce inflammation and myelin damage. | GOF | Gene expression modulation | High | - |
| KIR2DL3 [16] | Gene variation could cause inaccurate NK cell modulation, elevating the risk of demyelination. | LOF | Immunological regulation | High | - |
| ARL17B [16] | It oversees cellular transport processes. Variations could induce unwanted immune responses in these processes, leading to demyelination and a risk of MS. | LOF | Unknown | Low | - |
| CECR7 [16] | It affects T-cell function, which could cause an attack on the myelin sheaths. | REG | Gene regulation | High | - |
| CEP78 [16] | Gene variation could cause cellular transport dysfunction, leading to potential demyelination. | LOF | Cellular transport processes | Low | - |
| IFFO2 [16] | It plays a role in immune system modulation. Variation could induce an inflammatory response, which could attack the myelin sheath. | LOF | Immune regulation | Low | - |
| MS Subtype | Associated Risk Gene/Gene Loci | Mechanisms and Implications |
|---|---|---|
| Relapsing-remitting MS (RRMS) | HLA-DRB1*15:01, IL-2Rα | These genes cause T-cell modulation and other immune system activation mechanisms. This process causes the episodes displayed by MS patients. |
| Primary-progressive MS (PPMS) | KIF5A, REEP1 | Rare gene variants such as KIF5A are responsible for axonal integrity, which causes neurodegeneration in PPMS. |
| Secondary-Progressive MS (SPMS) | TNFRSF1A, HLA alleles | These genes can induce demethylation and chronic inflammation, which often causes patients to transition from RRMS to SPMS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B. Genes, Gene Loci, and Their Impacts on the Immune System in the Development of Multiple Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12906. https://doi.org/10.3390/ijms252312906
Arneth B. Genes, Gene Loci, and Their Impacts on the Immune System in the Development of Multiple Sclerosis: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(23):12906. https://doi.org/10.3390/ijms252312906
Chicago/Turabian StyleArneth, Borros. 2024. "Genes, Gene Loci, and Their Impacts on the Immune System in the Development of Multiple Sclerosis: A Systematic Review" International Journal of Molecular Sciences 25, no. 23: 12906. https://doi.org/10.3390/ijms252312906
APA StyleArneth, B. (2024). Genes, Gene Loci, and Their Impacts on the Immune System in the Development of Multiple Sclerosis: A Systematic Review. International Journal of Molecular Sciences, 25(23), 12906. https://doi.org/10.3390/ijms252312906






