Methoxyquinolone–Benzothiazole Hybrids as New Aggregation-Induced Emission Luminogens and Efficient Fluorescent Chemosensors for Cyanide Ions
Abstract
1. Introduction
2. Results
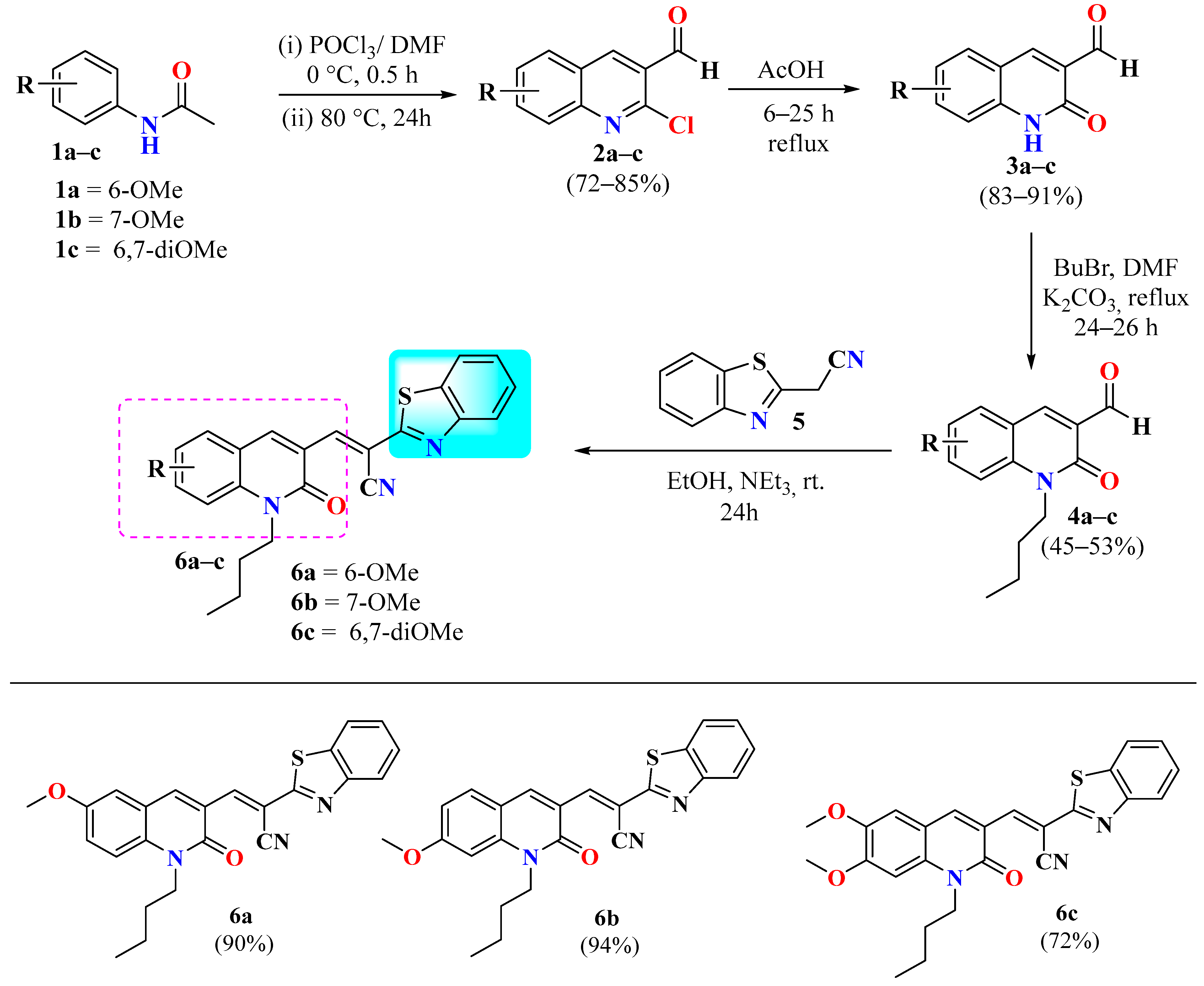
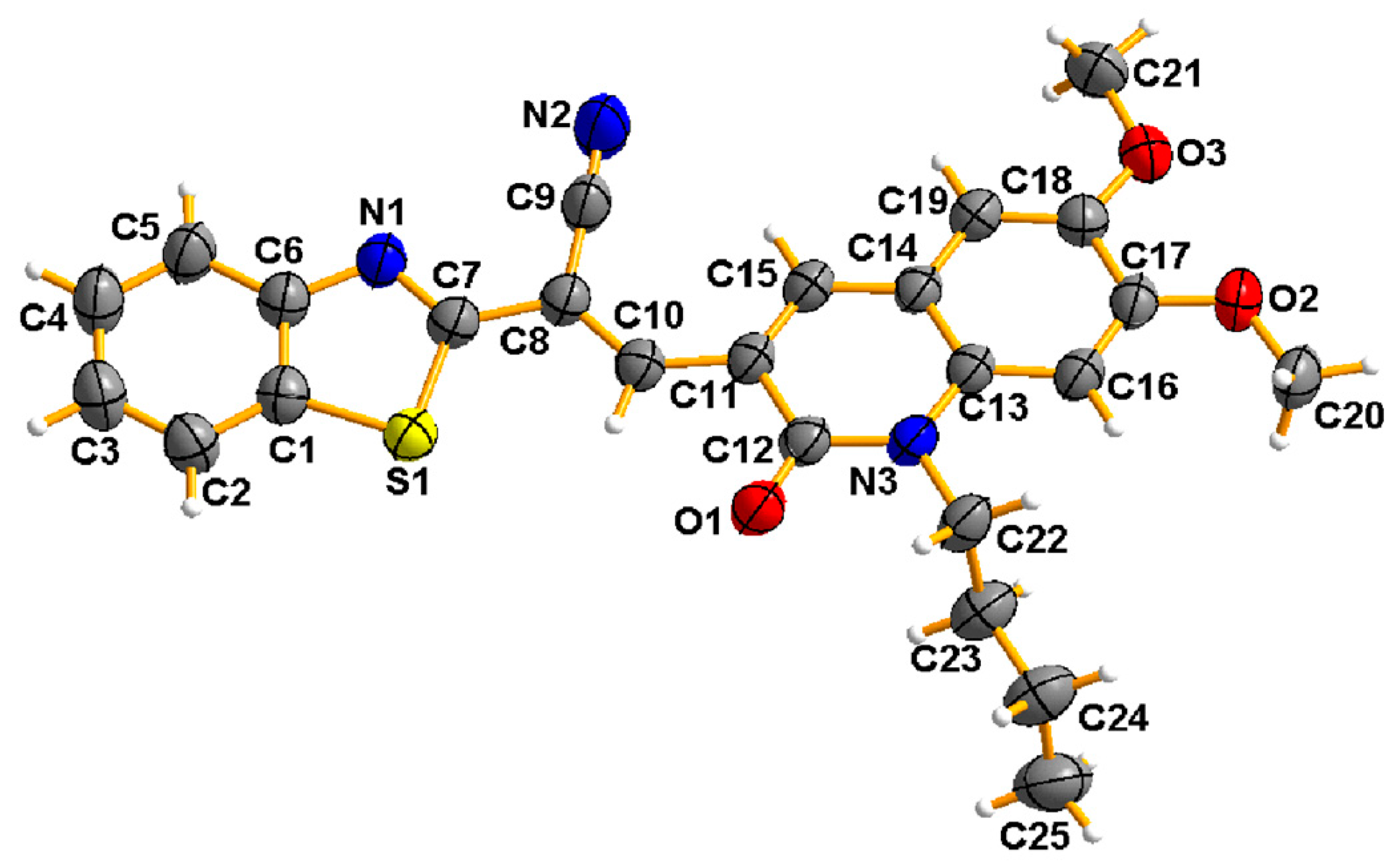
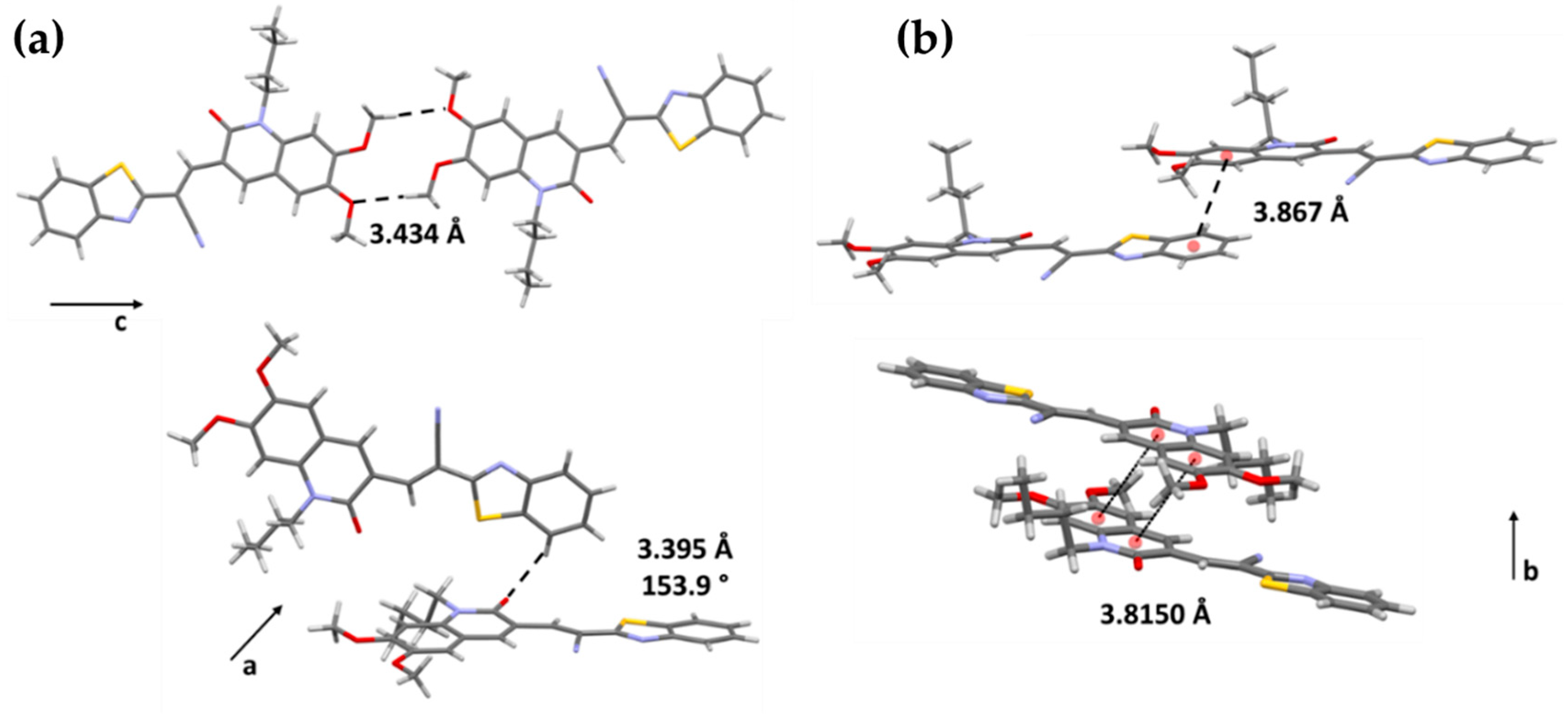
2.1. Molecular Design, Synthesis, and Structural Characterization
2.2. Photophysical Properties
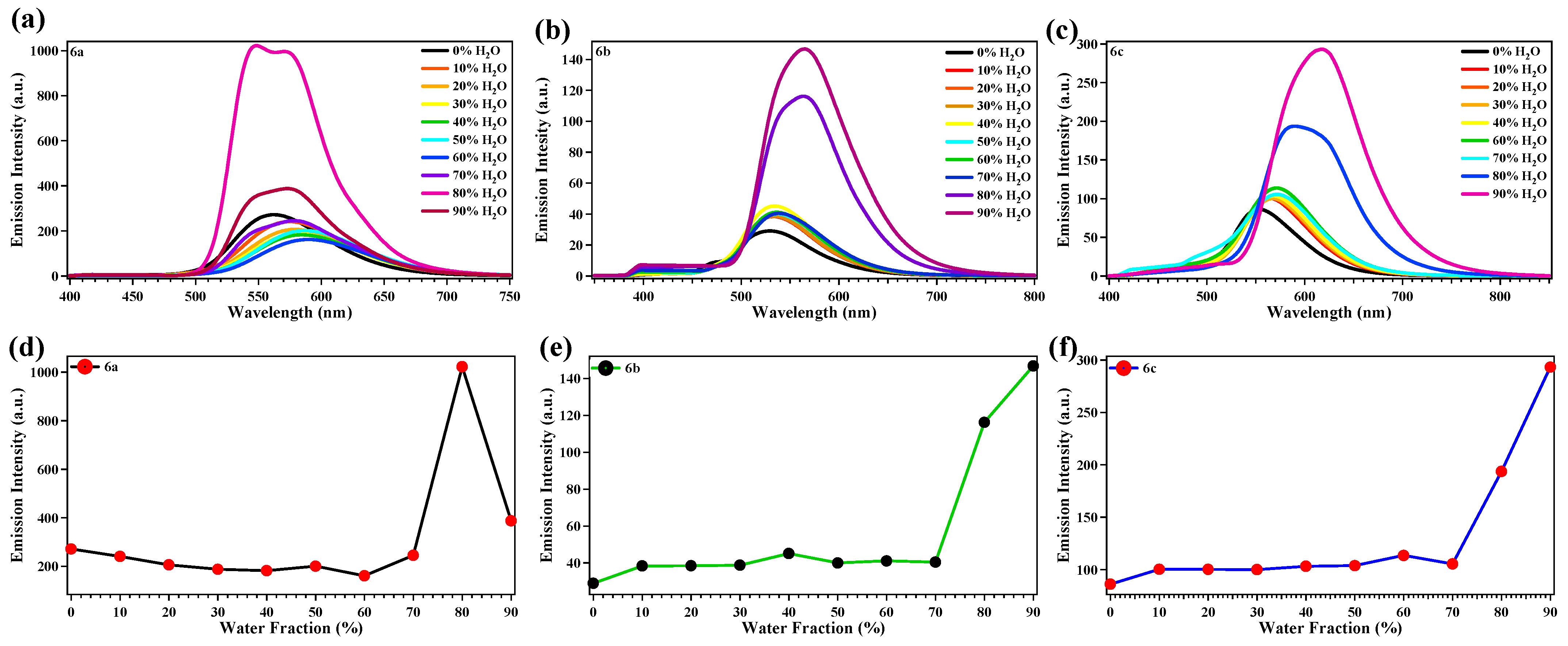
2.3. Aggregation-Induced Emission
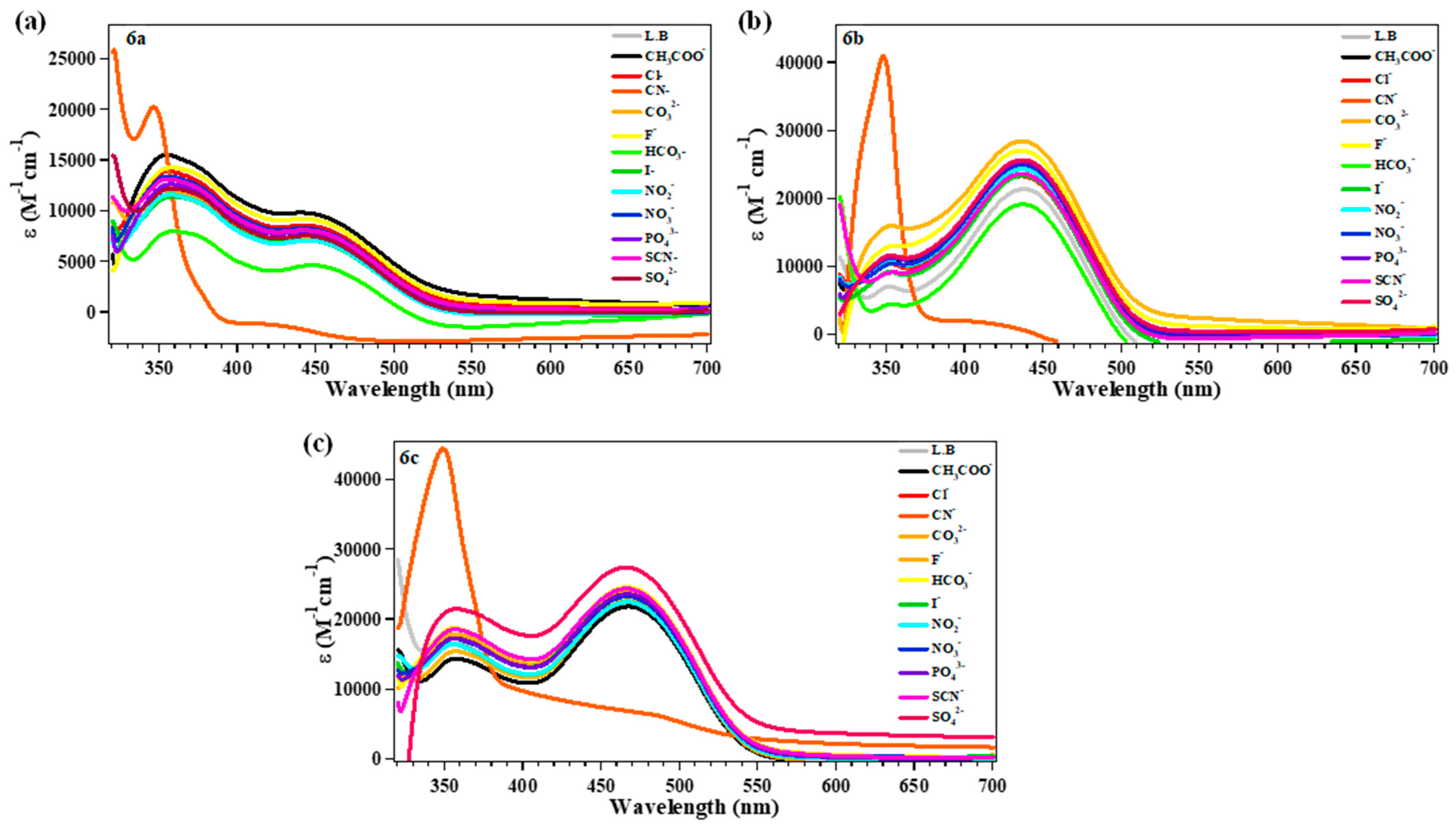
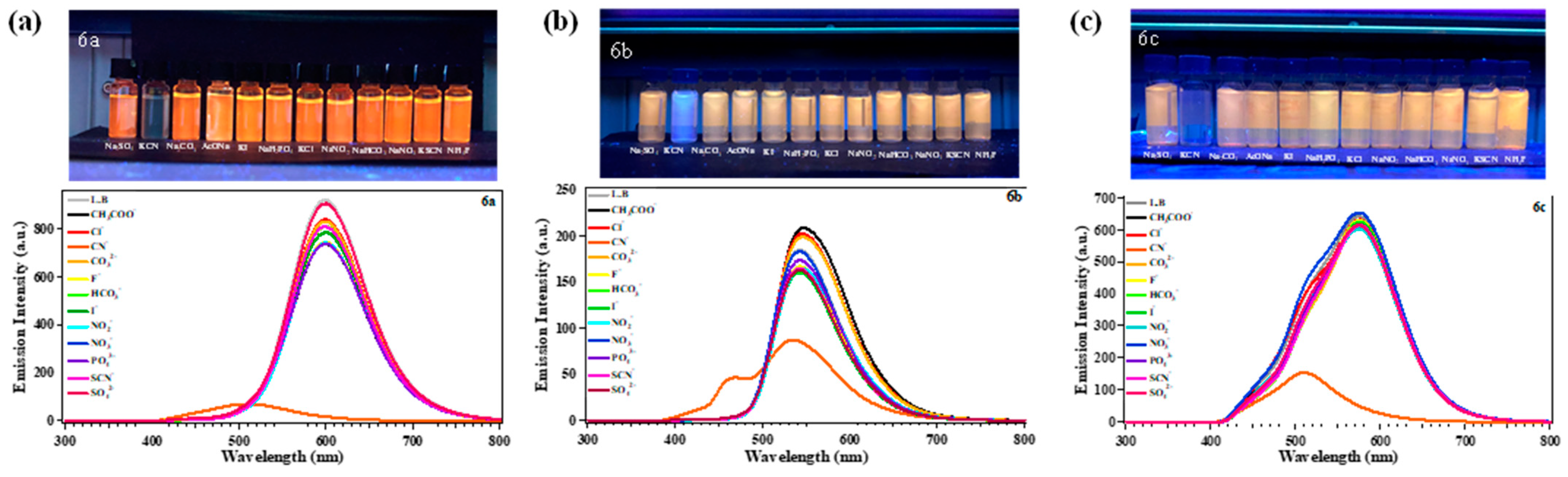
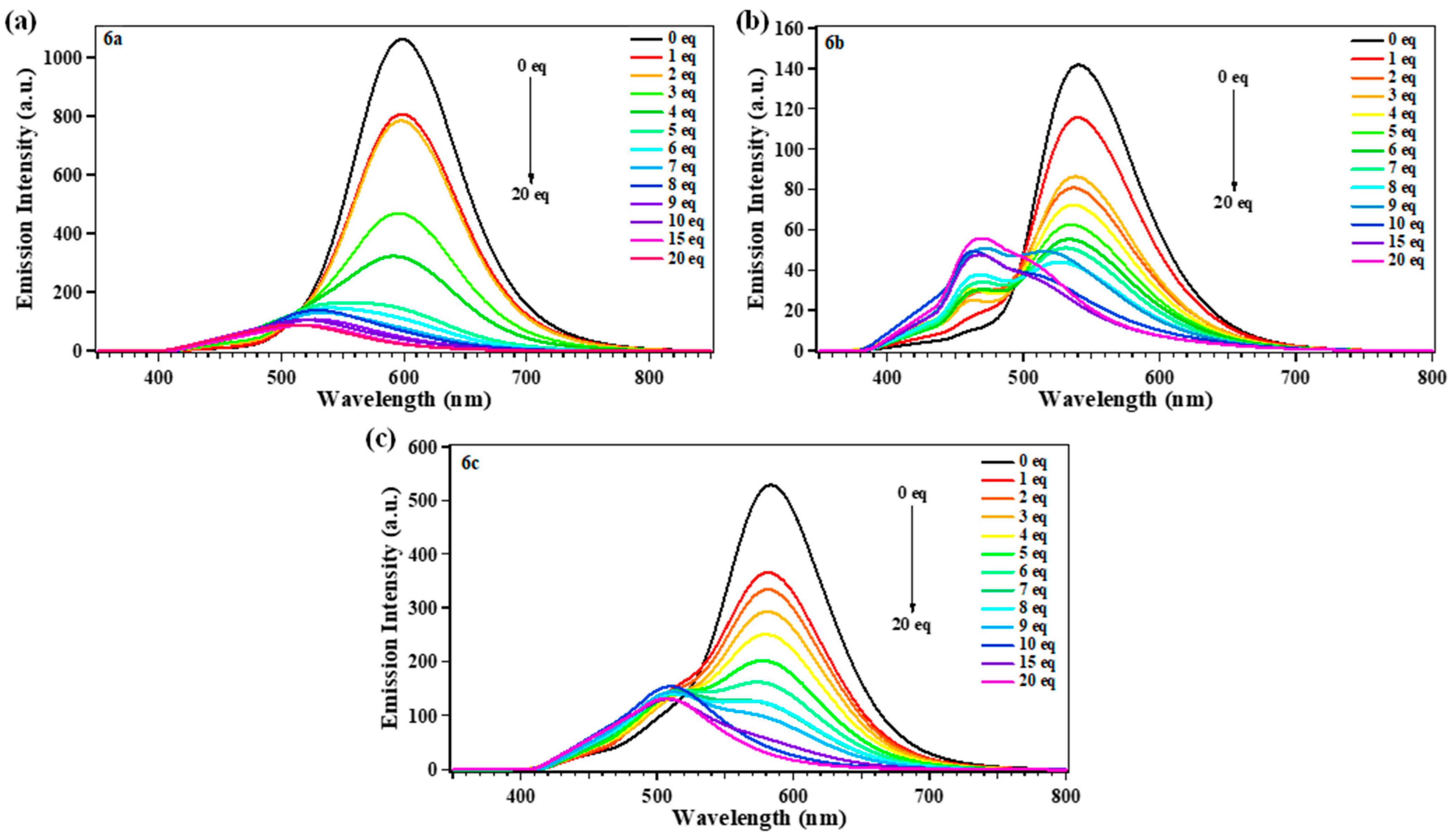
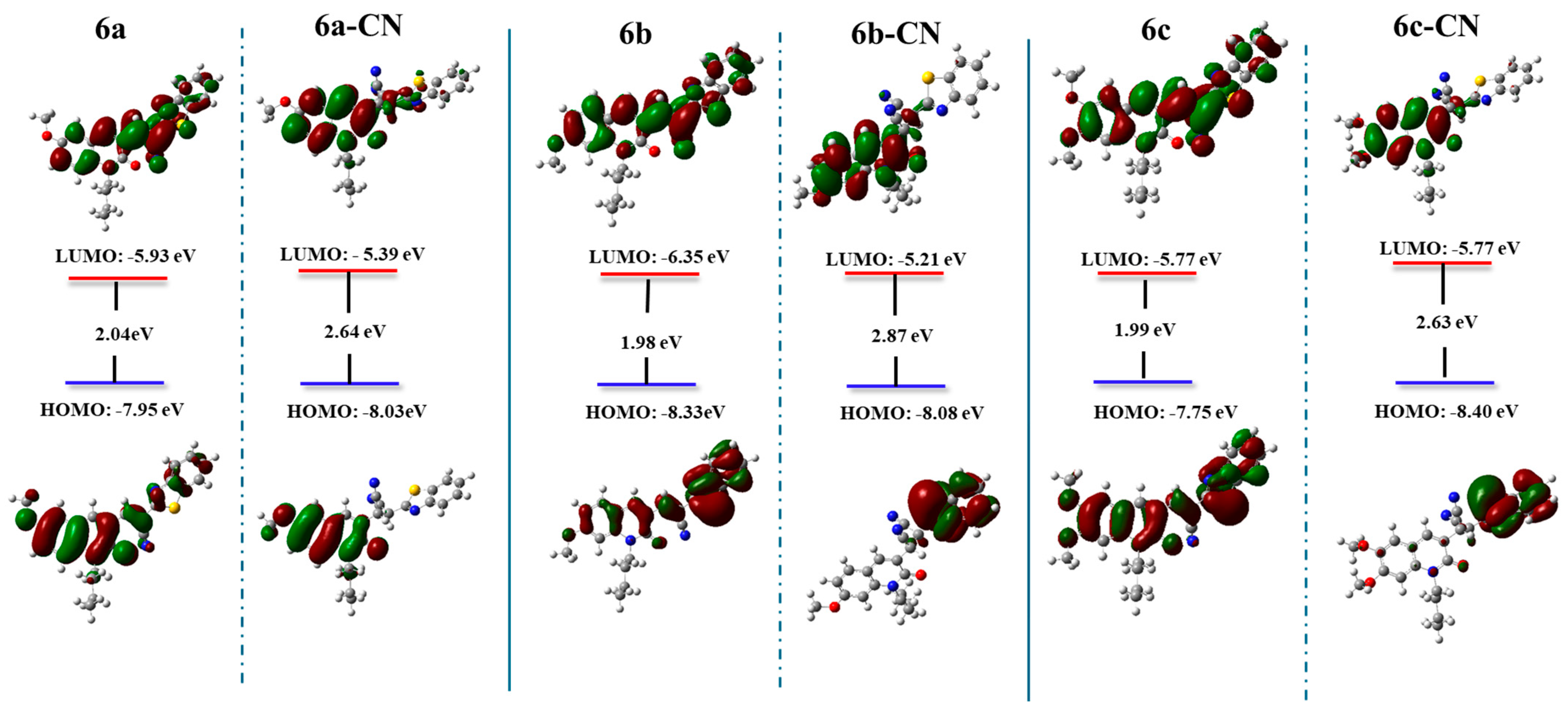
2.4. Sensing Response of 6a–c to Several Anions
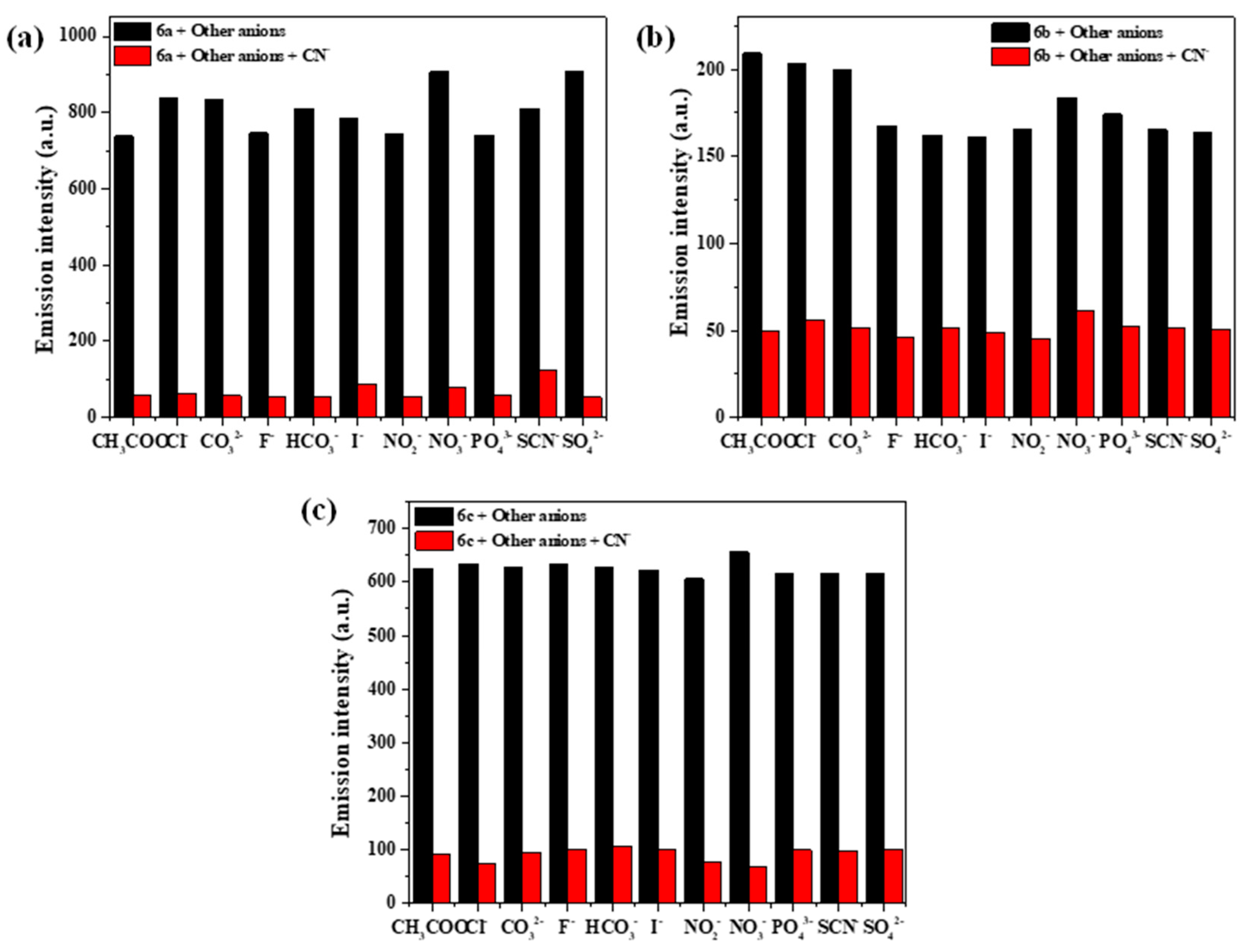
2.5. Selectivity and Competition Experiments for CN−
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. General Procedure for the Synthesis of 2a–c and 3a–c
3.3. General Procedure for the Synthesis of 5a–c
3.4. General Procedure for the Synthesis of Hybrids 6a–c
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dine, I.; Mulugeta, E.; Melaku, Y.; Belete, M. Recent Advances in the Synthesis of Pharmaceutically Active 4-Quinolone and Its Analogues: A Review. RSC Adv. 2023, 13, 8657–8682. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone Antibiotics: Occurrence, Mode of Action, Resistance, Environmental Detection, and Remediation—A Comprehensive Review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Frąckowiak, S.; Adamowicz, J.; Szeliski, K.; Rasmus, M.; Drewa, T.; Pokrywczyńska, M. Quinolones as a Potential Drug in Genitourinary Cancer Treatment—A Literature Review. Front. Oncol. 2022, 12, 890337. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Dhiman, P.; Arora, N.; Thanikachalam, P.V.; Monga, V. Recent Advances in the Synthetic and Medicinal Perspective of Quinolones: A Review. Bioorg. Chem. 2019, 92, 103291. [Google Scholar] [CrossRef]
- Naeem, A.; Badshah, S.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef]
- Stone, M.R.L.; Masi, M.; Phetsang, W.; Pagès, J.-M.; Cooper, M.A.; Blaskovich, M.A.T. Fluoroquinolone-Derived Fluorescent Probes for Studies of Bacterial Penetration and Efflux. Medchemcomm 2019, 10, 901–906. [Google Scholar] [CrossRef]
- Pal, T.; Mathai, T.; Mukherji, S. Colorimetric Chemosensor for Rapid Detection of Fluoroquinolone Load in Environmental Water Bodies, Urine, and Counterfeit Drug Testing. Biosens. Bioelectron. X 2023, 14, 100384. [Google Scholar] [CrossRef]
- Patil, S.R.; Nandre, J.P.; Patil, P.A.; Bothra, S.; Sahoo, S.K.; Klasek, A.; Rodríguez-López, J.; Mahulikar, P.P.; Patil, U.D. Quinolone Based Chemosensor for the Naked-Eye and Spectrophotometric Detection of Cu2+ in Aqueous Media. Inorg. Chem. Commun. 2014, 49, 59–62. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Chen, X.-G.; Li, H.; Feng, W.; Song, Q.-H. Quinolone-Based Fluorescent Probes for Distinguished Detection of Cys and GSH through Different Fluorescence Channels. New J. Chem. 2020, 44, 13781–13787. [Google Scholar] [CrossRef]
- Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini-Rev. Med. Chem. 2020, 20, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.P.; Rahman, M.A.; Nishad, S.; Maurya, S.K.; Anas, M.; Mujahid, M. Synthesis and Biological Activities of Benzothiazole Derivatives: A review. Intell. Pharm. 2023, 1, 122–132. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, D.; Singh, K.; Tyagi, R.; Yadav, Y.; Sagar, R. Recent Advances in the Synthesis of New Benzothiazole Based Anti-Tubercular Compounds. RSC Adv. 2023, 13, 21890–21925. [Google Scholar] [CrossRef] [PubMed]
- Rybczyński, P.; Bousquet, M.H.E.; Kaczmarek-Kędziera, A.; Jędrzejewska, B.; Jacquemin, D.; Ośmiałowski, B. Controlling the Fluorescence Quantum Yields of Benzothiazole-Difluoroborates by Optimal Substitution. Chem. Sci. 2022, 13, 13347–13360. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Indurthi, H.K.; Asati, P.; Saha, P.; Sharma, D.K. Benzothiazole Based Fluorescent Probes for the Detection of Biomolecules, Physiological Conditions, and Ions Responsible for Diseases. Dye. Pigment. 2022, 199, 110074. [Google Scholar] [CrossRef]
- Tang, L.; Zou, Y.; Zhong, K.; Bian, Y. A Novel Benzothiazole-Based Enaminone as a Fluorescent Probe for Highly Selective and Sensitive Detection of CN−. RSC Adv. 2016, 6, 48351–48356. [Google Scholar] [CrossRef]
- Mallesh, R.; Khan, J.; Pradhan, K.; Roy, R.; Jana, N.R.; Jaisankar, P.; Ghosh, S. Design and Development of Benzothiazole-Based Fluorescent Probes for Selective Detection of Aβ Aggregates in Alzheimer’s Disease. ACS Chem. Neurosci. 2022, 13, 2503–2516. [Google Scholar] [CrossRef]
- Maiti, A.; Manna, S.K.; Halder, S.; Mandal, M.; Karak, A.; Banik, D.; Jana, K.; Mahapatra, A.K. A Benzothiazole-Based Dual Reaction Site Fluorescent Probe for the Selective Detection of Hydrazine in Water and Live Cells. Org. Biomol. Chem. 2022, 20, 4949–4963. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, R.; Wang, X.; Qin, G.; Wu, F.; Wang, C.; Chen, M.; Wang, N.; Wang, Q.; Cao, D. Highly Sensitive Benzothiazole-Based Chemosensors for Detection and Bioimaging of Peroxynitrite in Living Cells. RSC Adv. 2022, 12, 27933–27939. [Google Scholar] [CrossRef]
- Hong, K.-I.; Kim, Y.; Lee, J.H.; Chu, K.H.; Jang, W.-D. Benzothiazole-Based Fluorescent Probe as a Simple and Effective Platform for Functional Mitochondria Imaging. Chem. Biomed. Imaging 2023, 1, 395–402. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Y.; Li, Y.; Li, Y.; Feng, Z.; Fan, X.; Qian, J.; Lin, H. A Historical Review of Aggregation-induced Emission from 2001 to 2020: A Bibliometric Analysis. Aggregate 2022, 3, e152. [Google Scholar] [CrossRef]
- Suman, G.R.; Pandey, M.; Chakravarthy, A.S.J. Review on New Horizons of Aggregation Induced Emission: From Design to Development. Mater. Chem. Front. 2021, 5, 1541–1584. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Naskar, B.; Dhara, A.; Maiti, D.K.; Kukułka, M.; Mitoraj, M.P.; Srebro-Hooper, M.; Prodhan, C.; Chaudhuri, K.; Goswami, S. Aggregation-Induced Emission-Based Sensing Platform for Selective Detection of Zn2+: Experimental and Theoretical Investigations. ChemPhysChem 2019, 20, 1630–1639. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Binder, W.H.; Zhu, J. Hydrogen-Bonded Polymer Nanomedicine with AIE Characteristic for Intelligent Cancer Therapy. ACS Macro. Lett. 2023, 12, 1384–1388. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, L.; Mao, L.; Hu, X.; Wei, Y.; Tan, L.; Chen, Y. AIE Molecules UV-Filtering Effect Improves the Photostability of Organic Solar Cells. Adv. Opt. Mater. 2022, 10, 2200968. [Google Scholar] [CrossRef]
- Balachandran, Y.L.; Jiang, X. Aggregation-Induced Fluorogens in Bio-Detection, Tumor Imaging, and Therapy: A Review. CCS Chem. 2022, 4, 420–436. [Google Scholar] [CrossRef]
- Rao Cheekatla, S.; Murale, D.P.; Gopala, L.; Lee, J. Sensing and Imaging Agents for Cyclooxygenase Enzyme. ChemMedChem 2024, e202400636. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593. [Google Scholar] [CrossRef]
- Chakravarty, S.; Roy Chowdhury, S.; Mukherjee, S. AIE Materials for Cancer Cell Detection, Bioimaging and Theranostics. Prog. Mol. Biol. Transl. Sci. 2021, 185, 19–44. [Google Scholar] [PubMed]
- Goshisht, M.K.; Tripathi, N. Fluorescence-Based Sensors as an Emerging Tool for Anion Detection: Mechanism, Sensory Materials and Applications. J. Mater. Chem. C. Mater. 2021, 9, 9820–9850. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, W.; Li, P.; Zhang, W.; Wang, X.; Tang, B. Versatile Fluorescent Probes for Imaging the Superoxide Anion in Living Cells and in Vivo. Angew. Chem. Int. Ed. 2020, 59, 4216–4230. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Chi, Y.; Ma, Y.; Jiang, T.; Wei, X.; Zhao, Q.; Shi, Z.; Shi, J. Novel Fluorescent Probes for the Fluoride Anion Based on Hydroxy-Substituted Perylene Tetra-(Alkoxycarbonyl) Derivatives. RSC Adv. 2018, 8, 14084–14091. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Davis, J.T.; Quesada, R. Anion Transport and Supramolecular Medicinal Chemistry. Chem. Soc. Rev. 2017, 46, 2497–2519. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, D.; Liu, Y.; Pan, H.; Chen, H.; Qu, X.; Li, H. A Dual-Channel and Fast-Response Fluorescent Probe for Selective Detection of HClO and Its Applications in Live Cells. Sens. Actuators. B. Chem. 2019, 299, 126937. [Google Scholar] [CrossRef]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and Fluorescent Anion Sensors: An Overview of Recent Developments in the Use of 1,8-Naphthalimide-Based Chemosensors. Chem. Soc. Rev. 2010, 39, 3936. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent Progress in the Development of Fluorometric and Colorimetric Chemosensors for Detection of Cyanide Ions. Chem. Soc. Rev. 2014, 43, 4312. [Google Scholar] [CrossRef]
- Peng, T.; Li, S.; Zhou, Y.; Liu, R.; Qu, J. Two Cyanoethylene-Based Fluorescence Probes for Highly Efficient Cyanide Detection and Practical Applications in Drinking Water and Living Cells. Talanta 2021, 234, 122615. [Google Scholar] [CrossRef]
- Yan, K.-C.; Steinbrueck, A.; Sedgwick, A.C.; James, T.D. Fluorescent Chemosensors for Ion and Molecule Recognition: The Next Chapter. Front. Sens. 2021, 2, 731928. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kim, H.; Pandey, B.; James, T.D.; Yoon, J.; Anslyn, E.V. Recent Advances in Fluorescent and Colorimetric Chemosensors for the Detection of Chemical Warfare Agents: A Legacy of the 21st Century. Chem. Soc. Rev. 2023, 52, 663–704. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Khan, S.; Shehzadi, S.A.; Gul, Z.; Al-Saidi, H.M.; Waheed Kamran, A.; Alhumaydhi, F.A. Recent Advances in Colorimetric and Fluorescent Chemosensors Based on Thiourea Derivatives for Metallic Cations: A Review. Dye. Pigment. 2022, 205, 110477. [Google Scholar] [CrossRef]
- Torre, P.; Saavedra, L.; Caballero, J.; Quiroga, J.; Alzate-Morales, J.; Cabrera, M.; Trilleras, J. A Novel Class of Selective Acetylcholinesterase Inhibitors: Synthesis and Evaluation of (E)-2-(Benzo[d]Thiazol-2-Yl)-3-Heteroarylacrylonitriles. Molecules 2012, 17, 12072–12085. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, P.; Osorio, E.; Alzate-Morales, J.H.; Caballero, J.; Trilleras, J.; Astudillo-Saavedra, L.; Brito, I.; Cárdenas, A.; Quiroga, J.; Gutiérrez, M. Ultrasound-Assisted Phase-Transfer Catalysis Method in an Aqueous Medium to Promote the Knoevenagel Reaction: Advantages over the Conventional and Microwave-Assisted Solvent-Free/Catalyst-Free Method. Ultrason. Sonochem. 2014, 21, 1666–1674. [Google Scholar] [CrossRef]
- Paramaguru, G.; Solomon, R.V.; Jagadeeswari, S.; Venuvanalingam, P.; Renganathan, R. Tuning the Photophysical Properties of 2-Quinolinone-Based Donor–Acceptor Molecules through N- versus O-Alkylation: Insights from Experimental and Theoretical Investigations. Eur. J. Org. Chem. 2014, 2014, 753–766. [Google Scholar] [CrossRef]
- Ganesan, P.; Chandiran, A.; Gao, P.; Rajalingam, R.; Grätzel, M.; Nazeeruddin, M.K. Molecular Engineering of 2-Quinolinone Based Anchoring Groups for Dye-Sensitized Solar Cells. J. Phys. Chem. C. 2014, 118, 16896–16903. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer US: Boston, MA, USA, 2006; pp. 1–26. ISBN 978-0-387-46312-4. [Google Scholar]
- Lakowicz, J.R. Solvent and Environmental Effects BT—Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer US: Boston, MA, USA, 2006. [Google Scholar]
- Mohd, Y.C.N.N.; Idris, A.; Zainal, A.Z.H.; Tajuddin, H.A.; Abdullah, Z. Intramolecular charge transfer-induced solvatochromism and large Stokes shifts of furocoumarins. Mater. Chem. Phys. 2022, 276, 125406. [Google Scholar] [CrossRef]
- Bakalska, R.; Todorova, M.; Sbirkova, H.; Shivachev, B.; Kolev, T. Comparing of the crystal structure and spectroscopic properties of some stilbazolium dyes with enlarged π-conjugated system I. Chromophores with p-dimethylamino group. Dye. Pigment. 2017, 136, 919–929. [Google Scholar] [CrossRef]
- Sachdeva, T.; Milton, M.D. AIEE Active Novel Red-Emitting D-π-A Phenothiazine Chalcones Displaying Large Stokes Shift, Solvatochromism and “Turn-on” Reversible Mechanofluorochromism. Dye. Pigment. 2020, 181, 108539. [Google Scholar] [CrossRef]
- Enbanathan, S.; Munusamy, S.; Ponnan, S.; Jothi, D.; Manoj Kumar, S.; Sathiyanarayanan, K.I. AIE Active Luminous Dye with a Triphenylamine Attached Benzothiazole Core as a Portable Polymer Film for Sensitively Detecting CN− Ions in Food Samples. Talanta 2023, 264, 124726. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Kumar, A.; Chowdhury, A.; Mukherjee, P.S. Aggregation-Induced Emission and White Luminescence from a Combination of π-Conjugated Donor–Acceptor Organic Luminogens. ACS Omega 2018, 3, 13757–13771. [Google Scholar] [CrossRef] [PubMed]
- Bouali, W.; Yaman, M.; Seferoğlu, N.; Seferoğlu, Z. Colorimetric and Fluorimetric Detection of CN– Ion Using a Highly Selective and Sensitive Chemosensor Derived from Coumarin-Hydrazone. J Photochem. Photobiol. A Chem. 2024, 448, 115227. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality Third Edition Incorporating The First And Second Addenda Volume 1 Recommendations Geneva 2008 WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2008; ISBN 9789241547611. [Google Scholar]
- Siddiqui, S.A.; Abdullah, M.M. Molecular Modeling and Simulation of Some Efficient Charge Transfer Materials Using Density Functional Theory. Mater Today Commun 2020, 22, 100788. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A. Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond-Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2006. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
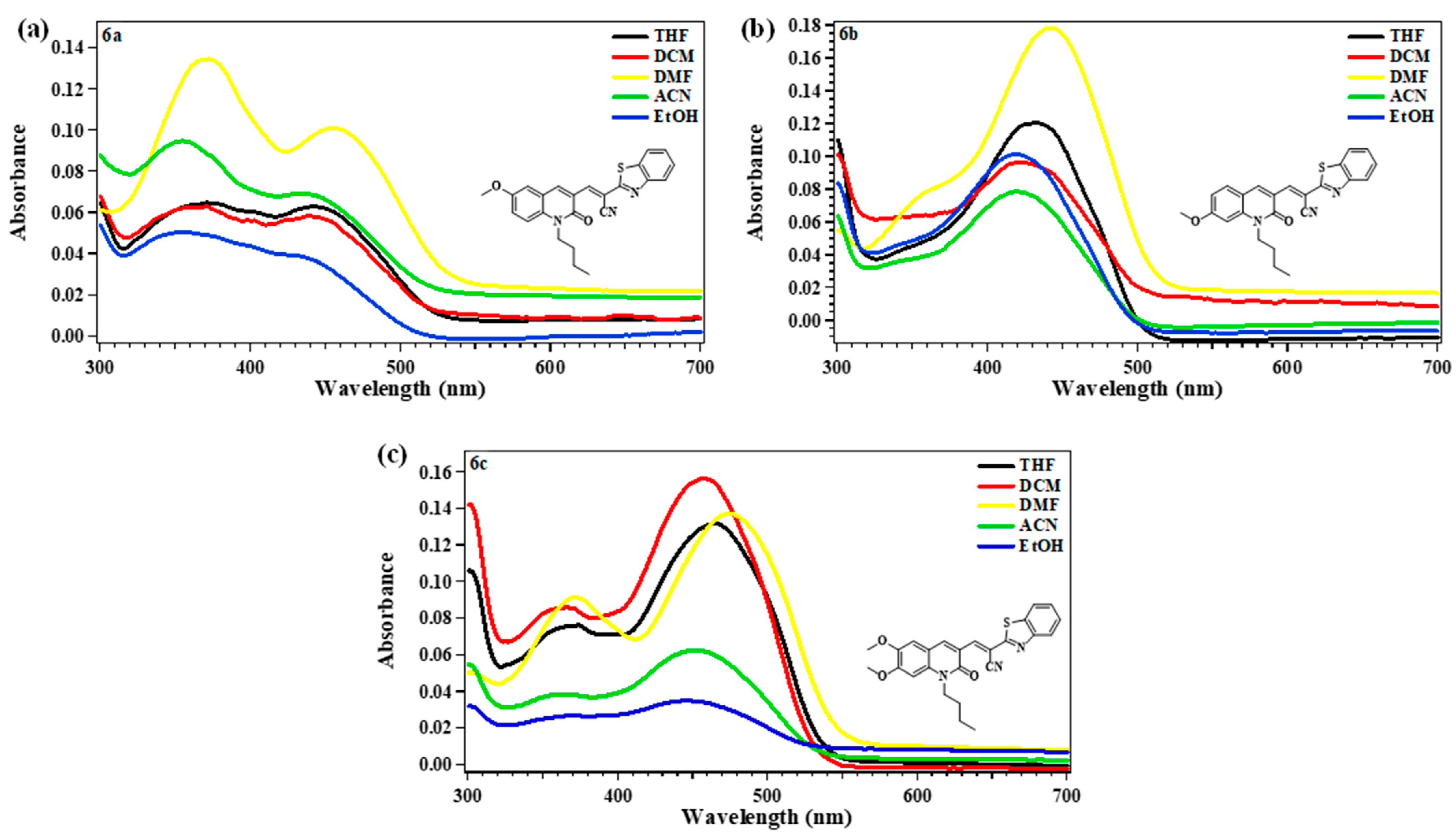
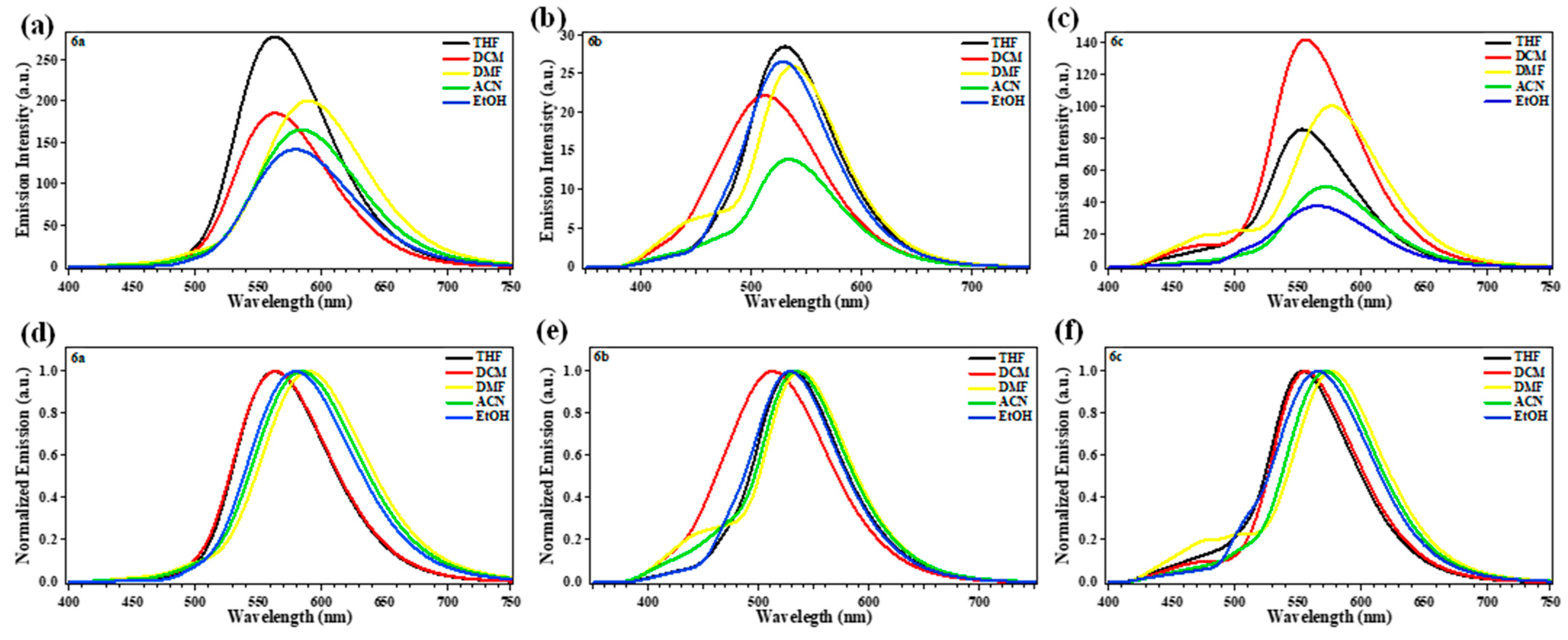

| Comp. | 6a | 6b | 6c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Solvents | Abs, nm (ε/M−1cm−1) | Em, nm | Stokes Shift nm (cm−1) | Abs, nm (ε/M−1cm−1) | Em, nm | Stokes Shift nm (cm−1) | Abs, nm (ε/M−1cm−1) | Em, nm | Stokes Shift nm (cm−1) |
| THF | 441 (12,600) | 563 | 122 (4913) | 431 (24,140) | 529 | 98 (4298) | 464 (26,380) | 553 | 89 (3468) |
| DCM | 438 (11,700) | 563 | 125 (5069) | 422 (19,360) | 512 | 90 (4165) | 456 (31,340) | 556 | 100 (3944) |
| DMF | 454 (20,240) | 590 | 136 (5077) | 442 (35,720) | 535 | 93 (3932) | 473 (27,480) | 576 | 103 (3780) |
| ACN | 433 (13,880) | 584 | 151 (5971) | 419 (15,760) | 532 | 113 (5069) | 449 (12,500) | 572 | 123 (4789) |
| EtOH | 438 (7620) | 578 | 140 (5530) | 418 (15,760) | 528 | 110 (4984) | 445 (7040) | 565 | 120 (4772) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutis-Ayala, M.; Trilleras, J.; D’Vries, R.; Macías, M.A.; Insuasty, A.; Abonia, R.; Quiroga, J.; Illicachi, L.A.; Márquez, E.; Insuasty, D. Methoxyquinolone–Benzothiazole Hybrids as New Aggregation-Induced Emission Luminogens and Efficient Fluorescent Chemosensors for Cyanide Ions. Int. J. Mol. Sci. 2024, 25, 12896. https://doi.org/10.3390/ijms252312896
Mutis-Ayala M, Trilleras J, D’Vries R, Macías MA, Insuasty A, Abonia R, Quiroga J, Illicachi LA, Márquez E, Insuasty D. Methoxyquinolone–Benzothiazole Hybrids as New Aggregation-Induced Emission Luminogens and Efficient Fluorescent Chemosensors for Cyanide Ions. International Journal of Molecular Sciences. 2024; 25(23):12896. https://doi.org/10.3390/ijms252312896
Chicago/Turabian StyleMutis-Ayala, Mario, Jorge Trilleras, Richard D’Vries, Mario A. Macías, Alberto Insuasty, Rodrigo Abonia, Jairo Quiroga, Luis A. Illicachi, Edgar Márquez, and Daniel Insuasty. 2024. "Methoxyquinolone–Benzothiazole Hybrids as New Aggregation-Induced Emission Luminogens and Efficient Fluorescent Chemosensors for Cyanide Ions" International Journal of Molecular Sciences 25, no. 23: 12896. https://doi.org/10.3390/ijms252312896
APA StyleMutis-Ayala, M., Trilleras, J., D’Vries, R., Macías, M. A., Insuasty, A., Abonia, R., Quiroga, J., Illicachi, L. A., Márquez, E., & Insuasty, D. (2024). Methoxyquinolone–Benzothiazole Hybrids as New Aggregation-Induced Emission Luminogens and Efficient Fluorescent Chemosensors for Cyanide Ions. International Journal of Molecular Sciences, 25(23), 12896. https://doi.org/10.3390/ijms252312896








