Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity
Abstract
1. Introduction
2. Results
2.1. Locomotor Activity of Animals
Analysis of Locomotor Activity Parameters
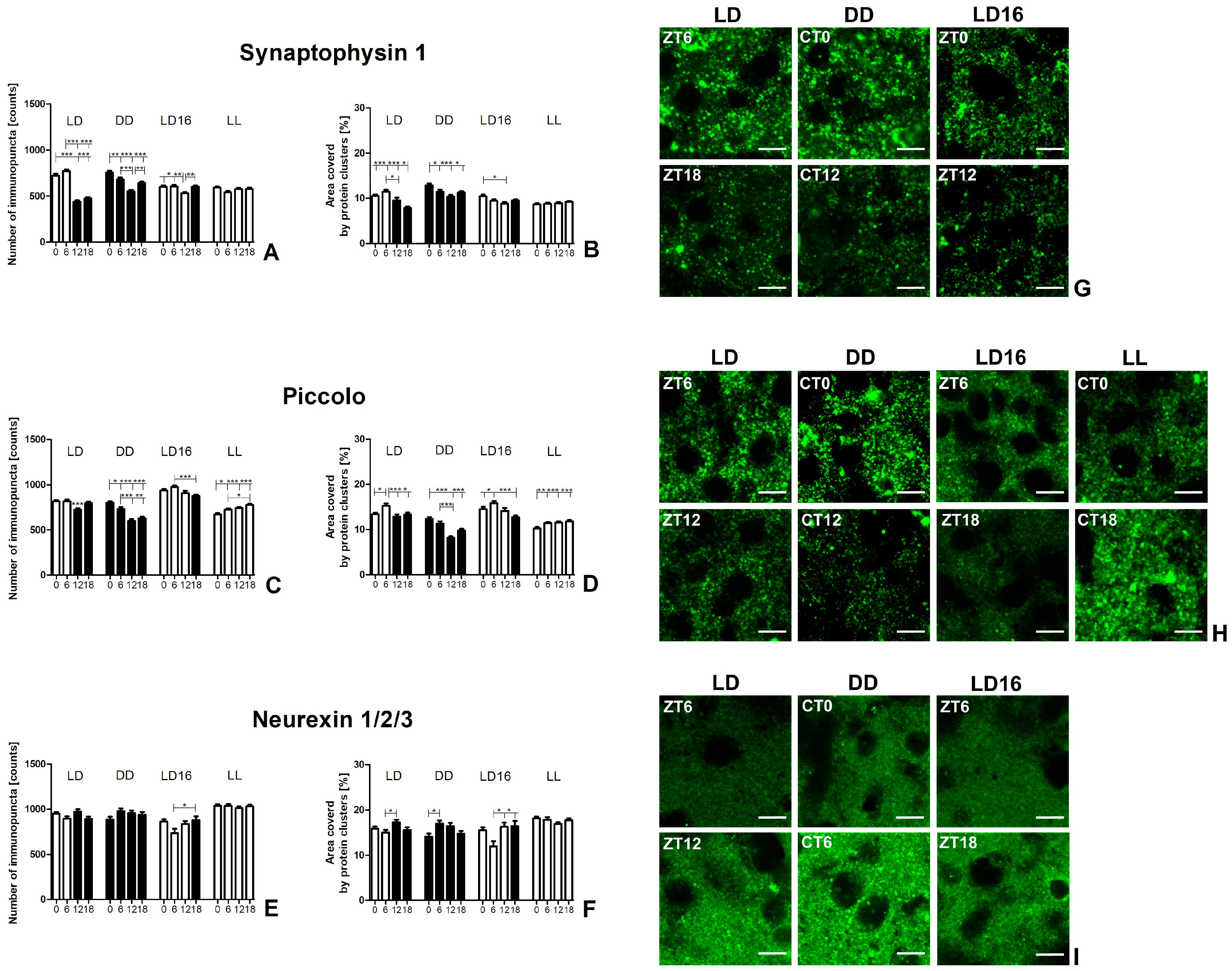
2.2. Immunohistochemical Results
2.2.1. Presynaptic Protein Expression
2.2.2. Postsynaptic Protein Expression
2.2.3. Density of the Excitatory Synapses
2.2.4. Participation of Synaptic Proteins in Excitatory Synapses
3. Discussion
3.1. Light Affects Locomotor Activity and Rhythm Robustness
3.2. Presynaptic Protein Levels Are Clock-Dependent and Modulated by Light
3.3. The Clock-Driven Expression of Postsynaptic Proteins Is Linked to Locomotor Activity and Significantly Modified by Light
3.4. Light Modifies Endogenous Changes in Excitatory Synapses
3.5. Postsynaptic Markers Reveal Distinct Roles of Glutamate Receptors in Circadian Synaptic Plasticity
3.6. Functional Implications and Possible Mechanisms of Circadian Changes in Excitatory Transmission
3.7. Limitations of the Study and Future Directions
4. Materials and Methods
4.1. Animals
4.2. Locomotor Activity Under Different Lighting Conditions
Parameters Related to Daily/Circadian Rhythmicity
4.3. Immunohistochemistry Procedure
4.3.1. Fixation and Sectioning
4.3.2. Double-Immunofluorescence Staining and Confocal Laser Scanning Microscopy
4.3.3. Image Acquiring
4.3.4. Quantitative Analysis of Synaptic Protein Expression and Colocalization Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Guilding, C.; Piggins, H.D. Challenging the Omnipotence of the Suprachiasmatic Timekeeper: Are Circadian Oscillators Present throughout the Mammalian Brain? Eur. J. Neurosci. 2007, 25, 3195–3216. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular Components of the Mammalian Circadian Clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef]
- Pilorz, V.; Helfrich-Förster, C.; Oster, H. The Role of the Circadian Clock System in Physiology. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 227–239. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Hannou, L.; Roy, P.; Ballester Roig, M.N.; Mongrain, V. Transcriptional Control of Synaptic Components by the Clock Machinery. Eur. J. Neurosci. 2020, 51, 241–267. [Google Scholar] [CrossRef]
- Shapiro-Reznik, M.; Jilg, A.; Lerner, H.; Earnest, D.J.; Zisapel, N. Diurnal Rhythms in Neurexins Transcripts and Inhibitory/Excitatory Synapse Scaffold Proteins in the Biological Clock. PLoS ONE 2012, 7, e37894. [Google Scholar] [CrossRef]
- Sarowar, T.; Chhabra, R.; Vilella, A.; Boeckers, T.M.; Zoli, M.; Grabrucker, A.M. Activity and Circadian Rhythm Influence Synaptic Shank3 Protein Levels in Mice. J. Neurochem. 2016, 887–895. [Google Scholar] [CrossRef]
- Frank, M.G. Renormalizing Synapses in Sleep: The Clock Is Ticking. Biochem. Pharmacol. 2021, 191, 114533. [Google Scholar] [CrossRef]
- Jasinska, M.; Pyza, E. Circadian Plasticity of Mammalian Inhibitory Interneurons. Neural Plast. 2017, 2017, 6373412. [Google Scholar] [CrossRef]
- Krzeptowski, W.; Hess, G.; Pyza, E. Circadian Plasticity in the Brain of Insects and Rodents. Front. Neural Circuits 2018, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, A.; Jordán-Álvarez, S.; Santana, E.; Jarabo, P.; Casas-Tintó, S.; Ferrús, A. Molecular Mechanisms That Change Synapse Number. J. Neurogenet. 2018, 32, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.E.; Miyanishi, K.; Takeda, H.; Islam, A.; Matsuoka, N.; Kubo, M.; Matsumoto, S.; Kunieda, T.; Nomoto, M.; Yano, H.; et al. Phagocytic Elimination of Synapses by Microglia during Sleep. Glia 2020, 68, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Smies, C.W.; Bodinayake, K.K.; Kwapis, J.L. Time to Learn: The Role of the Molecular Circadian Clock in Learning and Memory. Neurobiol. Learn. Mem. 2022, 193, 107651. [Google Scholar] [CrossRef]
- Lyons, L.C. Critical Role of the Circadian Clock in Memory Formation: Lessons from Aplysia. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef]
- Merrow, M.; Spoelstra, K.; Roenneberg, T. The Circadian Cycle: Daily Rhythms from Behaviour to Genes. EMBO Rep. 2005, 6, 930–935. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of Circadian Rhythms. Alcohol. Res. Health 2001, 25, 85–93. [Google Scholar]
- Hughes, A.T.L.; Piggins, H.D. Feedback Actions of Locomotor Activity to the Circadian Clock. Prog. Brain Res. 2012, 199, 305–336. [Google Scholar]
- von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. [Google Scholar] [CrossRef]
- Schröder, J.K.; Abdel-Hafiz, L.; Ali, A.A.H.; Cousin, T.C.; Hallenberger, J.; Rodrigues Almeida, F.; Anstötz, M.; Lenz, M.; Vlachos, A.; von Gall, C.; et al. Effects of the Light/Dark Phase and Constant Light on Spatial Working Memory and Spine Plasticity in the Mouse Hippocampus. Cells 2023, 12, 1758. [Google Scholar] [CrossRef]
- Tournier, B.B.; Menet, J.S.; Dardente, H.; Poirel, V.J.; Malan, A.; Masson-Pévet, M.; Pévet, P.; Vuillez, P. Photoperiod Differentially Regulates Clock Genes’ Expression in the Suprachiasmatic Nucleus of Syrian Hamster. Neuroscience 2003, 118, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, X.; Liang, Y.; Xue, T.; Wang, L.; Bao, J. Plasticity of Light-Induced Concurrent Glutamatergic and GABAergic Quantal Events in the Suprachiasmatic Nucleus. J. Biol. Rhythm. 2018, 33, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, T.; Weil, Z.M.; Nelson, R.J. Photoperiod Affects the Diurnal Rhythm of Hippocampal Neuronal Morphology of Siberian Hamsters. Chronobiol. Int. 2013, 30, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.; Rechtschaffen, A. Circadian Temperature and Wake Rhythms of Rats Exposed to Prolonged Continuous Illumination. Physiol. Behav. 1983, 31, 417–427. [Google Scholar] [CrossRef]
- Redlin, U. Neural Basis and Biological Function of Masking by Light in Mammals: Suppression of Melatonin and Locomotor Activity. Chronobiol. Int. 2001, 18, 737–758. [Google Scholar] [CrossRef]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant Light Desynchronizes Mammalian Clock Neurons. Nat. Neurosci. 2005, 8, 267–269. [Google Scholar] [CrossRef]
- Ma, W.-P.; Cao, J.; Tian, M.; Cui, M.-H.; Han, H.-L.; Yang, Y.-X.; Xu, L. Exposure to Chronic Constant Light Impairs Spatial Memory and Influences Long-Term Depression in Rats. Neurosci. Res. 2007, 59, 224–230. [Google Scholar] [CrossRef]
- Elbaz, I.; Zada, D.; Tovin, A.; Braun, T.; Lerer-Goldshtein, T.; Wang, G.; Mourrain, P.; Appelbaum, L. Sleep-Dependent Structural Synaptic Plasticity of Inhibitory Synapses in the Dendrites of Hypocretin/Orexin Neurons. Mol. Neurobiol. 2017, 54, 6581–6597. [Google Scholar] [CrossRef]
- Spiwoks-Becker, I.; Glas, M.; Lasarzik, I.; Vollrath, L. Mouse Photoreceptor Synaptic Ribbons Lose and Regain Material in Response to Illumination Changes. Eur. J. Neurosci. 2004, 19, 1559–1571. [Google Scholar] [CrossRef]
- Jasinska, M.; Grzegorczyk, A.; Woznicka, O.; Jasek, E.; Kossut, M.; Barbacka-Surowiak, G.; Litwin, J.A.; Pyza, E. Circadian Rhythmicity of Synapses in Mouse Somatosensory Cortex. Eur. J. Neurosci. 2015, 42, 2585–2594. [Google Scholar] [CrossRef]
- Honjoh, S.; de Vivo, L.; Okuno, H.; Bito, H.; Tononi, G.; Cirelli, C. Higher Arc Nucleus-to-Cytoplasm Ratio during Sleep in the Superficial Layers of the Mouse Cortex. Front. Neural Circuits 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.E.; Duricka, D.L.; Campbell, K.; Churchill, L.; Krueger, J.M. Homer1a and 1bc Levels in the Rat Somatosensory Cortex Vary with the Time of Day and Sleep Loss. Neurosci. Lett. 2004, 367, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Lu, J.; Fischer, D.; Saper, C.B. A Broad Role for Melanopsin in Nonvisual Photoreception. J. Neurosci. 2003, 23, 7093–7106. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.; Berson, D.M. Central Projections of Melanopsin-expressing Retinal Ganglion Cells in the Mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [PubMed]
- Ruby, N.F.; Brennan, T.J.; Xie, X.; Cao, V.; Franken, P.; Heller, H.C.; O’Hara, B.F. Role of Melanopsin in Circadian Responses to Light. Science 2002, 298, 2211–2213. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; van Doornen, L.J.P.; Buijs, R.M. Light and Diurnal Cycle Affect Autonomic Cardiac Balance in Human; Possible Role for the Biological Clock. Auton. Neurosci. 2004, 110, 44–48. [Google Scholar] [CrossRef]
- Chang, A.-M.; Scheer, F.A.J.L.; Czeisler, C.A.; Aeschbach, D. Direct Effects of Light on Alertness, Vigilance, and the Waking Electroencephalogram in Humans Depend on Prior Light History. Sleep. 2013, 36, 1239–1246. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Götz, T.; Cajochen, C. Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef]
- Vandewalle, G.; Balteau, E.; Phillips, C.; Degueldre, C.; Moreau, V.; Sterpenich, V.; Albouy, G.; Darsaud, A.; Desseilles, M.; Dang-Vu, T.T.; et al. Daytime Light Exposure Dynamically Enhances Brain Responses. Curr. Biol. 2006, 16, 1616–1621. [Google Scholar] [CrossRef]
- Jasińska, M.; Grzegorczyk, A.; Jasek, E.; Litwin, J.A.; Kossut, M.; Barbacka-Surowiak, G.; Pyza, E. Daily Rhythm of Synapse Turnover in Mouse Somatosensory Cortex. Acta Neurobiol. Exp. 2014, 74, 104–110. [Google Scholar] [CrossRef]
- Xiao, B.; Tu, J.C.; Petralia, R.S.; Yuan, J.P.; Doan, A.; Breder, C.D.; Ruggiero, A.; Lanahan, A.A.; Wenthold, R.J.; Worley, P.F. Homer Regulates the Association of Group 1 Metabotropic Glutamate Receptors with Multivalent Complexes of Homer-Related, Synaptic Proteins. Neuron 1998, 21, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.D.; Bear, M.F. New Views of Arc, a Master Regulator of Synaptic Plasticity. Nat. Neurosci. 2011, 14, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Von Der Ohe, C.G.; Garner, C.C.; Darian-Smith, C.; Heller, H.C. Synaptic Protein Dynamics in Hibernation. J. Neurosci. 2007, 27, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Delorme, T.C.; Srivastava, L.K.; Cermakian, N. Altered Circadian Rhythms in a Mouse Model of Neurodevelopmental Disorders Based on Prenatal Maternal Immune Activation. Brain Behav. Immun. 2021, 93, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Jud, C.; Schmutz, I.; Hampp, G.; Oster, H.; Albrecht, U. A Guideline for Analyzing Circadian Wheel-Running Behavior in Rodents under Different Lighting Conditions. Biol. Proced. Online 2005, 7, 101–116. [Google Scholar] [CrossRef]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between Circadian Rhythms and Stress. Neurobiol. Stress. 2017, 6, 57–67. [Google Scholar] [CrossRef]
- Refinetti, R. Comparison of Light, Food, and Temperature as Environmental Synchronizers of the Circadian Rhythm of Activity in Mice. J. Physiol. Sci. 2015. [Google Scholar] [CrossRef]
- González, M.M.C. Dim Light at Night and Constant Darkness: Two Frequently Used Lighting Conditions That Jeopardize the Health and Well-Being of Laboratory Rodents. Front. Neurol. 2018, 9, 609. [Google Scholar] [CrossRef]
- Gonzalez, M.M.C.; Aston-Jones, G. Light Deprivation Damages Monoamine Neurons and Produces a Depressive Behavioral Phenotype in Rats. Proc. Natl. Acad. Sci. USA 2008, 105, 4898–4903. [Google Scholar] [CrossRef]
- Aubrecht, T.G.; Jenkins, R.; Nelson, R.J. Dim Light at Night Increases Body Mass of Female Mice. Chronobiol. Int. 2015, 32, 557–560. [Google Scholar] [CrossRef]
- Feng, T.R.; Li, Z.; Li, S.X. Effects of Constant Light on the Circadian System in Rats. Austin J. Pharmacol. Ther. 2020, 8, 1121. [Google Scholar]
- Depres-Brummer, P.; Levi, F.; Metzger, G.; Touitou, Y. Light-Induced Suppression of the Rat Circadian System. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 268, R1111–R1116. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Sagara, M.; Inoué, S. Continuous Exposure to Dim Illumination Uncouples Temporal Patterns of Sleep, Body Temperature, Locomotion and Drinking Behavior in the Rat. Neurosci. Lett. 2000, 279, 185–189. [Google Scholar] [CrossRef]
- Sudo, M.; Sasahara, K.; Moriya, T.; Akiyama, M.; Hamada, T.; Shibata, S. Constant Light Housing Attenuates Circadian Rhythms of MPer2 MRNA AND MPER2 Protein Expression in the Suprachiasmatic Nucleus of Mice. Neuroscience 2003, 121, 493–499. [Google Scholar] [CrossRef]
- Cirelli, C.; Gutierrez, C.M.; Tononi, G. Extensive and Divergent Effects of Sleep and Wakefulness on Brain Gene Expression. Neuron 2004, 41, 35–43. [Google Scholar] [CrossRef]
- Brüning, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.K.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-Wake Cycles Drive Daily Dynamics of Synaptic Phosphorylation. Science 2019, 366, eaav3617. [Google Scholar] [CrossRef]
- Noya, S.B.; Colameo, D.; Brüning, F.; Spinnler, A.; Mircsof, D.; Opitz, L.; Mann, M.; Tyagarajan, S.K.; Robles, M.S.; Brown, S.A. The Forebrain Synaptic Transcriptome Is Organized by Clocks but Its Proteome Is Driven by Sleep. Science 2019, 366, aav2642. [Google Scholar] [CrossRef]
- Serrano, M.E.; Kim, E.; Petrinovic, M.M.; Turkheimer, F.; Cash, D. Imaging Synaptic Density: The Next Holy Grail of Neuroscience? Front. Neurosci. 2022, 16, 796129. [Google Scholar] [CrossRef]
- Uchigashima, M.; Cheung, A.; Suh, J.; Watanabe, M.; Futai, K. Differential Expression of Neurexin Genes in the Mouse Brain. J. Comp. Neurol. 2019, 527, 1940–1965. [Google Scholar] [CrossRef]
- White, E.L. Ultrastructure and Synaptic Contacts in Barrels of Mouse SI Cortex. Brain Res. 1976, 105, 229–251. [Google Scholar] [CrossRef]
- Evans, G.J.O.; Cousin, M.A. Tyrosine Phosphorylation of Synaptophysin in Synaptic Vesicle Recycling. Biochem. Soc. Trans. 2005, 33, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Aramendy, M.; Seibert, S.; Treppmann, P.; Richter, K.; Ahnert-Hilger, G.; Albrecht, U. Synaptophysin Is Involved in Resetting of the Mammalian Circadian Clock. J. Circadian Rhythm. 2013, 11, 11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gundelfinger, E.D.; Reissner, C.; Garner, C.C. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front. Synaptic Neurosci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Waites, C.L.; Leal-Ortiz, S.A.; Okerlund, N.; Dalke, H.; Fejtova, A.; Altrock, W.D.; Gundelfinger, E.D.; Garner, C.C. Bassoon and Piccolo Maintain Synapse Integrity by Regulating Protein Ubiquitination and Degradation. EMBO J. 2013, 32, 954–969. [Google Scholar] [CrossRef]
- Ivanova, D.; Dirks, A.; Fejtova, A. Bassoon and Piccolo Regulate Ubiquitination and Link Presynaptic Molecular Dynamics with Activity-Regulated Gene Expression. J. Physiol. 2016, 594, 5441–5448. [Google Scholar] [CrossRef]
- Spiwoks-Becker, I.; Maus, C.; tom Dieck, S.; Fejtová, A.; Engel, L.; Wolloscheck, T.; Wolfrum, U.; Vollrath, L.; Spessert, R. Active Zone Proteins Are Dynamically Associated with Synaptic Ribbons in Rat Pinealocytes. Cell Tissue Res. 2008, 333, 185. [Google Scholar] [CrossRef]
- Cooper, J.M.; Halter, K.A.; Prosser, R.A. Circadian Rhythm and Sleep-Wake Systems Share the Dynamic Extracellular Synaptic Milieu. Neurobiol. Sleep. Circadian Rhythm. 2018, 5, 15–36. [Google Scholar] [CrossRef]
- Reissner, C.; Runkel, F.; Missler, M. Neurexins. Genome Biol. 2013, 14, 213. [Google Scholar] [CrossRef]
- Rudenko, G. Dynamic Control of Synaptic Adhesion and Organizing Molecules in Synaptic Plasticity. Neural Plast. 2017, 2017, 6526151. [Google Scholar] [CrossRef]
- Kim, E.; Sheng, M. PDZ Domain Proteins of Synapses. Nat. Rev. Neurosci. 2004, 5, 771–781. [Google Scholar] [CrossRef]
- Xiao, B.; Cheng Tu, J.; Worley, P.F. Homer: A Link between Neural Activity and Glutamate Receptor Function. Curr. Opin. Neurobiol. 2000, 10, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Maret, S.; Dorsaz, S.; Gurcel, L.; Pradervand, S.; Petit, B.; Pfister, C.; Hagenbuchle, O.; O’Hara, B.F.; Franken, P.; Tafti, M. Homer1a Is a Core Brain Molecular Correlate of Sleep Loss. Proc. Natl. Acad. Sci. USA 2007, 104, 20090–20095. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, M.; Paigen, B.; Naidoo, N.; Pack, A.I. Analysis of the QTL for Sleep Homeostasis in Mice: Homer1a Is a Likely Candidate. Physiol. Genom. 2008, 33, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Nirujogi, R.S.; Roth, R.H.; Worley, P.F.; Pandey, A.; Huganir, R.L. Homer1a Drives Homeostatic Scaling-down of Excitatory Synapses during Sleep. Science 2017, 355, 511–515. [Google Scholar] [CrossRef]
- Lu, W.; Khatri, L.; Ziff, E.B. Trafficking of α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor (AMPA) Receptor Subunit GluA2 from the Endoplasmic Reticulum Is Stimulated by a Complex Containing Ca2+/Calmodulin-Activated Kinase II (CaMKII) and PICK1 Protein and by Release o. J. Biol. Chem. 2014, 289, 19218–19230. [Google Scholar] [CrossRef]
- Schneider Gasser, E.M.; Straub, C.J.; Panzanelli, P.; Weinmann, O.; Sassoè-Pognetto, M.; Fritschy, J.-M. Immunofluorescence in Brain Sections: Simultaneous Detection of Presynaptic and Postsynaptic Proteins in Identified Neurons. Nat. Protoc. 2006, 1, 1887–1897. [Google Scholar] [CrossRef]
- van Spronsen, M.; Hoogenraad, C.C. Synapse Pathology in Psychiatric and Neurologic Disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 207–214. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, E. The Postsynaptic Organization of Synapses. Cold Spring Harb. Perspect. Biol. 2011, 3, a005678. [Google Scholar] [CrossRef]
- McLeod, F.; Marzo, A.; Podpolny, M.; Galli, S.; Salinas, P. Evaluation of Synapse Density in Hippocampal Rodent Brain Slices. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Favuzzi, E.; Rico, B. Molecular Diversity Underlying Cortical Excitatory and Inhibitory Synapse Development. Curr. Opin. Neurobiol. 2018, 53, 8–15. [Google Scholar] [CrossRef]
- Lauterborn, J.C.; Scaduto, P.; Cox, C.D.; Schulmann, A.; Lynch, G.; Gall, C.M.; Keene, C.D.; Limon, A. Increased Excitatory to Inhibitory Synaptic Ratio in Parietal Cortex Samples from Individuals with Alzheimer’s Disease. Nat. Commun. 2021, 12, 2603. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, I.; Malinow, R. Postsynaptic Density 95 Controls AMPA Receptor Incorporation during Long-Term Potentiation and Experience-Driven Synaptic Plasticity. J. Neurosci. 2004, 24, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Opazo, P.; Sainlos, M.; Choquet, D. Regulation of AMPA Receptor Surface Diffusion by PSD-95 Slots. Curr. Opin. Neurobiol. 2012, 22, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Coley, A.A.; Gao, W.-J. PSD-95 Deficiency Disrupts PFC-Associated Function and Behavior during Neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef]
- Bredt, D.S.; Nicoll, R.A. AMPA Receptor Trafficking at Excitatory Synapses. Neuron 2003, 40, 361–379. [Google Scholar] [CrossRef]
- Meunier, C.N.J.; Chameau, P.; Fossier, P.M. Modulation of Synaptic Plasticity in the Cortex Needs to Understand All the Players. Front. Synaptic Neurosci. 2017, 9, 2. [Google Scholar] [CrossRef]
- Huemmeke, M.; Eysel, U.T.; Mittmann, T. Metabotropic Glutamate Receptors Mediate Expression of LTP in Slices of Rat Visual Cortex. Eur. J. Neurosci. 2002, 15, 1641–1645. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Li, X.-H.; Zhuo, M. NMDA Receptors and Synaptic Plasticity in the Anterior Cingulate Cortex. Neuropharmacology 2021, 197, 108749. [Google Scholar] [CrossRef]
- Lin, Y.; Skeberdis, V.A.; Francesconi, A.; Bennett, M.V.L.; Zukin, R.S. Postsynaptic Density Protein-95 Regulates NMDA Channel Gating and Surface Expression. J. Neurosci. 2004, 24, 10138–10148. [Google Scholar] [CrossRef]
- Craven, S.E.; El-Husseini, A.E.; Bredt, D.S. Synaptic Targeting of the Postsynaptic Density Protein PSD-95 Mediated by Lipid and Protein Motifs. Neuron 1999, 22, 497–509. [Google Scholar] [CrossRef]
- El-Husseini, A.E.-D.; Schnell, E.; Dakoji, S.; Sweeney, N.; Zhou, Q.; Prange, O.; Gauthier-Campbell, C.; Aguilera-Moreno, A.; Nicoll, R.A.; Bredt, D.S. Synaptic Strength Regulated by Palmitate Cycling on PSD-95. Cell 2002, 108, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Hessler, N.A.; Malinow, R. Activation of Postsynaptically Silent Synapses during Pairing-Induced LTP in CA1 Region of Hippocampal Slice. Nature 1995, 375, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, N.; Ferber, M.; Galante, R.J.; McShane, B.; Hu, J.H.; Zimmerman, J.; Maislin, G.; Cater, J.; Wyner, A.; Worley, P.; et al. Role of Homer Proteins in the Maintenance of Sleep-Wake States. PLoS ONE 2012, 7, e35174. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Chen, X.; Chen, S.; Tan, Y.; Rong, F.; Zhu, J.; Ma, W. Influence of SKF38393 on Changes of Gene Profile in Rat Prefrontal Cortex during Chronic Paradoxical Sleep Deprivation. Behav. Brain Res. 2016, 304, 60–66. [Google Scholar] [CrossRef]
- Henley, J.M.; Wilkinson, K.A. AMPA Receptor Trafficking and the Mechanisms Underlying Synaptic Plasticity and Cognitive Aging. Dialogues Clin. Neurosci. 2013, 15, 11–27. [Google Scholar] [CrossRef]
- Casimiro, T.M.; Nawy, S.; Carroll, R.C. Molecular Mechanisms Underlying Activity-Dependent AMPA Receptor Cycling in Retinal Ganglion Cells. Mol. Cell. Neurosci. 2013, 56, 384–392. [Google Scholar] [CrossRef][Green Version]
- Elmenhorst, D.; Mertens, K.; Kroll, T.; Oskamp, A.; Ermert, J.; Elmenhorst, E.; Wedekind, F.; Beer, S.; Coenen, H.H.; Bauer, A. Circadian Variation of Metabotropic Glutamate Receptor 5 Availability in the Rat Brain. J. Sleep. Res. 2016, 25, 754–761. [Google Scholar] [CrossRef]
- McCauley, J.P.; Petroccione, M.A.; D’Brant, L.Y.; Todd, G.C.; Affinnih, N.; Wisnoski, J.J.; Zahid, S.; Shree, S.; Sousa, A.A.; De Guzman, R.M.; et al. Circadian Modulation of Neurons and Astrocytes Controls Synaptic Plasticity in Hippocampal Area CA1. Cell Rep. 2020, 33, 108255. [Google Scholar] [CrossRef]
- Noguchi, J.; Matsuzaki, M.; Ellis-Davies, G.C.R.; Kasai, H. Spine-Neck Geometry Determines NMDA Receptor-Dependent Ca2+ Signaling in Dendrites. Neuron 2005, 46, 609–622. [Google Scholar] [CrossRef]
- Laperchia, C.; Imperatore, R.; Azeez, I.A.; Del Gallo, F.; Bertini, G.; Grassi-Zucconi, G.; Cristino, L.; Bentivoglio, M. The Excitatory/Inhibitory Input to Orexin/Hypocretin Neuron Soma Undergoes Day/Night Reorganization. Brain Struct. Funct. 2017, 222, 3847–3859. [Google Scholar] [CrossRef]
- Perez-Cruz, C.; Simon, M.; Czéh, B.; Flügge, G.; Fuchs, E. Hemispheric Differences in Basilar Dendrites and Spines of Pyramidal Neurons in the Rat Prelimbic Cortex: Activity- and Stress-Induced Changes. Eur. J. Neurosci. 2009, 29, 738–747. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini, A.E.-D.; Schnell, E.; Chetkovich, D.M.; Nicoll, R.A.; Bredt, D.S. PSD-95 Involvement in Maturation of Excitatory Synapses. Science 2000, 290, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Deller, T.; Korte, M.; Chabanis, S.; Drakew, A.; Schwegler, H.; Stefani, G.G.; Zuniga, A.; Schwarz, K.; Bonhoeffer, T.; Zeller, R.; et al. Synaptopodin-Deficient Mice Lack a Spine Apparatus and Show Deficits in Synaptic Plasticity. Proc. Natl. Acad. Sci. USA 2003, 100, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.; Harris, K.M. Do Thin Spines Learn to Be Mushroom Spines That Remember? Curr. Opin. Neurobiol. 2007, 17, 381–386. [Google Scholar] [CrossRef]
- Jasinska, M.; Jasek-Gajda, E.; Woznicka, O.; Lis, G.J.; Pyza, E.; Litwin, J.A. Circadian Clock Regulates the Shape and Content of Dendritic Spines in Mouse Barrel Cortex. PLoS ONE 2019, 14, e0225394. [Google Scholar] [CrossRef]
- Elibol-Can, B.; Kilic, E.; Yuruker, S.; Jakubowska-Dogru, E. Investigation into the Effects of Prenatal Alcohol Exposure on Postnatal Spine Development and Expression of Synaptophysin and PSD95 in Rat Hippocampus. Int. J. Dev. Neurosci. 2014, 33, 106–114. [Google Scholar] [CrossRef]
- Woods, G.F.; Oh, W.C.; Boudewyn, L.C.; Mikula, S.K.; Zito, K. Loss of PSD-95 Enrichment Is Not a Prerequisite for Spine Retraction. J. Neurosci. 2011, 31, 12129–12138. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Ellis-Davies, G.C.R.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic Spine Geometry Is Critical for AMPA Receptor Expression in Hippocampal CA1 Pyramidal Neurons. Nat. Neurosci. 2001, 4, 1086–1092. [Google Scholar] [CrossRef]
- Bourne, J.N.; Harris, K.M. Balancing Structure and Function at Hippocampal Dendritic Spines. Annu. Rev. Neurosci. 2008, 31, 47–67. [Google Scholar] [CrossRef]
- Fischer, M.; Kaech, S.; Wagner, U.; Brinkhaus, H.; Matus, A. Glutamate Receptors Regulate Actin-Based Plasticity in Dendritic Spines. Nat. Neurosci. 2000, 3, 887–894. [Google Scholar] [CrossRef]
- Chambille, I. Circadian Rhythm of AMPA Receptor GluR2/3 Subunit-Immunoreactivity in the Suprachiasmatic Nuclei of Syrian Hamster and Effect of a Light-Dark Cycle. Brain Res. 1999, 833, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Cirelli, C.; Pfister-Genskow, M.; Faraguna, U.; Tononi, G. Molecular and Electrophysiological Evidence for Net Synaptic Potentiation in Wake and Depression in Sleep. Nat. Neurosci. 2008, 11, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G.G.; Nelson, S.B. Hebb and Homeostasis in Neuronal Plasticity. Curr. Opin. Neurobiol. 2000, 10, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Pozo, K.; Goda, Y. Unraveling Mechanisms of Homeostatic Synaptic Plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef]
- Chung, S.; Son, G.H.; Kim, K. Circadian Rhythm of Adrenal Glucocorticoid: Its Regulation and Clinical Implications. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 581–591. [Google Scholar] [CrossRef]
- Cheifetz, P The Daily Rhythm of the Secretion of Corticotrophin and Corticosterone in Rats and Mice. J. Endocrinol. 1971, 49, xi–xii.
- Ishida, A.; Mutoh, T.; Ueyama, T.; Bando, H.; Masubuchi, S.; Nakahara, D.; Tsujimoto, G.; Okamura, H. Light Activates the Adrenal Gland: Timing of Gene Expression and Glucocorticoid Release. Cell Metab. 2005, 2, 297–307. [Google Scholar] [CrossRef]
- Liston, C.; Cichon, J.M.; Jeanneteau, F.; Jia, Z.; Chao, M.V.; Gan, W.-B. Circadian Glucocorticoid Oscillations Promote Learning- Dependent Synapse Formation and Maintenance. Nat. Neurosci. 2013, 16, 698–705. [Google Scholar] [CrossRef]
- Jasinska, M.; Woznicka, O.; Jasek-Gajda, E.; Lis, G.J.; Pyza, E.; Litwin, J.A. Circadian Changes of Dendritic Spine Geometry in Mouse Barrel Cortex. Front. Neurosci. 2020, 14, 578881. [Google Scholar] [CrossRef]
- Alamilla, J.; Aguilar-Roblero, R. Glutamate and GABA Neurotransmission from the Paraventricular Thalamus to the Suprachiasmatic Nuclei in the Rat. J. Biol. Rhythm. 2010, 25, 28–36. [Google Scholar] [CrossRef]
- Brown, L.A.; Fisk, A.S.; Pothecary, C.A.; Peirson, S.N. Telling the Time with a Broken Clock: Quantifying Circadian Disruption in Animal Models. Biology 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Tackenberg, M.C.; Hughey, J.J. The Risks of Using the Chi-Square Periodogram to Estimate the Period of Biological Rhythms. PLoS Comput. Biol. 2021, 17, e1008567. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. Non-Stationary Time Series and the Robustness of Circadian Rhythms. J. Theor. Biol. 2004, 227, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Seo, D.O.; Bell, E.; Von Gall, C.; Lee, D.C. Strong Resetting of the Mammalian Clock by Constant Light Followed by Constant Darkness. J. Neurosci. 2008, 28, 11839–11847. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.W.; Wicht, H. The Role of the Melatoninergic System in Light-Entrained Behavior of Mice. Int. J. Mol. Sci. 2017, 18, 530. [Google Scholar] [CrossRef]
- Jasinska, M.; Siucinska, E.; Cybulska-Klosowicz, A.; Pyza, E.; Furness, D.N.; Kossut, M.; Glazewski, S. Rapid, Learning-Induced Inhibitory Synaptogenesis in Murine Barrel Field. J. Neurosci. 2010, 30, 1176–1184. [Google Scholar] [CrossRef]
- Negoescu, A.; Labat-Moleur, F.; Lorimier, P.; Lamarcq, L.; Guillermet, C.; Chambaz, E.; Brambilla, E. F(Ab) Secondary Antibodies: A General Method for Double Immunolabeling with Primary Antisera from the Same Species. Efficiency Control by Chemiluminescence. J. Histochem. Cytochem. 1994, 42, 433–437. [Google Scholar] [CrossRef]
- Brouns, I.; Van Nassauw, L.; Van Genechten, J.; Majewski, M.; Scheuermann, D.W.; Timmermans, J.P.; Adriaensen, D. Triple Immunofluorescence Staining with Antibodies Raised in the Same Species to Study the Complex Innervation Pattern of Intrapulmonary Chemoreceptors. J. Histochem. Cytochem. 2002, 50, 575–582. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M.T.Z. (Eds.) Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies: Carlsbad, CA, USA, 2010. [Google Scholar]
- Silver, M.A.; Stryker, M.P. A Method for Measuring Colocalization of Presynaptic Markers with Anatomically Labeled Axons Using Double Label Immunofluorescence and Confocal Microscopy. J. Neurosci. Methods 2000, 94, 205–215. [Google Scholar] [CrossRef]
- Zinchuk, V.; Zinchuk, O.; Okada, T. Quantitative Colocalization Analysis of Multicolor Confocal Immunofluorescence Microscopy Images: Pushing Pixels to Explore Biological Phenomena. Acta Histochem. Cytochem. 2007, 40, 101–111. [Google Scholar] [CrossRef]
- Scriven, D.R.L.; Lynch, R.M.; Moore, E.D.W. Image Acquisition for Colocalization Using Optical Microscopy. Am. J. Physiol. Cell Physiol. 2008, 1119–1122. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A Guided Tour into Subcellular Colocalization Analysis in Light Microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]





| Parameter | Definition |
|---|---|
| Tau | The period of the daily (day–night) or circadian (subjective day–subjective night) rhythm. |
| Delta | The shift (phase advance or phase delay) of the activity onset. |
| Alpha | The duration of the activity phase; the time between the onset and offset of activity. |
| Rho | The duration of the rest phase; determined by the total cycle period (tau) and the activity phase (alpha). |
| Overall activity | The total number of wheel revolutions during one day–night or subjective day–subjective night cycle. |
| Qp | The robustness of the daily (day–night) or circadian (subjective day–subjective night) rhythm; an indication of the rhythm’s stability. |
| Night/subjective night activity | The number of wheel revolutions during the night (ZT12-ZT24) or subjective night (CT12-CT24). |
| Day/subjective day activity | The number of wheel revolutions during the day (ZT0-ZT12) or subjective day (CT0-CT12). |
| Activity phase | The number of wheel revolutions during the activity phase (alpha). |
| Rest phase | The number of wheel revolutions during the rest phase (rho). |
| Protein | Endogenous Effect (DD Conditions) | Effect of Light (12 h Light) | Effect of Prolonged Light (16 h Light) | Effect of Constant Light (24 h Light) | |
|---|---|---|---|---|---|
| Presynaptic proteins | Syp1 | increase during the day ↑CT0 | enhancing the cyclic changes ↑ZT6 | maintaining the cyclic changes ↑ZT0 | masking cyclic changes |
| Pic | increase during the day ↑CT0, ↑CT6 | maintaining the cyclic changes ↑ZT6 | maintaining the cyclic changes ↑ZT0, ↑ZT6 | decrease during the day ↓CT0 * | |
| NRXN | increase during the day ↑CT6 | decrease during the day ↓ZT6 | decrease during the day ↓ZT6 | masking cyclic changes | |
| Postsynaptic proteins | PSD95 | decrease at night ↓CT12 | increase at night ↑ZT12, ↑ZT18 | masking cyclic changes | masking cyclic changes |
| Hom1 | increase in the middle of the day and the night ↑CT6, ↑CT18 | masking cyclic changes | maintaining cyclic changes at night and an increase at the beginning of the day ↑ZT0, ↑ZT18 | maintaining cyclic changes ↑CT6, ↑CT18 * | |
| PICK1 | increase at the beginning of the day and in the middle of the night ↑CT0, ↑CT18 | maintaining during the day and masking cyclic changes at night ↑ZT0, ↑ZT6 * | masking cyclic changes | masking cyclic changes | |
| Synapse | Endogenous Effect (DD Conditions) | Effect of Light (12 h Light) | Effect of Prolonged Light (16 h Light) | Effect of Constant Light (24 h Light) |
|---|---|---|---|---|
| Syp1+/PSD95+ | increase during the day, and decrease at night ↑CT0, ↓CT12 | increase during the day ↑ZT0, ↑ZT6 | increase during the day ↑ZT0 | masking endogenic changes |
| Pic+/Hom1+ | decrease at night ↓CT12 | increase during the day ↑ZT0, ↑ZT6 | decrease at ZT12 ↓ZT12 | masking endogenic changes |
| NRXN+/PICK1+ | decrease followed by an increase at night ↓CT12 ↑CT18 | decrease at night ↓ZT18 | masking endogenic changes | masking endogenic changes |
| Summary of excitatory synapses | decrease in the night ↓CT12 | increase in the day and decrease at night ↑ZT0, ↑ZT6, ↓ZT18 | masking endogenic changes | masking endogenic changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, M.; Jasek-Gajda, E.; Ziaja, M.; Litwin, J.A.; Lis, G.J.; Pyza, E. Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity. Int. J. Mol. Sci. 2024, 25, 12870. https://doi.org/10.3390/ijms252312870
Jasińska M, Jasek-Gajda E, Ziaja M, Litwin JA, Lis GJ, Pyza E. Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity. International Journal of Molecular Sciences. 2024; 25(23):12870. https://doi.org/10.3390/ijms252312870
Chicago/Turabian StyleJasińska, Małgorzata, Ewa Jasek-Gajda, Marek Ziaja, Jan A. Litwin, Grzegorz J. Lis, and Elżbieta Pyza. 2024. "Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity" International Journal of Molecular Sciences 25, no. 23: 12870. https://doi.org/10.3390/ijms252312870
APA StyleJasińska, M., Jasek-Gajda, E., Ziaja, M., Litwin, J. A., Lis, G. J., & Pyza, E. (2024). Light-Modulated Circadian Synaptic Plasticity in the Somatosensory Cortex: Link to Locomotor Activity. International Journal of Molecular Sciences, 25(23), 12870. https://doi.org/10.3390/ijms252312870






