Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities
Abstract
1. Introduction
2. Methods
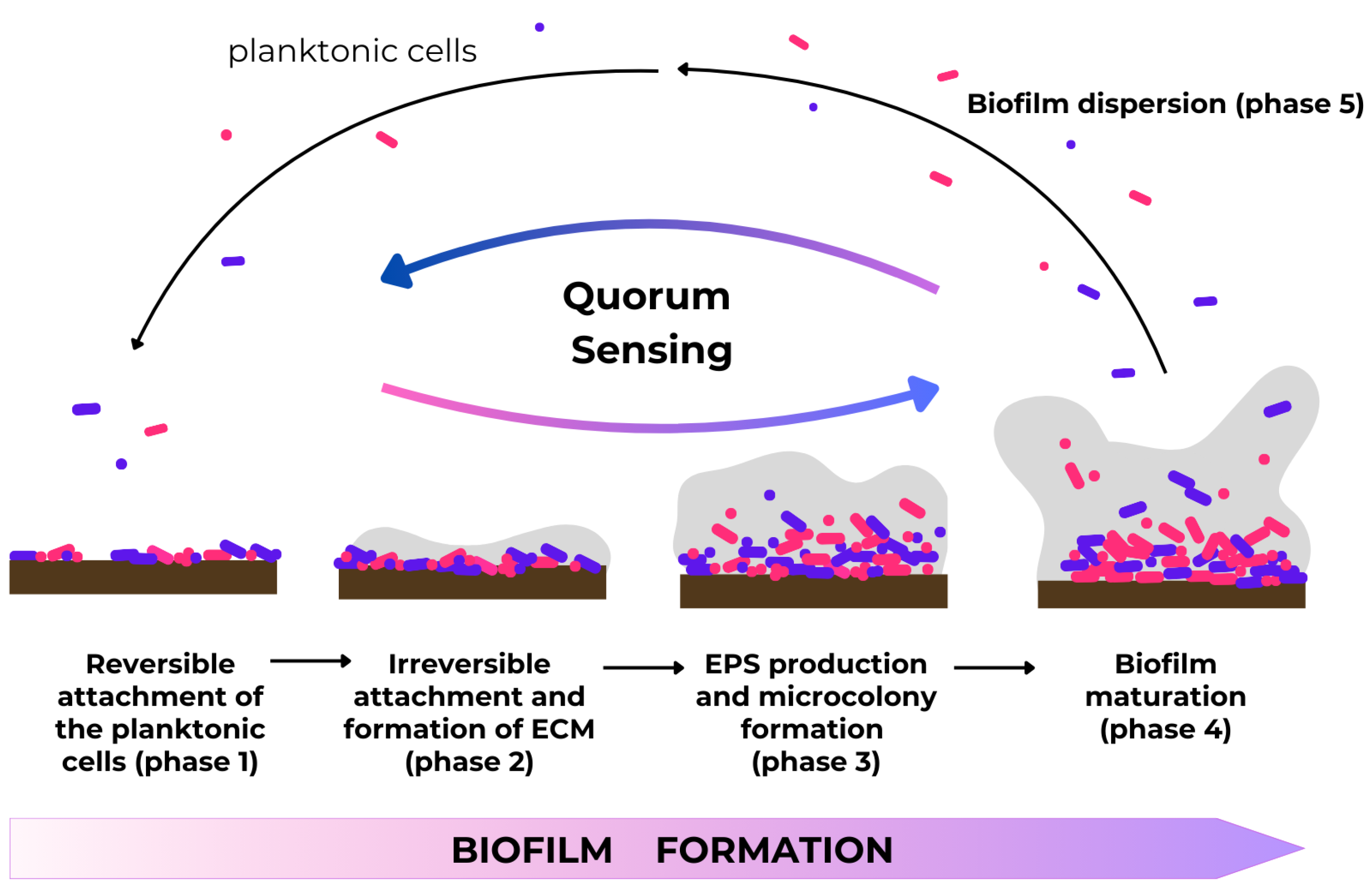
3. Biofilms and Their Medical Impacts
- intrauterine devices, e.g., as long-term contraception methods [27].
4. Quorum Sensing and Quorum-Sensing Molecules (QSMs)
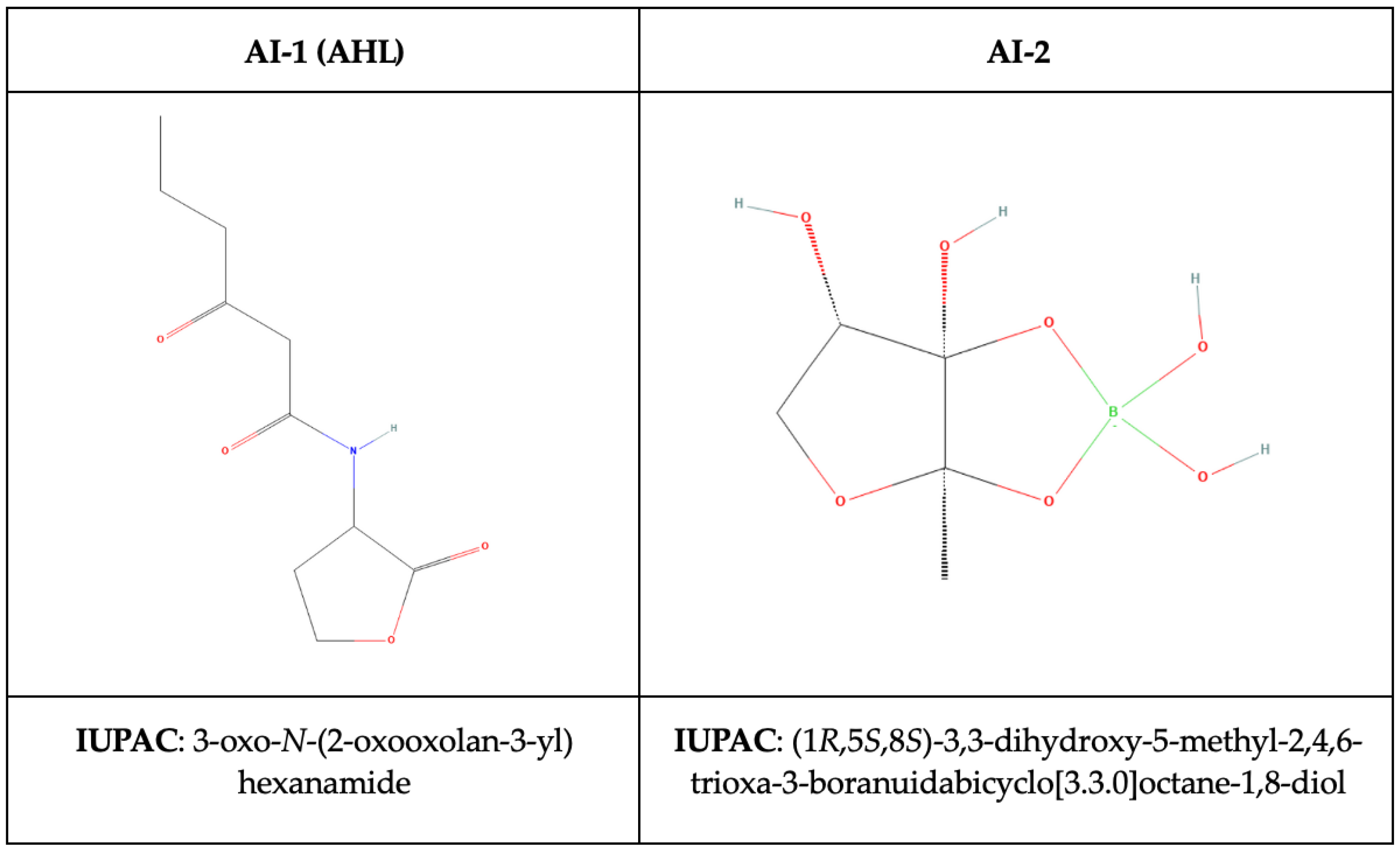
- N-Acyl homoserine lactones (AHL, autoinducer type-1, AI-1) specific to Gram-negative bacteria,
- autoinducer-2 molecules (AI-2), consisting of 4,5-dihydroxy-2,3-pentandedione (DPD) derivatives in both Gram-negative and Gram-positive bacteria,
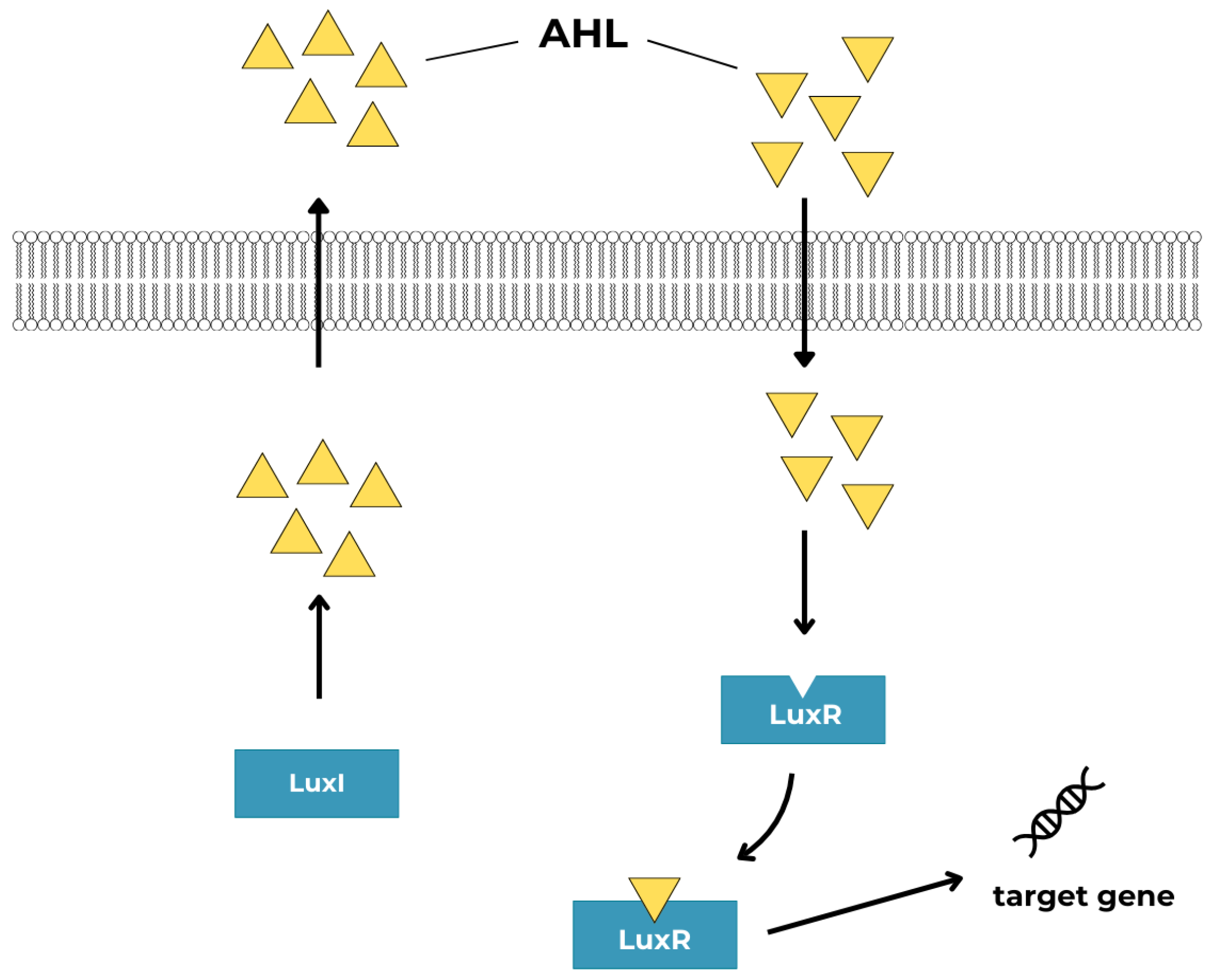
4.1. N-Acyl-Homoserine Lactones (AHLs)
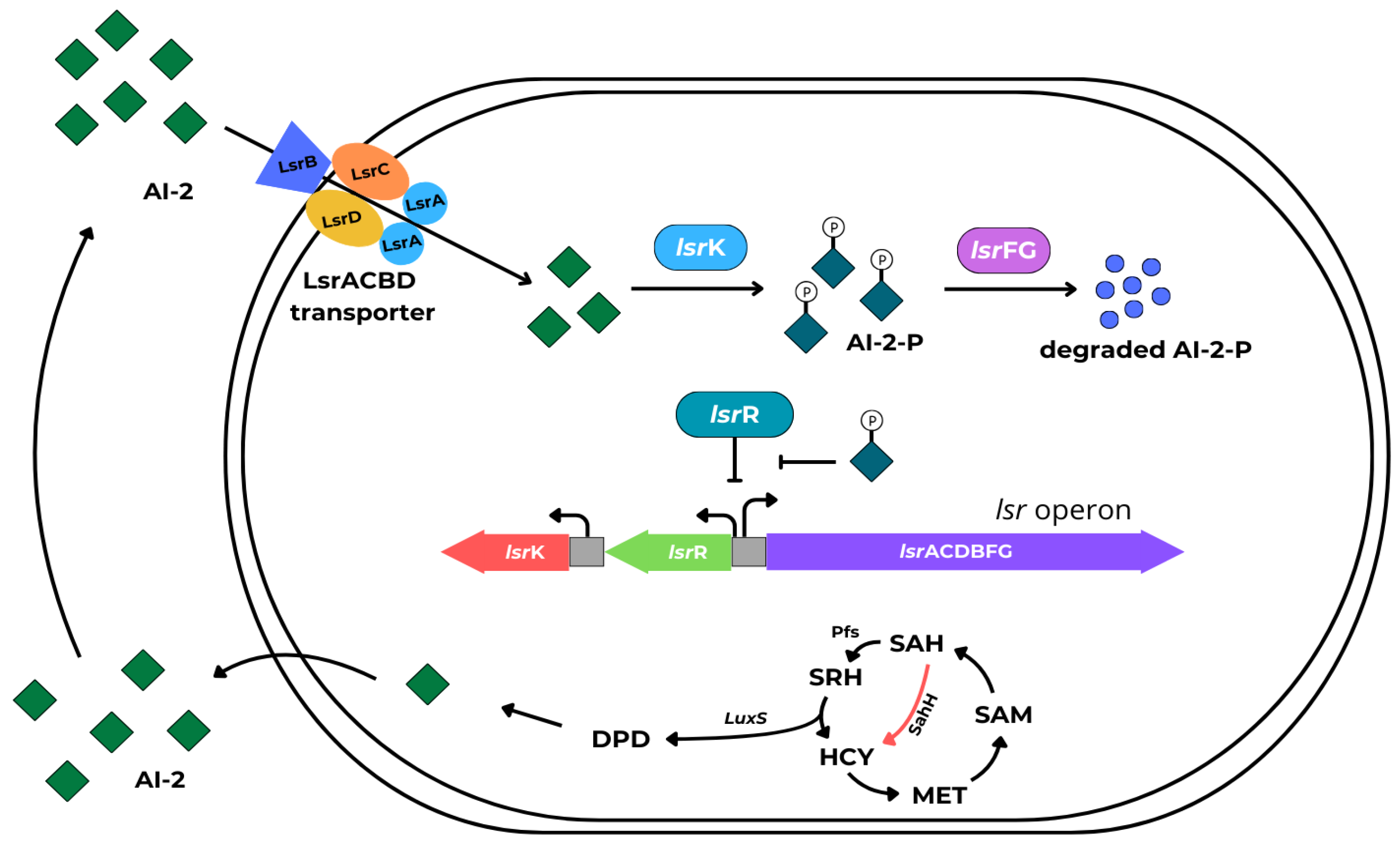
4.2. Autoinducer-2 (AI-2)
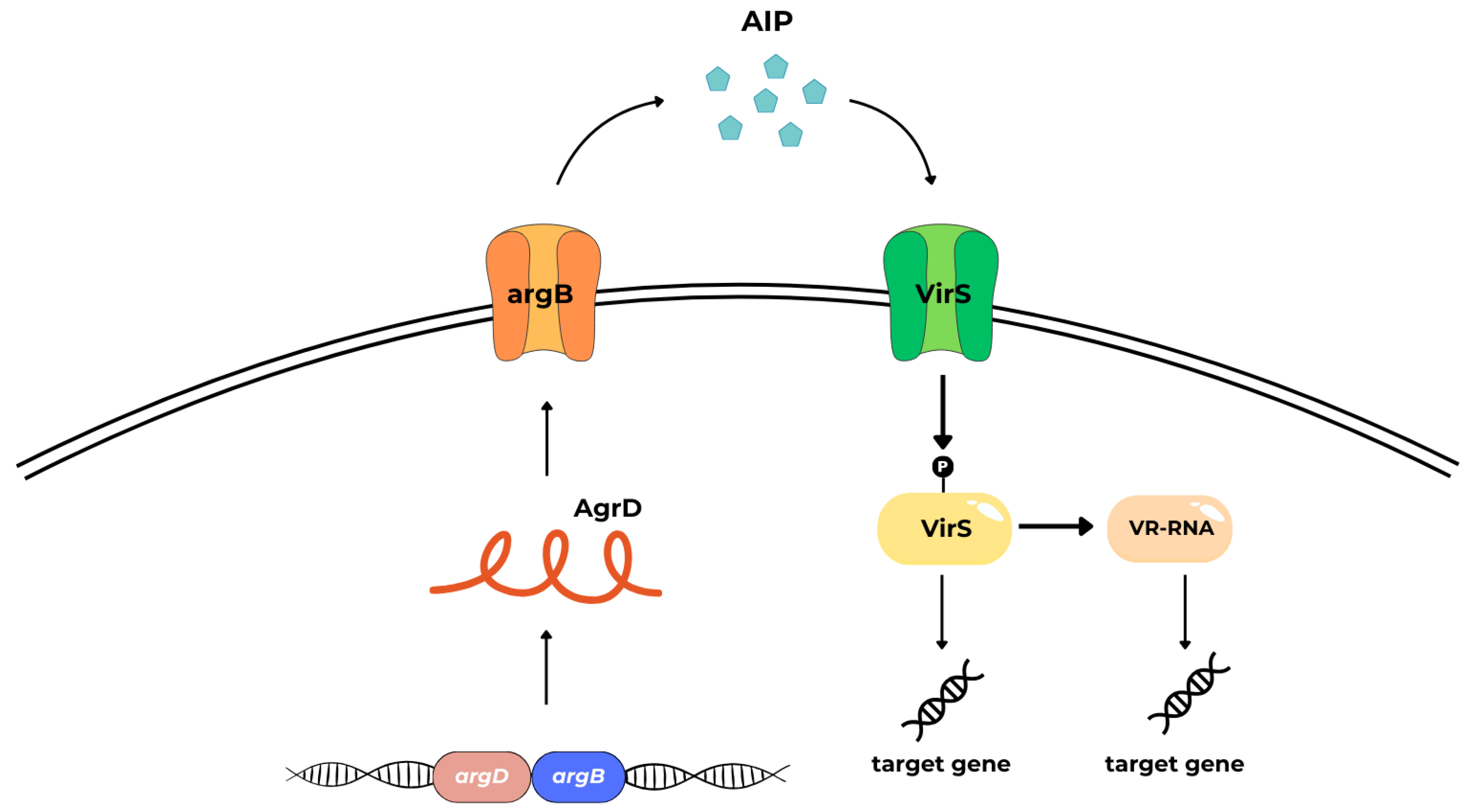
4.3. Autoinducing Peptides (AIPs)
- cytosolic response regulator acts as a transcription factor (AgrA),
- histidine kinase cell surface receptor (AgrC),
- ~45 residue pro-peptide precursor of the mature AIP (AgrD),
- transmembrane cysteine endopeptidase (AgrB) [39].
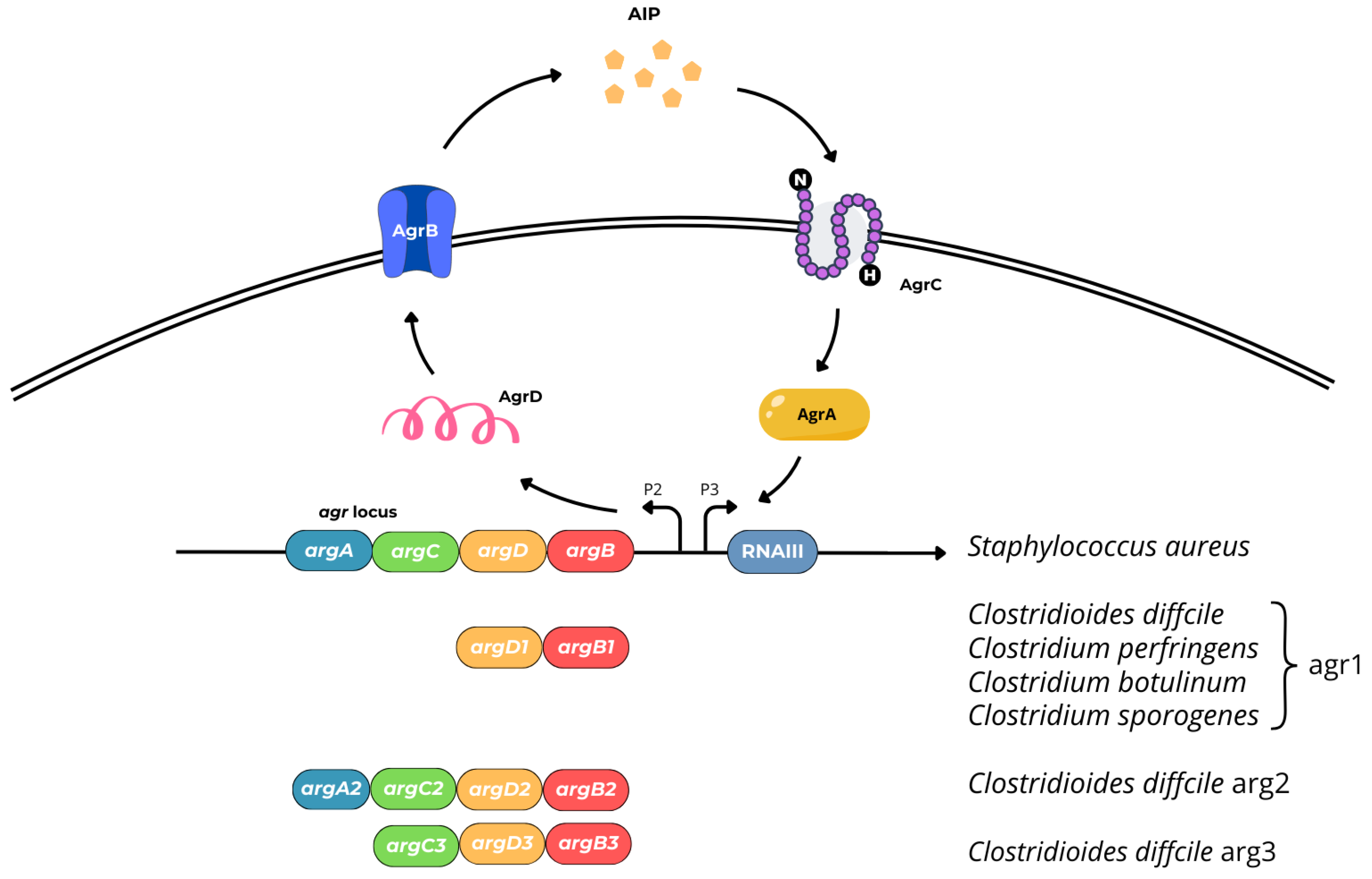
5. Prevention of Biofilm Formation by Quorum Quenching (QQ) and Perspectives of QQ Application
- inactivation or enzymatic degradation of signaling molecules.
- competition with signaling molecules; competing with inducers for the same binding site or binding the receptor noncompetitively.
- blocking of signal transduction cascades.
5.1. Inhibitors of AHL-Mediated Quorum Sensing
5.2. Inhibitors of AI-2-Mediated Quorum Sensing
5.3. Inhibitors of Agr-like Quorum Sensing
6. Summary, Constraints, and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.F. Anthony Van Leeuwenhoek, the First Bacteriologist. Sci. Mon. 1921, 12, 150–160. [Google Scholar]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J.W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef]
- Jendresen, M.D.; Glantz, P.O.; Baier, R.E.; Eick, J.D. Microtopography and clinical adhesiveness of an acid etched tooth surface. An in-vivo study. Acta Odontol. Scand. 1981, 39, 47–53. [Google Scholar] [CrossRef]
- Majewska, A.; Kierzkowska, M.; Kawecki, D. What we actually know about the pathogenicity of Bacteroides pyogenes. Med. Microbiol. Immunol. 2021, 210, 157–163. [Google Scholar] [CrossRef]
- Kierzkowska, M.; Majewska, A.; Kuthan, R.T.; Sawicka-Grzelak, A.; Mlynarczyk, G. A comparison of Api 20A vs MALDI-TOF MS for routine identification of clinically significant anaerobic bacterial strains to the species level. J. Microbiol. Methods 2013, 92, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Boyanova, L.; Justesen, U.S.; ESCMID Study Group of Anaerobic Infections. How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories. Clin. Microbiol. Infect. 2018, 24, 1139–1148. [Google Scholar] [CrossRef]
- Percival, S.L.; Malone, M.; Mayer, D.; Salisbury, A.M.; Schultz, G. Role of anaerobes in polymicrobial communities and biofilms complicating diabetic foot ulcers. Int. Wound J. 2018, 15, 776–782. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Vestby, L.K.; Gronseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jiang, W.; Jiang, Y.; Dong, J.; Song, Z.; Xu, J.; Zhou, W. Anti-biofilm activities of coumarin as quorum sensing inhibitor for Porphyromonas gingivalis. J. Oral. Microbiol. 2022, 14, 2055523. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Minarovits, J. Anaerobic bacterial communities associated with oral carcinoma: Intratumoral, surface-biofilm and salivary microbiota. Anaerobe 2021, 68, 102300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Urban, E.; Gajdacs, M.; Torkos, A. The incidence of anaerobic bacteria in adult patients with chronic sinusitis: A prospective, single-centre microbiological study. Eur. J. Microbiol. Immunol. (Bp) 2020, 10, 107–114. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontol. 2000 2022, 89, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Microbiology and management of joint and bone infections due to anaerobic bacteria. J. Orthop. Sci. 2008, 13, 160–169. [Google Scholar] [CrossRef]
- Varin-Simon, J.; Colin, M.; Velard, F.; Tang-Fichaux, M.; Ohl, X.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Cutibacterium acnes biofilm formation is influenced by bone microenvironment, implant surfaces and bacterial internalization. BMC Microbiol. 2024, 24, 270. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.P.; Sutter, V.L.; Finegold, S.M. Bone infections involving anaerobic bacteria. Medicine 1978, 57, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Hillier, S.L.; Bass, D.C.; Ness, R.B.; Evaluation, P.I.D.; Clinical Health study, i. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin. Infect. Dis. 2004, 39, 990–995. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Meyn, L.A.; Darville, T.; Macio, I.S.; Hillier, S.L. A Randomized Controlled Trial of Ceftriaxone and Doxycycline, With or Without Metronidazole, for the Treatment of Acute Pelvic Inflammatory Disease. Clin. Infect. Dis. 2021, 72, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Hesam Shariati, S.; Zali, M.R.; Asadzadeh Aghdaei, H.; Sarabi Asiabar, A.; Bokaie, S.; Nomanpour, B.; Sechi, L.A.; Feizabadi, M.M. Detection of enterotoxigenic Bacteroides fragilis in patients with ulcerative colitis. Gut Pathog. 2017, 9, 53. [Google Scholar] [CrossRef]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, S.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K.; et al. Bacteroidetes Species Are Correlated with Disease Activity in Ulcerative Colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.X.; Steed, L.L.; Marculescu, C.E.; Slone, H.S.; Woolf, S.K. Cutibacterium acnes Infection in Orthopedics: Microbiology, Clinical Findings, Diagnostic Strategies, and Management. Orthopedics 2020, 43, 52–61. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Karaiyagowder Govindarajan, D.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef]
- Hayward, C.; Brown, M.H.; Whiley, H. Hospital water as the source of healthcare-associated infection and antimicrobial-resistant organisms. Curr. Opin. Infect. Dis. 2022, 35, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A Brief Recap of Microbial Adhesion and Biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Enigk, K.; Jentsch, H.; Rodloff, A.C.; Eschrich, K.; Stingu, C.S. Activity of five antimicrobial peptides against periodontal as well as non-periodontal pathogenic strains. J. Oral. Microbiol. 2020, 12, 1829405. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Piątkowski, J. The Role of Human Oral Microbiome in Dental Biofilm Formation. In Microbial Biofilms; Dharumadurai, D., Nooruddin, T., Eds.; IntechOpen: Rijeka, Croatia, 2016; Chapter 16; pp. 329–382. [Google Scholar]
- Acemel, R.D.; Govantes, F.; Cuetos, A. Computer simulation study of early bacterial biofilm development. Sci. Rep. 2018, 8, 5340. [Google Scholar] [CrossRef]
- Okuda, K.I.; Nagahori, R.; Yamada, S.; Sugimoto, S.; Sato, C.; Sato, M.; Iwase, T.; Hashimoto, K.; Mizunoe, Y. The Composition and Structure of Biofilms Developed by Propionibacterium acnes Isolated from Cardiac Pacemaker Devices. Front. Microbiol. 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.K.B.; Ballard, J.D. Autoinducing peptide-based quorum signaling systems in Clostridioides difficile. Curr. Opin. Microbiol. 2022, 65, 81–86. [Google Scholar] [CrossRef]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodriguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral. Dis. 2022, 28, 307–313. [Google Scholar] [CrossRef]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 2018, 9, e02331-17. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Millette, M.; McFarland, L.V.; DuPont, H.L.; Lacroix, M. Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems. Microb. Pathog. 2021, 153, 104798. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Fteita, D.; Kononen, E.; Gursoy, M.; Ma, X.; Sintim, H.O.; Gursoy, U.K. Quorum sensing molecules regulate epithelial cytokine response and biofilm-related virulence of three Prevotella species. Anaerobe 2018, 54, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczynska, J.; Jedrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Ramachandra, S.S. Quorum Sensing and Quorum Quenching with a Focus on Cariogenic and Periodontopathic Oral Biofilms. Microorganisms 2022, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Scheres, N.; Lamont, R.J.; Crielaard, W.; Krom, B.P. LuxS signaling in Porphyromonas gingivalis-host interactions. Anaerobe 2015, 35, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.O.; Smith, J.A.; Wang, J.; Nakayama, S.; Yan, L. Paradigm shift in discovering next-generation anti-infective agents: Targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med. Chem. 2010, 2, 1005–1035. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Coquant, G.; Grill, J.P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11, 1827. [Google Scholar] [CrossRef]
- Muras, A.; Otero-Casal, P.; Blanc, V.; Otero, A. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: A paradigm revisited. Sci. Rep. 2020, 10, 9800. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, X.; Zheng, J.; Zou, Y.; Qiu, S.; Dai, Y. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef]
- Hernandez, P.; Sanchez, M.C.; Llama-Palacios, A.; Ciudad, M.J.; Collado, L. Strategies to Combat Caries by Maintaining the Integrity of Biofilm and Homeostasis during the Rapid Phase of Supragingival Plaque Formation. Antibiotics 2022, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Landman, C.; Grill, J.P.; Mallet, J.M.; Marteau, P.; Humbert, L.; Le Balc’h, E.; Maubert, M.A.; Perez, K.; Chaara, W.; Brot, L.; et al. Inter-kingdom effect on epithelial cells of the N-Acyl homoserine lactone 3-oxo-C12:2, a major quorum-sensing molecule from gut microbiota. PLoS ONE 2018, 13, e0202587. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Zhu, Y.; Bi, J.; Hao, H.; Hou, H. Effect of the luxI/R gene on AHL-signaling molecules and QS regulatory mechanism in Hafnia alvei H4. AMB Express 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Pumbwe, L.; Skilbeck, C.A.; Wexler, H.M. Presence of quorum-sensing systems associated with multidrug resistance and biofilm formation in Bacteroides fragilis. Microb. Ecol. 2008, 56, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Grellier, N.; Suzuki, M.T.; Brot, L.; Rodrigues, A.M.S.; Humbert, L.; Escoubeyrou, K.; Rainteau, D.; Grill, J.P.; Lami, R.; Seksik, P. Impact of IBD-Associated Dysbiosis on Bacterial Quorum Sensing Mediated by Acyl-Homoserine Lactone in Human Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 15404. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short-Chain N-Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, 86, e01941-19. [Google Scholar] [CrossRef]
- Sikdar, R.; Beauclaire, M.V.; Lima, B.P.; Herzberg, M.C.; Elias, M.H. N-acyl homoserine lactone signaling modulates bacterial community associated with human dental plaque. bioRxiv 2024. [Google Scholar] [CrossRef]
- Elmanfi, S.; Ma, X.; Sintim, H.O.; Kononen, E.; Syrjanen, S.; Gursoy, U.K. Quorum-sensing molecule dihydroxy-2,3-pentanedione and its analogs as regulators of epithelial integrity. J. Periodontal Res. 2018, 53, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zeng, C.; Wen, H.; Shi, Q.; Zhao, X.; Meng, Q.; Li, X.; Xiao, J. Discovery of AI-2 Quorum Sensing Inhibitors Targeting the LsrK/HPr Protein-Protein Interaction Site by Molecular Dynamics Simulation, Virtual Screening, and Bioassay Evaluation. Pharmaceuticals 2023, 16, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.G. SAM/SAH Analogs as Versatile Tools for SAM-Dependent Methyltransferases. ACS Chem. Biol. 2016, 11, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.J.; Sim, J.; Sim, J.; Lee, J.; Choi, B.K. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J. Microbiol. 2016, 54, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Donzella, M.; Nicolosi, G.; Santonocito, S.; Pesce, P.; Isola, G. Drugs for the Quorum Sensing Inhibition of Oral Biofilm: New Frontiers and Insights in the Treatment of Periodontitis. Pharmaceutics 2022, 14, 2740. [Google Scholar] [CrossRef]
- Wu, J.; Li, K.; Peng, W.; Li, H.; Li, Q.; Wang, X.; Peng, Y.; Tang, X.; Fu, X. Autoinducer-2 of Fusobacterium nucleatum promotes macrophage M1 polarization via TNFSF9/IL-1beta signaling. Int. Immunopharmacol. 2019, 74, 105724. [Google Scholar] [CrossRef]
- Jakubovics, N.S. Talk of the town: Interspecies communication in oral biofilms. Mol. Oral Microbiol. 2010, 25, 4–14. [Google Scholar] [CrossRef]
- Slater, R.T.; Frost, L.R.; Jossi, S.E.; Millard, A.D.; Unnikrishnan, M. Clostridioides difficile LuxS mediates inter-bacterial interactions within biofilms. Sci. Rep. 2019, 9, 9903. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Choi, Y.J.; Lee, S.H.; Jun, H.K.; Choi, B.K. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch. Oral Biol. 2013, 58, 17–27. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Wang, Q.; Sadiq, F.A.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W.; Li, H.; Lu, W. Transcriptome Analysis Reveals the Genes Involved in Bifidobacterium Longum FGSZY16M3 Biofilm Formation. Microorganisms 2021, 9, 385. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Jiang, Z. Immune induction identified by TMT proteomics analysis in Fusobacterium nucleatum autoinducer-2 treated macrophages. Expert. Rev. Proteom. 2020, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Lamont, R.J.; Demuth, D.R. Autoinducer 2 is required for biofilm growth of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect. Immun. 2007, 75, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Hasegawa, Y.; Park, Y.; Yeung, V.; Tribble, G.D.; Kuboniwa, M.; Demuth, D.R.; Lamont, R.J. LuxS involvement in the regulation of genes coding for hemin and iron acquisition systems in Porphyromonas gingivalis. Infect. Immun. 2006, 74, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N.A.; Kirke, D.F.; Williams, P.; Winzer, K.; Hardie, K.R.; Meyers, N.L.; Aduse-Opoku, J.; Curtis, M.A.; Camara, M. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology 2002, 148 Pt 3, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Beck, D.A.; Demuth, D.R.; Hackett, M.; Lamont, R.J. Deep sequencing of Porphyromonas gingivalis and comparative transcriptome analysis of a LuxS mutant. Front. Cell. Infect. Microbiol. 2012, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- McNab, R.; Ford, S.K.; El-Sabaeny, A.; Barbieri, B.; Cook, G.S.; Lamont, R.J. LuxS-based signaling in Streptococcus gordonii: Autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 2003, 185, 274–284. [Google Scholar] [CrossRef]
- Yuan, L.; Hillman, J.D.; Progulske-Fox, A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect. Immun. 2005, 73, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Namkung, J.U.; Ha, K.W.; Jun, H.K.; Kim, H.Y.; Choi, B.K. Inhibitory effect of d-arabinose on oral bacteria biofilm formation on titanium discs. Anaerobe 2022, 75, 102533. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontol. 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Ohtani, K.; Hayashi, H.; Shimizu, T. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol. Microbiol. 2002, 44, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.P.; Chung, W.O.; Lamont, R.J.; Demuth, D.R. Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS. Infect. Immun. 2001, 69, 7625–7634. [Google Scholar] [CrossRef] [PubMed]
- Ethapa, T.; Leuzzi, R.; Ng, Y.K.; Baban, S.T.; Adamo, R.; Kuehne, S.A.; Scarselli, M.; Minton, N.P.; Serruto, D.; Unnikrishnan, M. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J. Bacteriol. 2013, 195, 545–555. [Google Scholar] [CrossRef]
- Rubio-Mendoza, D.; Martinez-Melendez, A.; Maldonado-Garza, H.J.; Cordova-Fletes, C.; Garza-Gonzalez, E. Review of the Impact of Biofilm Formation on Recurrent Clostridioides difficile Infection. Microorganisms 2023, 11, 2525. [Google Scholar] [CrossRef]
- Polkade, A.V.; Mantri, S.S.; Patwekar, U.J.; Jangid, K. Quorum Sensing: An Under-Explored Phenomenon in the Phylum Actinobacteria. Front. Microbiol. 2016, 7, 131. [Google Scholar] [CrossRef][Green Version]
- Goldberg, E.; Amir, I.; Zafran, M.; Gophna, U.; Samra, Z.; Pitlik, S.; Bishara, J. The correlation between Clostridium-difficile infection and human gut concentrations of Bacteroidetes phylum and clostridial species. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 377–383. [Google Scholar] [CrossRef]
- Christiaen, S.E.; O’Connell Motherway, M.; Bottacini, F.; Lanigan, N.; Casey, P.G.; Huys, G.; Nelis, H.J.; van Sinderen, D.; Coenye, T. Autoinducer-2 plays a crucial role in gut colonization and probiotic functionality of Bifidobacterium breve UCC2003. PLoS ONE 2014, 9, e98111. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.J.; Verhoeven, T.L.; Shen, C.; Lambrichts, I.; Ceuppens, J.L.; Vanderleyden, J.; De Keersmaecker, S.C. Impact of luxS and suppressor mutations on the gastrointestinal transit of Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2008, 74, 4711–4718. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Szymanek-Majchrzak, K.; Młynarczyk, A.; Młynarczyk, G. Regulatory systems of gene expression in Staphylococcus aureus. Adv. Microbiol. 2009, 48, 7–22. [Google Scholar]
- Williams, P.; Hill, P.; Bonev, B.; Chan, W.C. Quorum-sensing, intra- and inter-species competition in the staphylococci. Microbiology 2023, 169, 001381. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.A.; Li, J.; Beingesser, J.; McClane, B.A.; Uzal, F.A. The Agr-Like Quorum-Sensing System Is Important for Clostridium perfringens Type A Strain ATCC 3624 To Cause Gas Gangrene in a Mouse Model. mSphere 2020, 5, e00500-20. [Google Scholar] [CrossRef] [PubMed]
- Polaske, T.J.; West, K.H.J.; Zhao, K.; Widner, D.L.; York, J.T.; Blackwell, H.E. Chemical and biomolecular insights into the Staphylococcus aureus agr quorum sensing system: Current progress and ongoing challenges. Isr. J. Chem. 2023, 63, e202200096. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Podkowik, M.; Perault, A.I.; Putzel, G.; Pountain, A.; Kim, J.; DuMont, A.L.; Zwack, E.E.; Ulrich, R.J.; Karagounis, T.K.; Zhou, C.; et al. Quorum-sensing agr system of Staphylococcus aureus primes gene expression for protection from lethal oxidative stress. Elife 2024, 12, RP89098. [Google Scholar] [CrossRef]
- Popoff, M.R.; Bruggemann, H. Regulatory Networks Controlling Neurotoxin Synthesis in Clostridium botulinum and Clostridium tetani. Toxins 2022, 14, 364. [Google Scholar] [CrossRef]
- Okada, Y.; Okugawa, S.; Ikeda, M.; Kobayashi, T.; Saito, R.; Higurashi, Y.; Moriya, K. Genetic diversity and epidemiology of accessory gene regulator loci in Clostridioides difficile. Access Microbiol. 2020, 2, acmi000134. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Vidal, J.E.; McClane, B.A. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect. Immun. 2011, 79, 2451–2459. [Google Scholar] [CrossRef]
- Darkoh, C.; Odo, C.; DuPont, H.L. Accessory Gene Regulator-1 Locus Is Essential for Virulence and Pathogenesis of Clostridium difficile. mBio 2016, 7, e01237-16. [Google Scholar] [CrossRef]
- Hargreaves, K.R.; Kropinski, A.M.; Clokie, M.R. What does the talking?: Quorum sensing signalling genes discovered in a bacteriophage genome. PLoS ONE 2014, 9, e85131. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.E.; Ma, M.; Saputo, J.; Garcia, J.; Uzal, F.A.; McClane, B.A. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol. Microbiol. 2012, 83, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Gohari, I.; Li, J.; McClane, B.A. Identifying the Basis for VirS/VirR Two-Component Regulatory System Control of Clostridium perfringens Beta-Toxin Production. J. Bacteriol. 2021, 203, e0027921. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Yuan, Y.; Hassan, S.; Wang, R.; Wang, Y.; Shimizu, T. Virulence gene regulation by the agr system in Clostridium perfringens. J. Bacteriol. 2009, 191, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, H.I.; Ohtani, K.; Okumura, K.; Hayashi, H.; Shimizu, T. Organization and transcriptional regulation of myo-inositol operon in Clostridium perfringens. FEMS Microbiol. Lett. 2004, 235, 289–295. [Google Scholar] [CrossRef]
- Hassan, S.; Ohtani, K.; Wang, R.; Yuan, Y.; Wang, Y.; Yamaguchi, Y.; Shimizu, T. Transcriptional regulation of hemO encoding heme oxygenase in Clostridium perfringens. J. Microbiol. 2010, 48, 96–101. [Google Scholar] [CrossRef]
- Ohtani, K. Gene regulation by the VirS/VirR system in Clostridium perfringens. Anaerobe 2016, 41, 5–9. [Google Scholar] [CrossRef]
- Vidal, J.E.; Chen, J.; Li, J.; McClane, B.A. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS ONE 2009, 4, e6232. [Google Scholar] [CrossRef]
- Ma, M.; Li, J.; McClane, B.A. Structure-function analysis of peptide signaling in the Clostridium perfringens Agr-like quorum sensing system. J. Bacteriol. 2015, 197, 1807–1818. [Google Scholar] [CrossRef]
- Ihekwaba, A.E.; Mura, I.; Walshaw, J.; Peck, M.W.; Barker, G.C. An Integrative Approach to Computational Modelling of the Gene Regulatory Network Controlling Clostridium botulinum Type A1 Toxin Production. PLoS Comput. Biol. 2016, 12, e1005205. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009, 56, 1–16. [Google Scholar] [CrossRef]
- Wang, L.H.; Weng, L.X.; Dong, Y.H.; Zhang, L.H. Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem. 2004, 279, 13645–13651. [Google Scholar] [CrossRef]
- Parga, A.; Muras, A.; Otero-Casal, P.; Arredondo, A.; Soler-Olle, A.; Alvarez, G.; Alcaraz, L.D.; Mira, A.; Blanc, V.; Otero, A. The quorum quenching enzyme Aii20J modifies in vitro periodontal biofilm formation. Front. Cell. Infect. Microbiol. 2023, 13, 1118630. [Google Scholar] [CrossRef]
- D’Aquila, P.; De Rose, E.; Sena, G.; Scorza, A.; Cretella, B.; Passarino, G.; Bellizzi, D. Quorum Quenching Approaches against Bacterial-Biofilm-Induced Antibiotic Resistance. Antibiotics 2024, 13, 619. [Google Scholar] [CrossRef]
- Kameswaran, S.; Gujjala, S.; Zhang, S.; Kondeti, S.; Mahalingam, S.; Bangeppagari, M.; Bellemkonda, R. Quenching and quorum sensing in bacterial bio-films. Res. Microbiol. 2024, 175, 104085. [Google Scholar] [CrossRef]
- Huang, J.; Shi, Y.; Zeng, G.; Gu, Y.; Chen, G.; Shi, L.; Hu, Y.; Tang, B.; Zhou, J. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview. Chemosphere 2016, 157, 137–151. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, E.R.; Abdel-Aziz, M.S.; Abdelmegeed, H.; Kirsch, G. Coumarins: Quorum Sensing and Biofilm Formation Inhibition. Molecules 2024, 29, 4534. [Google Scholar] [CrossRef]
- Ahmed, G.E.; Elshahid, Z.A.; El-Sawy, E.R.; Abdel-Aziz, M.S.; Abdel-Aziem, A. Synthesis, biofilm formation inhibitory, and inflammation inhibitory activities of new coumarin derivatives. Sci. Rep. 2024, 14, 9106. [Google Scholar] [CrossRef]
- Marquis, A.; Genovese, S.; Epifano, F.; Grenier, D. The plant coumarins auraptene and lacinartin as potential multifunctional therapeutic agents for treating periodontal disease. BMC Complement. Altern. Med. 2012, 12, 80. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.J.; Li, L.; Choi, B.K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- Hamada, S.; Minami, S.; Gomi, M. Heparinoid enhances the efficacy of a bactericidal agent by preventing Cutibacterium acnes biofilm formation via quorum sensing inhibition. J. Microorg. Control 2024, 29, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hao, L.; Liu, X.; Li, X.; Zhao, G. The Anti-Biofilm Properties of Phloretin and Its Analogs against Porphyromonas gingivalis and Its Complex Flora. Foods 2024, 13, 1994. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Sato, K.; Yukitake, H.; Kamaguchi, A.; Sasaki, Y.; Naito, M.; Nakayama, K. Identification of genes encoding glycosyltransferases involved in lipopolysaccharide synthesis in Porphyromonas gingivalis. Mol. Oral Microbiol. 2018, 33, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Rudin, L.; Bornstein, M.M.; Shyp, V. Inhibition of biofilm formation and virulence factors of cariogenic oral pathogen Streptococcus mutans by natural flavonoid phloretin. J. Oral Microbiol. 2023, 15, 2230711. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Ding, X.; Wang, J.; Zhan, X. Inhibitory effects of reuterin on biofilm formation, quorum sensing and virulence genes of Clostridium perfringens. LWT 2022, 162, 113421. [Google Scholar] [CrossRef]
- Singh, R.P.; Okubo, K.; Ohtani, K.; Adachi, K.; Sonomoto, K.; Nakayama, J. Rationale design of quorum-quenching peptides that target the VirSR system of Clostridium perfringens. FEMS Microbiol. Lett. 2015, 362, fnv188. [Google Scholar] [CrossRef]

| Biological Processes Associated with AI-2 in Biofilm Formed by Anaerobic Bacteria | References |
|---|---|
| LuxS signaling in P. gingivalis plays a significant role in interaction with fibroblasts. This may be through the downregulation of the gene PGN_0482, which encodes a putative outer membrane immunoreactive protein. | [47] |
| LuxS/AI-2 signaling mediates the interaction between P. gingivalis and periodontitis-associated species like Streptococcus gordonii. | [77] |
| The luxS gene from A. actinomycetemcomitans can complement a luxS mutation in P. gingivalis. | [84] |
| LuxS/AI-2 signaling in P. gingivalis regulates hemin acquisition, growth in hemin-limited conditions, and the expression of proteases and stress-related genes. In ΔluxS strains, genes encoding hemin binding protein (tlr) and (lysine-specific protease) kgp genes are downregulated, while gene hmuR encoding an outer-membrane hemin utilization receptor, fetB encoding heme-binding protein, feoB1 encoding ferrous iron transporter, and ftn ferritin-like proteins are upregulated. | [74,76] |
| LuxS signaling is involved in promoting the survival of P. gingivalis in the host by regulating its response to host-induced stress factors (H2O2, and high pH). In the luxS mutant, genes related to stress response (htrA, clpB, groEL, dnaK, and ahpF), coding outer membrane efflux protein, and immunoreactive antigen are upregulated. | [78] |
| In P. gingivalis, LuxS modulates protease levels and hemagglutination. luxS mutants exhibited a reduction in haemagglutinin titer and lower Rgp and Kgp proteases (gingipains) activity. | [75] |
| AI-2 from F. nucleatum enhanced single species biofilm formation of F. nucleatum, P. gingivalis, T. denticola, and T. forsythia, and promoted co-aggregation with red complex species (P. gingivalis, T. denticola, T. forsythia). AI-2 induces mRNA synthesis of adhesion molecules like FadA, RgpA, Msp, and BspA (representative adhesion molecules of these bacteria). | [70] |
| F. nucleatum AI-2 triggers inflammatory responses and promotes macrophage mobility and M1 polarization via the TNFSF9/TRAF1/p-AKT/IL-1β pathway. | [67,72] |
| AI-2 may induce prophages in C. difficile biofilms, leading to phage-mediated cell lysis and eDNA release, enhancing biofilm growth. | [69] |
| C. diffcile LuxS-mediated prophage induction. LuxS deficiency in C. difficile impairs prophage induction and biofilm formation in vitro. | [85,86] |
| C. perfringens AI-2 regulates toxin production. luxS activates pfoA transcription and theta-toxin production and possibly influences post-transcriptional regulation of the production of alpha and kappa toxins. | [82] |
| In C. acnes, AI-2 enhances virulence by increasing bacterial lipase activity. | [83,87] |
| C. difficile AI-2 in co-culture triggers selective metabolic responses in B. fragilis, downregulating carbon metabolism genes; genes related to alanine, aspartate, and glutamate metabolism; and involved in amino acid biosynthesis. | [69,88] |
| LuxS mutants of Lactobacillus rhamnosus GG and Bifidobacterium breve UCC2003 are less persistent in the murine gastrointestinal tract than wild strains, likely due to increased sensitivity to gastric fluid and impaired iron acquisition. | [89,90] |
| Potential QQ Inhibitors | Refs. |
|---|---|
| Inhibitors of AHL-Mediated Quorum Sensing | |
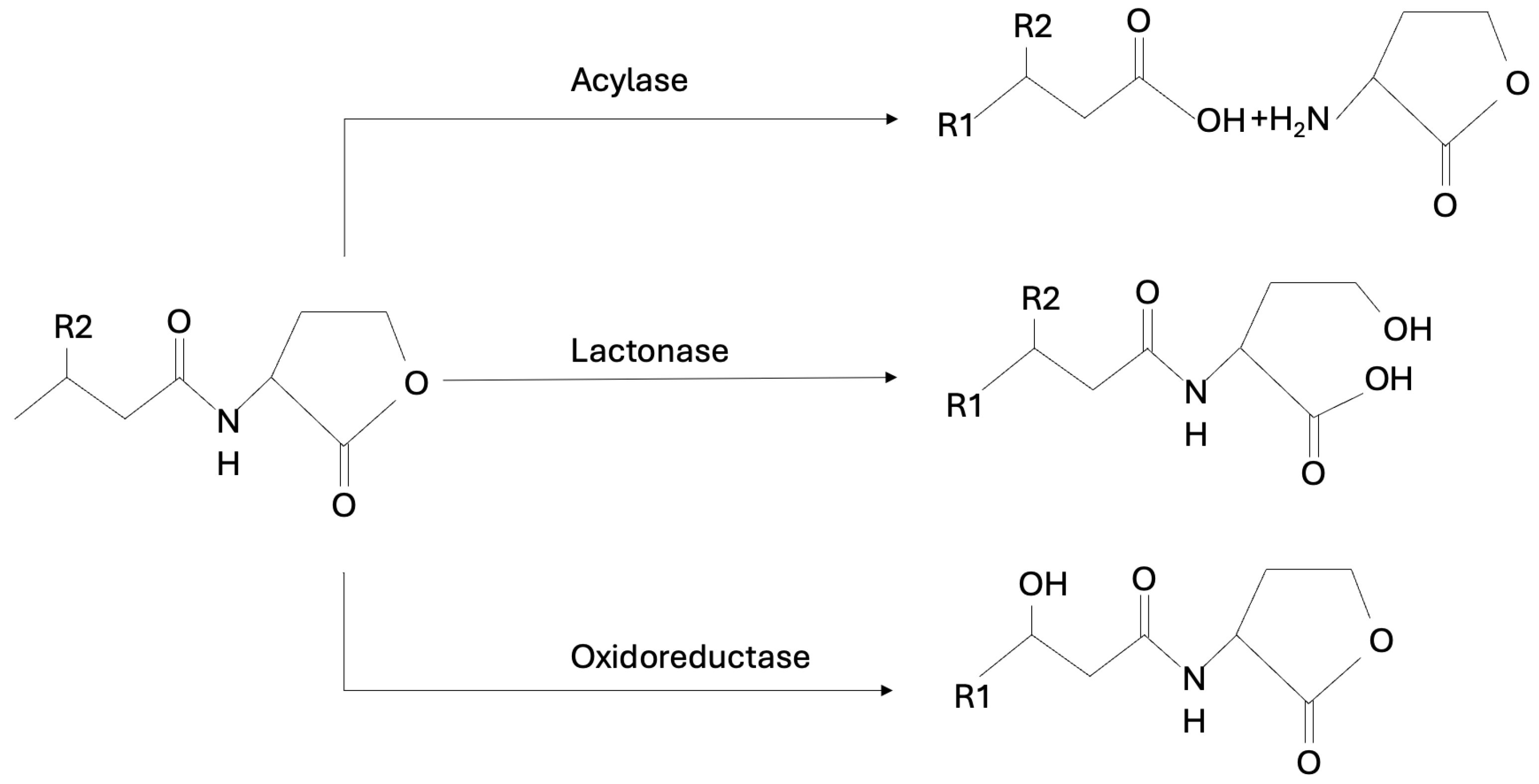
| AHL-degrading enzymes AHL-degrading enzymes are lactonases, acylases, and oxidoreductases. AHL-lactonase inhibits the QS process by hydrolyzing the lactone ring in the homoserine moiety of AHLs. | [51,62,112,115,116,117] |
| AHL-acylases hydrolyze the amide bond between the acyl side chain and the homoserine lactone in the AHL molecules producing the free fatty acid and the homoserine lactone. | |
| AHL oxidoreductases reduce or oxidize the acyl chain of AHLs. | |
| AHL analogs AHL-analogs inhibit AHL synthesis, alter protein expression, and slow the growth of P. gingivalis, offering the potential for periodontal disease treatment. | |
| AHL antagonists AHL antagonists compete with natural signaling molecules for receptor binding by blocking the interaction between LuxR and AHL and disrupting quorum sensing. | |
| Inhibitors of AI-2-mediated quorum sensing | |
| AI-2 analogs AI-2 analogs are D-ribose and D-galactose. These monosaccharides can block AI-2 receptors, reducing virulence gene expression and biofilm formation of periodontopathogens including A. actinomycetemcomitans, F. nucleatum, P. gingivalis, T. forsythia, and T. denticola. The production of bacterial adhesins was markedly reduced when the bacteria were grown in the presence of D-ribose. D-galactose reduces biofilm formation of F. nucleatum, P. gingivalis, and T. forsythia by blocking the AI-2 receptor. | [65,70,79] |
| D-arabinose reduces biofilm formation by potentially competing with salivary receptors or bacterial adhesins. | |
| Coumarin Coumarin inhibits biofilm formation by P. gingivalis through interaction with the heme-binding protein HmuY and causes its interspersal and dispersion in pre-formed and late-stages. | [13,118,119] |
| Lacinartin Lacinartin inhibits P. gingivalis growth, biofilm formation, and collagenase activity. Prevents adherence to oral epithelial cells and reduces inflammatory responses—secretion of IL-8 and TNF-α and matrix metalloproteinases (MMP-8 and MMP-9). | [120] |
| Brominated furanones Brominated furanones inactivate the LuxS enzyme, required for AI-2 synthesis. The bromofuranone analog, 3-(dibromomethylene) isobenzofuran-1(3H)-one derivative inhibits biofilm formation by F. nucleatum, P. gingivalis, and T. forsythia without killing the bacteria. | [70,121] |
| Heparinoids Glycosaminoglycans inhibit AI-2 activity, thereby reducing biofilm formation and lipase activity in C. acnes, additionally enhancing the bactericidal effects of isopropyl methylphenol on C. acnes biofilms. | [122] |
| Phlorizin and phloretin polyphenols Phlorizin and phloretin disrupt biofilm forming by P. gingivalis and suppress its QS by inhibition of glucosyltransferases GtfB and GtfC. | [123,124,125] |
| Reuterin Reuterin, produced by L. reuteri, suppresses biofilm formation of C. perfringens and downregulated QS-related genes like agrB and luxS, which decreases toxin production. | [126] |
| Inhibitors of Agr-like quorum sensing | |
| In C. perfringens, a partial agonist (Z-AIPCp-L2A/T5A) and a partial antagonist (Z-AIPCp-F4A/T5S), significantly reduces transcription of the theta-toxin gene (pfoA). | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. Int. J. Mol. Sci. 2024, 25, 12808. https://doi.org/10.3390/ijms252312808
Markowska K, Szymanek-Majchrzak K, Pituch H, Majewska A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. International Journal of Molecular Sciences. 2024; 25(23):12808. https://doi.org/10.3390/ijms252312808
Chicago/Turabian StyleMarkowska, Kinga, Ksenia Szymanek-Majchrzak, Hanna Pituch, and Anna Majewska. 2024. "Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities" International Journal of Molecular Sciences 25, no. 23: 12808. https://doi.org/10.3390/ijms252312808
APA StyleMarkowska, K., Szymanek-Majchrzak, K., Pituch, H., & Majewska, A. (2024). Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. International Journal of Molecular Sciences, 25(23), 12808. https://doi.org/10.3390/ijms252312808






