Suppression of Metastasis of Colon Cancer to Liver in Mouse Models by Pretreatment with Extracellular Vesicles Derived from Nanog-Overexpressing Colon-26 Cancer Cells
Abstract
1. Introduction
2. Results
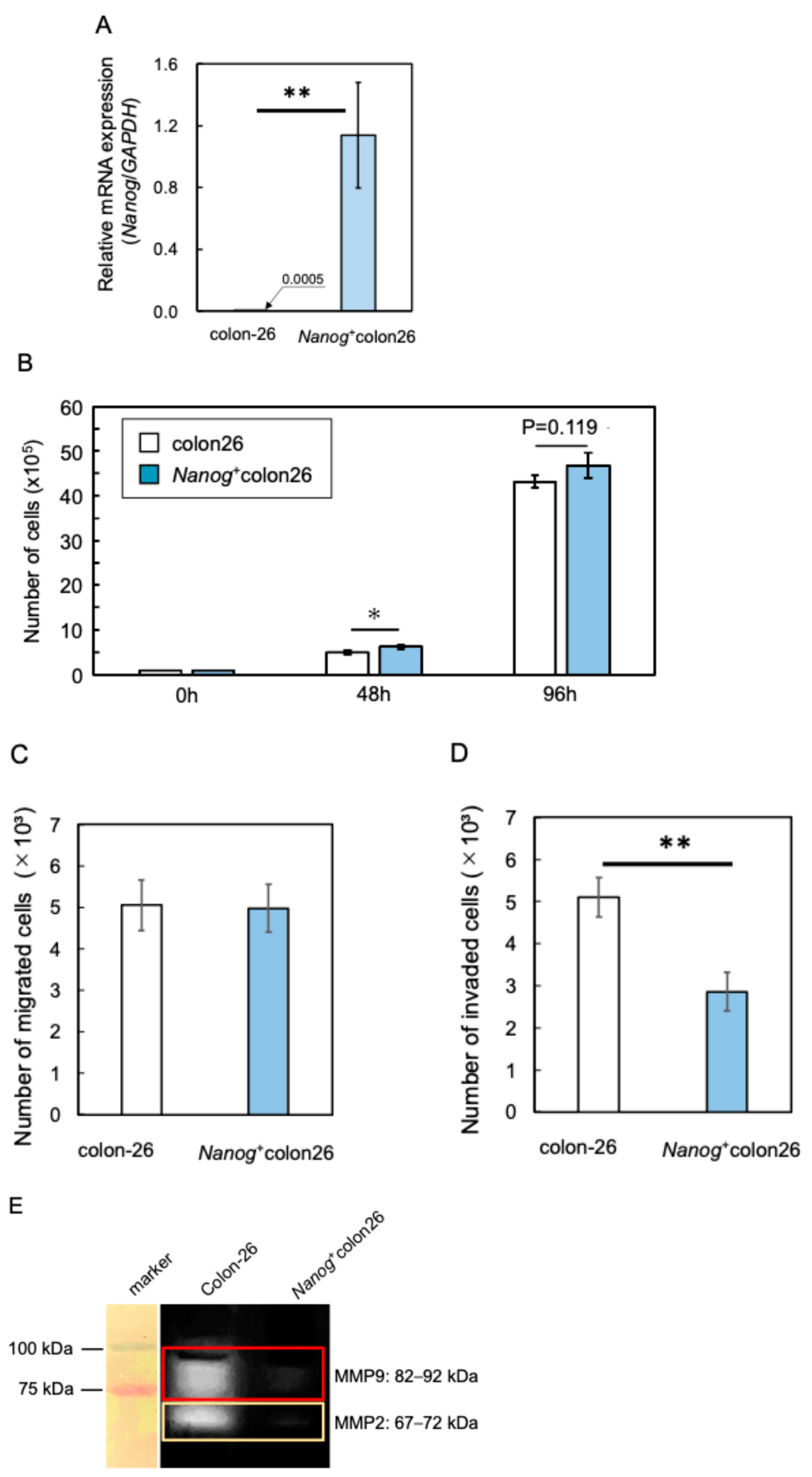
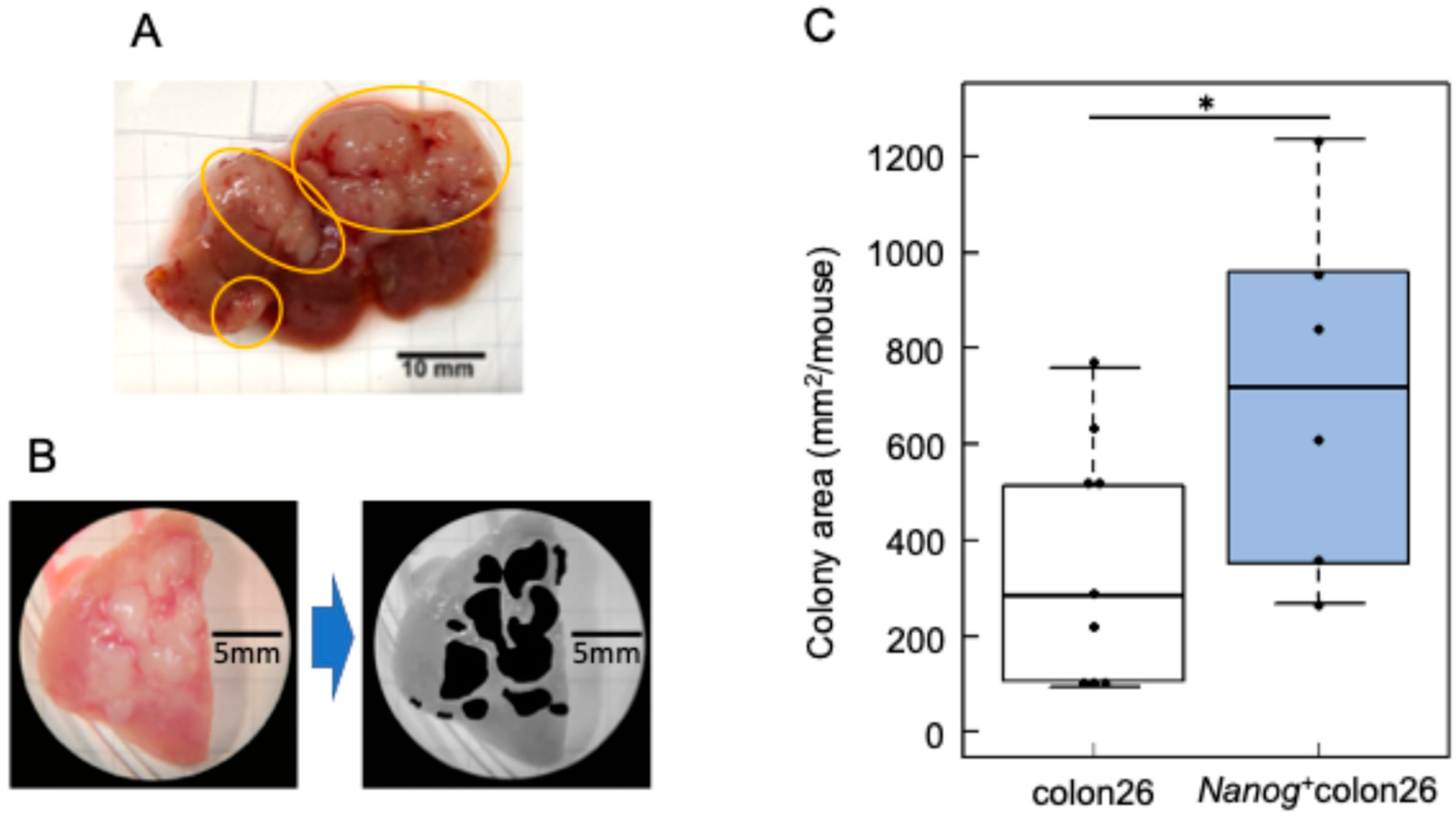
2.1. Properties of Nanog-Overexpressing Colon-26 (Nanog+colon26) Cells
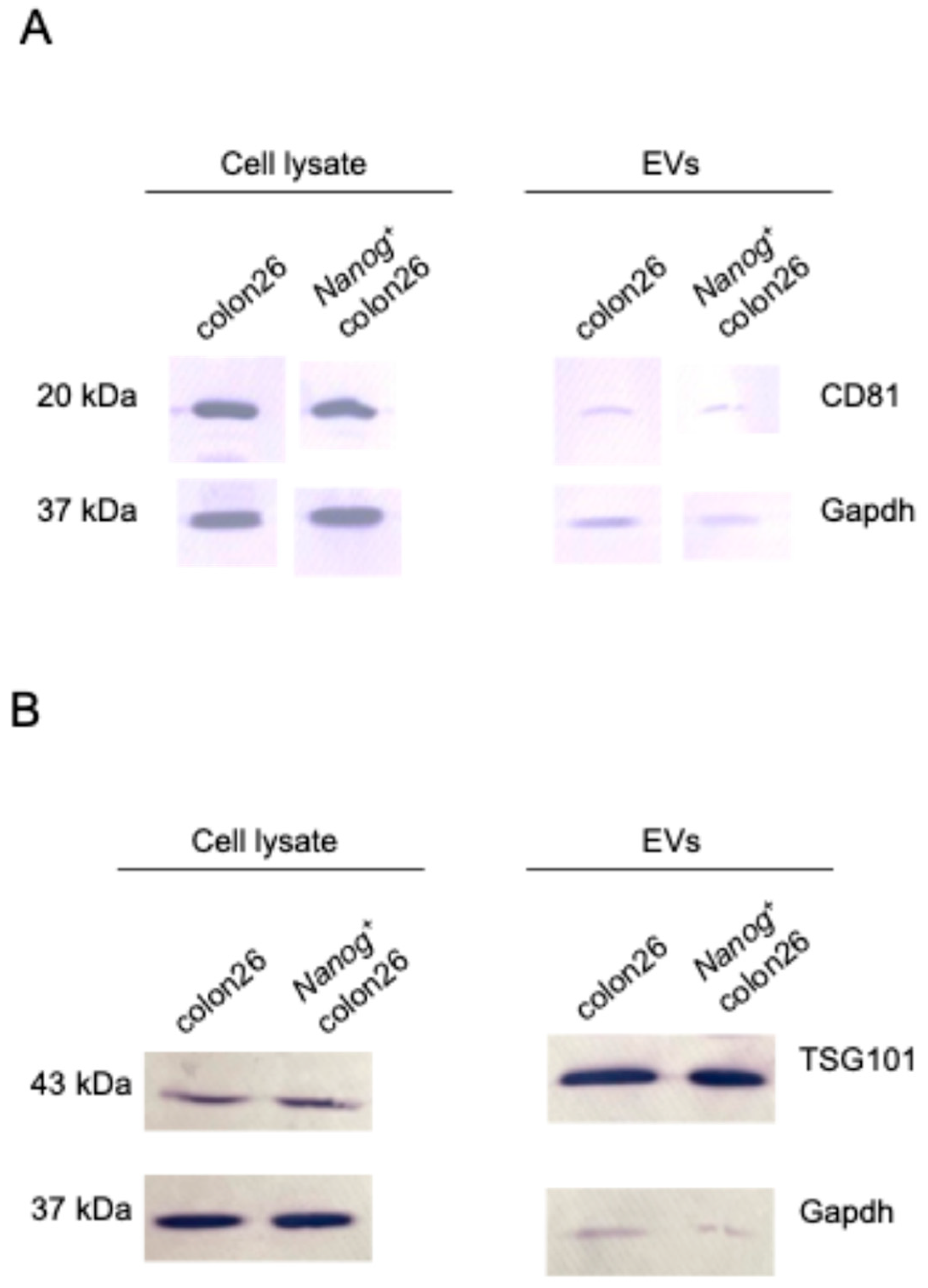
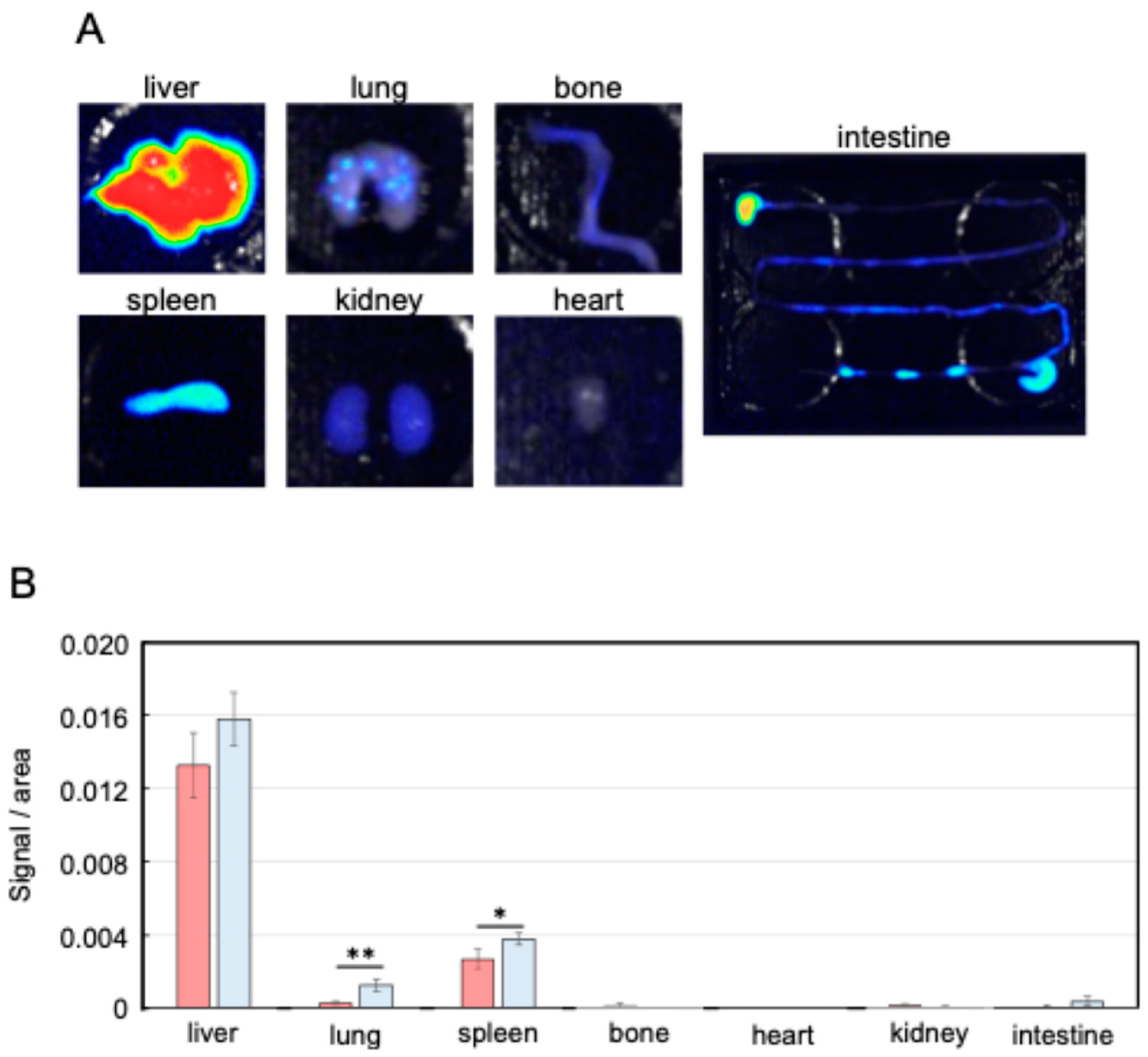
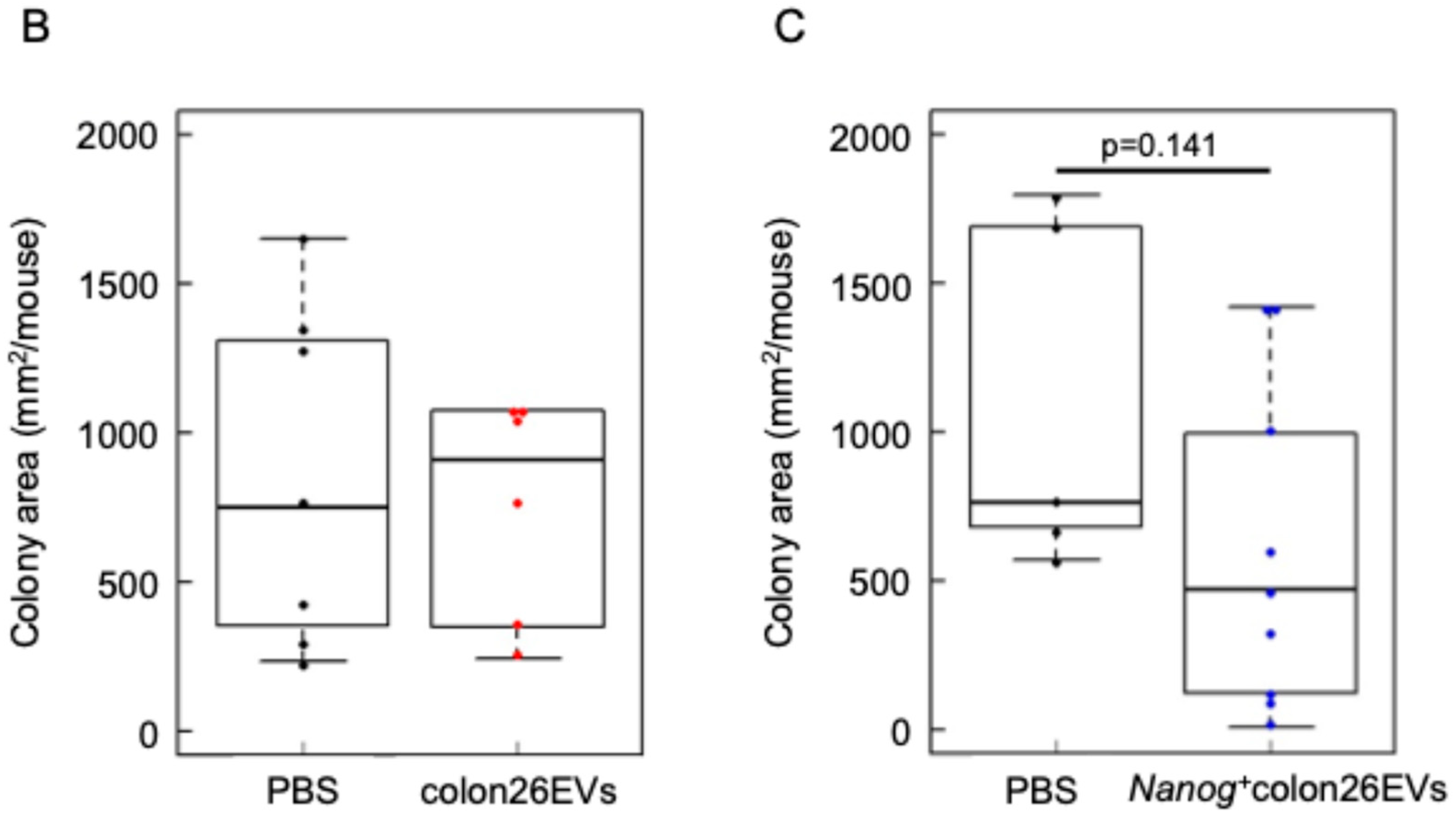
2.2. Metastasis Suppression Effect of Nanog+colon26EVs
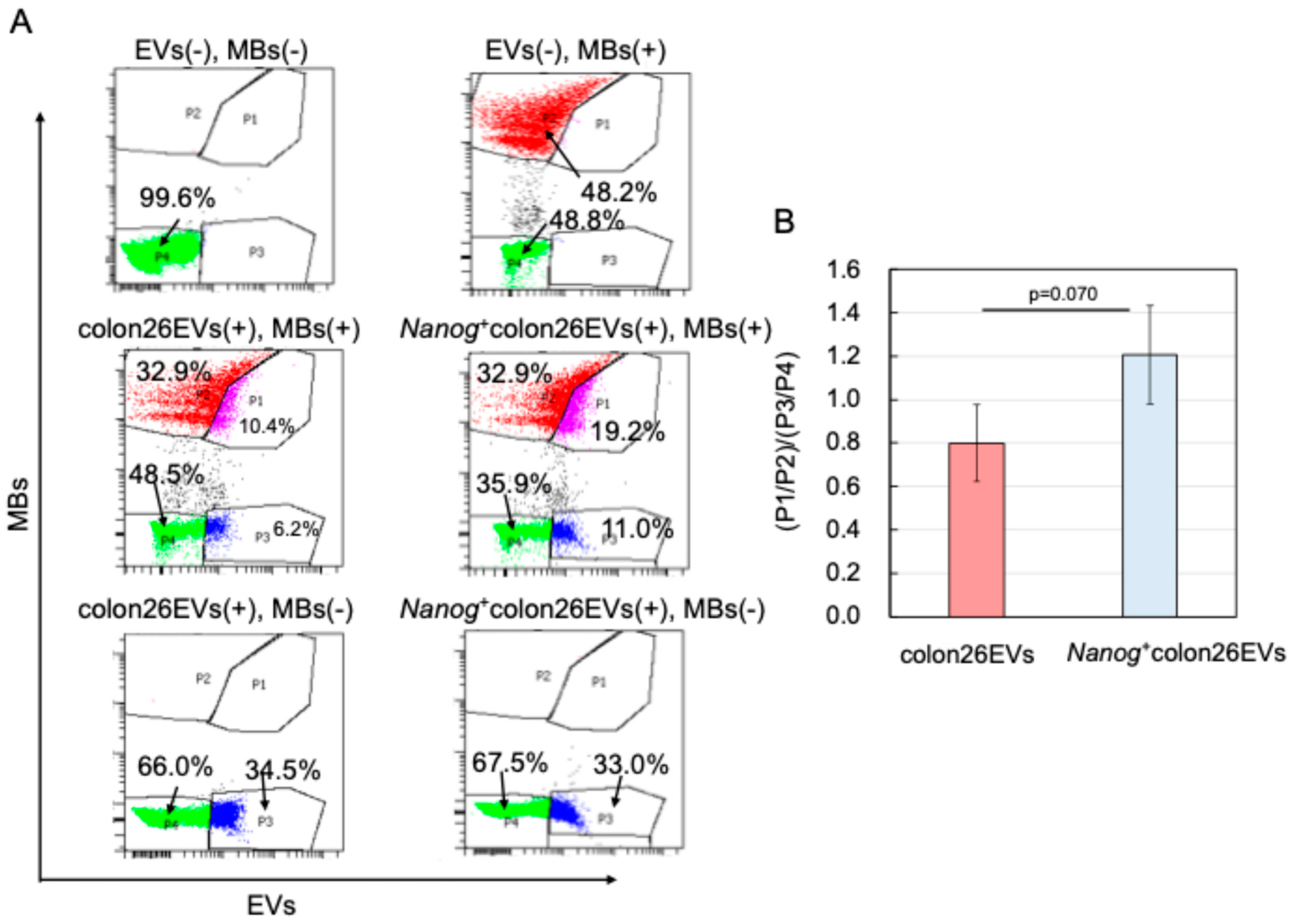
2.3. Effects of Nanog+colon26EVs on Macrophage Phagocytic Function
2.4. Effects on Macrophage Polarization
2.5. Expression of miRNAs and Their Target Genes in EVs
2.6. Factors Involved in the Activation of the Immune System
2.6.1. Results of the First Search
2.6.2. Results of the Second Search
2.6.3. Results of the Third Search
3. Material and Methods
3.1. Cell Culture
3.2. Animals
3.3. Generation of a Nanog-Overexpressing Colon Cancer Cell Line
3.4. Proliferation Activity
3.5. Migration Activity
3.6. Invasion Activity
3.7. Gelatine Zymography for Matrix Metalloproteinase (MMP) Activity
3.8. Quantification of Colon Metastasis to Liver
3.9. Preparation of EVs
3.10. Western Analysis of EVs Markers, Negative Marker, and Gapdh
3.11. Analysis of the Transfer of EVs to Various Organs in Mice
3.12. In Vivo Test of the Effects of EVs on Metastasis
3.13. Phagocytic Activity Test
3.14. Analysis of miRNAs and Their Target Genes
3.15. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Yamashita, M.; Ino, K.; Nawa, A.; Kikkawa, F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int. J. Oncol. 2007, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wattanawongdon, W.; Hahnvajanawong, C.; Namwat, N.; Kanchanawat, S.; Boonmars, T.; Jearanaikoon, P.; Leelayuwat, C.; Techasen, A.; Seubwai, W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 2015, 47, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wei, W.; Zhan, Z.; Xie, D.; Xie, Y.; Xiao, Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int. J. Mol. Med. 2018, 41, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Speers, C.; Zhao, S.; Liu, M.; Bartelink, H.; Pierce, L.J.; Feng, F.Y. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin. Cancer Res. 2015, 21, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Reyes, A.; Muñiz-Lino, M.A.; Romero-Garcia, S.; López-Camarillo, C.; Hernández-de la Cruz, O.N. Biological adaptations of tumor cells to radiation therapy. Front. Oncol. 2021, 11, 718636. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.R.; Wang, S.; Yan, S.C.; Zhang, Y.; Nelson, P.J.; Jia, H.L.; Qin, L.X.; Dong, Q.Z. Therapeutic Strategies Targeting Cancer Stem Cells and Their Microenvironment. Front. Oncol. 2019, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Kuşoğlu, A.; Avcı, Ç.B. Cancer Stem Cells: A Brief Review of Current Status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef]
- Lin, C.C.; Liao, T.T.; Yang, M.H. Immune Adaptation of Colorectal Cancer Stem Cells and Their Interaction with the Tumor Microenvironment. Front. Oncol. 2020, 10, 588542. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, A.; Snook, A.E.; Waldman, S.A. Stem Cells as Therapeutic Targets in Colorectal Cancer. Pers. Med. 2021, 18, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Cheng, Q.; Zheng, H.; Liu, J.; Liu, L.; Chen, Q. Targeting Stemness of Cancer Stem Cells to Fight Colorectal Cancers. Semin. Cancer Biol. 2022, 82, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Kumar, D.B.U.; Punj, V.; Xu, J.; Sher, L.; Tahara, S.M.; Hess, S.; Machida, K. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016, 23, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Rzemieniewska, N.; Bednarek, I. The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biol. Ther. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Saito, M. Novel roles of Nanog in cancer cells and their extracellular vesicles. Cells 2022, 11, 3881. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kishi, R.; Sasai, T.; Hatakenaka, T.; Matsuki, N.; Minagawa, S. Effect of Nanog overexpression on the metastatic potential of a mouse melanoma cell line B16-BL6. Mol. Cell. Biochem. 2021, 476, 2651–2661. [Google Scholar] [CrossRef]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Zuo, B.; Lu, Z.; Gao, X.; You, A.; Wu, C.; Du, Z.; Yin, H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology 2016, 64, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, K.; Figueira, R.L.; Antounians, L.; Lauriti, G.; Zani, A. Systematic review of extracellular vesicle-based treatments for lung injury: Are EVs a potential therapy for COVID-19? J. Extracell. Vesicles 2020, 9, 1795365. [Google Scholar] [CrossRef]
- Jamalkhah, M.; Asaadi, Y.; Azangou-Khyavy, M.; Khanali, J.; Soleimani, M.; Kiani, J.; Arefian, E. MSC-derived exosomes carrying a cocktail of exogenous interfering RNAs an unprecedented therapy in era of COVID-19 outbreak. J. Transl. Med. 2021, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signaling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Mitchell, M.I.; Ma, J.; Carter, C.L.; Loudig, O. Circulating exosome cargoes contain functionally diverse cancer biomarkers: From biogenesis and function to purification and potential translational utility. Cancers 2022, 14, 3350. [Google Scholar] [CrossRef]
- Kar, R.; Dhar, R.; Mukherjee, S.; Nag, S.; Gorai, S.; Mukerjee, N.; Mukherjee, R.; Vatsa, R.; Jadhav, M.C.; Ghosh, A.; et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater. Sci. Eng. 2023, 9, 577–594. Available online: https://pubs.acs.org/doi/10.1021/acsbiomaterials.2c01329# (accessed on 4 October 2024). [CrossRef]
- Karn, V.; Ahmed, S.; Tsai, L.-W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular vesicle-based therapy for COVID-19: Promises, challenges and future prospects. Biomedicines 2021, 9, 1373. [Google Scholar] [CrossRef]
- Vecchio, F.D.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef] [PubMed]
- Rossowska, J.; Anger, N.; Wegierek, K.; Szczygieł, A.; Mierzejewska, J.; Milczarek, M.; Szermer-Olearnik, B.; Pajtasz-Piasecka, E. Antitumor potential of extracellular vesicles released by genetically modified murine colon carcinoma cells with overexpression of interleukin-12 and shRNA for TGF-b1. Front. Immunol. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Dhasmana, A.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. miR-205: A potential biomedicine for cancer therapy. Cells 2020, 9, 1957. [Google Scholar] [CrossRef] [PubMed]
- Hatakenaka, T.; Matsuki, N.; Minagawa, S.; May, C.K.S.; Saito, M. Anti-metastatic function of extracellular vesicles derived from Nanog-overexpressing melanoma. Curr. Oncol. 2022, 29, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.S.M.; Henmi, T.; Saito, M. Comparative study of metastasis suppression effects of extracellular vesicles derived from anaplastic cell lines, Nanog-overexpressing melanoma, and induced pluripotent stem cells. Int. J. Mol. Sci. 2023, 24, 17206. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory; Cancer Tomorrow. WHO International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.fr/en (accessed on 4 October 2024).
- Arlt, F.; Stein, U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int. J. Biochem. Cell Biol. 2009, 41, 2356–2359. [Google Scholar] [CrossRef]
- Merck, Sharp; Dohme LLC. Study of Pembrolizumab (MK-3475) vs Standard Therapy in Participants with Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Stage IV Colorectal Carcinoma (MK-3475-177/KEYNOTE-177). In Clinical Trials gov. ID NCT02563002.; 2024. Available online: https://clinicaltrials.gov/study/NCT02563002 (accessed on 4 October 2024).
- Descarpentrie, J.; Araúzo-Bravo, M.J.; He, Z.; François, A.; González, Á.; Garcia-Gallastegi, P.; Badiola, I.; Evrard, S.; Pernot, S.; Creemers, J.W.M.; et al. Role of furin in colon cancer stem cells malignant phenotype and expression of LGR5 and NANOG in KRAS and BRAF-mutated colon tumors. Cancers 2022, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, R.; Wang, H.; Yang, Z.; Zhang, H.; Zhang, Y.; Wang, M.; Wang, H.; Lin, J.; Zhao, Q.; et al. Nanog mediated by FAO/ACLY signaling induces cellular dormancy in colorectal cancer cells. Cell Death Dis. 2022, 13, 159. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Giannatale, A.D.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Shi, R.; Zhang, D.; Kang, X.; Zhang, L.; Yuan, J.; Zhang, X.; Miao, H. Dietary fats suppress the peritoneal seeding of colorectal cancer cells through the TLR4/Cxcl10 axis in adipose tissue macrophages. Signal Transduct. Targeted Ther. 2020, 5, 239. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.S.M.; Hatakenaka, T.; Matsuki, N.; Minagawa, S.; Asami, K.; Henmi, T.; Morimoto, A.; Saito, M. Moderate hyperglycemia suppresses melanoma metastasis to the liver. Exp. Anim. 2023, 72, 183–192. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, S.; Zeng, L.; Liu, J.; Kang, R.; Yang, M.; Cao, L.; Wang, H.; Billiar, T.R.; Jiang, J.; et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 2018, 24, 366–378. [Google Scholar] [CrossRef] [PubMed]



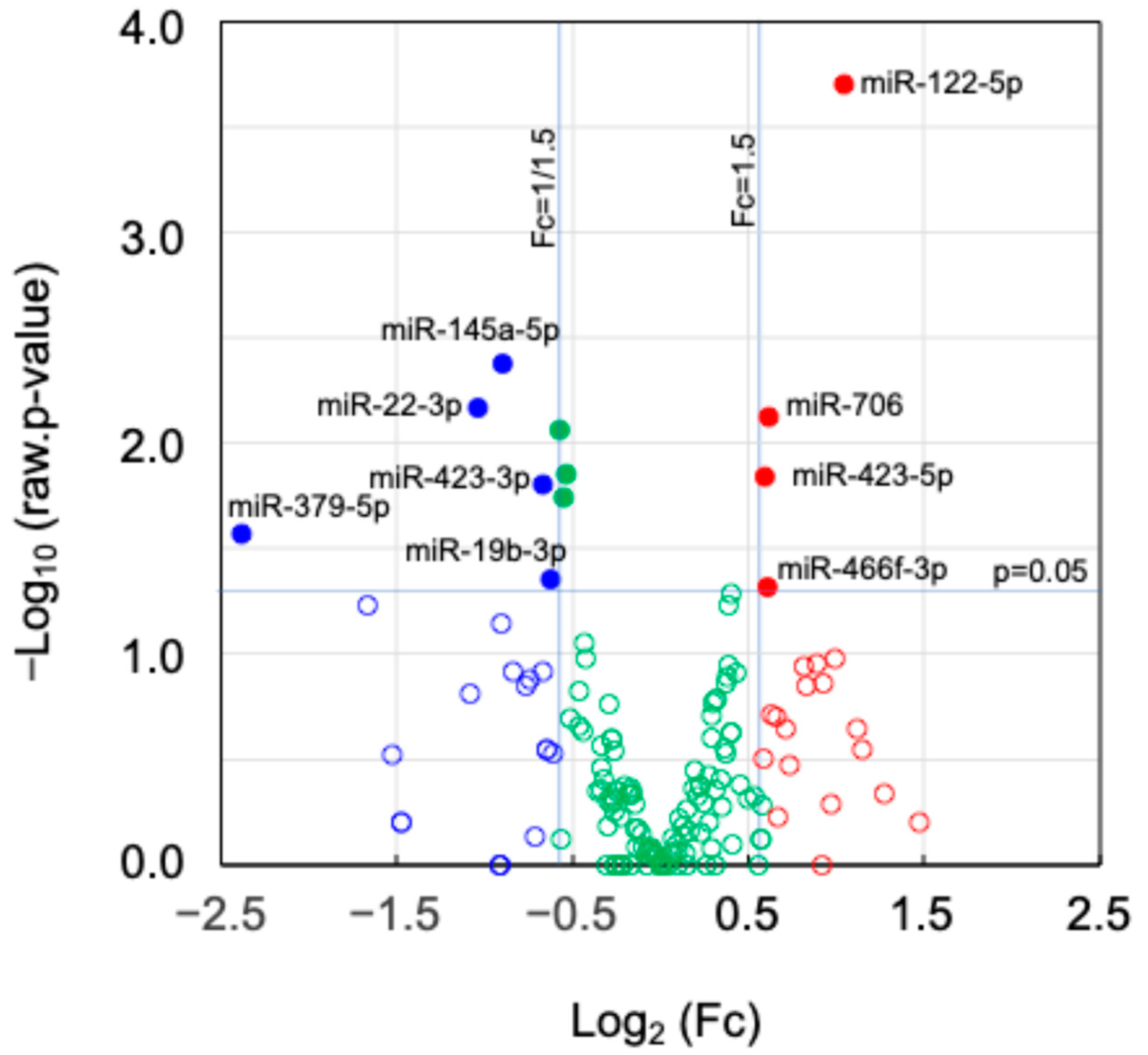
| miRNA | Log2(Fc) | Number of Target Genes | |||
|---|---|---|---|---|---|
| p < 0.05 CWCS ≤ −0.40 | Targeted by Each miRNA Among Top30 Genes | ||||
| Nanog+colon26EVs/ colon26EVs | miR-122-5p | 1.04 | 29 | 321 | 2 |
| miR-706 | 0.62 | 62 | 3 | ||
| miR-466f-3p | 0.61 | 146 | 6 | ||
| miR-423-5p | 0.59 | 84 | 2 | ||
| miR-379-5p | −2.38 | 7 | 302 | 0 | |
| miR-22-3p | −1.04 | 103 | 8 | ||
| miR-145a-5p | −0.89 | 48 | 4 | ||
| miR-423-3p | −0.67 | 8 | 1 | ||
| miR-19b-3p | −0.62 | 136 | 4 | ||
| Sum | 623 | 30 | |||
| Rank | Gene | miRNA | Predicted Effects | Rank | Gene | miRNA | Rank | Gene | miRNA |
| 1 | Actb | miR-145a-5p | cell motility and contraction→up/no | 11 | Csf1r | miR-22-3p | 21 | Mecp2 | miR-22-3p |
| 2 | Ins1 | miR-466f-3p | blood glucose decrease→down | 12 | Sirt1 | miR-22-3p | 22 | Mlxipl | miR-706 |
| 3 | Fgf17 | miR-423-5p | normal brain development→down | 13 | H3f3b | miR-22-3p | 23 | Socs1 | miR-19b-3p |
| 4 | Fgf16 | miR-466f-3p | normal heart development→down | 14 | Angpt2 | miR-145a-5p | 24 | Hnrnph2 | miR-145a-5p |
| 5 | Grb2 | miR-466f-3p | linking of cell growth factor receptor and Ras signaling→down→growth inhibition | 15 | Cdkn1b | miR-706 | 25 | Pkm | miR-122-5p |
| 6 | Ngf | miR-423-5p | normal nerve growth→down | 16 | Ppara | miR-22-3p | 26 | Prkcd | miR-466f-3p |
| 7 | Erbb3 | miR-22-3p | development of various organs and myeloid cell differentiation→up/no | 17 | Slc2a4 | miR-466f-3p | 27 | Arrb1 | miR-22-3p |
| 8 | Fgfr2 | miR-423-3p | cell proliferation, differentiation, migration, apoptosis→up/no | 18 | Mycn | miR-19b-3p | 28 | Sgk1 | miR-19b-3p |
| 9 | Kitl | miR-466f-3p | cell survival, proliferation, stem cell maintenance→down | 19 | Ccnd2 | miR-19b-3p | 29 | Hnrnpf | miR-706 |
| 10 | Esr1 | miR-22-3p | cellular proliferation and differentiation→up/no | 20 | G6pc3 | miR-122-5p | 30 | Actg1 | miR-145a-5p |
| Pathway | Description | Count in Network | Strength | |
|---|---|---|---|---|
| KE | mmu04010 | MAPK signaling pathway | 20 of 287 | 0.40 |
| mmu04014 | Ras signaling pathway | 17 of 225 | 0.44 | |
| mmu04015 | Rap1 signaling pathway | 18 of 208 | 0.50 | |
| mmu04068 | FoxO signaling pathway | 15 of 129 | 0.63 | |
| mmu04151 | PI3K-Akt signaling pathway | 23 of 353 | 0.38 | |
| mmu04152 | AMPK signaling pathway | 11 of 124 | 0.51 | |
| mmu04510 | Focal adhesion | 17 of 225 | 0.45 | |
| mmu04930 | Type II diabetes mellitus | 7 of 48 | 0.73 | |
| mmu04931 | Insulin resistance | 10 of 109 | 0.52 | |
| mmu04960 | Aldosterone-regulated sodium reabsorption | 6 of 38 | 0.76 | |
| WK | WP2841 | Focal adhesion: PI3K-Akt-mTOR signaling pathway | 24 of 316 | 0.44 |
| WP85 | Focal adhesion | 15 of 185 | 0.47 | |
| Rank | Name | Score | miRNA | Rank | Name | Score | miRNA | Rank | Name | Score | miRNA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ins1 | 1500 | miR-466f-3p | 9 | Angpt2 | 48 | miR-145a-5p | 15 | Rab14 | 2 | miR-466f-3p |
| 2 | Fgf17 | 1490 | miR-423-5p | 10 | Pik3cd | 6 | miR-22-3p | 15 | Efna3 | 2 | miR-423-5p |
| 3 | Fgf16 | 1488 | miR-466f-3p | 11 | Slc2a4 | 5 | miR-466f-3p | 15 | Pfkfb2 | 2 | miR-466f-3p |
| 4 | Grb2 | 1450 | miR-466f-3p | 11 | Pdpk1 | 5 | miR-466f-3p | 15 | Fgf12 | 2 | miR-706 |
| 5 | Kitl | 1440 | miR-466f-3p | 13 | Cdkn1b | 4 | miR-706 | 21 | Gys1 | 1 | miR-122-5p |
| 5 | Ngf | 1440 | miR-423-5p | 13 | Pdgfd | 4 | miR-145a-5p | 21 | Ppp2r5e | 1 | miR-19b-3p |
| 7 | Fgfr2 | 751 | miR-423-3p | 15 | Ddit4 | 2 | miR-423-5p | 21 | Itgb8 | 1 | miR-145a-5p |
| 8 | Csf1r | 749 | miR-22-3p | 15 | Rab10 | 2 | miR-706 | 21 | Thbs3 | 1 | miR-423-5p |
| Keyword | Gene | miRNA | Predicted Effects |
|---|---|---|---|
| Macrophage | Maf1 | miR-122-5p | Required for monocytic, macrophage, osteoclast, and islet beta cell differentiation.→down |
| Pkm | miR-122-5p | Promotes in a STAT1-dependent manner, the expression of the immune checkpoint protein PD-L1 in ARNTL/BMAL1-deficient macrophages, consequently suppresses immune activity.→down | |
| Ocln | miR-122-5p | Regulation of the tight junction and macrophage adhesion and spreading →down | |
| Ifi204 | miR-466f-3p | Inhibits cell growth and may be involved in macrophage differentiation.→down | |
| Il31 | miR-22-3p | May function in skin immunity, enhances myeloid progenitor cell survival in vitro, and induces amyloid A protein expression in macrophages.→up/no | |
| Csf1r | miR-22-3p | Regulation of survival, proliferation and differentiation of macrophages and monocytes and plays an important role in innate immunity and in inflammatory processes.→up/no | |
| Pdgfd | miR-145a-5p | Plays an important role in wound healing and Induces macrophage recruitment.→up/no | |
| Phagocytosis | Il15ra | miR-466f-3p | Required for IL15-induced phagocytosis in neutrophils→down |
| Gulp1 | miR-19b-3p | Required for efficient phagocytosis of apoptotic cells.→up/no |
| miRNAs | Genes | Most Relevant Keyword |
|---|---|---|
| miR-122-5p | Pkm | Immune checkpoint |
| miR-466f-3p | Ins1 | Pre-diabetes condition |
| miR-22-3p | Erbb3 | Cell growth and differentiation |
| Esr1 | ||
| Csf1r | ||
| Pik3cd | B-cell | |
| miR-145a-5p | Pdgfd | Macrophage |
| miR-423-3p | Fgfr2 | Cell growth and differentiation |
| miR-19b-3p | Gulp1 | Phagocyte |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henmi, T.; Matsuoka, H.; Katayama, N.; Saito, M. Suppression of Metastasis of Colon Cancer to Liver in Mouse Models by Pretreatment with Extracellular Vesicles Derived from Nanog-Overexpressing Colon-26 Cancer Cells. Int. J. Mol. Sci. 2024, 25, 12794. https://doi.org/10.3390/ijms252312794
Henmi T, Matsuoka H, Katayama N, Saito M. Suppression of Metastasis of Colon Cancer to Liver in Mouse Models by Pretreatment with Extracellular Vesicles Derived from Nanog-Overexpressing Colon-26 Cancer Cells. International Journal of Molecular Sciences. 2024; 25(23):12794. https://doi.org/10.3390/ijms252312794
Chicago/Turabian StyleHenmi, Takuya, Hideaki Matsuoka, Noa Katayama, and Mikako Saito. 2024. "Suppression of Metastasis of Colon Cancer to Liver in Mouse Models by Pretreatment with Extracellular Vesicles Derived from Nanog-Overexpressing Colon-26 Cancer Cells" International Journal of Molecular Sciences 25, no. 23: 12794. https://doi.org/10.3390/ijms252312794
APA StyleHenmi, T., Matsuoka, H., Katayama, N., & Saito, M. (2024). Suppression of Metastasis of Colon Cancer to Liver in Mouse Models by Pretreatment with Extracellular Vesicles Derived from Nanog-Overexpressing Colon-26 Cancer Cells. International Journal of Molecular Sciences, 25(23), 12794. https://doi.org/10.3390/ijms252312794






