Degradation and/or Dissociation of Neurodegenerative Disease-Related Factor Amyloid-β by a Suspension Containing Calcium Hydrogen Carbonate Mesoscopic Crystals
Abstract
1. Introduction
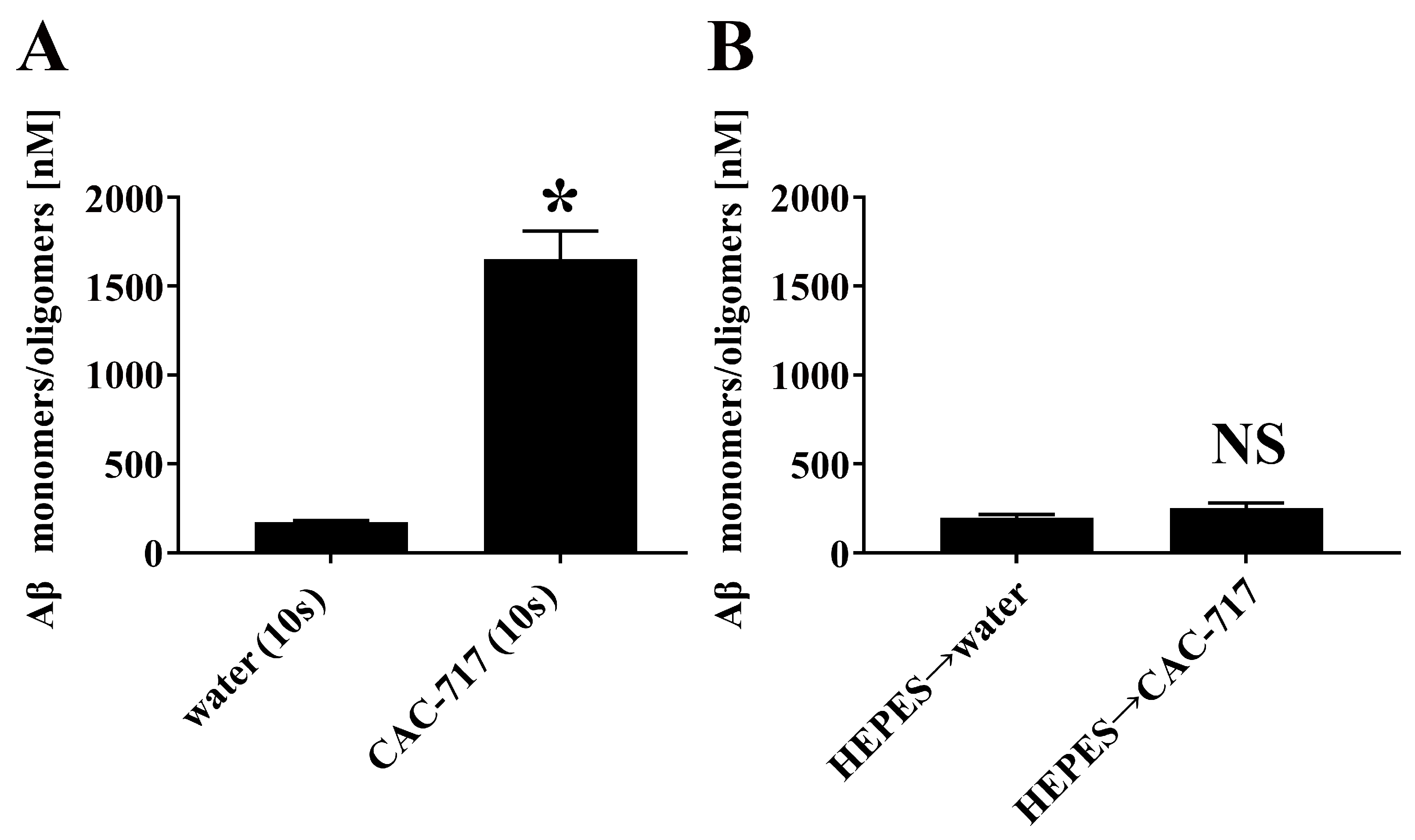
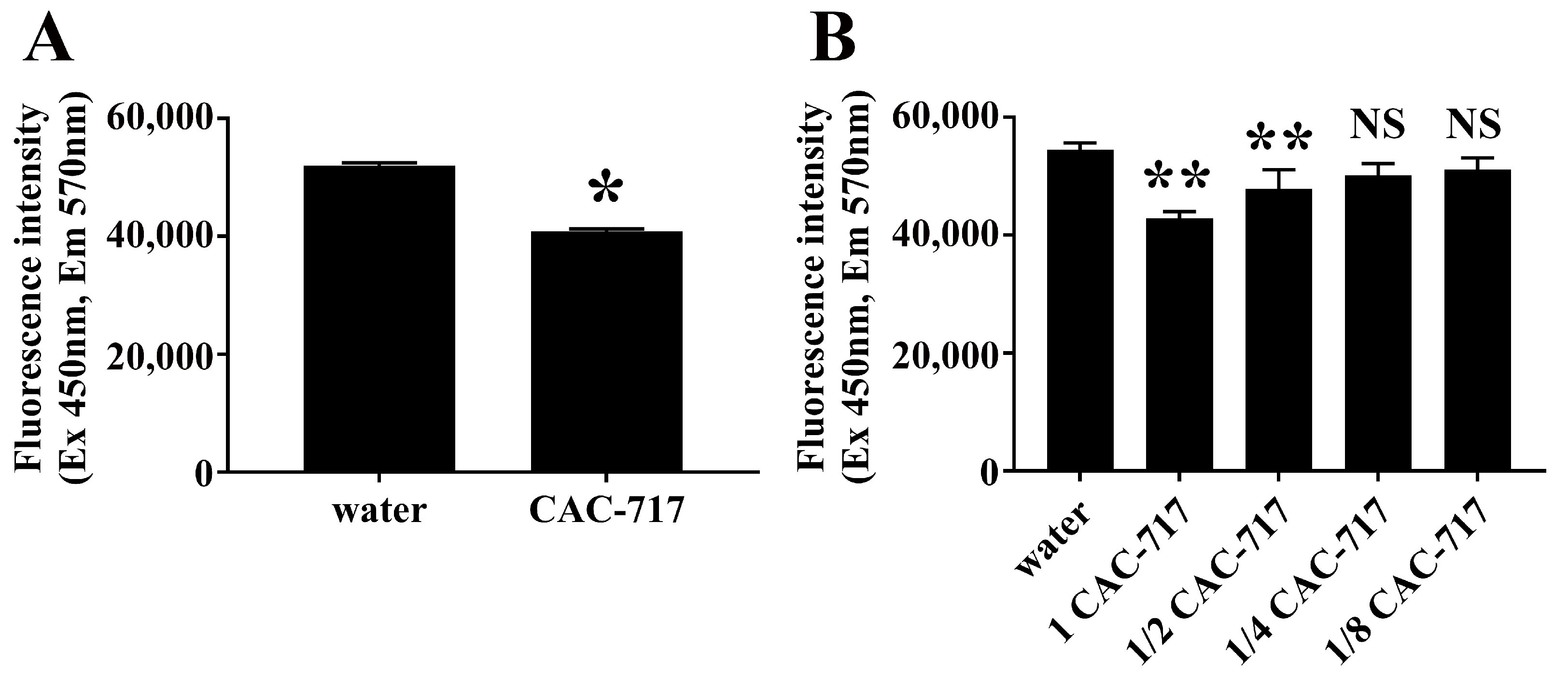
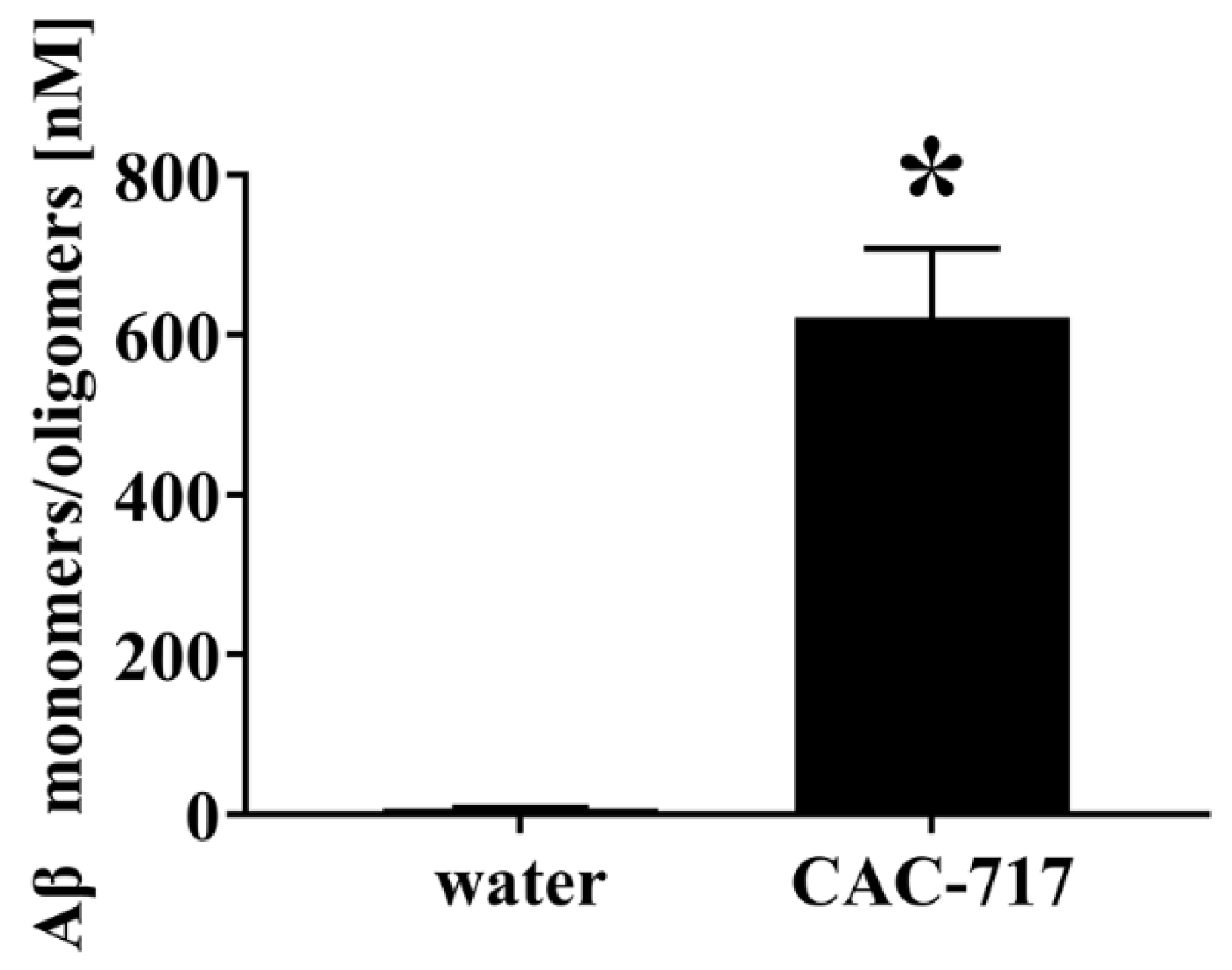
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents for the Formation of Aβ Aggregates
4.2. Degradation of Aβ Aggregates by CAC-717
4.3. Inhibition of Aβ Aggregation by CAC-717
4.4. Analysis of Aggregates Using Thioflavin T
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Haroutunian, V.; Mohs, R.; Davis, K.L.; Davies, P.; Greengard, P.; Buxbaum, J.D. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 2000, 283, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Solis, E., Jr.; Hascup, K.N.; Hascup, E.R. Alzheimer’s Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. J. Alzheimer’s Dis. 2020, 76, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Zetterberg, H. Amyloid-related biomarkers for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef]
- Sakudo, A.; Ano, Y.; Onodera, T.; Nitta, K.; Shintani, H.; Ikuta, K.; Tanaka, Y. Fundamentals of prions and their inactivation (review). Int. J. Mol. Med. 2011, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Catania, M.; Di Fede, G. One or more β-amyloid(s)? New insights into the prion-like nature of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2020, 175, 213–237. [Google Scholar] [PubMed]
- Prusiner, S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013, 47, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.I.; Paras, N.A.; Prusiner, S.B. Expanding spectrum of prion diseases. Emerg. Top. Life Sci. 2020, 4, 155–167. [Google Scholar] [PubMed]
- Scheckel, C.; Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018, 19, 405–418. [Google Scholar] [CrossRef]
- Kumar, A.; Pate, K.M.; Moss, M.A.; Dean, D.N.; Rangachari, V. Self-propagative replication of Aβ oligomers suggests potential transmissibility in Alzheimer disease. PLoS ONE 2014, 9, e111492. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Robertson, N.P. Transmissible amyloid protein: Evidence from iatrogenic CJD. J. Neurol. 2018, 265, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.L.; Barria, M.A. Prion Diseases: A Unique Transmissible Agent or a Model for Neurodegenerative Diseases? Biomolecules 2021, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Groveman, B.R.; Caughey, B. Prions and the potential transmissibility of protein misfolding diseases. Annu. Rev. Microbiol. 2013, 67, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Johnson, B.S.; King, O.D.; Gitler, A.D.; Shorter, J. Prion-like disorders: Blurring the divide between transmissibility and infectivity. J. Cell Sci. 2010, 123, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef]
- Ashe, K.H.; Aguzzi, A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion 2013, 7, 55–59. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Rising, A.; Johansson, J.; Jaudzems, K.; Westermark, P. Transmissible amyloid. J. Intern. Med. 2016, 280, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Farmer, S.F.; Hyare, H.; Jaunmuktane, Z.; Mead, S.; Ryan, N.S.; Schott, J.M.; Werring, D.J.; Rudge, P.; Collinge, J. Iatrogenic Alzheimer’s disease in recipients of cadaveric pituitary-derived growth hormone. Nat. Med. 2024, 30, 394–402. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Evidence for iatrogenic transmission of Alzheimer’s disease. Nat. Med. 2024, 30, 344–345. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Sakudo, A.; Iwamaru, Y.; Yokoyama, T.; Haritani, M.; Sugiura, K.; Shimakura, H.; Haga, T.; Onishi, R.; Furusaki, K. Calcium bicarbonate as an antimicrobial, antiviral, and prion-inhibiting agent (Review). Biomed. Rep. 2022, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Haritani, M.; Furusaki, K.; Onishi, R.; Onodera, T. Electrically Charged Disinfectant Containing Calcium Hydrogen Carbonate Mesoscopic Crystals as a Potential Measure to Control Xanthomonas campestris pv. campestris on Cabbage Seeds. Microorganisms 2020, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Kawamoto, M.; Miyazaki, S.; Onishi, R.; Furusaki, K.; Osaki, M.; Kirisawa, R.; Sakudo, A.; Onodera, T. Evaluation of calcium hydrogen carbonate mesoscopic crystals as a disinfectant for influenza A viruses. J. Vet. Med. Sci. 2017, 79, 939–942. [Google Scholar] [CrossRef]
- Sakudo, A.; Yamashiro, R.; Haritani, M.; Furusaki, K.; Onishi, R.; Onodera, T. Inactivation of Non-Enveloped Viruses and Bacteria by an Electrically Charged Disinfectant Containing Meso-Structure Nanoparticles via Modification of the Genome. Int. J. Nanomed. 2020, 15, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Shimakura, H.; Gen-Nagata, F.; Haritani, M.; Furusaki, K.; Kato, Y.; Yamashita-Kawanishi, N.; Le, D.T.; Tsuzuki, M.; Tohya, Y.; Kyuwa, S.; et al. Inactivation of human norovirus and its surrogate by the disinfectant consisting of calcium hydrogen carbonate mesoscopic crystals. FEMS Microbiol. Lett. 2019, 366, fnz235. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Nishimura, T.; Uwamino, Y.; Kosaki, K.; Furusaki, K.; Onishi, R.; Onodera, T.; Haritani, M.; Sugiura, K.; Kirisawa, R.; et al. Virucidal Effect of the Mesoscopic Structure of CAC-717 on Severe Acute Respiratory Syndrome Coronavirus-2. Microorganisms 2021, 9, 2096. [Google Scholar] [CrossRef] [PubMed]
- Kirisawa, R.; Kato, R.; Furusaki, K.; Onodera, T. Universal Virucidal Activity of Calcium Bicarbonate Mesoscopic Crystals That Provides an Effective and Biosafe Disinfectant. Microorganisms 2022, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Iwamaru, Y.; Furusaki, K.; Haritani, M.; Onishi, R.; Imamura, M.; Yokoyama, T.; Yoshikawa, Y.; Onodera, T. Inactivation of Scrapie Prions by the Electrically Charged Disinfectant CAC-717. Pathogens 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Howlett, D.R.; Perry, A.E.; Godfrey, F.; Swatton, J.E.; Jennings, K.H.; Spitzfaden, C.; Wadsworth, H.; Wood, S.J.; Markwell, R.E. Inhibition of fibril formation in beta-amyloid peptide by a novel series of benzofurans. Biochem. J. 1999, 340, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Hartley, D.M.; Balakhaneh, D.; Aggarwal, A.; Teichberg, S.; Callaway, D.J. New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer’s disease. J. Biol. Chem. 2002, 277, 42881–42890. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Frydman-Marom, A.; Rechter, M.; Gazit, E. Inhibition of amyloid fibril formation and cytotoxicity by hydroxyindole derivatives. Biochemistry 2006, 45, 4727–4735. [Google Scholar] [CrossRef]
- Sievers, S.A.; Karanicolas, J.; Chang, H.W.; Zhao, A.; Jiang, L.; Zirafi, O.; Stevens, J.T.; Münch, J.; Baker, D.; Eisenberg, D. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature 2011, 475, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Howlett, D.R.; George, A.R.; Owen, D.E.; Ward, R.V.; Markwell, R.E. Common structural features determine the effectiveness of carvedilol, daunomycin and rolitetracycline as inhibitors of Alzheimer beta-amyloid fibril formation. Biochem. J. 1999, 343, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Török, M.; Abid, M.; Mhadgut, S.C.; Török, B. Organofluorine inhibitors of amyloid fibrillogenesis. Biochemistry 2006, 45, 5377–5383. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; MacKenzie, L.; Maleeff, B.; Hurle, M.R.; Wetzel, R. Selective inhibition of Abeta fibril formation. J. Biol. Chem. 1996, 271, 4086–4092. [Google Scholar] [CrossRef]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef]
- Iwamaru, Y.; Furusaki, K.; Sugiura, K.; Haritani, M.; Onodera, T. Ceramic absorbed with calcium bicarbonate mesoscopic crystals partially inactivate scrapie prions. Microbiol. Immunol. 2023, 67, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Bag, S.; Sarkar, M.; Dasgupta, S.; Torok, M. Structural Features of Small Molecule Amyloid-Beta Self-Assembly Inhibitors. Curr. Bioact. Compd. 2013, 9, 37–63. [Google Scholar] [CrossRef]
- ISO 10993-10; Biological Evaluation of Medical Devices-Part 10: Test for Irritation and Skin Sensitization. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaya, N.; Sakudo, A.; Kanda, T.; Furusaki, K.; Onishi, R.; Onodera, T.; Yoshikawa, Y. Degradation and/or Dissociation of Neurodegenerative Disease-Related Factor Amyloid-β by a Suspension Containing Calcium Hydrogen Carbonate Mesoscopic Crystals. Int. J. Mol. Sci. 2024, 25, 12761. https://doi.org/10.3390/ijms252312761
Iwaya N, Sakudo A, Kanda T, Furusaki K, Onishi R, Onodera T, Yoshikawa Y. Degradation and/or Dissociation of Neurodegenerative Disease-Related Factor Amyloid-β by a Suspension Containing Calcium Hydrogen Carbonate Mesoscopic Crystals. International Journal of Molecular Sciences. 2024; 25(23):12761. https://doi.org/10.3390/ijms252312761
Chicago/Turabian StyleIwaya, Nodoka, Akikazu Sakudo, Takuya Kanda, Koichi Furusaki, Rumiko Onishi, Takashi Onodera, and Yasuhiro Yoshikawa. 2024. "Degradation and/or Dissociation of Neurodegenerative Disease-Related Factor Amyloid-β by a Suspension Containing Calcium Hydrogen Carbonate Mesoscopic Crystals" International Journal of Molecular Sciences 25, no. 23: 12761. https://doi.org/10.3390/ijms252312761
APA StyleIwaya, N., Sakudo, A., Kanda, T., Furusaki, K., Onishi, R., Onodera, T., & Yoshikawa, Y. (2024). Degradation and/or Dissociation of Neurodegenerative Disease-Related Factor Amyloid-β by a Suspension Containing Calcium Hydrogen Carbonate Mesoscopic Crystals. International Journal of Molecular Sciences, 25(23), 12761. https://doi.org/10.3390/ijms252312761






