Molecular Characteristics of Circ_002156 and Its Effects on Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells
Abstract
1. Introduction
2. Results
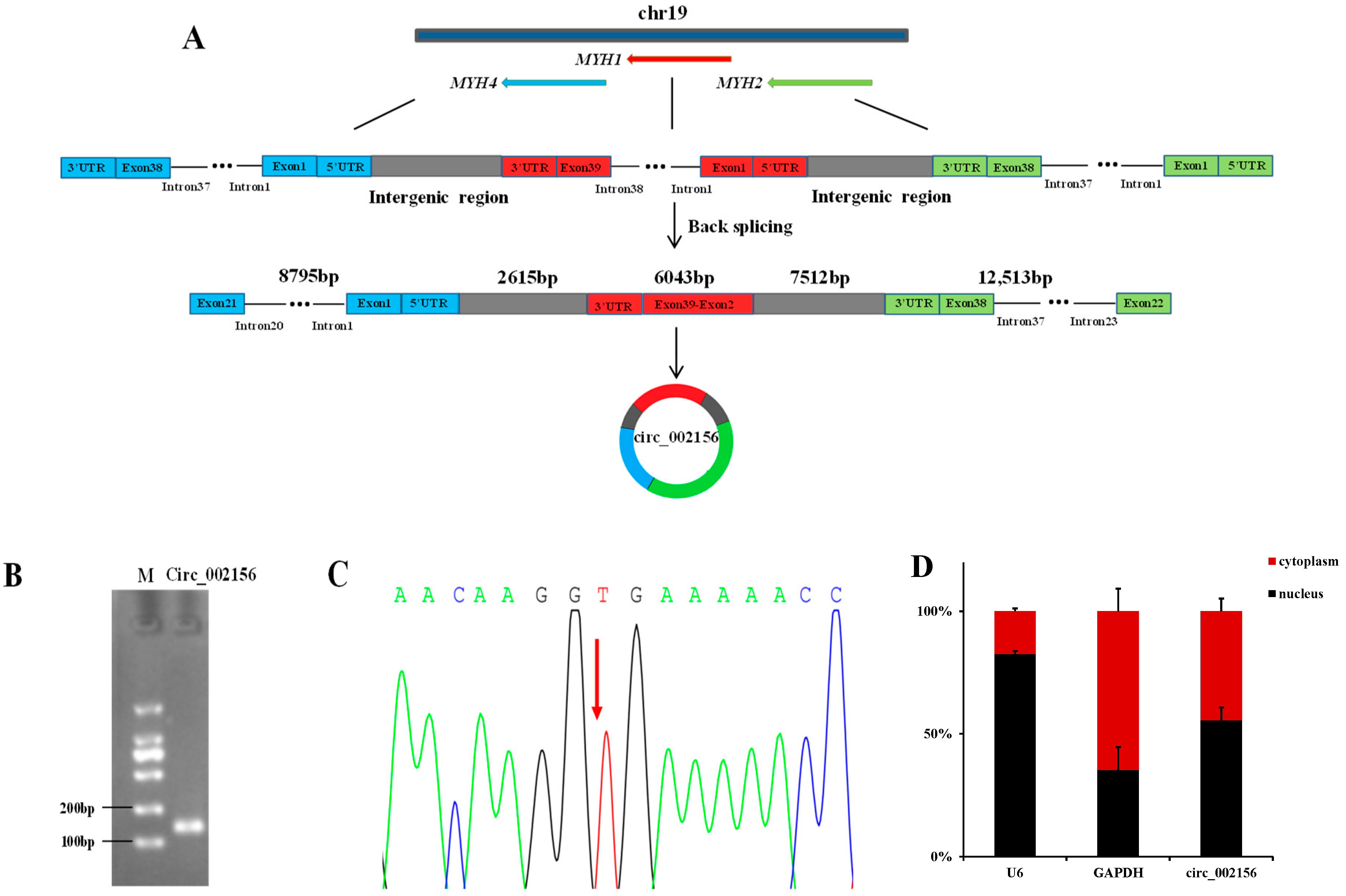
2.1. Characteristics and Cellular Localization of Caprine Circ_002156
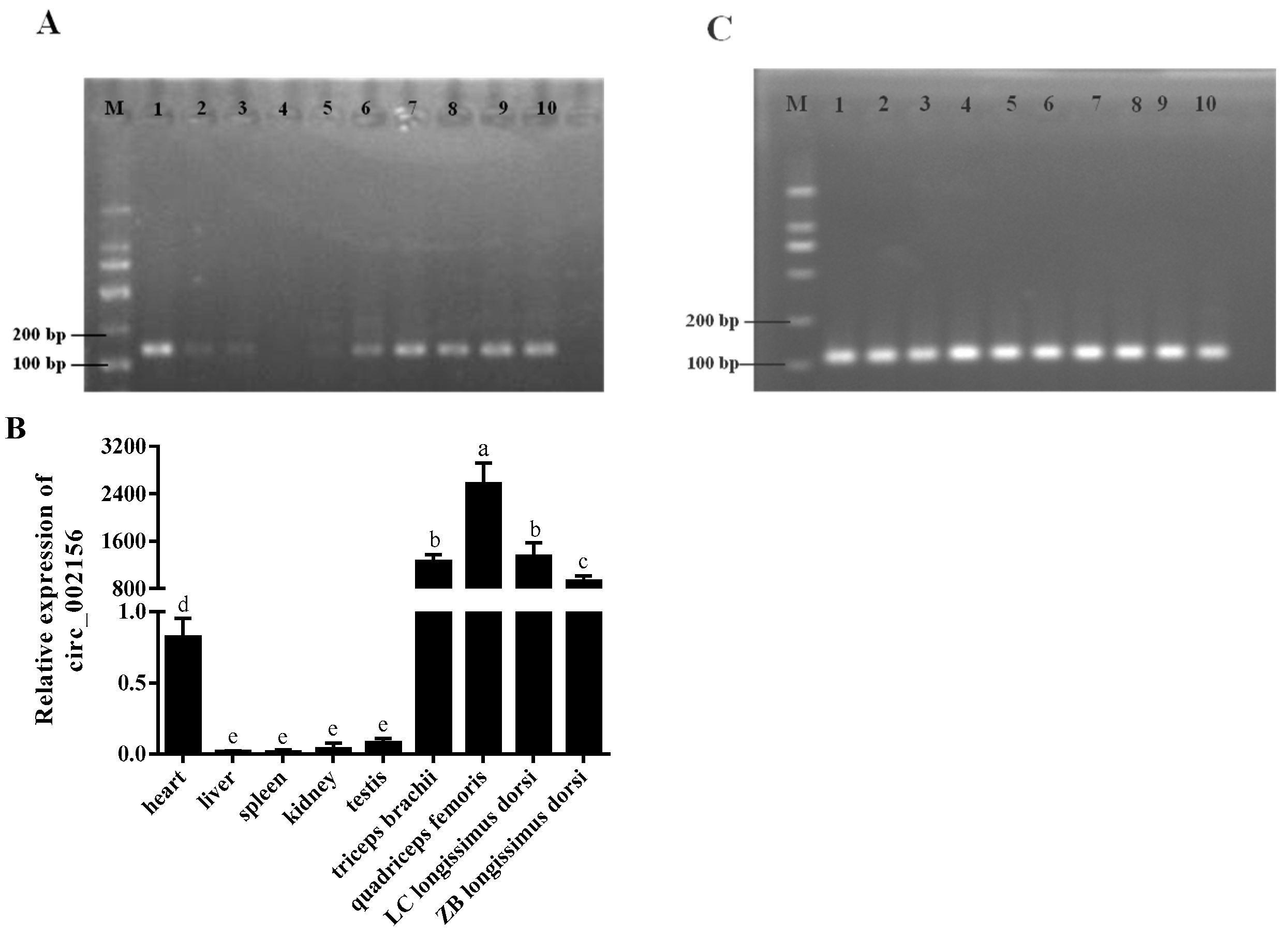
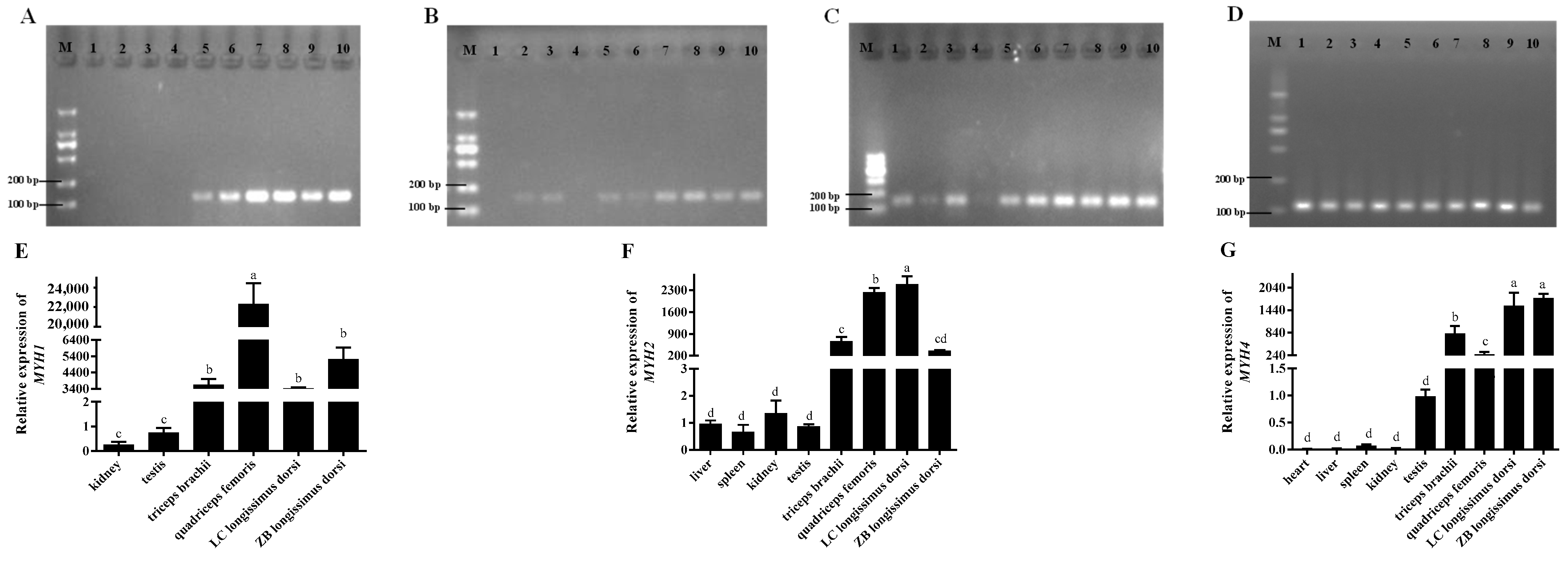
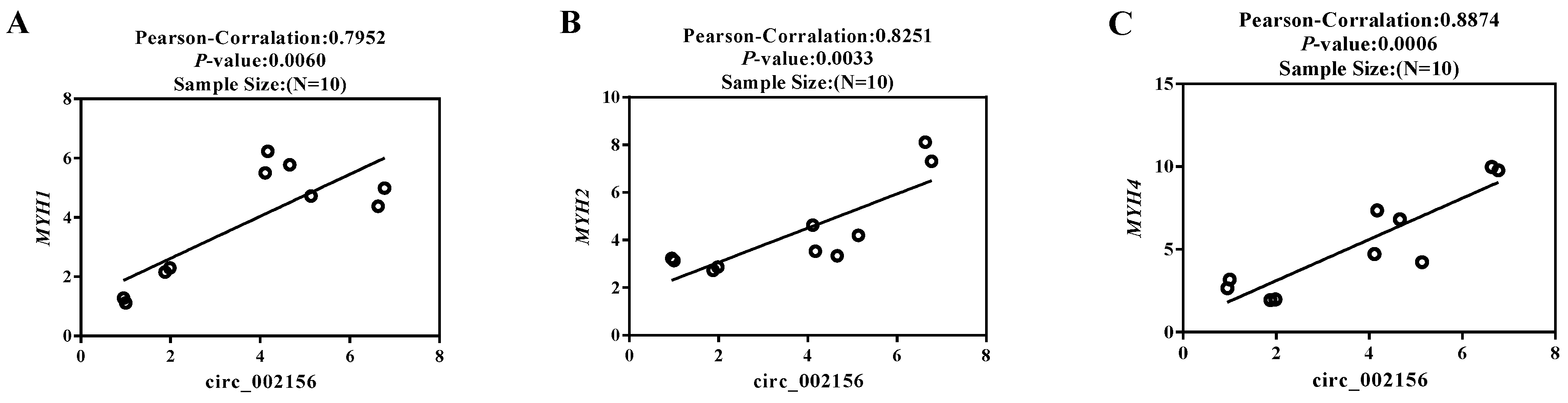
2.2. The Tissue Expression Characteristics of Circ_002156 and Its Parent Genes
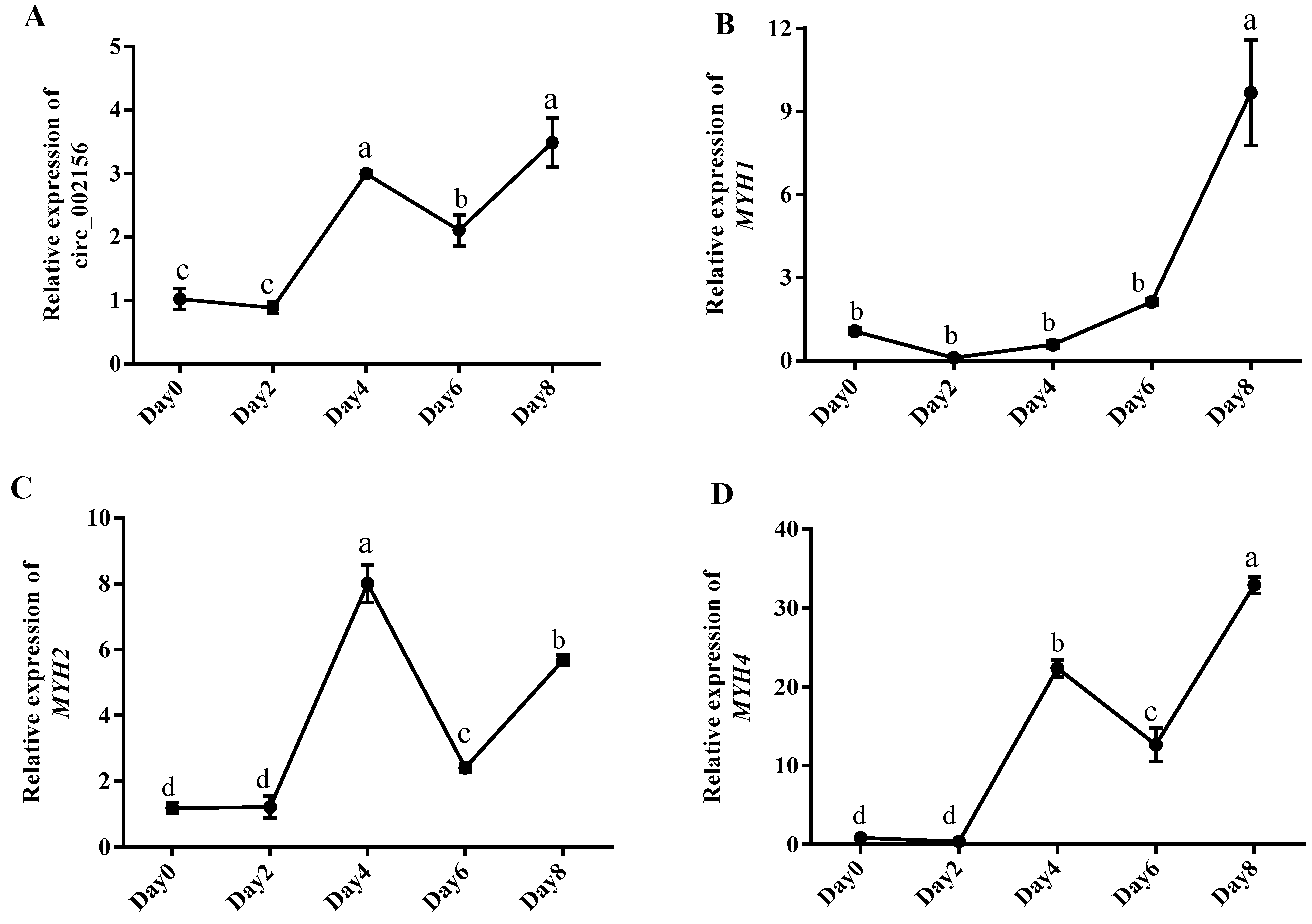
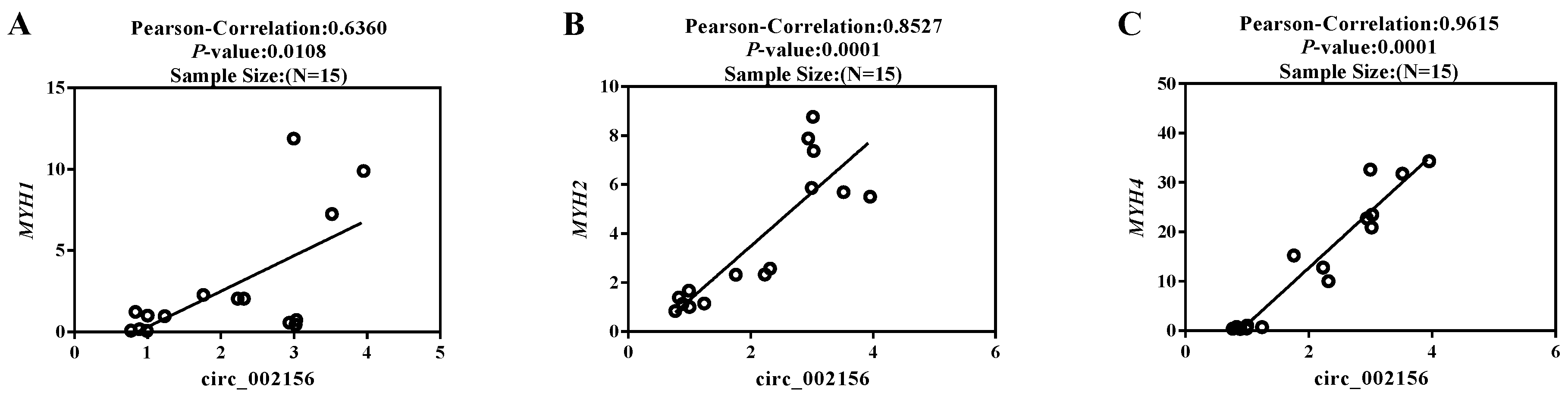
2.3. The Expression Levels of Circ_002156 and Its Parent Genes in Caprine SMSCs
2.4. Circ_002156 Inhibits Viability of SMSCs
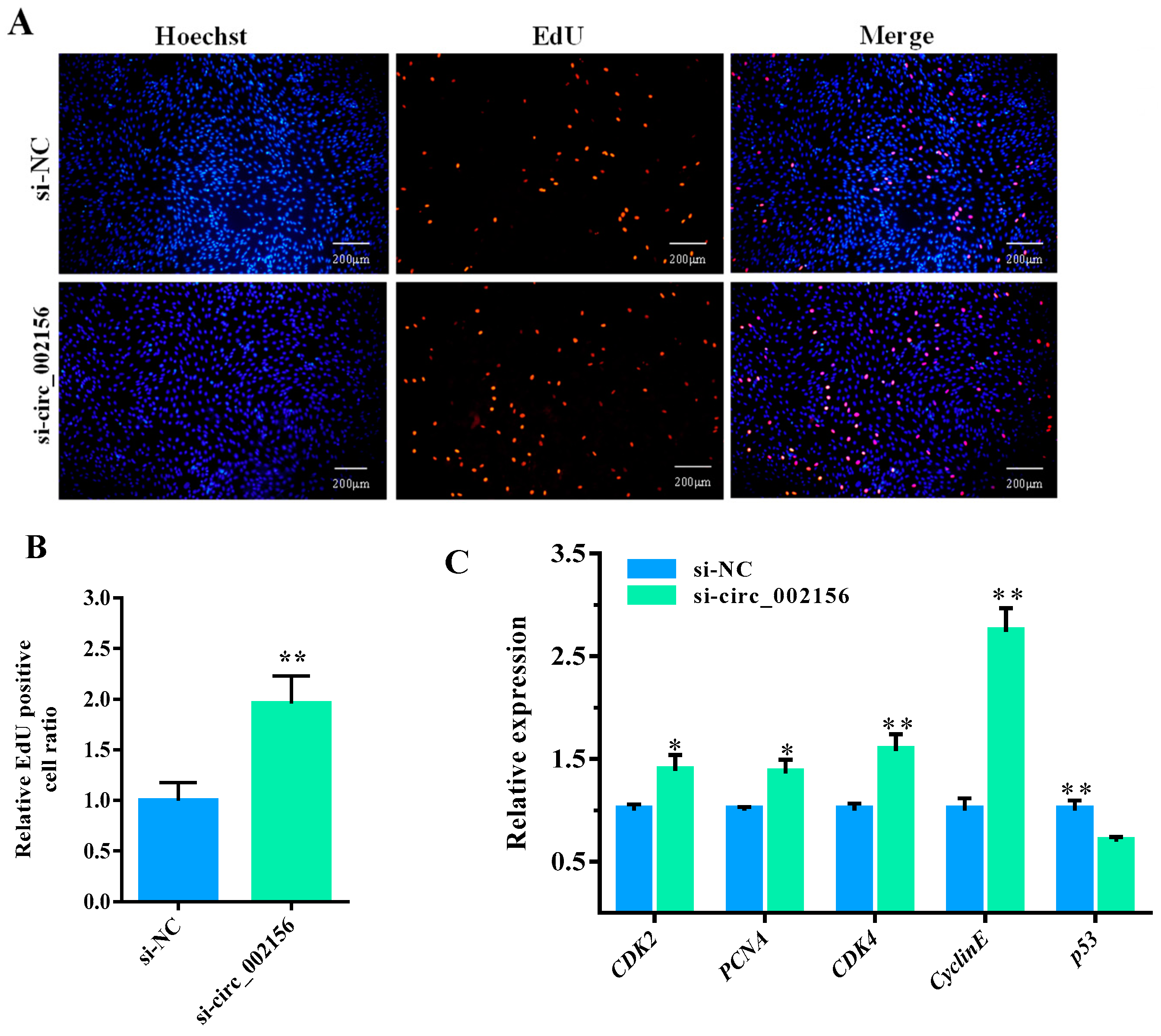
2.5. Circ_002156 Inhibits Proliferation of SMSCs
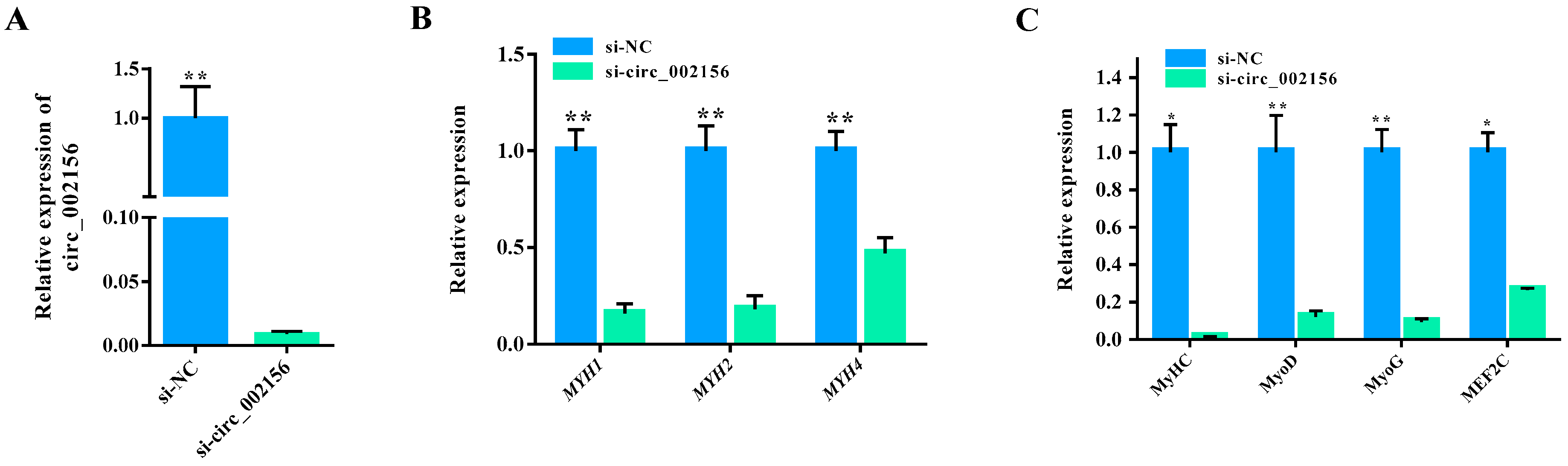
2.6. Circ_002156 Promotes Differentiation of SMSCs
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Cell Culture
4.3. The Authenticity Validation of Circ_002156
4.4. Cellular Localization of Circ_002156 in SMSCs
4.5. RNA Isolation and RT-qPCR
4.6. Transfection of Circ_002156 into SMSCs
4.7. Viability and Proliferation of SMSCs Detected
4.8. Effect of Circ_002156 on Differentiation of SMSCs
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, K.K.; Kjems, J.; Hansen, T.B. Circular RNAs: Identification, biogenesis and function. Biochim. Biophys. Acta. 2016, 1859, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell. 2018, 71, 428–442. [Google Scholar] [CrossRef]
- Wei, X.; Li, H.; Yang, J.; Hao, D.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Lin, F.; et al. Circular RNA profiling reveals an abundant circLMO7 that regulates myoblasts differentiation and survival by sponging miR-378a-3p. Cell. Death. Dis. 2017, 8, e3153. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Yang, J.; Dong, D.; Hao, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Ma, Y.; et al. circFGFR4 Promotes Differentiation of Myoblasts via Binding miR-107 to Relieve Its Inhibition of Wnt3a. Mol. Ther. Nucleic. Acids. 2018, 11, 272–283. [Google Scholar] [CrossRef]
- Ouyang, H.; Chen, X.; Li, W.; Li, Z.; Nie, Q.; Zhang, X. Circular RNA circSVIL Promotes Myoblast Proliferation and Differentiation by Sponging miR-203 in Chicken. Front. Genet. 2018, 9, 172. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Y.; Deng, K.; Liang, Y.; Zhang, G.; Gao, X.; El-Samahy, M.A.; Zhang, Y.; Deng, M.; Wang, F. Circular RNA circUSP13 sponges miR-29c to promote differentiation and inhibit apoptosis of goat myoblasts by targeting IGF1. FASEB J. 2022, 36, e22097. [Google Scholar] [CrossRef]
- Zhen, H.; Shen, J.; Wang, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Li, M.; Shi, B.; et al. Characteristics and Expression of circ_003628 and Its Promoted Effect on Proliferation and Differentiation of Skeletal Muscle Satellite Cells in Goats. Animals 2022, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Z.; Deng, K.; Kang, Z.; Guo, J.; Zhang, G.; Zhang, Y.; Wang, F. CircUBE3A promotes myoblasts proliferation and differentiation by sponging miR-28-5p to enhance expression. Int. J. Biol. Macromol. 2023, 226, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, J.; Zhen, H.; Liu, Y.; Li, L.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; et al. MicroRNA-381 Regulates Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells by Targeting PTEN and JAG2. Int. J. Mol. Sci. 2022, 23, 13587. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Xie, J.; Gao, T.; Cai, C.; Jiang, S.; Bi, H.; Xie, S.; Cui, W. Targeted myostatin loss-of-function mutation increases type II muscle fibers in Meishan pigs. J. Integr. Agric. 2022, 21, 188–198. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, X.; Zhou, L.; Yang, J.; Yang, B.; Ma, H.; Xie, X.; Huang, Y.; Fang, S.; Xiao, S.; et al. Genome-wide association analysis reveals genetic loci and candidate genes for meat quality traits in Chinese Laiwu pigs. Mamm. Genome 2015, 26, 181–190. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, D.; Chen, S.; Wang, L.; Li, Y.; Ma, X.; Song, X.; Liu, X.; Li, W.; Liang, J.; et al. Genome-wide association analysis of meat quality traits in a porcine Large White × Minzhu intercross population. Int. J. Biol. Sci. 2012, 8, 580–595. [Google Scholar] [CrossRef]
- Hitachi, K.; Kiyofuji, Y.; Yamaguchi, H.; Nakatani, M.; Inui, M.; Tsuchida, K. Simultaneous loss of skeletal muscle myosin heavy chain IIx and IIb causes severe skeletal muscle hypoplasia in postnatal mice. FASEB J. 2023, 37, e22692. [Google Scholar] [CrossRef]
- Oldfors, A.; Tajsharghi, H.; Darin, N.; Lindberg, C. Myopathies associated with myosin heavy chain mutations. Acta. Myol. 2004, 23, 90–96. [Google Scholar]
- Brown, D.M.; Parr, T.; Brameld, J.M. Myosin heavy chain mRNA isoforms are expressed in two distinct cohorts during C2C12 myogenesis. J. Muscle. Res. Cell Motil. 2012, 32, 383–390. [Google Scholar] [CrossRef]
- Egner, I.M.; Bruusgaard, J.C.; Gundersen, K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 2016, 143, 2898–2906. [Google Scholar] [CrossRef]
- Shen, J.; Hao, Z.; Luo, Y.; Zhen, H.; Liu, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Zhao, Z.; et al. Deep Small RNA Sequencing Reveals Important miRNAs Related to Muscle Development and Intramuscular Fat Deposition in Longissimus dorsi Muscle from Different Goat Breeds. Front. Vet. Sci. 2022, 9, 911166. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [PubMed]
- Wu, J.; Yue, B. Regulation of myogenic cell proliferation and differentiation during mammalian skeletal myogenesis. Biomed. Pharmacother. 2024, 174, 116563. [Google Scholar] [CrossRef] [PubMed]
- Lovel, A.G.; Mitchell, D.M. PCNA Staining of Retinal Cryosections to Assess Microglial/Macrophage Proliferation. Methods Mol. Biol. 2023, 2636, 389–400. [Google Scholar] [PubMed]
- Martínez-Alonso, D.; Malumbres, M. Mammalian cell cycle cyclins. Semin. Cell. Dev. Biol. 2020, 107, 28–35. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Ren, L.; Wei, J.; Zhu, Y.; Duan, J.; Jing, L.; Sun, Z.; Zhou, X. Fine particulate matters induce apoptosis via the ATM/P53/CDK2 and mitochondria apoptosis pathway triggered by oxidative stress in rat and GC-2spd cell. Ecotoxicol. Environ. Saf. 2019, 180, 280–287. [Google Scholar] [CrossRef]
- Yin, H.; Shen, X.; Zhao, J.; Cao, X.; He, H.; Han, S.; Chen, Y.; Cui, C.; Wei, Y.; Wang, Y.; et al. Circular RNA CircFAM188B Encodes a Protein That Regulates Proliferation and Differentiation of Chicken Skeletal Muscle Satellite Cells. Front. Cell. Dev. Biol. 2020, 8, 522588. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Sun, H.S.; Tsai, S.J. Circular RNA-new member of noncoding RNA with novel functions. Exp. Biol. Med. 2017, 242, 1136–1141. [Google Scholar] [CrossRef]
- Zhuang, X.; Lin, Z.; Xie, F.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Identification of circRNA-associated ceRNA networks using longissimus thoracis of pigs of different breeds and growth stages. BMC Genom. 2022, 23, 294. [Google Scholar] [CrossRef]
- Elnour, I.E.; Wang, X.; Zhansaya, T.; Akhatayeva, Z.; Khan, R.; Cheng, J.; Hung, Y.; Lan, X.; Lei, C.; Chen, H. Circular RNA circMYL1 Inhibit Proliferation and Promote Differentiation of Myoblasts by Sponging miR-2400. Cells 2021, 10, 176. [Google Scholar] [CrossRef]
- Sassoon, D.A. Myogenic regulatory factors: Dissecting their role and regulation during vertebrate embryogenesis. Dev. Biol. 1993, 156, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 2020, 9, e60445. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Wu, C.C.; Li, N.; Weng, T.H. Post-transcriptional regulation of myogenic transcription factors during muscle development and pathogenesis. J. Muscle. Res. Cell. Motil. 2024, 45, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Wang, X.; Cao, X.; Dong, D.; Shen, X.; Cheng, J.; Jiang, R.; Yang, Z.; Peng, S.; Huang, Y.; Lan, X.; et al. Circular RNA TTN Acts As a miR-432 Sponge to Facilitate Proliferation and Differentiation of Myoblasts via the IGF2/PI3K/AKT Signaling Pathway. Mol. Ther. Nucleic. Acids. 2019, 18, 966–980. [Google Scholar] [CrossRef]
- Shen, J.; Zhen, H.; Li, L.; Zhang, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Li, M.; et al. Identification and characterization of circular RNAs in Longissimus dorsi muscle tissue from two goat breeds using RNA-Seq. Mol. Genet. Genom. 2022, 297, 817–831. [Google Scholar] [CrossRef]


| circRNA | Nuclei/Myotube |
|---|---|
| si-NC | 45.976 ± 1.100 ** |
| si-circ_002156 | 28.562 ± 2.276 |
| Name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| circ_002156 | F: CTGAACTGACGGCCAAGAAG | R: TCATCCAGACCTGCCATCTC |
| MYH2 | F: CTGGCTGGAGAAGAACAAGG | R: CACCGTCTGGAAAGAAGAGC |
| MYH1 | F: ATGCGGGAACAGTGGACTAC | R: GCACCAGAGAACAGGAAAGC |
| MYH4 | F: TCAAGGGGAGATCACAGTCC | R: GAGGTAGGCAGCCTTGTCAG |
| CDK2 | F: GACCAGCTCTTCCGGATCTT | R: ACAAGCTCCGTCCATCTTCA |
| CDK4 | F: ACTTTGTGGCCCTCAAGAGT | R: CCTGAGGTCTTGGTCCACAT |
| PCNA | F: GAACCTCACCAGCATGTCCAA | R: TTCACCAGAAGGCATCTTTACT |
| CyclinE | F: CTCCCTGATTCCCACACCTG | R: CATAAGATGCTTGTCCCTCA |
| p53 | F: CGGCTTGCAGAAACCTCTTT | R: CCCTTTTCTACCTCCTGCCA |
| MYHC | F: CCACATCTTCTCCATCTCTG | R: GGTTCCTCCTTCTTCTTCTC |
| MYOD | F: GTGCAAACGCAAGACGACTA | R: GCTGGTTTGGGTTGCTAGAC |
| MYOG | F: CGTGGGCGTGTAAGGTGT | R: GGCGCTCTATGTACTGGATGG |
| MEF2C | F: ATCCTGATGCAGACGATTCAG | R: GGTGGAACAGCACACAATCTT |
| β-tubulin | F: AGCGTATCTCAGAGCAGTTC | R: AATCCTCTTCCTCTTCTGCG |
| GAPDH | F: ACACTGAGGACCAGGTTGTG | R: GACAAAGTGGTCGTTGAGGG |
| β-actin | F: AGCCTTCCTTCCTGGGCATGGA | R: GGACAGCACCGTGTTGGCGTAA |
| U6 | F: GGAACGATACAGAGAAGATTAGC | R: TGGAACGCTTCACGAATTTGCG |
| si-circ_002156 | F: GAGAACAAGGUGAAAAACCTT | R: TGGAACGCTTCACGAATTTGCG |
| si-NC | F: UUCUCCGAACGUGUCACGUTT | R: GGUUUUUCACCUUGUUCUCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Shen, J.; Hao, Z.; Zhen, H.; Wu, X.; Wang, J.; Li, M.; Ren, C.; Liu, Y.; Zhao, Y.; et al. Molecular Characteristics of Circ_002156 and Its Effects on Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells. Int. J. Mol. Sci. 2024, 25, 12745. https://doi.org/10.3390/ijms252312745
Gu Y, Shen J, Hao Z, Zhen H, Wu X, Wang J, Li M, Ren C, Liu Y, Zhao Y, et al. Molecular Characteristics of Circ_002156 and Its Effects on Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells. International Journal of Molecular Sciences. 2024; 25(23):12745. https://doi.org/10.3390/ijms252312745
Chicago/Turabian StyleGu, Yuanhua, Jiyuan Shen, Zhiyun Hao, Huimin Zhen, Xinmiao Wu, Jiqing Wang, Mingna Li, Chunyan Ren, Yuan Liu, Yuan Zhao, and et al. 2024. "Molecular Characteristics of Circ_002156 and Its Effects on Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells" International Journal of Molecular Sciences 25, no. 23: 12745. https://doi.org/10.3390/ijms252312745
APA StyleGu, Y., Shen, J., Hao, Z., Zhen, H., Wu, X., Wang, J., Li, M., Ren, C., Liu, Y., Zhao, Y., Yang, P., & Wang, X. (2024). Molecular Characteristics of Circ_002156 and Its Effects on Proliferation and Differentiation of Caprine Skeletal Muscle Satellite Cells. International Journal of Molecular Sciences, 25(23), 12745. https://doi.org/10.3390/ijms252312745






