Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation
2.2. Neuroprotective Activity
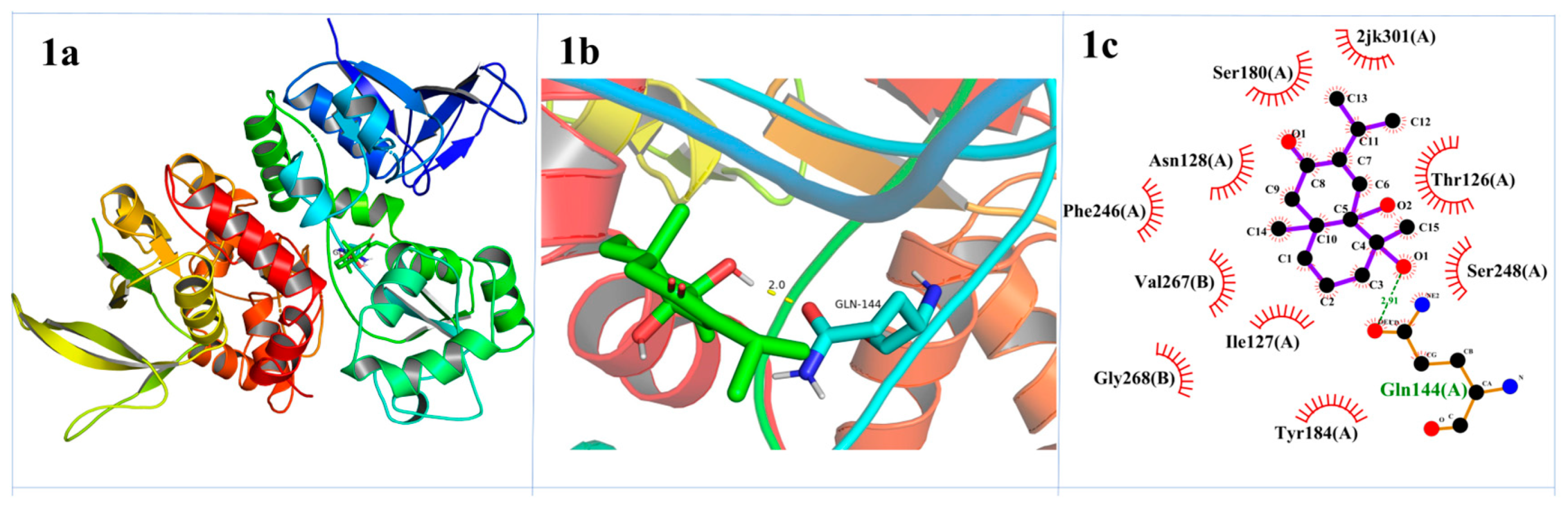
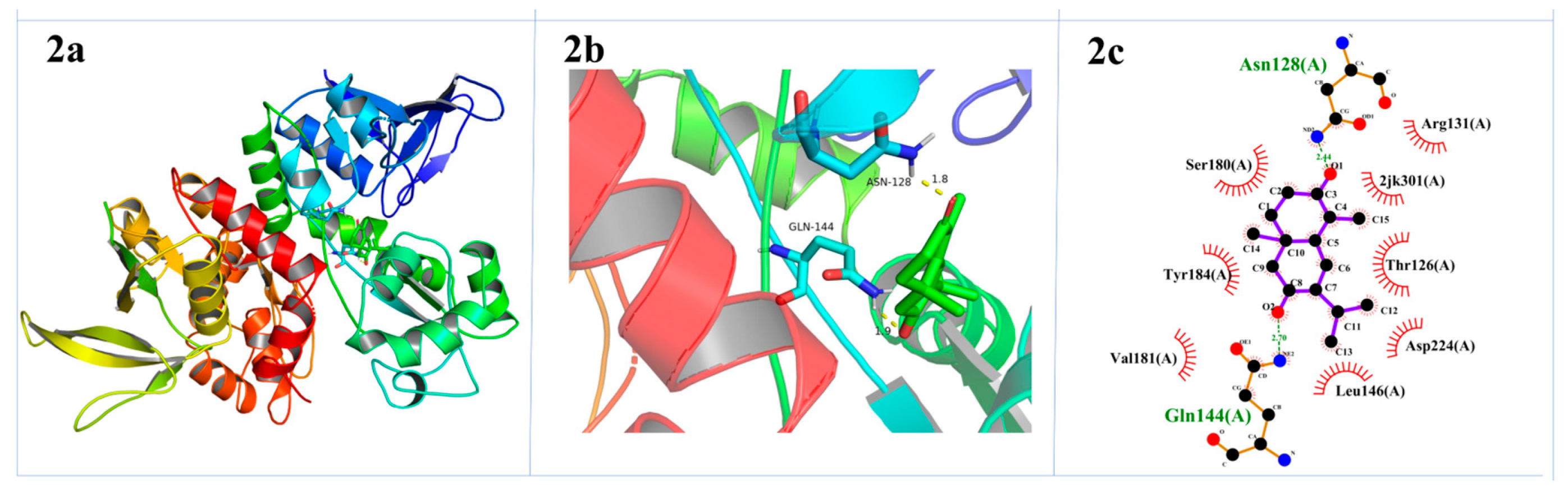
2.3. Molecular Docking Study
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. X-Ray Crystallographic Analysis of Compounds 1, 2 and 4
3.5. Theoretical ECD Calculation
3.6. Cell Viability Assay
3.7. Molecular Docking Study
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Shonberg, J.; Kaindl, J.; Clark, M.J.; Stößel, A.; Maul, L.; Mayer, D.; Hübner, H.; Hirata, K.; Venkatakrishnan, A.J.; et al. Constrained catecholamines gain β2AR selectivity through allosteric effects on pocket dynamics. Nat. Commun. 2023, 14, 2138. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Yin, F.C.; Wang, X.B.; Kong, L.Y. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Lamaida, N.; Cerciello, A. 852 Dapaglifozin in patients with cardiac heart failure. Eur. Heart J. Suppl. 2022, 24, suac121.150. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Flora. Flora of China; Science Press: Beijing, China, 1982; p. 176. [Google Scholar]
- Sun, X.X.; Xu, J.; Liu, J.; Guo, J.T.; Zhu, X.T. Optimization of the extraction process of Cinnamomum migao oil and its ameliorative effects on myocardial ischemia. J. Food. Saf. Qual. 2024, 15, 262–273. [Google Scholar]
- Sun, X.H.; Ye, J.Q. Effects of oil of Cinnamomum migao (CV-3) on isolated smooth muscles. Eur. J. Pharmacol. 1990, 183, 554. [Google Scholar] [CrossRef]
- Gong, W.X.; Zhou, Y.Z.; Qin, X.M.; Du, G.H. Involvement of mitochondrial apoptotic pathway and MAPKs/NF-κB inflammatory pathway in the neuroprotective effect of atractylenolide III in corticosterone-induced PC12 cells. Chin. J. Nat. Med. 2019, 17, 264–274. [Google Scholar] [CrossRef]
- Wang, S.N.; Jin, D.Q.; Xie, C.F.; Wang, H.; Wang, M.C.; Xu, J.; Guo, Y.Q. Isolation, characterization, and neuroprotective activities of sesquiterpenes from Petasites japonicus. Food Chem. 2013, 141, 2075–2082. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, X.Q.; Ren, J.L.; Liu, P.L.; Yan, Q.; Chen, Z.M. Cuparene-type sesquiterpenes with neuroprotective activities from the edible mushroom Flammulina filiformis. Fitoterapia 2024, 179, 106235. [Google Scholar] [CrossRef]
- Ma, L.F.; Lou, S.Q.; Chen, H.Y.; Luo, D.; Guo, L.; Chen, N.Y.; Wu, R.; Fang, L.; Zhan, Z.J. Highly oxidized guaiane 12(8), 15(6)-dilactones with neuroprotective activities from the roots of Lindera aggregata (Sims) Kosterm. Phytochemistry 2024, 224, 114150. [Google Scholar] [CrossRef]
- Muhammad, I.; Hassan, S.S.U.; Xu, W.J.; Tu, G.L.; Yu, H.J.; Xiao, X.; Yan, K.; Jin, H.Z.; Bungau, S. An extensive pharmacological evaluation of novel anti-nociceptive and IL-6 targeted anti-inflammatory guaiane-type sesquiterpenoids from Cinnamomum migao H. W. Li through in-depth in-vitro, ADMET, and molecular docking studies. Biomed. Pharmacother. 2023, 164, 114946. [Google Scholar] [CrossRef]
- Muhammad, I.; Luo, W.; Shoaib, M.R.; Li, G.L.; Hassan, S.S.U.; Yang, Z.H.; Xiao, X.; Tu, G.L.; Yan, S.K.; Ma, X.P.; et al. Guaiane-type sesquiterpenoids from Cinnamomum migao H. W. Li: And their anti-inflammatory activities. Phytochemistry 2021, 190, 112850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, L.S.; Wang, L.; Liu, H.D.; Gao, M.; Chen, F.J.; Yang, J.; Li, Q.J.; Yang, X.S. Cinnamigones A–C, three highly oxidized guaiane-type sesquiterpenes with neuroprotective activity from Cinnamomum migao. Phytochemistry 2023, 212, 113728. [Google Scholar] [CrossRef]
- Morita, H.; Simizu, K.; Takizawa, H.; Aiyama, R.; Itokawa, H. Studies on Chemical Conversion of Alpinenone to Furopelargone B. Chem. Pharm. Bull. 1988, 36, 3156–3160. [Google Scholar] [CrossRef][Green Version]
- Kiem, P.V.; Minh, C.V.; Nhiem, N.X.; Cuc, N.T.; Quang, N.V.; Anh, H.L.T.; Tai, B.H.; Yen, P.H.; Hoai, N.T.; Ho, K.Y.; et al. Muurolane-type sesquiterpenes from marine sponge Dysidea cinerea. Magn. Reson. Chem. 2014, 52, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Yu, M.T. Four New Sesquiterpenes from the Heartwood of Juniperus formosana var. concolor. Chem. Pharm. Bull. 1999, 47, 1017–1019. [Google Scholar] [CrossRef]
- Zan, K.; Shi, S.P.; Fu, Q.; Chen, X.Q.; Zhou, S.X.; Xiao, M.T.; Tu, P.F. New Sesquiterpenoids from Artemisia anomala. Helv. Chim. Acta. 2010, 93, 2000–2006. [Google Scholar] [CrossRef]
- Muraoka, O.; Fujimoto, M.; Tanabe, G.; Kubo, M.; Minematsu, T.; Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Absolute stereostructures of novel norcadinane and trinoreudesmane-type sesquiterpenes with nitric oxide production inhibitory activity from Alpinia oxyphylla. Bioorg. Med. Chem. Lett. 2001, 11, 2217–2220. [Google Scholar] [CrossRef]
- Soliman, H.S.M.; El-Dib, R.; Shalaby, N.M.M.; Duddeck, H.; Simon, A.; Tóth, G. Isolation and structure determination of compounds from Stachys yemenensis hedge. Nat. Prod. Commun. 2007, 2, 977–980. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Zhong, X.M.; Li, Z.Y.; Huang, Z. Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother. Res. 2011, 25, 681–685. [Google Scholar] [CrossRef]
- Yang, L.S.; Yang, Q.; Wang, E.H.; Yang, J.; Li, Q.J.; Cao, J.F.; Wang, L.; Liao, X.; Yang, Y.; Yang, X.S. Synthesis of novel 1-phenyl-benzopyrrolizidin-3-one derivatives and evaluation of their cytoneuroprotective effects against NMDA-induced injury in PC12 cells. Bioorg. Med. Chem. 2022, 59, 116675. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXT. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Lou, H.Y.; Chen, M.J.; Yi, P.; Jin, J.; Zeng, Y.R.; Gu, W.; Hu, Z.X.; Yang, J.; Hao, X.J.; Yuan, C.M. Hyperkouytones A—O, new polyprenylated acylphloroglucinols from Hypericum kouytchense with multidrug resistance reversal activity. Chin. J. Chem. 2024, 42, 3293–3307. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H.A. A simple method of estimating 50 percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
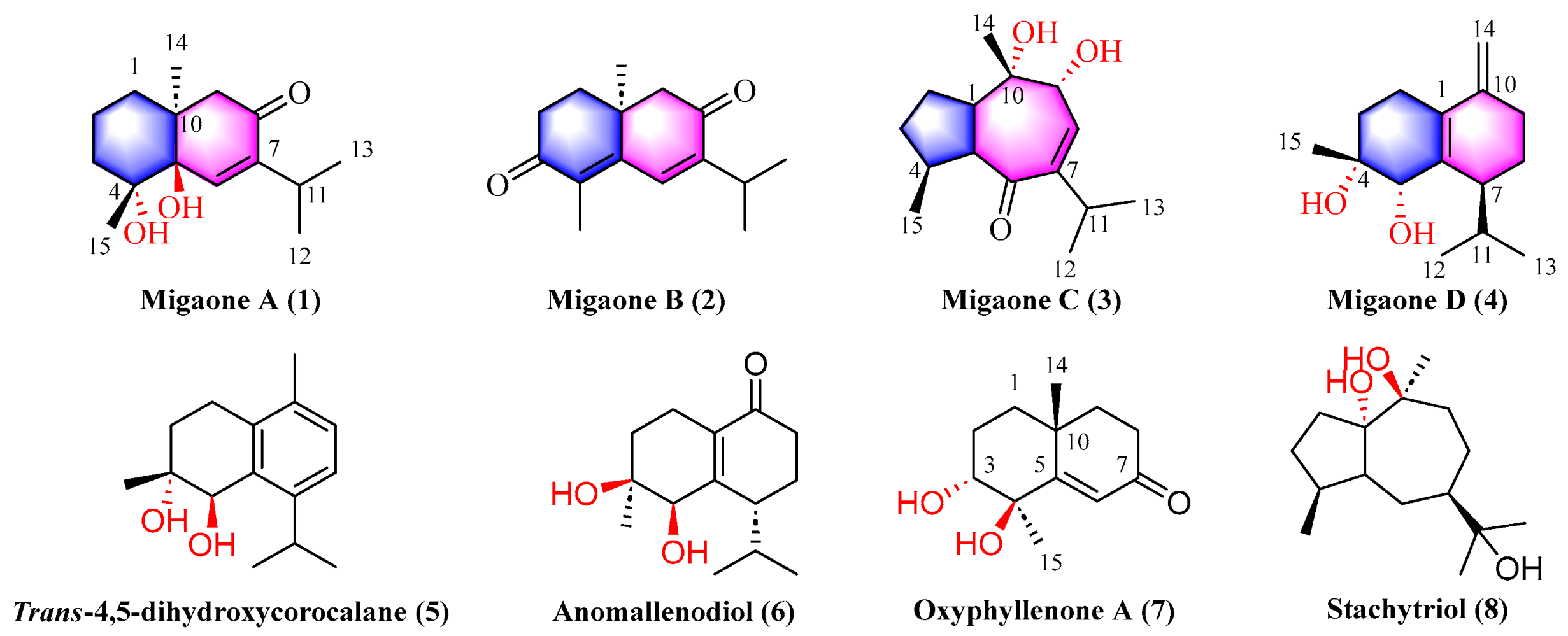
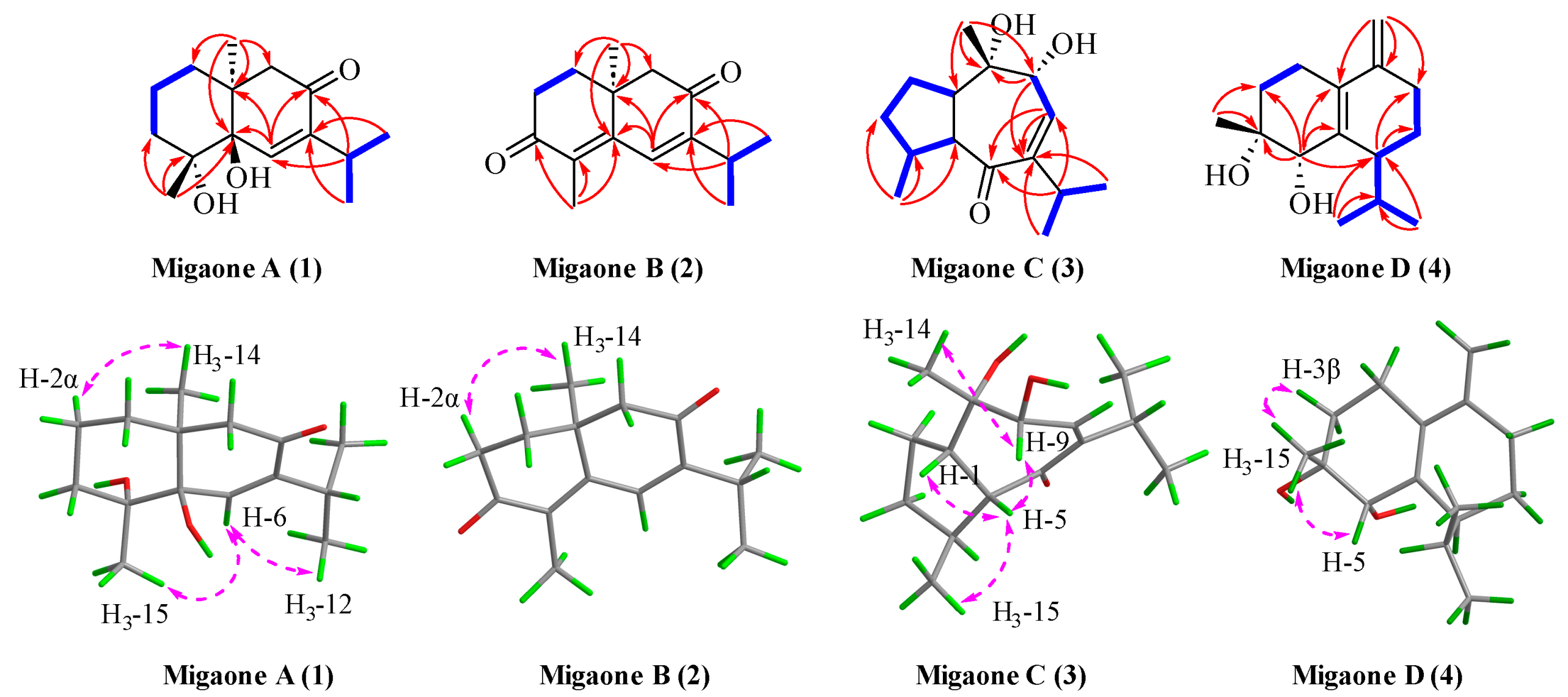
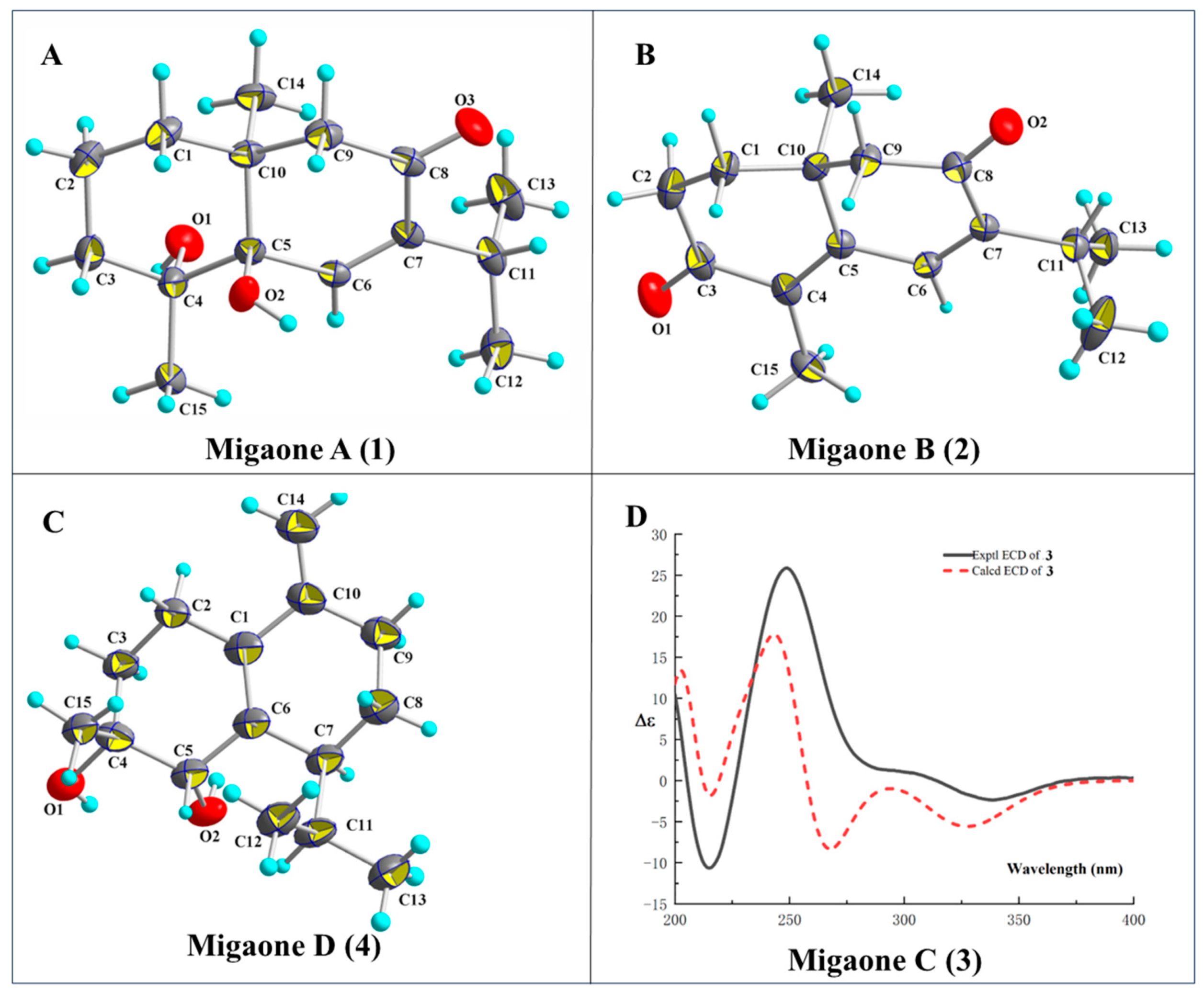

| No. | Migaone A (1) a | Migaone B (2) a | Migaone C (3) b | Migaone D (4) b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 33.0 | 1.85, m | 35.9 | 2.02, td (14.0, 5.3) | 51.5 | 2.15, m | 132.4 | |
| 1.20, m | 1.82, ddd (13.3, 5.3, 2.3) | |||||||
| 2 | 17.4 | 1.85, m | 33.7 | 2.62, ddd(17.8, 14.5, 5.2) | 26.2 | 1.78, m | 25.6 | 2.30, m |
| 1.50, m | 2.55, ddd (17.9, 5.2, 2.3) | 1.70, m | 2.24, m | |||||
| 3 | 36.3 | 1.97, m | 198.6 | 35.1 | 1.70, m | 31.9 | 1.86, m | |
| 1.50, m | 1.26, m | 1.52, m | ||||||
| 4 | 74.4 | 133.3 | 37.5 | 2.23, m | 71.4 | |||
| 5 | 73.8 | 152.1 | 62.0 | 1.93, dd (10.7, 7.8) | 73.2 | 3.78, s | ||
| 6 | 139.7 | 6.87, s | 133.6 | 7.16, s | 207.3 | 139.1 | ||
| 7 | 148.5 | 148.8 | 147.6 | 43.1 | 2.43, m | |||
| 8 | 199.8 | 197.9 | 141.5 | 6.16, dd (4.6, 1.2) | 24.0 | 1.70, m | ||
| 1.52, m | ||||||||
| 9 | 51.2 | 2.91, m | 53.0 | 2.45, m | 76.0 | 4.17, d (4.6) | 31.5 | 2.43, m |
| 1.98, m | 2.24, m | |||||||
| 10 | 41.3 | 37.5 | 76.0 | 146.0 | ||||
| 11 | 26.4 | 2.90, m | 27.2 | 3.02, p (6.9) | 30.1 | 2.82, m | 29.7 | 2.17, m |
| 12 | 22.1 | 1.05, d (6.9) | 22.0 | 1.11, d (6.9) | 22.1 | 1.04, d, (6.9) | 18.2 | 0.76, d (6.9) |
| 13 | 21.5 | 1.07, d (6.9) | 21.8 | 1.14, d (6.9) | 22.6 | 0.97, d, (6.9) | 21.9 | 0.97, d (6.9) |
| 14 | 23.6 | 1.23, s | 23.9 | 1.25, s | 19.9 | 1.24, s | 108.5 | 4.92, s |
| 4.74, s | ||||||||
| 15 | 26.7 | 1.37, s | 11.2 | 1.97, s | 21.3 | 1.09, d (6.7) | 24.7 | 1.15, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Chen, F.; Yang, L.; Peng, M.; Pan, X.; Lou, H.; Yang, J.; Yang, X.; Li, Q. Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells. Int. J. Mol. Sci. 2024, 25, 12693. https://doi.org/10.3390/ijms252312693
Zhou L, Chen F, Yang L, Peng M, Pan X, Lou H, Yang J, Yang X, Li Q. Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells. International Journal of Molecular Sciences. 2024; 25(23):12693. https://doi.org/10.3390/ijms252312693
Chicago/Turabian StyleZhou, Lang, Faju Chen, Lishou Yang, Mei Peng, Xiong Pan, Huayong Lou, Juan Yang, Xiaosheng Yang, and Qiji Li. 2024. "Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells" International Journal of Molecular Sciences 25, no. 23: 12693. https://doi.org/10.3390/ijms252312693
APA StyleZhou, L., Chen, F., Yang, L., Peng, M., Pan, X., Lou, H., Yang, J., Yang, X., & Li, Q. (2024). Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells. International Journal of Molecular Sciences, 25(23), 12693. https://doi.org/10.3390/ijms252312693






