Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions
Abstract
1. Introduction
2. Technology Innovation for Renal Autoantibody Discovery
2.1. Indirect Approaches
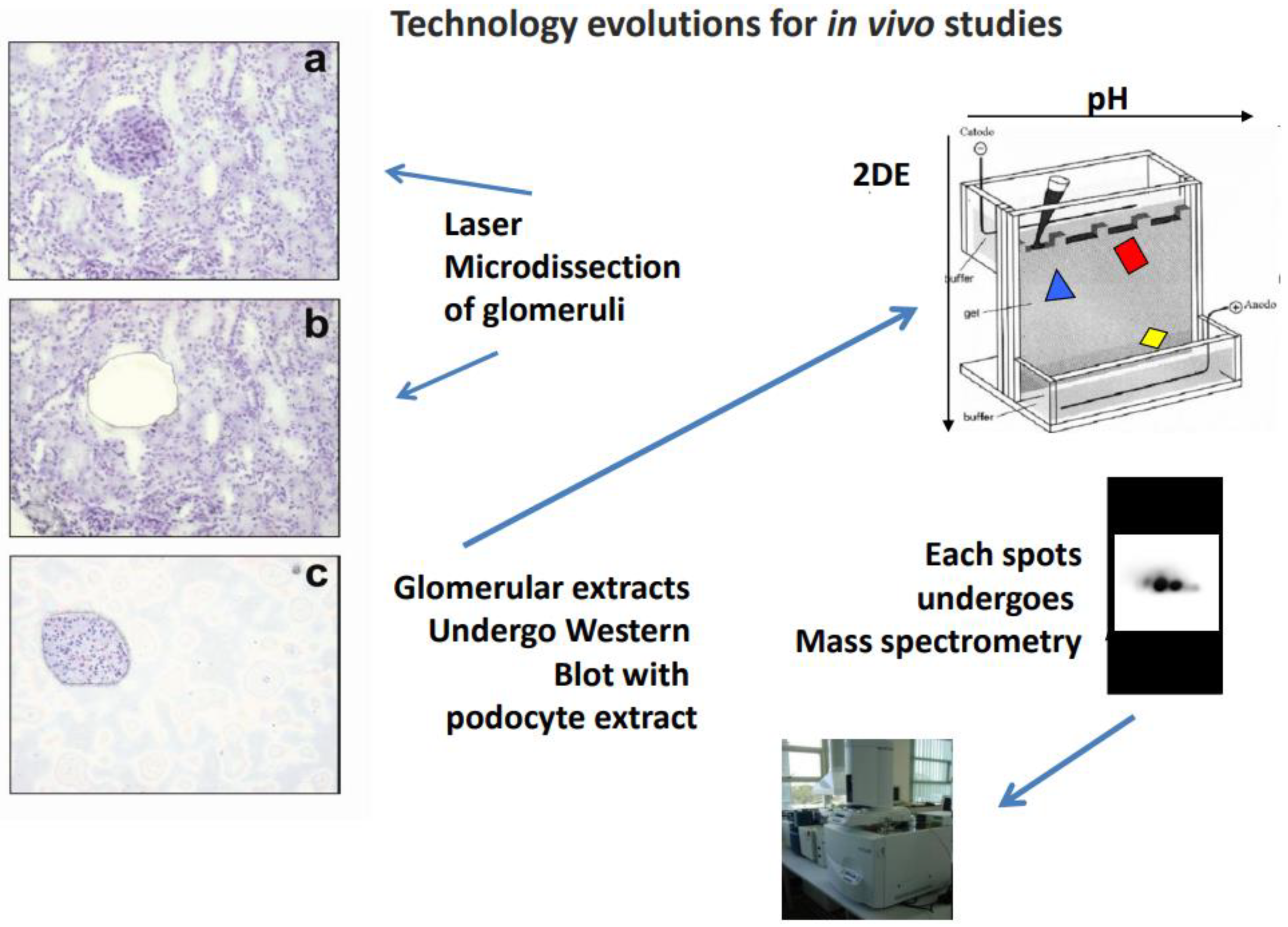
2.2. Laser Microdissection and Characterization of Microeluates
2.3. Limited Proteolysis of Tissue
2.4. Magnetic Beads’ Immunoprecipitation for ‘Highly Reactive Antibodies’
2.5. High-Resolution Microscopy
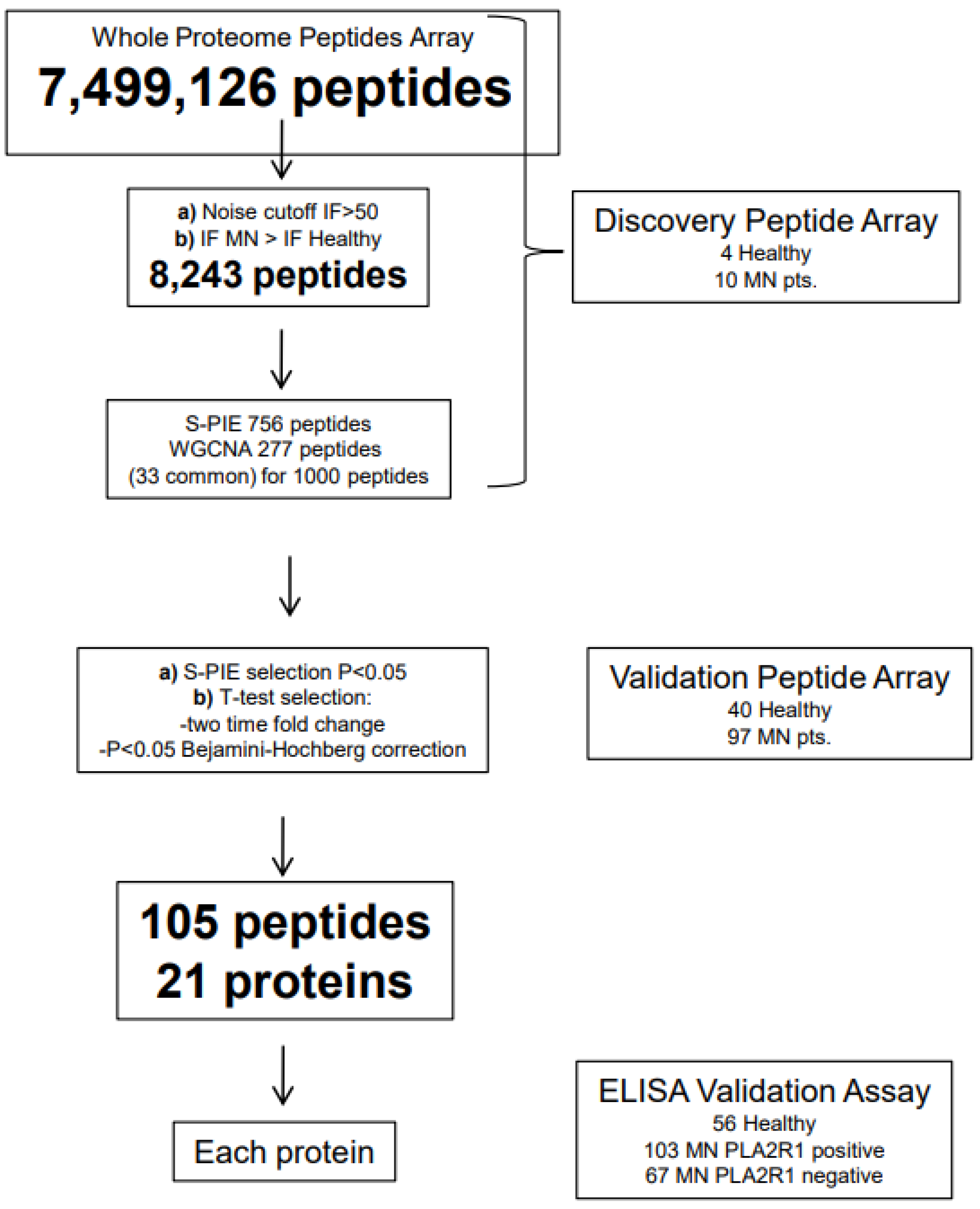
2.6. Peptide Arrays
2.7. Protein Array
2.8. Miscellaneous Techniques
3. Analysis of Cell-Producing Antibodies
4. Applications in Glomerular Autoimmunity
4.1. Goodpasture Syndrome
4.2. Membranous Nephropathy
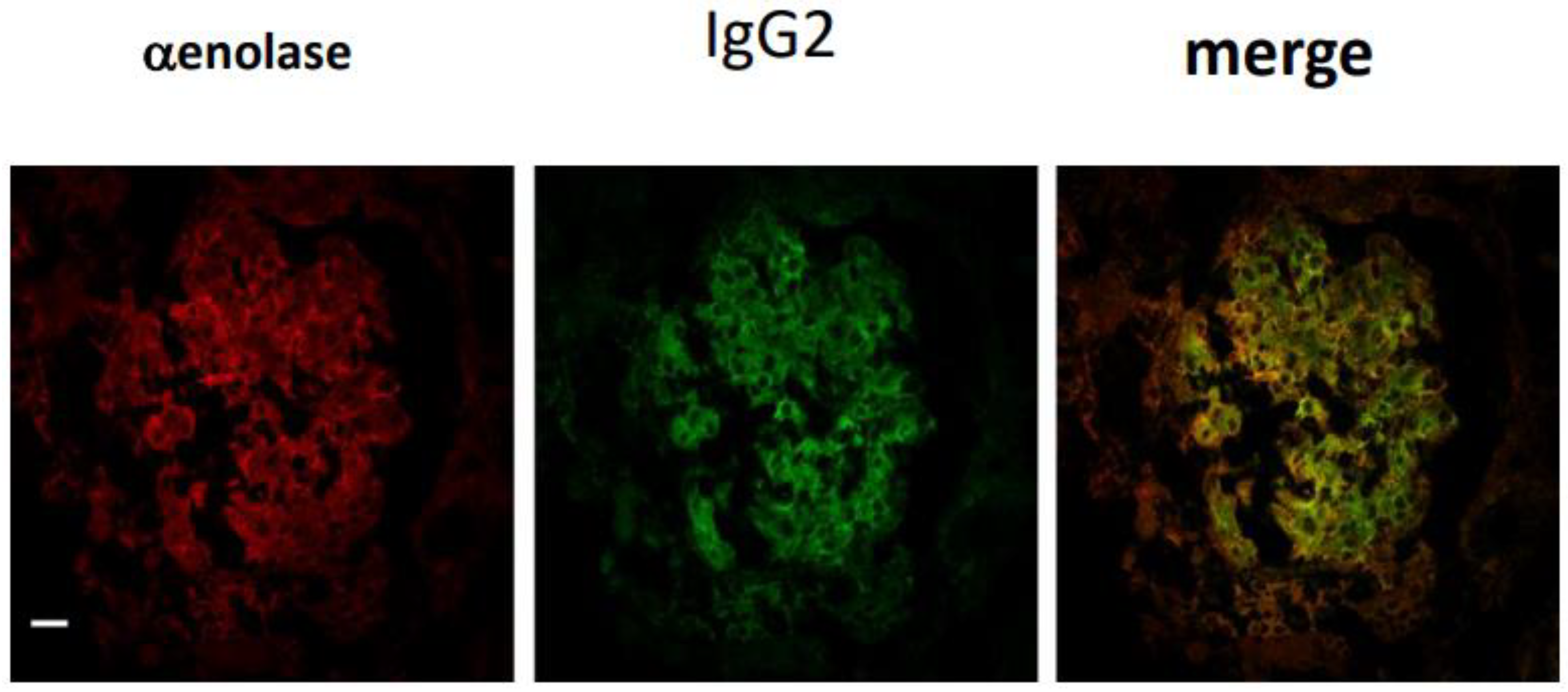
4.3. Lupus Nephritis
4.4. Minimal Change Nephropathy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Beck, L.H., Jr.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Prunotto, M.; Carnevali, M.L.; Candiano, G.; Murtas, C.; Bruschi, M.; Corradini, E.; Trivelli, A.; Magnasco, A.; Petretto, A.; Santucci, L.; et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J. Am. Soc. Nephrol. 2010, 21, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Emmi, G.; Silvestri, E.; Bruschi, G.; Ciucciarelli, L.; Squatrito, D.; Vaglio, A.; Taddei, N.; Abbate, R.; Emmi, L.; et al. Neutrophil Activation Promotes Fibrinogen Oxidation and Thrombus Formation in Behcet Disease. Circulation 2016, 133, 302–311. [Google Scholar] [CrossRef]
- Ghiggeri, G.M.; Seitz-Polski, B.; Justino, J.; Zaghrini, C.; Payré, C.; Brglez, V.; Dolla, G.; Sinico, A.; Scolari, F.; Vaglio, A.; et al. Multi-Autoantibody Signature and Clinical Outcome in Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2020, 15, 1762–1776. [Google Scholar] [CrossRef]
- Caza, T.N.; Storey, A.J.; Hassen, S.I.; Herzog, C.; Edmondson, R.D.; Arthur, J.M.; Kenan, D.J.; Larsen, C.P. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int. 2023, 103, 593–606. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, M.C.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P. Exostosin 1/Exostosin 2-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2019, 30, 1123–1136. [Google Scholar] [CrossRef]
- Sethi, S.; Debiec, H.; Madden, B.; Charlesworth, M.C.; Morelle, J.; Gross, L.; Ravindran, A.; Buob, D.; Jadoul, M.; Fervenza, F.C.; et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020, 97, 163–174. [Google Scholar] [CrossRef]
- Sethi, S.; Debiec, H.; Madden, B.; Vivarelli, M.; Charlesworth, M.C.; Ravindran, A.; Gross, L.; Ulinski, T.; Buob, D.; Tran, C.L.; et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020, 98, 1253–1264. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Gross, L.; Negron, V.C.; Charlesworth, C.; Debiec, H.; Ronco, P.M.; Fervenza, F.C. Protocadherin 7-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2020, 31, 26. [Google Scholar] [CrossRef]
- Sethi, S.; Theis, J.D.; Palma, L.M.P.; Madden, B. From Patterns to Proteins: Mass Spectrometry Comes of Age in Glomerular Disease. J. Am. Soc. Nephrol. 2024, 35, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabadi, L.F.; Caza, T.; Trivin-Avillach, C.; Rodan, A.R.; Andeen, N.; Hayashi, N.; Williams, B.; Revelo, M.P.; Clayton, F.; Abraham, J.; et al. Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, L.; Machalitza, M.; Wiech, T.; Gröne, H.-J.; Lassé, M.; Rinschen, M.M.; Ferru, N.; Bräsen, J.H.; Drömann, F.; Rob, P.M.; et al. Netrin G1 Is a Novel Target Antigen in Primary Membranous Nephropathy. J. Am. Soc. Nephrol. 2022, 33, 1823–1831. [Google Scholar] [CrossRef]
- Le Quintrec, M.; Teisseyre, M.; Bec, N.; Delmont, E.; Szwarc, I.; Perrochia, H.; Machet, M.C.; Chauvin, A.; Mavroudakis, N.; Taieb, G.; et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int. 2021, 100, 1240–1249. [Google Scholar] [CrossRef]
- Caza, T.N.; Hassen, S.I.; Kuperman, M.; Sharma, S.G.; Dvanajscak, Z.; Arthur, J.; Edmondson, R.; Storey, A.; Herzog, C.; Kenan, D.J.; et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021, 100, 171–181. [Google Scholar] [CrossRef]
- Caza, T.N.; Hassen, S.I.; Kenan, D.J.; Storey, A.; Arthur, J.M.; Herzog, C.; Edmondson, R.D.; Bourne, T.D.; Beck, L.H.; Larsen, C.P. Transforming Growth Factor Beta Receptor 3 (TGFBR3)-Associated Membranous Nephropathy. Kidney360 2021, 2, 1275–1286. [Google Scholar] [CrossRef]
- Raglianti, V.; Angelotti, M.L.; Cirillo, L.; Ravaglia, F.; Landini, S.; Palazzo, V.; Melica, M.E.; Antonelli, G.; Conte, C.; Buti, E.; et al. Anti-slit diaphragm antibodies on kidney biopsy identify pediatric patients with steroid-resistant nephrotic syndrome responsive to second-line immunosuppressants. Kidney Int. 2024, in press. [Google Scholar] [CrossRef]
- Bruschi, M.; Cavalli, A.; Moll, S.; Candiano, G.; Scapozza, L.; Patel, J.J.; Tan, J.C.; Lo, K.C.; Angeletti, A.; Ghiggeri, G.M.; et al. Discovery of anti-Formin-like 1 protein (FMNL1) antibodies in membranous nephropathy and other glomerular diseases. Sci. Rep. 2022, 12, 13659. [Google Scholar] [CrossRef]
- Omenn, G.S.; Lane, L.; Overall, C.M.; Corrales, F.J.; Schwenk, J.M.; Paik, Y.-K.; Van Eyk, J.E.; Liu, S.; Pennington, S.; Snyder, M.P.; et al. Progress on Identifying and Characterizing the Human Proteome: 2019 Metrics from the HUPO Human Proteome Project. J. Proteome Res. 2019, 18, 4098–4107. [Google Scholar] [CrossRef]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13, 942. [Google Scholar] [CrossRef]
- Cantarelli, C.; Jarque, M.; Angeletti, A.; Manrique, J.; Hartzell, S.; O’donnell, T.; Merritt, E.; Laserson, U.; Perin, L.; Donadei, C.; et al. A Comprehensive Phenotypic and Functional Immune Analysis Unravels Circulating Anti-Phospholipase A2 Receptor Antibody Secreting Cells in Membranous Nephropathy Patients. Kidney Int. Rep. 2020, 5, 1764–1776. [Google Scholar] [CrossRef] [PubMed]
- Manrique, J.; Chan, E.; Hartzell, S.; Yu, S.M.W.; Cantarelli, C.; Fernandez, L.F.; Slon, M.F.; Purroy, C.; Tassiulas, I.; Azzi, J.; et al. Circulating B Cells, Plasma Cells, and Treg Associate with ANCA Levels in ANCA-Associated Vasculitis. Kidney Int. Rep. 2021, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Larman, H.B.; Laserson, U.; Querol, L.; Verhaeghen, K.; Solimini, N.L.; Xu, G.J.; Klarenbeek, P.L.; Church, G.M.; Hafler, D.A.; Plenge, R.M.; et al. PhIP-Seq characterization of autoantibodies from patients with multiple sclerosis, type 1 diabetes and rheumatoid arthritis. J. Autoimmun. 2013, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mandel-Brehm, C.; Dubey, D.; Kryzer, T.J.; O’Donovan, B.D.; Tran, B.; Vazquez, S.E.; Sample, H.A.; Zorn, K.C.; Khan, L.M.; Bledsoe, I.O.; et al. Kelch-like Protein 11 Antibodies in Seminoma-Associated Paraneoplastic Encephalitis. N. Engl. J. Med. 2019, 381, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Wieslander, J.; Barr, J.F.; Butkowski, R.J.; Edwards, S.J.; Bygren, P.; Heinegård, D.; Hudson, B.G. Goodpasture antigen of the glomerular basement membrane: Localization to noncollagenous regions of type IV collagen. Proc. Natl. Acad. Sci. USA 1984, 81, 3838–3842. [Google Scholar] [CrossRef]
- Wieslander, J.; Bygren, P.; Heinegard, D. Isolation of the specific glomerular basement membrane antigen involved in Goodpasture syndrome. Proc. Natl. Acad. Sci. USA 1984, 81, 1544–1548. [Google Scholar] [CrossRef]
- Butkowski, R.J.; Wieslander, J.; Wisdom, B.J.; Barr, J.F.; Noelken, M.E.; Hudson, B.G. Properties of the globular domain of type IV collagen and its relationship to the Goodpasture antigen. J. Biol. Chem. 1985, 260, 3739–3747. [Google Scholar] [CrossRef]
- Butkowski, R.J.; Langeveld, J.P.; Wieslander, J.; Hamilton, J.; Hudson, B.G. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J. Biol. Chem. 1987, 262, 7874–7877. [Google Scholar] [CrossRef]
- Saus, J.; Wieslander, J.; Langeveld, J.P.; Quinones, S.; Hudson, B.G. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J. Biol. Chem. 1988, 263, 13374–13380. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, Z.; Yang, R.; Jia, X.Y.; Zhang, Y.; Zhao, M.H. Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int. 2009, 76, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Netzer, K.O.; Leinonen, A.; Boutaud, A.; Borza, D.B.; Todd, P.; Gunwar, S.; Langeveld, J.P.; Hudson, B.G. The goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17-31 and 127-141 of the NC1 domain. J. Biol. Chem. 1999, 274, 11267–11274. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, M.-H.; Jia, X.-Y.; Wang, M.; Hu, S.-Y.; Wang, S.-X.; Yu, F.; Brown, K.L.; Hudson, B.G.; Pedchenko, V. Antibodies to alpha5 chain of collagen IV are pathogenic in Goodpasture’s disease. J. Autoimmun. 2016, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-R.; Jia, X.-Y.; Luo, W.; Olaru, F.; Cui, Z.; Zhao, M.-H.; Borza, D.-B. Laminin-521 is a Novel Target of Autoantibodies Associated with Lung Hemorrhage in Anti-GBM Disease. J. Am. Soc. Nephrol. 2021, 32, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, M.; Kodama, Y.; Sekiguchi, K.; Kuwada, T.; Tomono, T.; Kuriyama, K.; Yamazaki, H.; Morita, T.; Marui, S.; Sogabe, Y.; et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci. Transl. Med. 2018, 10, eaaq0997. [Google Scholar] [CrossRef]
- Ooi, J.D.; Chang, J.; O’Sullivan, K.M.; Pedchenko, V.; Hudson, B.G.; Vandenbark, A.A.; Fugger, L.; Holdsworth, S.R.; Kitching, A.R. The HLA-DRB1*15:01-restricted Goodpasture’s T cell epitope induces GN. J. Am. Soc. Nephrol. 2013, 24, 419–431. [Google Scholar] [CrossRef]
- Xie, L.; Cui, Z.; Chen, F.; Pei, Z.; Hu, S.; Gu, Q.; Jia, X.; Zhu, L.; Zhou, X.; Zhang, H.; et al. The susceptible HLA class II alleles and their presenting epitope(s) in Goodpasture’s disease. Immunology 2017, 151, 395–404. [Google Scholar] [CrossRef]
- Gu, Q.-H.; Huynh, M.; Shi, Y.; Jia, X.-Y.; Luo, J.-J.; Jiang, T.-J.; Cui, Z.; Ooi, J.D.; Kitching, A.R.; Zhao, M.-H. Experimental Antiglomerular Basement Membrane GN Induced by a Peptide from Actinomyces. J. Am. Soc. Nephrol. 2020, 31, 1282–1295. [Google Scholar] [CrossRef]
- Gu, Q.; Xie, L.; Jia, X.; Ma, R.; Liao, Y.; Cui, Z.; Zhao, M. Fever and prodromal infections in anti-glomerular basement membrane disease. Nephrology 2018, 23, 476–482. [Google Scholar] [CrossRef]
- Hu, S.-Y.; Jia, X.-Y.; Gu, Q.-H.; Yu, C.-Y.; Cheng, X.-Y.; Jin, Q.-Z.; Zhou, F.-D.; Cui, Z.; Zhao, M.-H. T cell responses to peptides of Goodpasture autoantigen in patients with anti-glomerular basement membrane disease. Nephrology 2018, 23, 345–350. [Google Scholar] [CrossRef]
- Seitz-Polski, B.; Debiec, H.; Rousseau, A.; Dahan, K.; Zaghrini, C.; Payré, C.; Esnault, V.L.; Lambeau, G.; Ronco, P. Phospholipase A2 Receptor 1 Epitope Spreading at Baseline Predicts Reduced Likelihood of Remission of Membranous Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Seitz-Polski, B.; Dolla, G.; Payré, C.; Girard, C.A.; Polidori, J.; Zorzi, K.; Birgy-Barelli, E.; Jullien, P.; Courivaud, C.; Krummel, T.; et al. Epitope Spreading of Autoantibody Response to PLA2R Associates with Poor Prognosis in Membranous Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.; Lam, V.; Waldman, M.; Glassock, R.J.; Zhu, Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2015, 26, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Fresquet, M.; Jowitt, T.A.; Gummadova, J.; Collins, R.; O’cualain, R.; McKenzie, E.A.; Lennon, R.; Brenchley, P.E. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J. Am. Soc. Nephrol. 2015, 26, 302–313. [Google Scholar] [CrossRef]
- Sethi, S. New ‘Antigens’ in Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 268–278. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.; Moura, M.C.; Nasr, S.H.; Klomjit, N.; Gross, L.; Negron, V.; Charlesworth, M.C.; Alexander, M.P.; Leung, N.; et al. Hematopoietic Stem Cell Transplant-Membranous Nephropathy Is Associated with Protocadherin FAT1. J. Am. Soc. Nephrol. 2022, 33, 1033–1044. [Google Scholar] [CrossRef]
- Tomas, N.M.; Hoxha, E.; Reinicke, A.T.; Fester, L.; Helmchen, U.; Gerth, J.; Bachmann, F.; Budde, K.; Koch-Nolte, F.; Zahner, G.; et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J. Clin. Investig. 2016, 126, 2519–2532. [Google Scholar] [CrossRef]
- Buelli, S.; Perico, L.; Galbusera, M.; Abbate, M.; Morigi, M.; Novelli, R.; Gagliardini, E.; Tentori, C.; Rottoli, D.; Sabadini, E.; et al. Mitochondrial-dependent Autoimmunity in Membranous Nephropathy of IgG4-Related Disease. EBioMedicine 2015, 2, 456–466. [Google Scholar] [CrossRef]
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef]
- Acronim, O.B.O.T.F.; Gensous, N.; Marti, A.; Barnetche, T.; Blanco, P.; Lazaro, E.; Seneschal, J.; Truchetet, M.-E.; Duffau, P.; Richez, C. Predictive biological markers of systemic lupus erythematosus flares: A systematic literature review. Arthritis Res. Ther. 2017, 19, 238. [Google Scholar]
- Font, J.; Cervera, R.; Ramos-Casals, M.; García-Carrasco, M.; Sentís, J.; Herrero, C.; del Olmo, J.A.; Darnell, A.; Ingelmo, M. Clusters of clinical and immunologic features in systemic lupus erythematosus: Analysis of 600 patients from a single center. Semin. Arthritis Rheum. 2004, 33, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Moroni, G.; Sinico, R.A.; Franceschini, F.; Fredi, M.; Vaglio, A.; Cavagna, L.; Petretto, A.; Pratesi, F.; Migliorini, P.; et al. Serum IgG2 antibody multicomposition in systemic lupus erythematosus and lupus nephritis (Part 1): Cross-sectional analysis. Rheumatology 2021, 60, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Moroni, G.; Sinico, R.A.; Franceschini, F.; Fredi, M.; Vaglio, A.; Cavagna, L.; Petretto, A.; Pratesi, F.; Migliorini, P.; et al. Serum IgG2 antibody multi-composition in systemic lupus erythematosus and in lupus nephritis (Part 2): Prospective study. Rheumatology 2021, 60, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, A.; Vaglio, A.; Bruschi, M.; Sinico, R.A.; Cavagna, L.; Moroni, G.; Franceschini, F.; Allegri, L.; Pratesi, F.; Migliorini, P.; et al. Multi-antibody composition in lupus nephritis: Isotype and antigen specificity make the difference. Autoimmun. Rev. 2015, 14, 692–702. [Google Scholar] [CrossRef]
- Watts, A.J.; Keller, K.H.; Lerner, G.; Rosales, I.; Collins, A.B.; Sekulic, M.; Waikar, S.S.; Chandraker, A.; Riella, L.V.; Alexander, M.P.; et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J. Am. Soc. Nephrol. 2022, 33, 238–252. [Google Scholar] [CrossRef]
- Hengel, F.E.; Dehde, S.; Lassé, M.; Zahner, G.; Seifert, L.; Schnarre, A.; Kretz, O.; Demir, F.; Pinnschmidt, H.O.; Grahammer, F.; et al. Autoantibodies Targeting Nephrin in Podocytopathies. N. Engl. J. Med. 2024, 391, 422–433. [Google Scholar] [CrossRef]
- Shirai, Y.; Miura, K.; Ishizuka, K.; Ando, T.; Kanda, S.; Hashimoto, J.; Hamasaki, Y.; Hotta, K.; Ito, N.; Honda, K.; et al. A multi-institutional study found a possible role of anti-nephrin antibodies in post-transplant focal segmental glomerulosclerosis recurrence. Kidney Int. 2024, 105, 608–617. [Google Scholar] [CrossRef]
- Ravani, P.; Rossi, R.; Bonanni, A.; Quinn, R.R.; Sica, F.; Bodria, M.; Pasini, A.; Montini, G.; Edefonti, A.; Belingheri, M.; et al. Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial. J. Am. Soc. Nephrol. 2015, 26, 2259–2266. [Google Scholar] [CrossRef]
- Ravani, P.; Magnasco, A.; Edefonti, A.; Murer, L.; Rossi, R.; Ghio, L.; Benetti, E.; Scozzola, F.; Pasini, A.; Dallera, N.; et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2011, 6, 1308–1315. [Google Scholar] [CrossRef]
- Basu, B.; Angeletti, A.; Islam, B.; Ghiggeri, G.M. New and Old Anti-CD20 Monoclonal Antibodies for Nephrotic Syndrome. Where We Are? Front. Immunol. 2022, 13, 805697. [Google Scholar] [CrossRef]
- Yu, C.-C.; Fornoni, A.; Weins, A.; Hakroush, S.; Maiguel, D.; Sageshima, J.; Chen, L.; Ciancio, G.; Faridi, M.H.; Behr, D.; et al. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 2013, 369, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Kestilä, M.; Lenkkeri, U.; Männikkö, M.; Lamerdin, J.; McCready, P.; Putaala, H.; Ruotsalainen, V.; Morita, T.; Nissinen, M.; Herva, R.; et al. Positionally cloned gene for a novel glomerular protein—Nephrin—Is mutated in congenital nephrotic syndrome. Mol. Cell 1998, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Patrakka, J.; Ruotsalainen, V.; Reponen, P.; Qvist, E.; Laine, J.; Holmberg, C.; Tryggvason, K.; Jalanko, H. Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: Role of nephrin. Transplantation 2002, 73, 394–403. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruschi, M.; Candiano, G.; Petretto, A.; Angeletti, A.; Meroni, P.L.; Prunotto, M.; Ghiggeri, G.M., on behalf of the Zeus Consortium. Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions. Int. J. Mol. Sci. 2024, 25, 12659. https://doi.org/10.3390/ijms252312659
Bruschi M, Candiano G, Petretto A, Angeletti A, Meroni PL, Prunotto M, Ghiggeri GM on behalf of the Zeus Consortium. Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions. International Journal of Molecular Sciences. 2024; 25(23):12659. https://doi.org/10.3390/ijms252312659
Chicago/Turabian StyleBruschi, Maurizio, Giovanni Candiano, Andrea Petretto, Andrea Angeletti, Pier Luigi Meroni, Marco Prunotto, and Gian Marco Ghiggeri on behalf of the Zeus Consortium. 2024. "Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions" International Journal of Molecular Sciences 25, no. 23: 12659. https://doi.org/10.3390/ijms252312659
APA StyleBruschi, M., Candiano, G., Petretto, A., Angeletti, A., Meroni, P. L., Prunotto, M., & Ghiggeri, G. M., on behalf of the Zeus Consortium. (2024). Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions. International Journal of Molecular Sciences, 25(23), 12659. https://doi.org/10.3390/ijms252312659





