Eleutherin and Isoeleutherin Activity against Staphylococcus aureus and Escherichia coli Strain’s: Molecular Docking and Antibacterial Evaluation
Abstract
1. Introduction
2. Results
2.1. Chemical Studies
2.2. Antibacterial Activity of Eleutherin and Isoeleutherin
2.3. Target Proteins Involved in the Eleutherin and Isoeleutherin Action
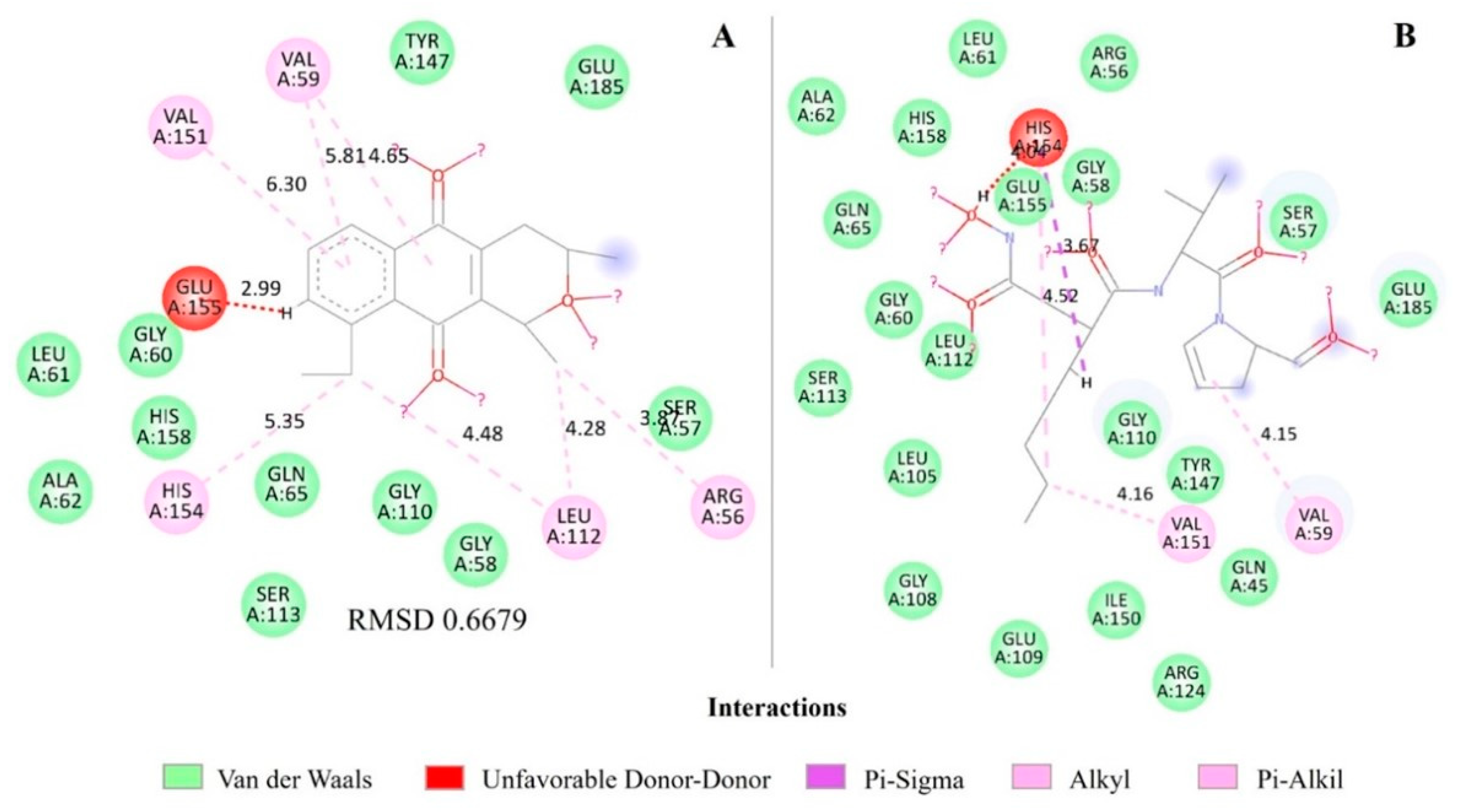
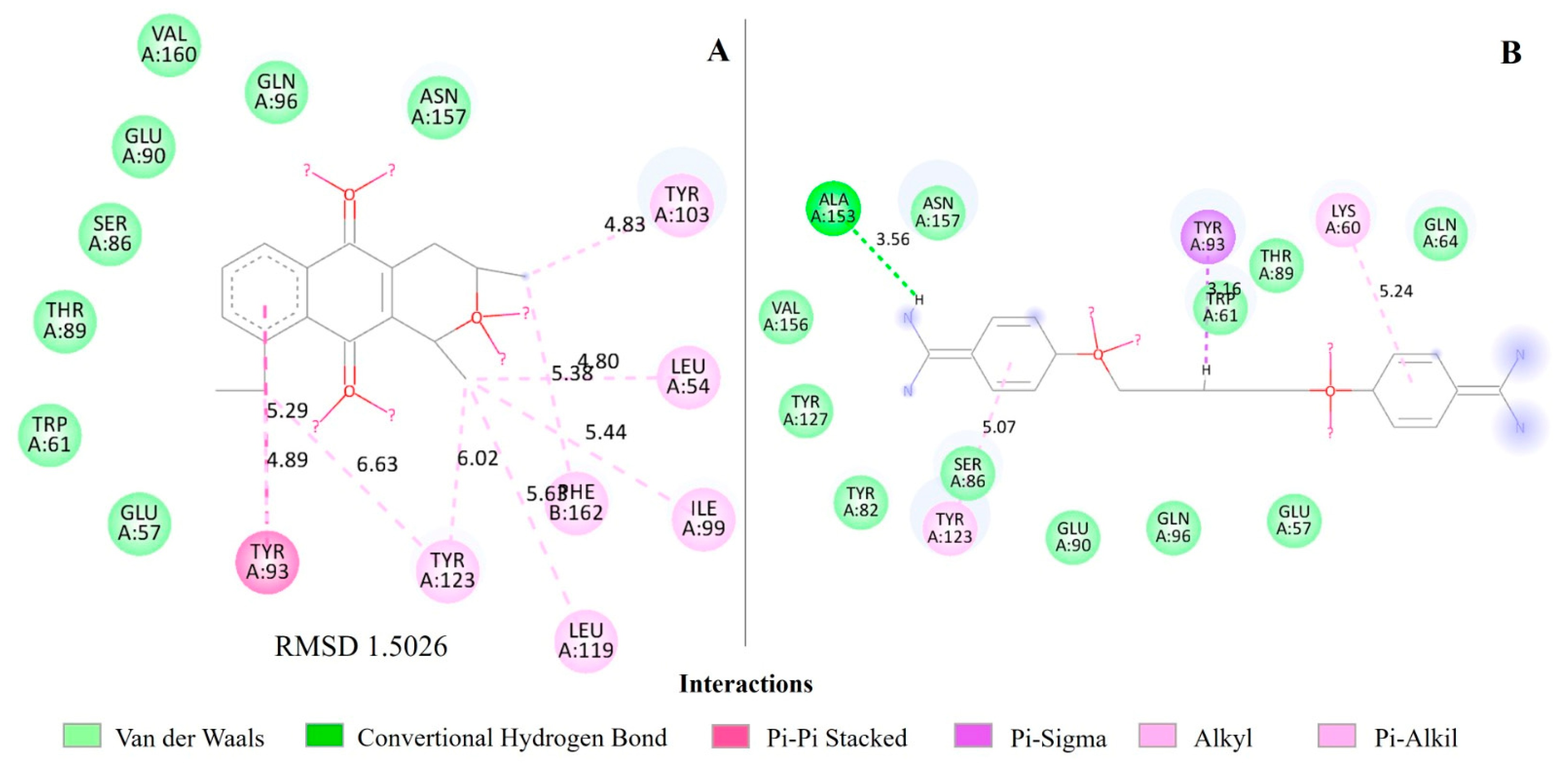
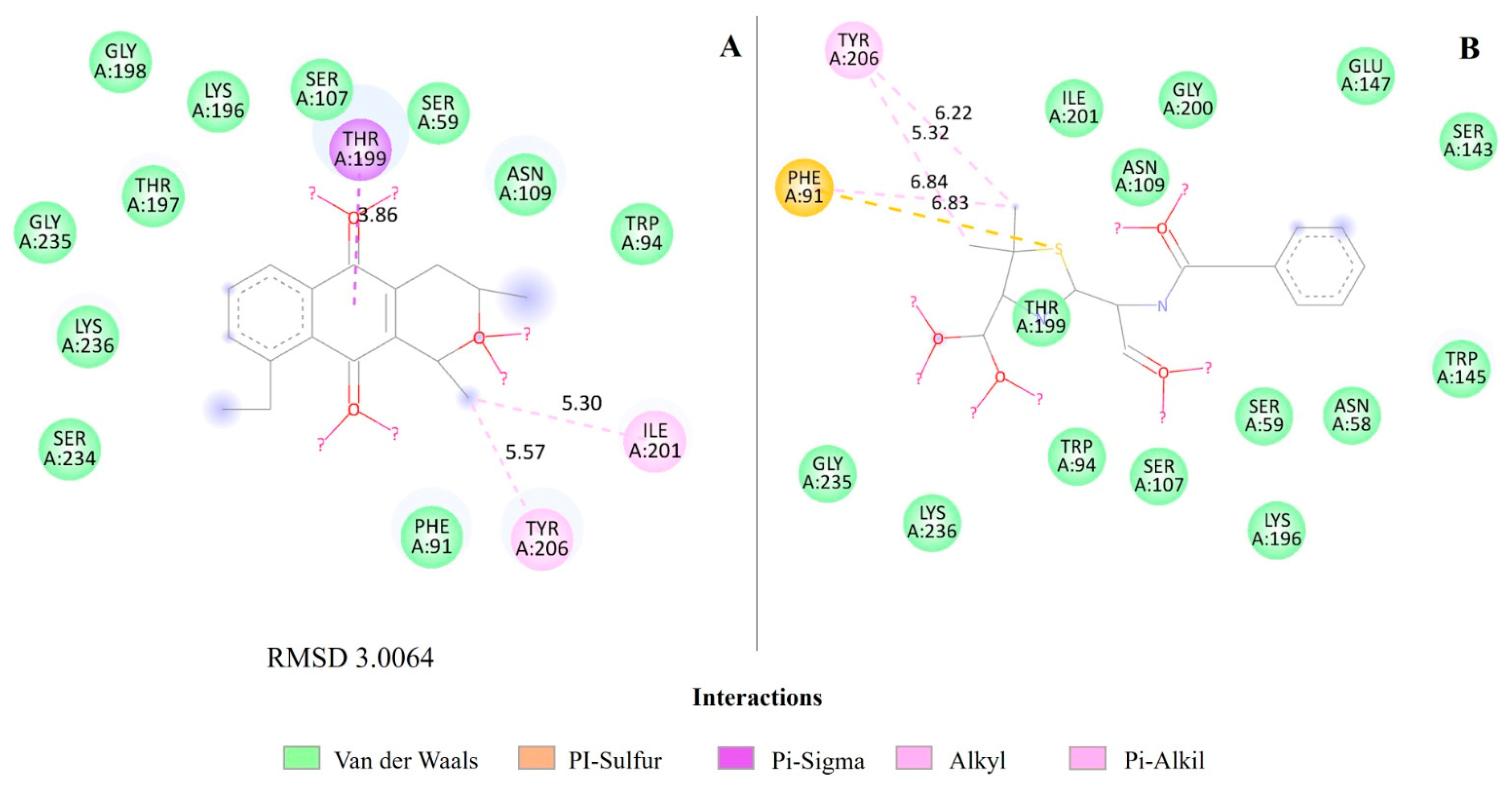
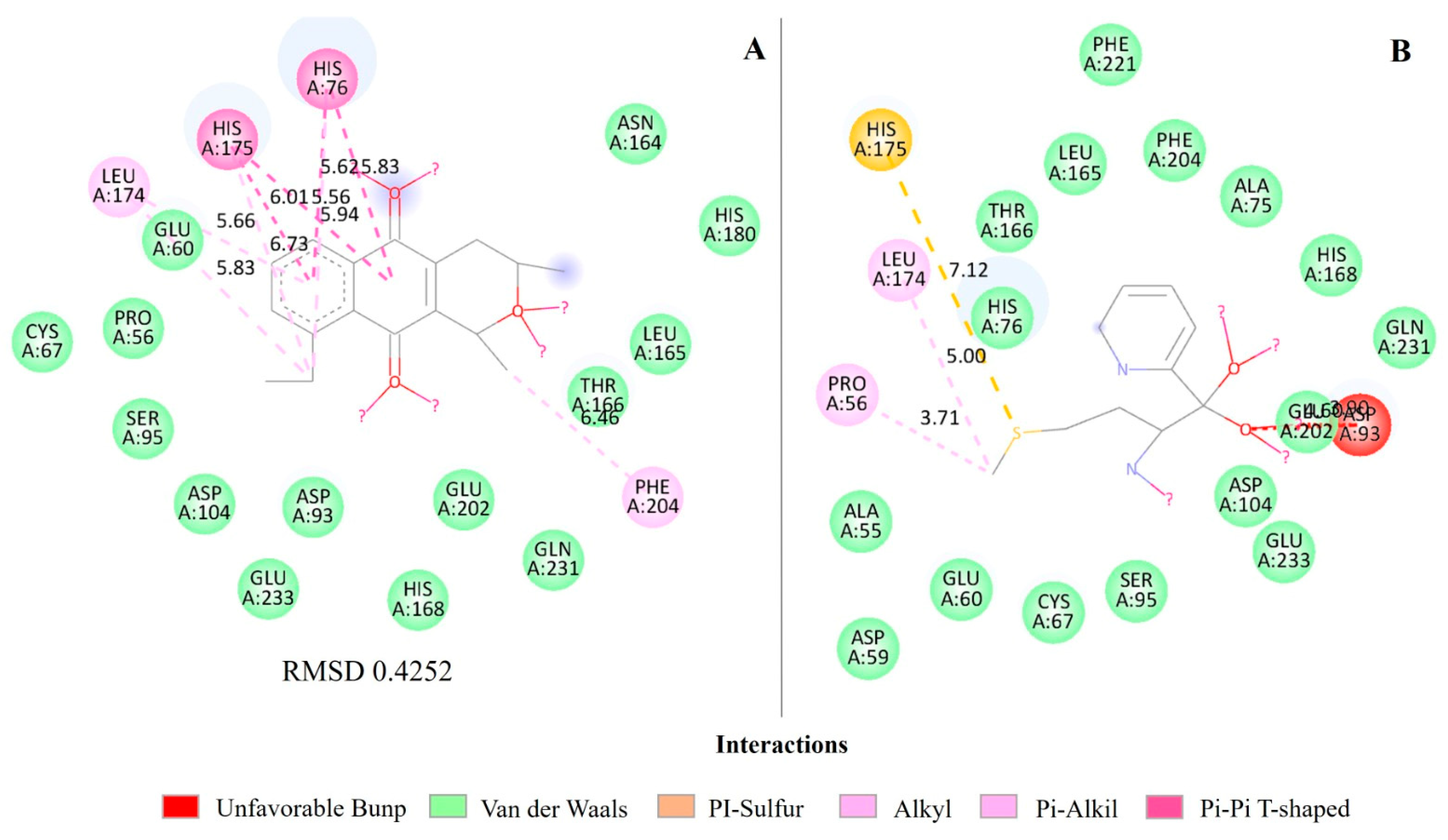
2.4. Molecular Docking of Eleutherin and Isoeleutherin in S. aureus Proteins
3. Discussion
4. Materials and Methods
4.1. Chemical Studies
4.2. Antimicrobial Activity
4.3. Target Proteins Involved in Eleutherin and Isoeleutherin Activity
4.4. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prameela, R.; Swamy, J.; Venkaiah, M. Eleutherine bulbosa (Mill.) Urb. (Iridaceae): A New Distributional Record to the Flora of Eastern Ghats, India. Trop. Plant Res. 2018, 5, 303–305. [Google Scholar] [CrossRef]
- Borges, E.S.; Galucio, N.C.D.R.; Veiga, A.S.S.; Busman, D.V.; Lins, A.L.F.D.A.; Bahia, M.D.O.; Rissino, J.D.; Correa, R.M.D.S.; Burbano, R.M.R.; Marinho, A.M.R.; et al. Botanical Studies, Antimicrobial Activity and cytotoxicity of Eleutherine Bulbosa (Mill.) Urb. Res. Soc. Dev. 2020, 9, e3369119992. [Google Scholar] [CrossRef]
- Couto, C.L.L.; Moraes, D.F.C.; Maria do Socorro, S.C.; Amaral, F.M.; Guerra, R. Eleutherine bulbous (Mill.) Urb.: A Review Study. J. Med. Plants Res. 2016, 10, 286–297. [Google Scholar] [CrossRef]
- Vale, V.V.; Cruz, J.N.; Viana, G.M.R.; Póvoa, M.M.; Brasil, D.D.S.B.; Dolabela, M.F. Naphthoquinones Isolated from Eleutherine plicata Herb: In Vitro Antimalarial Activity and Molecular Modeling to Investigate Their Binding Modes. Med. Chem. Res. 2020, 29, 487–494. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Hossan, S.; Roy, P. Ethnomedicinal Applications of Plants by the Traditional Healers of the Marma Tribe of Naikhongchhari, Bandarban District, Bangladesh. Adv. Nat. Appl. Sci. 2009, 3, 392–401. [Google Scholar]
- Laura Gadelha Castro, A.; Correa-Barbosa, J.; Santos de Campos, P.; Franzen Matte, B.; Lazzaron Lamers, M.; Edson de Sousa Siqueira, J.; Moacir do Rosario Marinho, A.; Chagas Monteiro, M.; Vieira Vale, V.; Fâni Dolabela, M. Antitumoral Activity of Eleutherine plicata Herb. and Its Compounds. Int. J. Dev. Res. 2020, 11, 21154. [Google Scholar] [CrossRef]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Galeffi, C.; Chifundera, K.; Nicoletti, M. Polyketides from Eleutherine Bulbosa. Nat. Prod. Res. 2010, 24, 1578–1586. [Google Scholar] [CrossRef]
- Malheiros, L.C.d.S.; Barbosa, J.C.P.d.M.; Barbosa, W.L.R. Eleutherine Plicata—Quinones and Antioxidant Activity. In Phytochemicals—Isolation, Characterisation and Role in Human Health; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Gomes, A.R.Q.; Cruz, J.N.; Castro, A.L.G.; Cordovil Brigido, H.P.; Varela, E.L.P.; Vale, V.V.; Carneiro, L.A.; Ferreira, G.G.; Percario, S.; Dolabela, M.F. Participation of Oxidative Stress in the Activity of Compounds Isolated from Eleutherine Plicata Herb. Molecules 2023, 28, 5557. [Google Scholar] [CrossRef]
- Castro, A.L.G.; Cruz, J.N.; Sodré, D.F.; Correa-Barbosa, J.; Azonsivo, R.; de Oliveira, M.S.; de Sousa Siqueira, J.E.; da Rocha Galucio, N.C.; de Oliveira Bahia, M.; Burbano, R.M.R.; et al. Evaluation of the Genotoxicity and Mutagenicity of Isoeleutherin and Eleutherin Isolated from Eleutherine Plicata Herb. Using Bioassays and in Silico Approaches. Arab. J. Chem. 2021, 14, 103084. [Google Scholar] [CrossRef]
- Liu, C.; Shen, G.N.; Luo, Y.H.; Piao, X.J.; Jiang, X.Y.; Meng, L.Q.; Wang, Y.; Zhang, Y.; Wang, J.R.; Wang, H.; et al. Novel 1,4-Naphthoquinone Derivatives Induce Apoptosis via ROS-Mediated P38/MAPK, Akt and STAT3 Signaling in Human Hepatoma Hep3B Cells. Int. J. Biochem. Cell Biol. 2018, 96, 9–19. [Google Scholar] [CrossRef]
- Mazel, D.; Pochet, S.; Marlière, P. Genetic Characterization of Polypeptide Deformylase, a Distinctive Enzyme of Eubacterial Translation. EMBO J. 1994, 13, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; O’Dwyer, K.M.; Palmer, L.M.; Ambrad, J.D.; Ingraham, K.A.; So, C.; Lonetto, M.A.; Biswas, S.; Rosenberg, M.; Holmes, D.J.; et al. Characterization of a Novel Fucose-Regulated Promoter (PfcsK) Suitable for Gene Essentiality and Antibacterial Mode-of-Action Studies in Streptococcus Pneumoniae. J. Bacteriol. 2003, 185, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Hye, L.K.; Lee, S.K.; Kim, H.W.; Kim, H.W.; Jae, Y.L.; Mikami, B.; Se, W.S. Crystal Structure of Peptide Deformylase from Staphylococcus Aureus in Complex with Actinonin, a Naturally Occurring Antibacterial Agent. Proteins: Struct. Funct. Bioinform. 2004, 57, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, S.; Brown, M.H.; Roberts, N.J.; Paulsen, I.T.; Skurray, R.A. QacR Is a Repressor Protein That Regulates Expression of the Staphylococcus Aureus Multidrug Efflux Pump QacA. J. Biol. Chem. 1998, 273, 18665–18673. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, F.R.M. Presença de Staphylococcus aureus Resistentes à (MRSA) em Queijos de Coalho Produzidos no do Ceará e Seu Perfil de Resistência e Genes de. Esse; Universidade Federal do Ceará: Fortaleza, Brazil, 2022. [Google Scholar]
- Chai, S.C.; Wang, W.L.; Ding, D.R.; Ye, Q.Z. Growth Inhibition of Escherichia Coli and Methicillin-Resistant Staphylococcus Aureus by Targeting Cellular Methionine Aminopeptidase. Eur. J. Med. Chem. 2011, 46, 3537–3540. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant Staphylococcus Aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. Medchemcomm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections; StatPearls: St. Petersburg, Russia, 2023. [Google Scholar]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. Coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Hong, J.H.; Yu, E.S.; Han, A.R.; Nam, J.W.; Seo, E.K.; Hwang, E.S. Isoeleutherin and Eleutherinol, Naturally Occurring Selective Modulators of Th Cell-Mediated Immune Responses. Biochem. Biophys. Res. Commun. 2008, 371, 278–282. [Google Scholar] [CrossRef]
- Macêdo, L.d.S. Efeito Sinérgico Entre a β-Lapachona e Agentes Antimicrobianos Frente Às Cepas de Staphylococcus aureus Multirresistentes. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2010. [Google Scholar]
- Ifesan, B.O.T.; Hamtasin, C.; Mahabusarakam, W.; Voravuthikunchai, S.P. Inhibitory Effect of Eleutherine Americana Merr. Extract on Staphylococcus aureus Isolated from Food. J. Food Sci. 2009, 74, M31–M36. [Google Scholar] [CrossRef]
- Dias, P.L.D.P.; de Novais, V.P.; Schmidt, R.B.; Ely, E.; Carmago, S.; Carlos, F.; Silva, D.A. Análise da Atividade Antibacteriana dos Bulbos de Eleutherine plicata herb. (iridaceae) Antibacterial Activity Analysis of the Bulbs of Eleutherine plicata Herb. (IRIDACEAE). Braz. J. Surg. Clin. Res.–BJSCR 2018, 23, 7–11. [Google Scholar]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of Some Plants Used in the Brazilian Folk Medicine for the Treatment of Infectious Diseases. Mem. Inst. Oswaldo. Cruz. 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.d.S. Estudos Farmacognósticos, Fitoquímicos e Atividade Antimicrobiana de Eleutherine plicata Herb. Ph.D. Thesis, Federal University of Pará, Belém, Brazil, 2012. [Google Scholar]
- Lee, S.J.; Lee, S.J.; Lee, S.K.; Yoon, H.J.; Lee, H.H.; Kim, K.K.; Lee, B.J.; Lee, B.I.; Suh, S.W. Structures of Staphylococcus aureus Peptide Deformylase in Complex with Two Classes of New Inhibitors. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Chetri, S. The Culmination of Multidrug-Resistant Efflux Pumps vs. Meager Antibiotic Arsenal Era: Urgent Need for an Improved New Generation of EPIs. Front. Microbiol. 2023, 14, 1149418. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Q.; Yu, Y.; Zheng, Q. A Thermodynamic and Kinetic Study on Electrochemical Esterification in Aroma-Enhanced Distilled Liquor (Baijiu). Catalysts 2023, 13, 478. [Google Scholar] [CrossRef]
- Alexander, J.A.N.; Worrall, L.J.; Hu, J.; Vuckovic, M.; Satishkumar, N.; Poon, R.; Sobhanifar, S.; Rosell, F.I.; Jenkins, J.; Chiang, D.; et al. Structural Basis of Broad-Spectrum β-Lactam Resistance in Staphylococcus Aureus. Nature 2023, 613, 375–382. [Google Scholar] [CrossRef]
- Bronowska, A.K.; Bronowska, A.K. Thermodynamics of Ligand-Protein Interactions: Implications for Molecular Design. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Nolan, T.; Singh, N.; McCurdy, C.R. Ligand Macromolecule Interactions: Theoretical Principles of Molecular Recognition. In Ligand—Macromolecular Interactions in Drug Discovery; Methods in Molecular Biology; Humana: Totowa, NJ, USA, 2009; Volume 572, pp. 13–29. [Google Scholar]
- Wankowicz, S.A.; de Oliveira, S.H.P.; Hogan, D.W.; van den Bedem, H.; Fraser, J.S. Ligand Binding Remodels Protein Side-Chain Conformational Heterogeneity. eLife 2022, 11, e74114. [Google Scholar] [CrossRef]
- Esadze, A.; Chen, C.; Zandarashvili, L.; Roy, S.; Pettitt, B.M.; Iwahara, J. Changes in Conformational Dynamics of Basic Side Chains upon Protein–DNA Association. Nucleic Acids Res. 2016, 44, 6961–6970. [Google Scholar] [CrossRef]
- Boudreau, M.A.; Fishovitz, J.; Llarrull, L.I.; Xiao, Q.; Mobashery, S. Phosphorylation of BlaR1 in Manifestation of Antibiotic Resistance in Methicillin-Resistant Staphylococcus Aureus and Its Abrogation by Small Molecules. ACS Infect. Dis. 2015, 1, 454–459. [Google Scholar] [CrossRef]
- Morris, R.; Black, K.A.; Stollar, E.J. Uncovering Protein Function: From Classification to Complexes. Essays Biochem. 2022, 66, 255–285. [Google Scholar] [CrossRef]
- Helgren, T.R.; Wangtrakuldee, P.; Staker, B.L.; Hagen, T.J. Advances in Bacterial Methionine Aminopeptidase Inhibition. Curr. Top. Med. Chem. 2015, 16, 397–414. [Google Scholar] [CrossRef]
- Che, X.; Hu, J.; Wang, L.; Zhu, Z.; Xu, Q.; Lv, J.; Fu, Z.; Sun, Y.; Sun, J.; Lin, G.; et al. Expression, Purification, and Activity Assay of Peptide Deformylase from Escherichia Coli and Staphylococcus Aureus. Mol. Cell. Biochem. 2011, 357, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.M.; Schuman, J.T.; Skurray, R.A.; Brown, M.H.; Brennan, R.G.; Schumacher, M.A. QacR-Cation Recognition Is Mediated by a Redundancy of Residues Capable of Charge Neutralization. Biochemistry 2008, 47, 8122–8129. [Google Scholar] [CrossRef] [PubMed]
- Llarrull, L.I.; Toth, M.; Champion, M.M.; Mobashery, S. Activation of BlaR1 Protein of Methicillin-Resistant Staphylococcus Aureus, Its Proteolytic Processing, and Recovery from Induction of Resistance. J. Biol. Chem. 2011, 286, 38148–38158. [Google Scholar] [CrossRef] [PubMed]
- Roderick, S.L.; Matthews, B.W. Structure of the Cobalt-Dependent Methionine Aminopeptidase from Escherichia Coli: A New Type of Proteolytic Enzyme. Biochemistry 1993, 32, 3907–3912. [Google Scholar] [CrossRef] [PubMed]
- Lowther, W.T.; Orville, A.M.; Madden, D.T.; Lim, S.; Rich, D.H.; Matthews, B.W. Escherichia Coli Methionine Aminopeptidase: Implications of Crystallographic Analyses of the Native, Mutant, and Inhibited Enzymes for the Mechanism of Catalysis. Biochemistry 1999, 38, 7678–7688. [Google Scholar] [CrossRef]
- Ghai, I.; Ghai, S. Understanding Antibiotic Resistance via Outer Membrane Permeability. Infect. Drug. Resist. 2018, 11, 523–530. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, J.S.; Bin Park, S.; Lee, A.R.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. The Importance of Porins and β-Lactamase in Outer Membrane Vesicles on the Hydrolysis of β-Lactam Antibiotics. Int. J. Mol. Sci. 2020, 21, 2822. [Google Scholar] [CrossRef]
- Turner, N.A.; Harris, J.; Russell, A.D.; Lloyd, D. Microbial Differentiation and Changes in Susceptibility to Antimicrobial Agents. J. Appl. Microbiol. 2000, 89, 751–759. [Google Scholar] [CrossRef]
- Vale, V.V.; Vilhena, T.C.; Trindade, R.C.S.; Ferreira, M.R.C.; Percário, S.; Soares, L.F.; Pereira, W.L.A.; Brandão, G.C.; Oliveira, A.B.; Dolabela, M.F.; et al. Anti-Malarial Activity and Toxicity Assessment of Himatanthus articulatus, a Plant Used to Treat Malaria in the Brazilian Amazon. Malar. J. 2015, 14, 132. [Google Scholar] [CrossRef]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical And Laboratory Standards Institute: Wayne, PA, USA, 2003; Volume 1, ISBN 1562388363. [Google Scholar]
- CLSI. M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition, 2nd ed.; Clinical And Laboratory Standards Institute: Wayne, PA, USA, 2012; ISBN 1-56238-784-7. [Google Scholar]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 Update: A Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C. Molecular Recognition of Receptor Sites Using a Genetic Algorithm with a Description of Desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Annamala, M.K.; Inampudi, K.K.; Guruprasad, L. Docking of Phosphonate and Trehalose Analog Inhibitors into M. Tuberculosis Mycolyltransferase Ag85C: Comparison of the Two Scoring Fitness Functions GoldScore and ChemScore, in the GOLD Software. Bioinformation 2007, 1, 339–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical Scoring Functions: I. The Development of a Fast Empirical Scoring Function to Estimate the Binding Affinity of Ligands in Receptor Complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
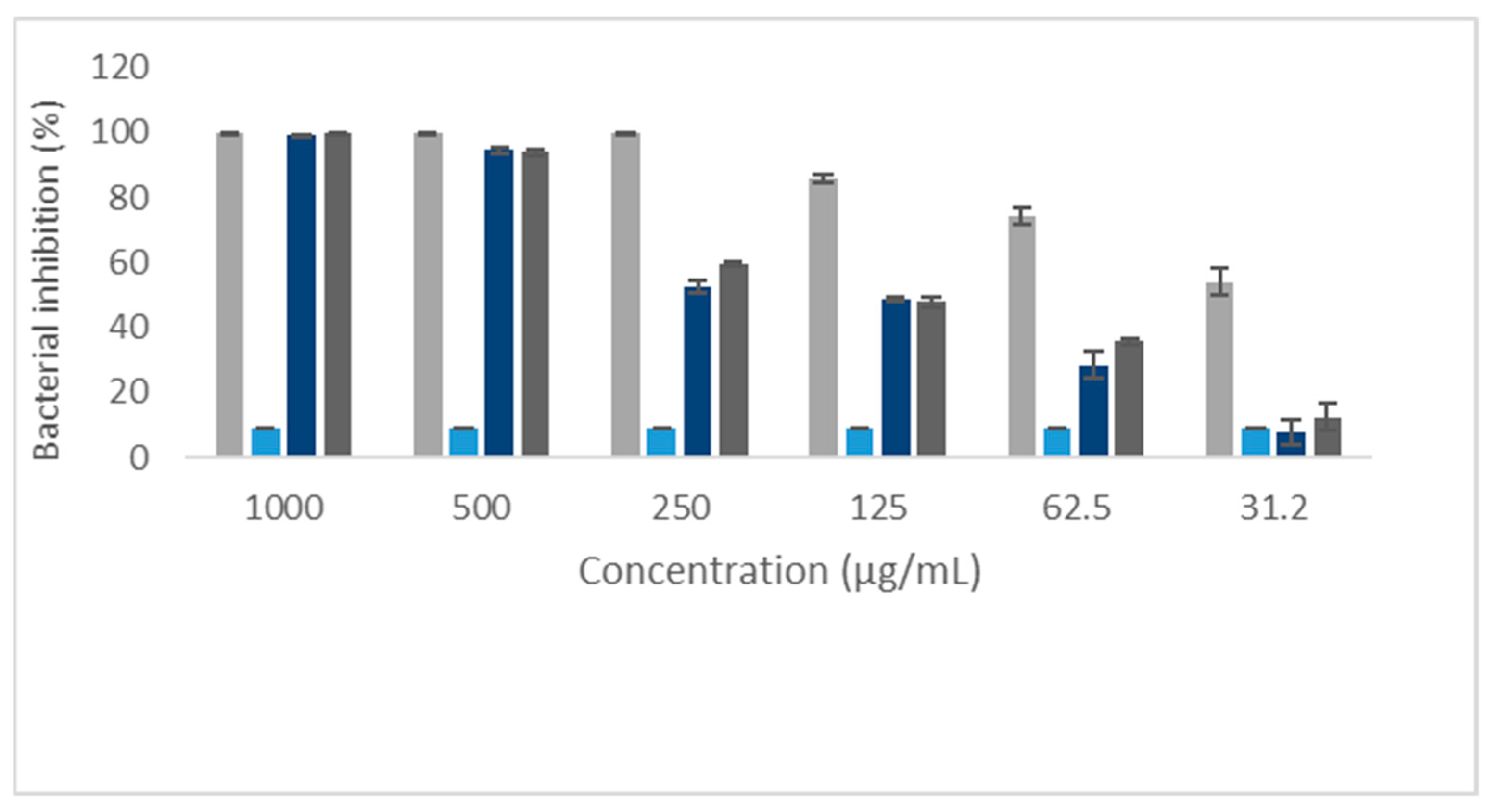

| Concentration (µg/mL) | Staphylococcus aureus | Escherichia coli | ||
|---|---|---|---|---|
| Inhibition (%) | ||||
| Eleutherin | Isoeleutherin | Eleutherin | Isoeleutherin | |
| 1000 | 99.7 | 99.4 | 30.2 | 30.0 |
| 500 | 94.0 | 94.4 | 30.0 | 30.2 |
| 250 | 59.5 | 52.5 | 30.3 | 29.6 |
| 125 | 47.7 | 48.7 | 27.0 | 27.4 |
| 62.5 | 35.6 | 28.4 | 25.8 | 25.2 |
| 31.2 | 12.5 | 7.8 | 23.1 | 22.8 |
| Compounds | Staphylococcus aureus | Escherichia coli | ||||
|---|---|---|---|---|---|---|
| MIC (µg/mL) | IC50 (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | IC50 (µg/mL) | MBC (µg/mL) | |
| Eleutherin | 1000 | 165.00 | 1000 | >1000 | >1000 | >1000 |
| Isoeleutherin | 1000 | 172.90 | 1000 | >1000 | >1000 | >1000 |
| Chloramphenicol | 250 | 78.2 | - | 125 | 71.33 | - |
| PDB Code | Target Name | Score Adjustment | |
|---|---|---|---|
| Eleutherin | 1Q1Y | Peptide deformylase (PDF) | 1.56 |
| 1RKW | Regulator QacR | 1.37 | |
| 1XA7 | Regulatory protein BlaR1 | 1.07 | |
| Isoeleutherin | 1QXY | Methionine aminopeptidase (MetAP) | 2.48 |
| Staphylococcus aureus Target | Compound | CS | ΔG | GS | WdWExt |
|---|---|---|---|---|---|
| Peptide deformylase (PDF) | Eleutherin | 23.00 | −25.47 | 28.58 | 20.62 |
| Actinonin * | 28.45 | −29.46 | 62.07 | 41.12 | |
| Regulator QacR | Eleutherin | 33.79 | −33.86 | 29.53 | 21.30 |
| Pentamidine * | 36.58 | −36.62 | 56.31 | 39.71 | |
| Regulatory protein BlaR1 | Eleutherin | 28.67 | −28.71 | 35.19 | 21.68 |
| Benzylpenicillin * | 27.01 | −27.39 | 35.01 | 22.72 | |
| Methionine aminopeptidase (MetAP) | Isoeleutherin | 21.79 | −22.33 | 33.72 | 33.72 |
| Ketoheterocycle 618 * | 23.00 | −24.49 | 45.79 | 29.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, M.L.C.; Adido, H.E.F.; Martins de Brito, A.K.; Chagas, C.K.S.; Castro, A.L.G.; Ferreira, G.G.; Nascimento, P.H.C.; Padilha, W.R.d.S.; Sarmento, R.M.; Garcia, V.V.; et al. Eleutherin and Isoeleutherin Activity against Staphylococcus aureus and Escherichia coli Strain’s: Molecular Docking and Antibacterial Evaluation. Int. J. Mol. Sci. 2024, 25, 12583. https://doi.org/10.3390/ijms252312583
Bastos MLC, Adido HEF, Martins de Brito AK, Chagas CKS, Castro ALG, Ferreira GG, Nascimento PHC, Padilha WRdS, Sarmento RM, Garcia VV, et al. Eleutherin and Isoeleutherin Activity against Staphylococcus aureus and Escherichia coli Strain’s: Molecular Docking and Antibacterial Evaluation. International Journal of Molecular Sciences. 2024; 25(23):12583. https://doi.org/10.3390/ijms252312583
Chicago/Turabian StyleBastos, Mírian Letícia Carmo, Houéfa Egidia Fallon Adido, Ananda Karolyne Martins de Brito, Cristian Kallahan Silva Chagas, Ana Laura Gadelha Castro, Gleison Gonçalves Ferreira, Pedro Henrique Costa Nascimento, Walice Rans da Silva Padilha, Rosana Moura Sarmento, Viviane Vasconcelos Garcia, and et al. 2024. "Eleutherin and Isoeleutherin Activity against Staphylococcus aureus and Escherichia coli Strain’s: Molecular Docking and Antibacterial Evaluation" International Journal of Molecular Sciences 25, no. 23: 12583. https://doi.org/10.3390/ijms252312583
APA StyleBastos, M. L. C., Adido, H. E. F., Martins de Brito, A. K., Chagas, C. K. S., Castro, A. L. G., Ferreira, G. G., Nascimento, P. H. C., Padilha, W. R. d. S., Sarmento, R. M., Garcia, V. V., Marinho, A. M. d. R., Marinho, P. S. B., Rocha de Oliveira, J. A., Vale, V. V., Percário, S., & Dolabela, M. F. (2024). Eleutherin and Isoeleutherin Activity against Staphylococcus aureus and Escherichia coli Strain’s: Molecular Docking and Antibacterial Evaluation. International Journal of Molecular Sciences, 25(23), 12583. https://doi.org/10.3390/ijms252312583









