The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review
Abstract
1. Introduction
Chronic Inflammation and Tumorigenesis
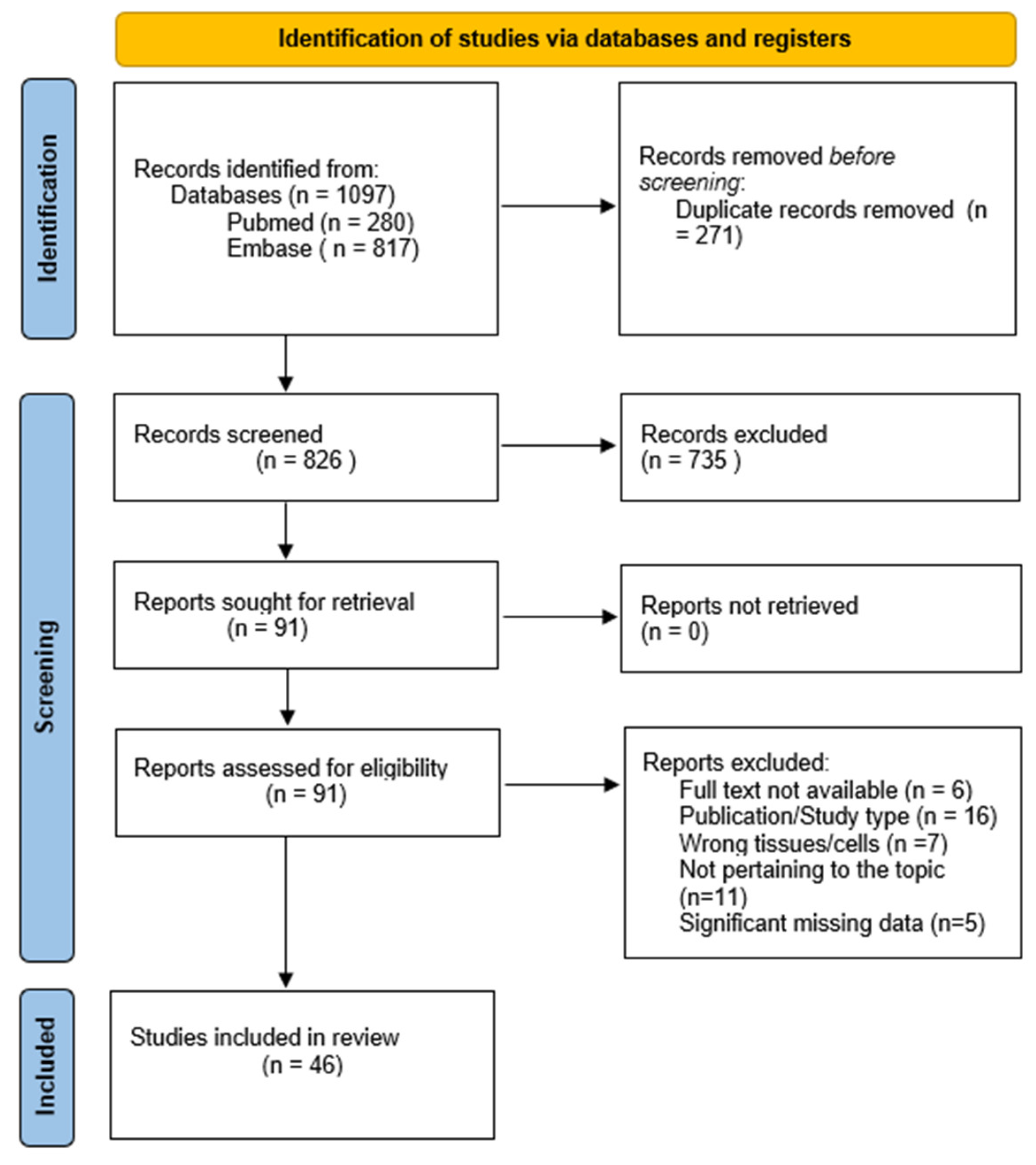
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
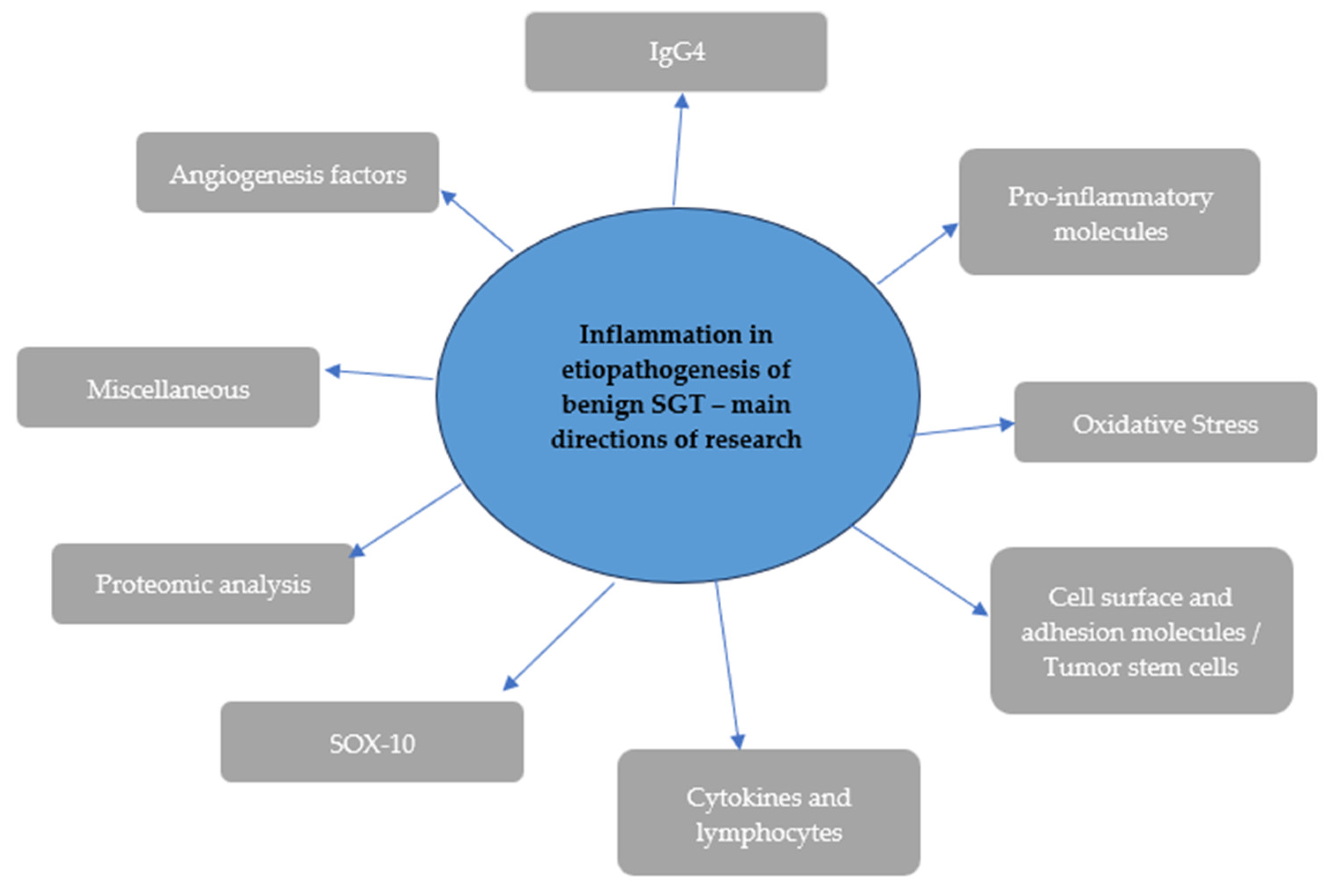
4. Discussion
4.1. Angiogenesis Factors
4.2. IgG4
4.3. Pro-Inflammatory Molecules
4.4. Oxidative Stress
4.5. Cell Surface and Adhesion Molecules/Tumor Stem Cells
4.6. Cytokines and Lymphocytes
4.7. SOX-10
4.8. Proteomic Analysis
4.9. Miscellaneous
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shah, J.; Bhuvanesh, S.; Patel, S.; Wong, R. Jatin Shah’s Head and Neck Surgery and Oncology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 7, pp. 489–555. [Google Scholar]
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Khaja, S.F.; Flack, A.B.; Day, T.A. Benign Parotid Tumors. Otolaryngol. Clin. N. Am. 2016, 49, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.J. Frequency and Histopathology by Site, Major Pathologies, Symptoms and Signs of Salivary Gland Neoplasms. Adv. Otorhinolaryngol. 2016, 78, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, H.; Liu, Y.; Zhang, X.; Qiu, Y.; Huang, D. Modifiable Factors for Benign Salivary Gland Neoplasms: A Mendelian Randomization Study. Oral. Dis. 2024, 30, 2245–2253. [Google Scholar] [CrossRef]
- Li, T.I.; Chiang, M.T.; Chiu, K.C.; Lai, C.H.; Liu, S.Y.; Shieh, Y.S. The Association of Betel Quid, Alcohol, and Cigarettes with Salivary Gland Tumor-A Case-Control Study. J. Dent. Sci. 2017, 12, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Tretiakow, D.; Skorek, A. Regarding the “Review of Surgical Techniques and Guide for Decision Making in the Treatment of Benign Parotid Tumors. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3537–3538. [Google Scholar] [CrossRef] [PubMed]
- Tretiakow, D.; Mikaszewski, B.; Skorek, A. The Role of Fine needle Aspiration Biopsy (FNAB) in the Diagnostic Management of Parotid Gland Masses with Emphasis on Potential Pitfalls. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2939–2940. [Google Scholar] [CrossRef]
- Nakaguro, M. Diagnostic Clues and Pitfalls in Salivary Gland Fine-Needle Aspiration Cytology. Semin. Diagn. Pathol. 2024, 41, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Reerds, S.T.H.; Van Engen-Van Grunsven, A.C.H.; van den Hoogen, F.J.A.; Takes, R.P.; Marres, H.A.M.; Honings, J. Accuracy of Parotid Gland FNA Cytology and Reliability of the Milan System for Reporting Salivary Gland Cytopathology in Clinical Practice. Cancer Cytopathol. 2021, 129, 719–728. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, M.; Tretiakow, D.; Skorek, A.; Szydłowski, K.; Stodulski, D.; Mikaszewski, B.; Odroniec, A.; Musiał, N.; Thiel, M.; Czaplewska, P.; et al. Comparison of Peptidomes Extracted from Healthy Tissue and Tumor Tissue of the Parotid Glands and Saliva Samples. Int. J. Mol. Sci. 2024, 25, 8799. [Google Scholar] [CrossRef] [PubMed]
- Decock, J.; Comito, G.; Zaravinos, A. Editorial: Tumor Microenvironment, Inflammation, and Resistance to Immunotherapies. Front. Oncol. 2023, 13, 1215332. [Google Scholar] [CrossRef]
- Niu, T.; Zhou, F. Inflammation and Tumor Microenvironment. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 1899–1913. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Loy, A.H.C.; Putti, T.C.; Tan, L.K.S. Cyclooxygenase-2 Expression in Warthin’s Tumour. J. Laryngol. Otol. 2005, 119, 515–518. [Google Scholar] [CrossRef]
- Andreadis, D.; Epivatianos, A.; Mireas, G.; Nomikos, A.; Poulopoulos, A.; Yiotakis, J.; Barbatis, C. Immunohistochemical Detection of E-Cadherin in Certain Types of Salivary Gland Tumours. J. Laryngol. Otol. 2006, 120, 298–304. [Google Scholar] [CrossRef]
- Pantelis, A.; Wenghoefer, M.; Haas, S.; Merkelbach-Bruse, S.; Pantelis, D.; Jepsen, S.; Bootz, F.; Winter, J. Down Regulation and Nuclear Localization of Human β-Defensin-1 in Pleomorphic Adenomas of Salivary Glands. Oral. Oncol. 2009, 45, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Tampouris, A.I.; Kandiloros, D.; Giotakis, I.; Gakiopoulou, H.; Lazaris, A.C. The Role of the VEGF-C/-D/Flt-4 Autocrine Loop in the Pathogenesis of Salivary Neoplasms. Pathol. Res. Pract. 2012, 208, 151–156. [Google Scholar] [CrossRef]
- Liu, G.X.; Lan, J.; Sun, Y.; Hu, Y.J.; Jiang, G.S. Expression of the Chemokine CCL28 in Pleomorphic Adenoma and Adenolymphoma of the Human Salivary Glands. Exp. Ther. Med. 2012, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, N.; Epivatianos, A.; Sakas, L.; Andreadis, D.; Markopoulos, A.; Antoniades, K. Expression of N-Cadherin in Salivary Gland Tumors. Med. Princ. Pract. 2012, 22, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Carlesimo, M.; Mari, E.; La Pietra, M.; Orsini, D.; Pranteda, G.; Pranteda, G.; Grimaldi, M.; Arcese, A. Occurrence of Salivary Gland Tumours in Two Patients Treated with Biological Agents. Int. J. Immunopathol. Pharmacol. 2012, 25, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Jour, G.; West, K.; Ghali, V.; Shank, D.; Ephrem, G.; Wenig, B.M. Differential Expression of P16INK4A and Cyclin D1 in Benign and Malignant Salivary Gland Tumors: A Study of 44 Cases. Head Neck Pathol. 2013, 7, 224–231. [Google Scholar] [CrossRef]
- Andisheh Tadbir, A.; Khademi, B.; Malekzadeh, M.; Mardani, M.; Khademi, B. Upregulation of Serum Vascular Endothelial Growth Factor in Patients with Salivary Gland Tumor. Patholog Res. Int. 2013, 2013, 740582. [Google Scholar] [CrossRef]
- Aga, M.; Kondo, S.; Yamada, K.; Sawada-Kitamura, S.; Yagi-Nakanishi, S.; Endo, K.; Wakisaka, N.; Murono, S.; Kawano, M.; Yoshizaki, T. Warthin’s Tumor Associated with IgG4-Related Disease. Auris Nasus Larynx 2013, 40, 514–517. [Google Scholar] [CrossRef]
- Ohtomo, R.; Mori, T.; Shibata, S.; Tsuta, K.; Maeshima, A.M.; Akazawa, C.; Watabe, Y.; Honda, K.; Yamada, T.; Yoshimoto, S.; et al. SOX10 Is a Novel Marker of Acinus and Intercalated Duct Differentiation in Salivary Gland Tumors: A Clue to the Histogenesis for Tumor Diagnosis. Mod. Pathol. 2013, 26, 1041–1050. [Google Scholar] [CrossRef]
- Donadio, E.; Giusti, L.; Seccia, V.; Ciregia, F.; da Valle, Y.; Dallan, I.; Ventroni, T.; Giannaccini, G.; Sellari-Franceschini, S.; Lucacchini, A. New Insight into Benign Tumours of Major Salivary Glands by Proteomic Approach. PLoS ONE 2013, 8, e71874. [Google Scholar] [CrossRef]
- Ianez, R.C.F.; Coutinho-Camillo, C.M.; Buim, M.E.; Pinto, C.A.L.; Soares, F.A.; Lourenço, S.V. CD24 and CD44 in Salivary Gland Pleomorphic Adenoma and in Human Salivary Gland Morphogenesis: Differential Markers of Glandular Structure or Stem Cell Indicators? Histopathology 2013, 62, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Karbanová, J.; Laco, J.; Marzesco, A.M.; Janich, P.; Voborníková, M.; Mokrý, J.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Human PROMININ-1 (CD133) Is Detected in Both Neoplastic and Non-Neoplastic Salivary Gland Diseases and Released into Saliva in a Ubiquitinated Form. PLoS ONE 2014, 9, e98927. [Google Scholar] [CrossRef]
- Reshma, V.; Rao, K.; Priya, N.; Umadevi, H.; Smitha, T.; Sheethal, H. Expression of Maspin in Benign and Malignant Salivary Gland Tumors: An Immunohistochemical Study. Indian J. Dent. Res. 2014, 25, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Aga, M.; Kondo, S.; Yamada, K.; Wakisaka, N.; Yagi-Nakanishi, S.; Tsuji, A.; Endo, K.; Murono, S.; Ito, M.; Muramatsu, M.; et al. Immunoglobulin Class Switching to IgG4 in Warthin Tumor and Analysis of Serum IgG4 Levels and IgG4-Positive Plasma Cells in the Tumor. Hum. Pathol. 2014, 45, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Faur, A.C.; Lazar, E.; Cornianu, M. Vascular Endothelial Growth Factor (VEGF) Expression and Microvascular Density in Salivary Gland Tumours. APMIS 2014, 122, 418–426. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Takizawa, Y.; Hayasaka, T.; Masaki, N.; Kusama, Y.; Su, J.; Mineta, H.; Setou, M. Increased Phosphatidylcholine (16:0/16:0) in the Folliculus Lymphaticus of Warthin Tumor. Anal. Bioanal. Chem. 2014, 406, 5815–5825. [Google Scholar] [CrossRef]
- Andisheh-Tadbir, A.; Ashraf, M.J.; Khademi, B.; Ahmadi, S. Clinical Implication of CD166 Expression in Salivary Gland Tumor. Tumor Biol. 2015, 36, 2793–2799. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Zhang, Y.; Wang, L.; Liu, Y.; Fan, L.; Zhu, J.; Xu, X.; Huang, G.; Li, X.; et al. PRDMI Expression on the Epithelial Component but Not on Ectopic Lymphoid Tissues of Warthin Tumour. Oral Dis. 2015, 21, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, M.R.; Khademi, B.; Faghih, Z.; Ghaderi, A.; Erfani, N. Immune Regulatory Cells and IL17-Producing Lymphocytes in Patients with Benign and Malignant Salivary Gland Tumors. Immunol. Lett. 2015, 164, 109–116. [Google Scholar] [CrossRef]
- Jaafari-Ashkavandi, Z.; Ashraf, M.J.; Nazhvani, A.D.; Azizi, Z. Caveolin-1 Overexpression in Benign and Malignant Salivary Gland Tumors. Tumor Biol. 2016, 37, 1863–1869. [Google Scholar] [CrossRef]
- Fonseca, F.P.; Bingle, L.; Santos-Silva, A.R.; Lopes, M.A.; de Almeida, O.P.; de Andrade, B.A.B.; Mariano, F.V.; Kowalski, L.P.; Rangel, A.L.C.A.; Martins, M.D.; et al. Semaphorins and Neuropilins Expression in Salivary Gland Tumors. J. Oral Pathol. Med. 2016, 45, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Khademi, B.; Tajvarpour, M.; Mojtahedi, Z.; Haghshenas, M.R.; Erfani, N. T-Helper Type 1 and 2 Cytokine Levels in Patients with Benign and Malignant Salivary Gland Tumors. Iran. J. Immunol. 2016, 13, 9–15. [Google Scholar] [PubMed]
- Kim, J.; Song, J.S.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y.; Cho, K.J. Increased Immunoglobulin G4-Positive Plasma Cells in Lymphadenoma of the Salivary Gland: An Immunohistochemical Comparison among Lymphoepithelial Lesions. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, M.R.; Khademi, B.; Ashraf, M.J.; Ghaderi, A.; Erfani, N. Helper and Cytotoxic T-Cell Subsets (Th1, Th2, Tc1, and Tc2) in Benign and Malignant Salivary Gland Tumors. Oral. Dis. 2016, 22, 566–572. [Google Scholar] [CrossRef]
- Zare, R.; Malekzadeh, M.; Hashemi, M.; Khademi, B.; Andishe-Tadbir, A. Investigation of IL-33 Serum Levels in Patients with Benign and Malignant Salivary Gland Tumors. Cancer Biomark. 2018, 23, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Misiolek, M.; Orecka, B.; Czecior, E.; Adamczyk-Sowa, M. Serum Levels of Selected Adipocytokines in Benign and Malignant Parotid Gland Tumor Patients. Cytokine 2018, 106, 40–44. [Google Scholar] [CrossRef]
- Sowa, P.; Misiolek, M.; Pasinski, B.; Bartosz, G.; Soszynski, M.; Adamczyk-Sowa, M.; Sadowska-Bartosz, I. Oxidative Stress Markers Patients with Parotid Gland Tumors: A Pilot Study. Biomed. Res. Int. 2018, 2018, 4340871. [Google Scholar] [CrossRef]
- Błochowiak, K.J.; Sokalski, J.; Bodnar, M.B.; Trzybulska, D.; Marszałek, A.K.; Witmanowski, H. Expression of VEGF165b, VEGFR1, VEGFR2 and CD34 in Benign and Malignant Tumors of Parotid Glands. Adv. Clin. Exp. Med. 2018, 27, 83–90. [Google Scholar] [CrossRef]
- Tenório, J.D.R.; da Silva, L.P.; Xavier, M.G.D.A.; Santana, T.; do Nascimento, G.J.F.; Sobral, A.P.V. Differential Expression of Cyclooxygenase-2 and Cyclin D1 in Salivary Gland Tumors. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2341–2347. [Google Scholar] [CrossRef]
- Andisheh-Tadbir, A.; Ashraf, M.J.; Gudarzi, A.; Zare, R. Evaluation of Glypican-3 Expression in Benign and Malignant Salivary Gland Tumors. J. Oral Biol. Craniofac Res. 2019, 9, 63–66. [Google Scholar] [CrossRef]
- Aslan, F.; Küçük, Ü. RANK and RANKL Expression in Salivary Gland Tumors. Ear Nose Throat J. 2020, 99, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Baněčková, M.; Uro-Coste, E.; Ptáková, N.; Šteiner, P.; Stanowska, O.; Benincasa, G.; Colella, G.; Vondrák, J.; Michal, M.; Leivo, I.; et al. What Is Hiding behind S100 Protein and SOX10 Positive Oncocytomas? Oncocytic Pleomorphic Adenoma and Myoepithelioma with Novel Gene Fusions in a Subset of Cases. Hum. Pathol. 2020, 103, 52–62. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; de Sousa Lopes, M.L.D.; Sarmento, A.S.C.; de Albuquerque Borges, M.; de Moura, S.R.S.; Sobral, A.P.V.; de Souza, L.B. Increased Expression of ALDH-1 Is Associated with Clinical Parameters of Salivary Glands Neoplasms. Exp. Mol. Pathol. 2020, 117, 104552. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, K.; Oishi, N.; Kawai, M.; Odate, T.; Tahara, I.; Inoue, T.; Kasai, K.; Kondo, T. Expressions of Cxcl12, Cxcl10 and Ccl18 in Warthin Tumors Characterized Pathologically by Having a Lymphoid Stroma with Germinal Centers. Histol. Histopathol. 2021, 36, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kurose, N.; Guo, X.; Shioya, A.; Kitamura, M.; Tsuji, H.; Yamada, S. The Potential Role of Follicular Helper T Cells and Helper T Cells Type 1 in Warthin Tumour. Pathol. Res. Pract. 2021, 220, 153386. [Google Scholar] [CrossRef]
- Sahin, A.; Kars, A.; Kilic, K.; Ucar, H.B.; Sakat, M.S. Efficacy of the Systemic Immune Inflammation Index in Malignant and Benign Parotid Neoplasms. Cureus 2022, 14, e31878. [Google Scholar] [CrossRef]
- Haghshenas, M.R.; Erfani, N.; Khansalar, S.; Khademi, B.; Ashraf, M.J.; Razmkhah, M.; Ghaderi, A. Proteomics Study of Mesenchymal Stem Cell-Like Cells Obtained from Tumor Microenvironment of Patients with Malignant and Benign Salivary Gland Tumors. Cell J. 2022, 24, 196–203. [Google Scholar] [CrossRef]
- Laohavisudhi, F.; Chunchai, T.; Ketchaikosol, N.; Thosaporn, W.; Chattipakorn, N.; Chattipakorn, S.C. Evaluation of CD44s, CD44v6, CXCR2, CXCL1, and IL-1β in Benign and Malignant Tumors of Salivary Glands. Diagnostics 2022, 12, 1275. [Google Scholar] [CrossRef]
- Gaonkar, P.P.; Patankar, S.R.; Sridharan, G. Assessment of Angiogenesis Using Endoglin in Salivary Gland Tumors—An Immunohistochemical Study. J. Cancer Res. Ther. 2022, 18, 623–628. [Google Scholar] [CrossRef]
- Abbate, V.; Barone, S.; Orabona, G.D.; Bonavolontà, P.; Galdiero, M.R.; Cristinziano, L.; Modestino, L.; Iaconetta, G.; Califano, L. Role of Inflammation in Benign Salivary Gland Tumor Etiopathogenesis: An Evaluation through the Blood Inflammatory Biomarkers. Eurasian J. Med. Oncol. 2022, 6, 150–155. [Google Scholar] [CrossRef]
- Sowa, P.; Kasperczyk, S.; Dadok, A.; Misiołek, M.; Adamczyk-Sowa, M. Low-Intensity Whole-Body Oxidative Stress in Patients Withparotid Gland Tumors. Otolaryngol. Pol. 2022, 77, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, Z.; Khan, M.M.; Tariq, S.F.; Alam, S.; Arbab, K.N.; Khan, A.A.; Khalid, N.; Abdullah, L.; Jabbar, R. Association of Vascular Endothelial Growth Factor (VEGF) in Salivary Gland Tumors. Pak. J. Med. Health Sci. 2023, 17, 156–159. [Google Scholar] [CrossRef]
- Abbate, V.; Barone, S.; Borriello, G.; Troise, S.; Bonavolontà, P.; Pacella, D.; Vaira, L.A.; Turri-Zanoni, M.; Cuéllar, C.N.; Califano, L.; et al. Diagnostic Performance of Inflammatory Biomarkers and Cytological Analysis in Salivary Gland Tumors. Head Neck 2023, 45, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Tateda, Y.; Suzuki, T.; Sato, T.; Izuhara, K.; Ise, K.; Shimada, H.; Murakami, K.; Murakami, K.; Nakamura, Y.; Ohta, N. Expression of Periostin in Benign Salivary Gland Tumors. Tohoku J. Exp. Med. 2024, 262, 105–113. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The Role of VEGF in Cancer-Induced Angiogenesis and Research Progress of Drugs Targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Heloterä, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Düwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabañas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial Endoglin Is Involved in Inflammation: Role in Leukocyte Adhesion and Transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef]
- Meurer, S.K.; Weiskirchen, R. Endoglin: An “Accessory” Receptor Regulating Blood Cell Development and Inflammation. Int. J. Mol. Sci. 2020, 21, 9247. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among Smoking, Oxidative Stress, Inflammation, Macromolecular Damage, and Cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Wigner, P.; Grębowski, R.; Bijak, M.; Saluk-Bijak, J.; Szemraj, J. The Interplay between Oxidative Stress, Inflammation and Angiogenesis in Bladder Cancer Development. Int. J. Mol. Sci. 2021, 22, 4483. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Toka, F.N.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Chang, C.C.; Wang, C.S.; Lin, K.H. Association between Inflammation and Function of Cell Adhesion Molecules Influence on Gastrointestinal Cancer Development. Cells 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tan, Y.; Li, F.; Han, Y.; Zhang, S.; Lin, X. CD44: A Cancer Stem Cell Marker and Therapeutic Target in Leukemia Treatment. Front. Immunol. 2024, 15, 1354992. [Google Scholar] [CrossRef]
- Hou, W.; Kong, L.; Hou, Z.; Ji, H. CD44 Is a Prognostic Biomarker and Correlated with Immune Infiltrates in Gastric Cancer. BMC Med. Genom. 2022, 15, 225. [Google Scholar] [CrossRef]
- Mesrati, M.H.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Cushing, K.; Higgins, P.D.R. Management of Crohn Disease: A Review. JAMA 2021, 325, 69–80. [Google Scholar] [CrossRef]
- Briani, C.; Visentin, A. Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies. Neurotherapeutics 2022, 19, 874–884. [Google Scholar] [CrossRef]
- Maximus, P.S.; Al Achkar, Z.; Hamid, P.F.; Hasnain, S.S.; Peralta, C.A. Adipocytokines: Are They the Theory of Everything? Cytokine 2020, 133, 155144. [Google Scholar] [CrossRef]
- Sy, A.L.; Hoang, M.P. SOX10. J. Clin. Pathol. 2023, 76, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Bancaro, N.; Calì, B.; Troiani, M.; Elia, A.R.; Arzola, R.A.; Attanasio, G.; Lai, P.; Crespo, M.; Gurel, B.; Pereira, R.; et al. Apolipoprotein E Induces Pathogenic Senescent-like Myeloid Cells in Prostate Cancer. Cancer Cell 2023, 41, 602–619.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.R.; Mao, X.R.; Wang, X.F.; Zheng, Y.; Wang, Y.P.; Zhou, Y.N. Role of Annexin A Family in Tumorigenesis and Chemoresistance of Gastric Cancer. Neoplasma 2022, 69, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Ji, X.; Yan, L.; Lian, S.; Chen, Z.; Luo, Y. Roles of S100A8, S100A9 and S100A12 in Infection, Inflammation and Immunity. Immunology 2024, 171, 365–376. [Google Scholar] [CrossRef]
- Ghosh, S.K.; McCormick, T.S.; Weinberg, A. Human Beta Defensins and Cancer: Contradictions and Common Ground. Front. Oncol. 2019, 9, 314. [Google Scholar] [CrossRef]
- Tang, S.; Ling, Z.; Jiang, J.; Gu, X.; Leng, Y.; Wei, C.; Cheng, H.; Li, X. Integrating the Tumor-Suppressive Activity of Maspin with P53 in Retuning the Epithelial Homeostasis: A Working Hypothesis and Applicable Prospects. Front. Oncol. 2022, 12, 1037794. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the Tumor Microenvironment: From Cancer Progression to Treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Kim, S.J. Immunological Function of Blimp-1 in Dendritic Cells and Relevance to Autoimmune Diseases. Immunol. Res. 2015, 63, 113–120. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, P.; Wu, H.; Liang, Y.; Xu, R.; Lu, H.; Chen, Q. Caveolin-1 Is a Prognostic Marker and Suppresses the Proliferation of Breast Cancer. Transl. Cancer Res. 2021, 10, 3797–3810. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Z.; Pan, X.; Zhang, J.; Wang, X.; Wang, M.; Li, H.; Yan, M.; Chen, W. Caveolin-1 Promotes Cancer Progression via Inhibiting Ferroptosis in Head and Neck Squamous Cell Carcinoma. J. Oral. Pathol. Med. 2022, 51, 52–62. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Lei, Y.; Wang, G.; Liu, M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 824208. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Y.; Zhang, Y.; Wang, L.; Chen, G. Glypican-3 Promotes Cell Proliferation and Tumorigenesis through up-Regulation of β-Catenin Expression in Lung Squamous Cell Carcinoma. Biosci. Rep. 2019, 39, BSR20181147. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast Differentiation by RANKL and OPG Signaling Pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK-RANKL-OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef] [PubMed]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and Function in Cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef]
- Sonnenberg-Riethmacher, E.; Miehe, M.; Riethmacher, D. Periostin in Allergy and Inflammation. Front. Immunol. 2021, 12, 722170. [Google Scholar] [CrossRef]
| Study | Number of Patients with Benign SGT | Studied Molecule | Effect on Benign Tumor Tissues or Serum |
|---|---|---|---|
| Loy et al., 2005 [20] | 21 | COX-2 | Overexpression/ upregulation |
| Andreadis et al., 2006 [21] | 54 | E-cadherin | Strong overexpression |
| Pantelis et al., 2008 [22] | 5 | Human beta-defensin 1/2/3 | Decreased expression of hBD-1, no significant difference regarding hBD-2 and hBD-3 |
| Tampouris et al., 2011 [23] | 20 | VEGF-C/VEGF-D/ VEGFR-3 (flt-4) | All 3 strongly expressed in PA, VEGF-C/D moderately expressed or not expressed in other benign tumors flt-4 strongly expressed in all benign tumors |
| Liu et al., 2012 [24] | 72 | Chemokine CCL28 | Decreased expression |
| Kehagias et al., 2012 [25] | 18 | N-cadherin | Expressed in some cases of Warthin’s tumor, not expressed in other benign samples |
| Carlesimo et al., 2012 [26] | 2 | TNF-alpha | Not analyzed in the tissue |
| Jour et al., 2013 [27] | 14 | Cyclin D1 and p16INK4A | Almost all benign tumors expressed p16 and cyclin D1 |
| Andisheh Tadbir et al., 2013 [28] | 31 | VEGF | Higher concentration in serum |
| Aga et al., 2013 [29] | 1 | IgG4 | Serum and tissue levels increased |
| Ohtomo et al., 2013 [30] | 14 | SOX-10 | Present in all PA, ME, absent in some of WT |
| Donadio et al., 2013 [31] | 36 | 26 proteins | Characteristic separate chains of proteins for PA and WT |
| Ianez et al., 2013 [32] | 101 | CD24/CD44 | CD24/CD44 positive by immunochemistry, CD44, also by PCR |
| Karbanová et al., 2014 [33] | 10 | Prominin-1 (CD133) | Overexpressed |
| Reshma et al., 2014 [34] | 15 | Maspin | Overexpressed |
| Aga et al., 2014 [35] | 37 | IgG4 | Serum and tissue levels increased in some of WT, mRNA overexpressed in some of WT, not increased/expressed in PA |
| Faur et al., 2014 [36] | 20 | VEGF | Moderately positive VEGF expression |
| He et al., 2014 [37] | 3 | Phosphatidylocholine | Increased in WT |
| Andisheh-Tadbir et al., 2014 [38] | 15 | CD166 | Overexpressed |
| Wang et al., 2015 [39] | 40 | PRDM1 | Pverexpressed |
| Haghshenas et al., 2015 [40] | 19 | IL-17-producing lymphocytes and CTLA4+ lymphocytes | Increased concentration in serum |
| Jaafari-Ashkavandi et al., 2015 [41] | 15 | Caveolin-1 | overexpressed |
| Fonseca et al., 2015 [42] | 120 | Semaphorins and neutrophilins | No significant difference vs. control group |
| Khademi et al., 2016 [43] | 50 | INF y, IL-4 | No significant difference vs. control group |
| Kim et al., 2016 [44] | 8 | IgG4 | Increased concentration in serum |
| Haghshenas et al., 2016 [45] | 15 | Th1, Th2, Tc1, Tc2 lymphocytes | No significant difference vs. control group |
| Zare et al., 2018 [46] | 14 | IL-33 | Slightly overexpressed |
| Sowa et al., 2018 [47] | 51 | Adipocytokines | Serum levels of adiponectin and visfatin elevated, leptin elevated in males, IL-6 no difference |
| Sowa et al., 2018 [48] | 26 | Protein oxidation products | Serum level of Total Antioxidant Capacity of Blood Serum and thiol groups decreased, Advanced oxidation protein products increased |
| Błochowiak et al., 2018 [49] | 45 | VEGF165b, VEGFR1, VEGFR2 and CD34 | No significant difference vs. control group |
| Tenorio et al., 2018 [50] | 38 | COX-2, cyclin D1 | Underexpressed vs. malignant tumors |
| Andisheh-Tadbir et al., 2019 [51] | 17 | Glypican-3 (GPC-3) | Overexpressed |
| Aslan et al., 2020 [52] | 38 | RANK, RANKL | Underexpressed vs. malignant tumors |
| Baneckova et al., 2020 [53] | 89 | S1009, SOX-10 | Expressed in 10% of oncocytic cases of PA/ME, negative in oncocytoma |
| Da Silva et al., 2020 [54] | 51 | ALDH-1 | Expressed in parenchyma of all benign tumors |
| Mochizuki et al., 2021 [55] | 40 | CXCL10, CXCL12, CCL18 | WT mostly positive for expression, PA mostly negative |
| Kobayashi et al., 2021 [56] | 64 | Th, Tfh | Different for solid-type and cyst-type tumor |
| Sahin et al., 2022 [57] | 185 | Systemic Immune-Inflammation Index (SII) | Lower vs. malignant tumors |
| Haghshenas et al., 2022 [58] | 5 | MSC cells | Many cell expressed—CD44, CD73, CD90, CD105, and CD166, heat shock protein 70 (Hsp70), keratin, type II cytoskeletal 7 (CK-7), |
| Laohavisudhi et al., 2022 [59] | 13 | CD44s, CD44v6, CXCR2, CXCL1, and IL-1β | CD44s, CD44v6, CXCR2—increased in benign tumors |
| Gaonkar et al., 2022 [60] | 15 | Endoglin | Higher expression vs. control group, lower vs. malignant tumors |
| Abbate et al., 2022 [61] | 191 | NLR, SII, PRL | All markers significantly increased vs. control group |
| Sowa et al., 2022 [62] | 52 | Oxidative stress markers | Plasma lipofuscin increased in all benign tumors, Cu-Zn SOD decreased in WT |
| Jabbar et al., 2023 [63] | 30 | VEGF | Overexpression |
| Abbate et al., 2023 [64] | 140 | Inflammatory biomarkers SII, SIRI, PLR, and NLR | SIRI showed highest accuracy in determining malignancy, decreased vs. malignant tumors |
| Tateda et al., 2024 [65] | 38 | Periostin | Overexpression in 32 out of 38 benign tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowski, K.; Puchalski, M.; Ołdziej, S.; Kasprzyk-Tryk, A.; Skorek, A.; Tretiakow, D. The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 12558. https://doi.org/10.3390/ijms252312558
Szydłowski K, Puchalski M, Ołdziej S, Kasprzyk-Tryk A, Skorek A, Tretiakow D. The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review. International Journal of Molecular Sciences. 2024; 25(23):12558. https://doi.org/10.3390/ijms252312558
Chicago/Turabian StyleSzydłowski, Konrad, Michał Puchalski, Stanisław Ołdziej, Agnieszka Kasprzyk-Tryk, Andrzej Skorek, and Dmitry Tretiakow. 2024. "The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review" International Journal of Molecular Sciences 25, no. 23: 12558. https://doi.org/10.3390/ijms252312558
APA StyleSzydłowski, K., Puchalski, M., Ołdziej, S., Kasprzyk-Tryk, A., Skorek, A., & Tretiakow, D. (2024). The Impact of Inflammation on the Etiopathogenesis of Benign Salivary Gland Tumors: A Scoping Review. International Journal of Molecular Sciences, 25(23), 12558. https://doi.org/10.3390/ijms252312558









