Advances in the Regulation of Neural Function by Infrared Light
Abstract
1. Introduction
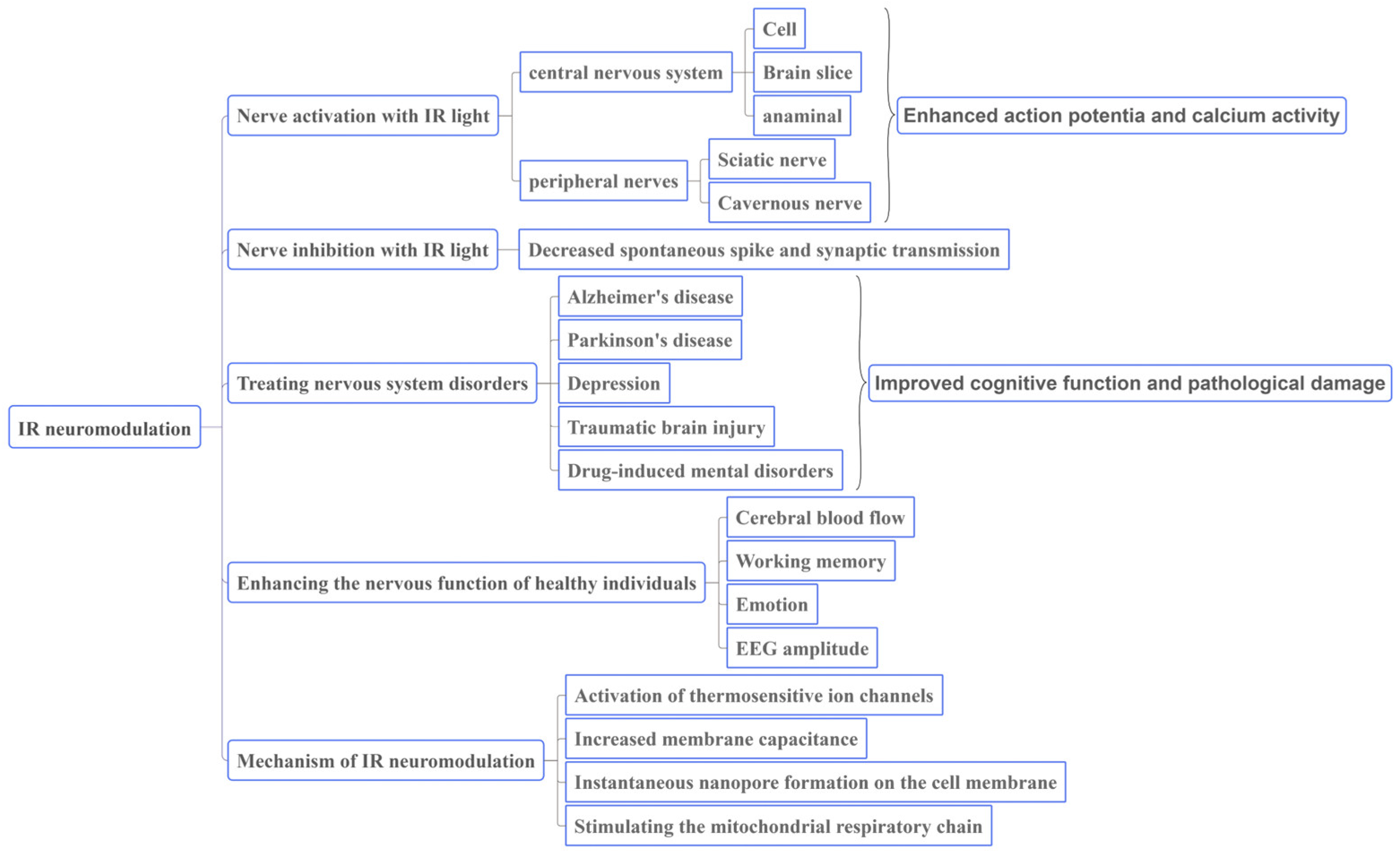
2. Roles of IR light in Neural Regulation
3. IR Stimulation Can Be Used to Regulate Neural Function and Improve Health
3.1. IR Light Can Regulate Neural Activity
3.1.1. Stimulating Effect on the Central Nervous System
3.1.2. Stimulation of Peripheral Nerves
3.2. Nerve Inhibition with IR Light
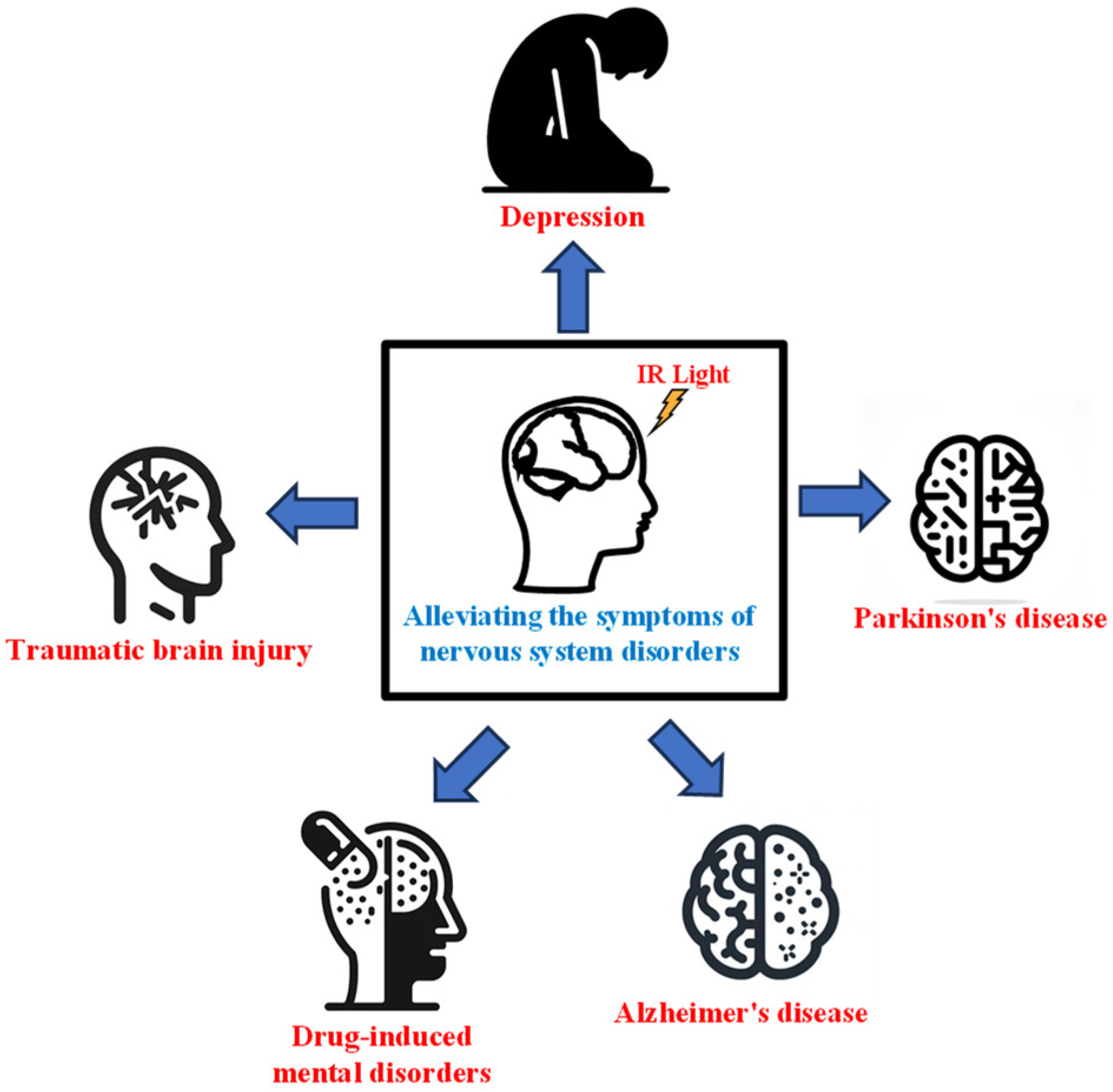
3.3. IR Light Can Be Used to Treat Nervous System Disorders
3.3.1. IR Light for Treating Alzheimer’s Disease
3.3.2. IR Light for Treating Parkinson’s Disease
3.3.3. IR Light for Treating Depression
3.3.4. IR Light for Treating Traumatic Brain Injury
3.3.5. IR Light for Alleviating Drug-Induced Mental Disorders
3.4. IR Light Can Enhance the Nervous Function of Healthy Individuals
4. Mechanism of IR Light in Regulating Neural Function
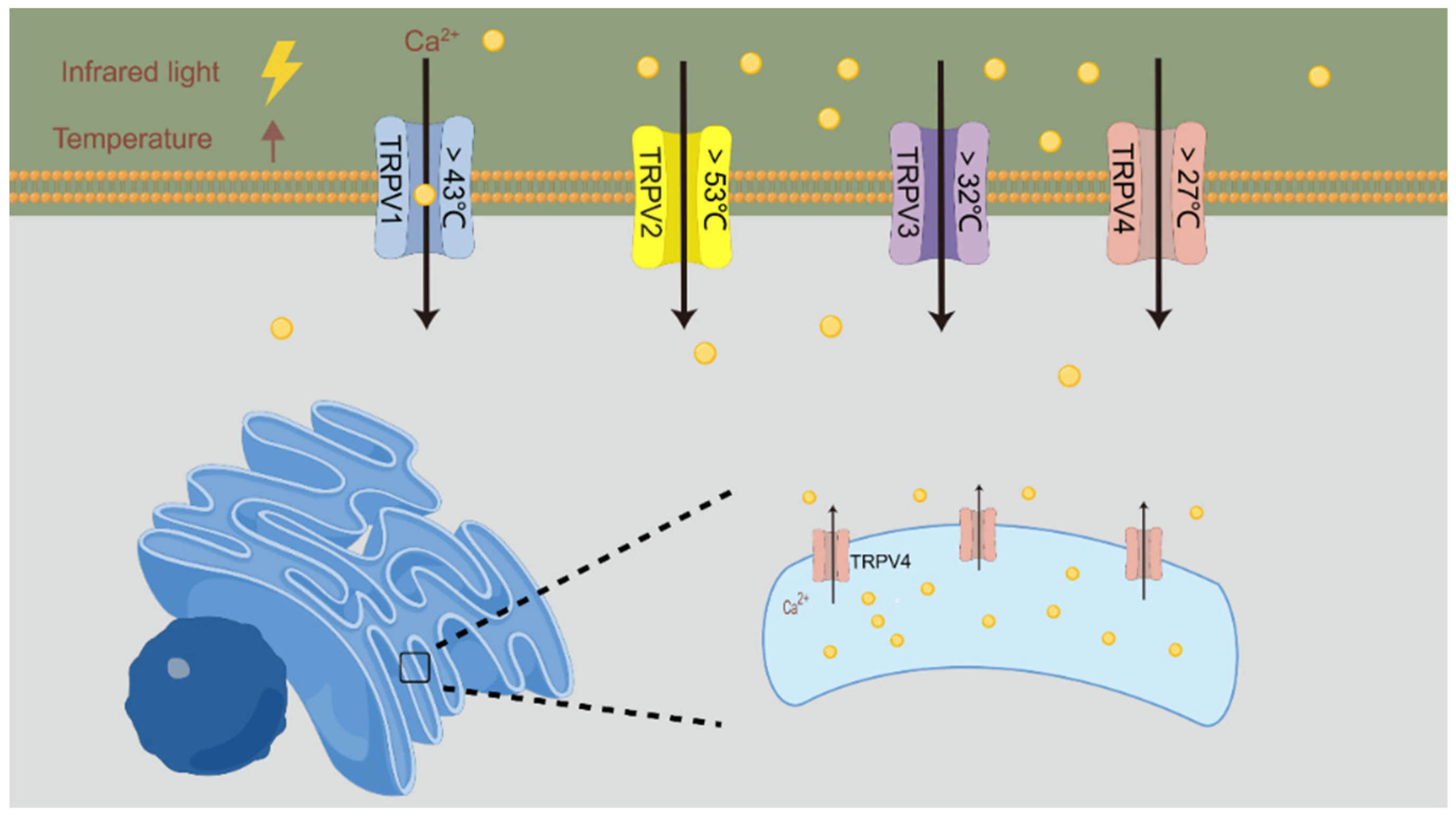
4.1. Activation of Thermosensitive Ion Channels
4.2. Increased Membrane Capacitance
4.3. Instantaneous Nanopore Formation on the Cell Membrane
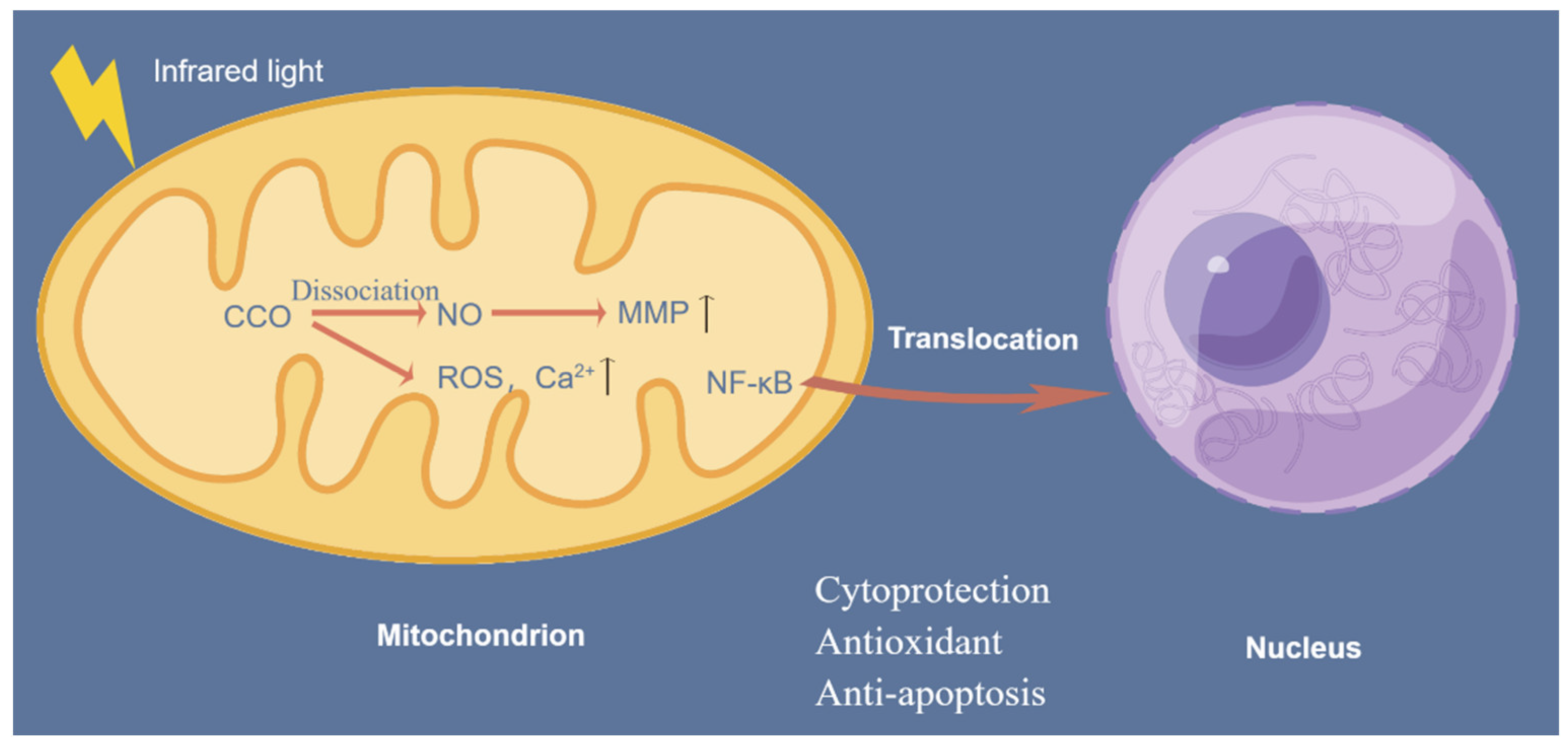
4.4. Stimulating the Mitochondrial Respiratory Chain
5. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Zhou, J.; Li, F. Functional Near-Infrared Spectroscopy: An Emerging Functional Neuroimaging Technology. J. Psychol. Sci. 2011, 34, 943–949. [Google Scholar]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.E.; Lee, Y.M.; Park, C.H.; Chung, J.H. Effects of infrared radiation and heat on human skin aging in vivo. J. Investig. Dermatol. Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H. Low-Level Laser Irradiation Precondition for Cardiac Regenerative Therapy. Photomed. Laser Surg. 2016, 34, 572–579. [Google Scholar] [CrossRef]
- Rojas, J.C.; Gonzalez-Lima, F. Low-level light therapy of the eye and brain. Eye Brain 2011, 3, 49–67. [Google Scholar] [CrossRef]
- Berman, M.H.; Nichols, T.W. Treatment of Neurodegeneration: Integrating Photobiomodulation and Neurofeedback in Alzheimer’s Dementia and Parkinson’s: A Review. Photobiomodul Photomed. Laser Surg. 2019, 37, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Huck, C.W. Biomolecular and bioanalytical applications of infrared spectroscopy—A review. Anal. Chim. Acta 2020, 1133, 150–177. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, L.; Peng, R.Y. Research progress in the effects of terahertz waves on biomacromolecules. Mil. Med. Res. 2021, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Choi, H.J.; Jeong, H. Tunable metasurfaces for visible and SWIR applications. Nano Converg. 2020, 7, 3. [Google Scholar] [CrossRef]
- Escobedo, R.; Miranda, R.; Martínez, J. Infrared Irradiation: Toward Green Chemistry, a Review. Int. J. Mol. Sci. 2016, 17, 453. [Google Scholar] [CrossRef]
- Levy, R.M. Neuromodulation: The “Not-so-Hidden” Cure for the Opioid Crisis. Neuromodulation 2017, 20, 519–524. [Google Scholar] [CrossRef]
- Drossman, D.A.; Tack, J.; Ford, A.C.; Szigethy, E.; Törnblom, H.; Van Oudenhove, L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology 2018, 154, 1140–1171.e1141. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Ibbotson, M.R.; Thompson, A.C.; Wise, A.K.; Fallon, J.B. Optical stimulation of neural tissue. Healthc. Technol. Lett. 2020, 7, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.P.; Matic, A.I.; Wells, J.D.; Jansen, E.D.; Walsh, J.T., Jr. Neural stimulation with optical radiation. Laser Photon Rev. 2011, 5, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Aplin, F.P.; Fridman, G.Y. Implantable Direct Current Neural Modulation: Theory, Feasibility, and Efficacy. Front. Neurosci. 2019, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Qureshi, A.A.; Kozin, E.D.; Lee, D.J. Concepts in Neural Stimulation: Electrical and Optical Modulation of the Auditory Pathways. Otolaryngol. Clin. N. Am. 2020, 53, 31–43. [Google Scholar] [CrossRef]
- Dautrebande, M.; Doguet, P.; Gorza, S.-P.; Delbeke, J.; Nonclercq, A. Peripheral nerve recruitment curve using near-infrared stimulation. In Proceedings of the Conference on Optogenetics and Optical Manipulation, San Francisco, CA, USA, 27–28 January 2018. [Google Scholar]
- Bonmassar, G.; Lee, S.W.; Freeman, D.K.; Polasek, M.; Fried, S.I.; Gale, J.T. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 2012, 3, 921. [Google Scholar] [CrossRef]
- Goetz, S.M.; Deng, Z.D. The development and modelling of devices and paradigms for transcranial magnetic stimulation. Int. Rev. Psychiatry 2017, 29, 115–145. [Google Scholar] [CrossRef]
- Cardin, J.A.; Carlén, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.H.; Moore, C.I. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 2010, 5, 247–254. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Packer, A.M.; Roska, B.; Häusser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 2013, 16, 805–815. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Mu, Y.; Huang, L.; Hu, Y.; Chen, Z.; Yang, Y.; Huang, X.; Fu, Y.; Xi, Y.; Lin, S.; et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron 2022, 110, 1712–1727.e1717. [Google Scholar] [CrossRef] [PubMed]
- Teudt, I.U.; Nevel, A.E.; Izzo, A.D.; Walsh, J.T., Jr.; Richter, C.P. Optical stimulation of the facial nerve: A new monitoring technique? Laryngoscope 2007, 117, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the brain: A new paradigm. J. Opt. 2017, 19, 013003. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Wade, S.A.; Cadusch, P.J.; Brown, W.G.; Stoddart, P.R. Modeling of the temporal effects of heating during infrared neural stimulation. J. Biomed. Opt. 2013, 18, 035004. [Google Scholar] [CrossRef]
- Cayce, J.M.; Friedman, R.M.; Jansen, E.D.; Mahavaden-Jansen, A.; Roe, A.W. Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. Neuroimage 2011, 57, 155–166. [Google Scholar] [CrossRef]
- Song, P.; Li, S.; Hao, W.; Wei, M.; Liu, J.; Lin, H.; Hu, S.; Dai, X.; Wang, J.; Wang, R.; et al. Corticospinal excitability enhancement with simultaneous transcranial near-infrared stimulation and anodal direct current stimulation of motor cortex. Clin. Neurophysiol. 2021, 132, 1018–1024. [Google Scholar] [CrossRef]
- Bao, W. Surface Enhanced Infrared Absorption Spectroscopy and Its Applications; Nanjing University: Nanjing, China, 2015. [Google Scholar]
- Geng, J.; Li, S.; Wang, S.; Huang, C.; Lv, Y.; Hu, R.; Qu, J.; Liu, L. Stimulating Ca2+ photoactivation of nerve cells by near-infrared light. Acta Phys. Sin. 2020, 69, 336–344. [Google Scholar] [CrossRef]
- Tian, L.; Wang, J.; Wei, Y.; Lu, J.; Xu, A.; Xia, M. Short-wavelength infrared laser activates the auditory neurons: Comparing the effect of 980 vs. 810 nm wavelength. Lasers Med. Sci. 2017, 32, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.D.; Richter, C.P.; Jansen, E.D.; Walsh, J.T., Jr. Laser stimulation of the auditory nerve. Lasers Surg. Med. 2006, 38, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, Z.; Chai, Y.; Zhu, Z.; Wu, K.; Ji, W.; Li, D.; Xiao, Y.; Mao, L.; Chang, C.; et al. Nonthermal and reversible control of neuronal signaling and behavior by midinfrared stimulation. Proc. Natl. Acad. Sci. USA 2021, 118, e2015685118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Y.; Liang, S.; Liao, X.; Li, T.; Qiao, Z.; Chang, C.; Jia, H.; Chen, X. Non-invasive, opsin-free mid-infrared modulation activates cortical neurons and accelerates associative learning. Nat. Commun. 2021, 12, 2730. [Google Scholar] [CrossRef] [PubMed]
- Cayce, J.M.; Kao, C.C.; Malphrus, J.D.; Konrad, P.E.; Mahadevan-Jansen, A.; Jansen, E.D. Infrared Neural Stimulation of Thalamocortical Brain Slices. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 565–572. [Google Scholar] [CrossRef]
- Cury, J.; Vande Perre, L.; Smets, H.; Stumpp, L.; Vespa, S.; Vanhoestenberghe, A.; Doguet, P.; Delbeke, J.; El Tahry, R.; Gorza, S.P.; et al. Infrared neurostimulation inex-vivorat sciatic nerve using 1470 nm wavelength. J. Neural Eng. 2021, 18, 056018. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Kao, C.; Jansen, E.D.; Konrad, P.; Mahadevan-Jansen, A. Application of infrared light for in vivo neural stimulation. J. Biomed. Opt. 2005, 10, 064003. [Google Scholar] [CrossRef]
- Wells, J.D.; Thomsen, S.; Whitaker, P.; Jansen, E.D.; Kao, C.C.; Konrad, P.E.; Mahadevan-Jansen, A. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg. Med. 2007, 39, 513–526. [Google Scholar] [CrossRef]
- Tozburun, S.; Cilip, C.M.; Lagoda, G.A.; Burnett, A.L.; Fried, N.M. Continuous-wave infrared optical nerve stimulation for potential diagnostic applications. J. Biomed. Opt. 2010, 15. [Google Scholar] [CrossRef][Green Version]
- Fried, N.M.; Lagoda, G.A.; Scott, N.J.; Su, L.M.; Burnett, A.L. Laser stimulation of the cavernous nerves in the rat prostate, in vivo: Optimization of wavelength, pulse energy, and pulse repetition rate. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 2777–2780. [Google Scholar] [CrossRef]
- Xia, Q.; Nyberg, T. Inhibition of cortical neural networks using infrared laser. J. Biophotonics 2019, 12, e201800403. [Google Scholar] [CrossRef]
- Feng, H.-J.; Kao, C.; Gallagher, M.J.; Jansen, E.D.; Mahadevan-Jansen, A.; Konrad, P.E.; Macdonald, R.L. Alteration of GABAergic neurotransmission by pulsed infrared laser stimulation. J. Neurosci. Methods 2010, 192, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.S.; Zângaro, R.A.; Parreira, R.B.; Kerppers, I.I. The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med. Sci. 2015, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Khuman, J.; Zhang, J.; Park, J.; Carroll, J.D.; Donahue, C.; Whalen, M.J. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J. Neurotrauma 2012, 29, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S. Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med. Sci. 2016, 31, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, F.; Massri, N.E.; Darlot, F.; Torres, N.; Johnstone, D.M.; Chabrol, C.; Costecalde, T.; Stone, J.; Mitrofanis, J.; Benabid, A.L.; et al. 810nm near-infrared light offers neuroprotection and improves locomotor activity in MPTP-treated mice. Neurosci. Res. 2015, 92, 86–90. [Google Scholar] [CrossRef]
- Wu, X.; Alberico, S.L.; Moges, H.; De Taboada, L.; Tedford, C.E.; Anders, J.J. Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg. Med. 2012, 44, 227–232. [Google Scholar] [CrossRef]
- Morries, L.D.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159–2175. [Google Scholar] [CrossRef]
- El Khoury, H.; Mitrofanis, J.; Henderson, L.A. Exploring the Effects of Near Infrared Light on Resting and Evoked Brain Activity in Humans Using Magnetic Resonance Imaging. Neuroscience 2019, 422, 161–171. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; Qi, X.; Berman, M.H.; Dougal, G.; Dayawansa, S.; Wu, E.; Yi, S.S.; Stevens, A.B.; Huang, J.H. Transcranial Near Infrared Light Stimulations Improve Cognition in Patients with Dementia. Aging Dis. 2021, 12, 954–963. [Google Scholar] [CrossRef]
- Gutiérrez-Menéndez, A.; Cid-Duarte, S.; Banqueri, M.; Martínez, J.A.; Méndez, M.; Arias, J.L. Photobiomodulation effects on active brain networks during a spatial memory task. Physiol. Behav. 2021, 230, 113291. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.W.; Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013, 230, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.J.; Maddox, W.T.; Gonzalez-Lima, F. Improving executive function using transcranial infrared laser stimulation. J. Neuropsychol. 2017, 11, 14–25. [Google Scholar] [CrossRef]
- Michalikova, S.; Ennaceur, A.; van Rensburg, R.; Chazot, P.L. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiol. Learn. Mem. 2008, 89, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Yaji, T.; Ohta, T.; Tsukiyama, K.; Nakamura, K. Dissociation of β-Sheet Stacking of Amyloid β Fibrils by Irradiation of Intense, Short-Pulsed Mid-infrared Laser. Cell Mol. Neurobiol. 2018, 38, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Kimura, S.; Kato, Y.; Kohno, M. Effects of far infrared light on Alzheimer’s disease-transgenic mice. PLoS ONE 2021, 16, e0253320. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.N.; Sharma, N.; Shin, E.J.; Nguyen, B.T.; Nguyen, P.T.; Jeong, J.H.; Cho, E.H.; Lee, Y.J.; Kim, N.H.; Jang, C.G.; et al. Exposure to far-infrared ray attenuates methamphetamine-induced impairment in recognition memory through inhibition of protein kinase C δ in male mice: Comparison with the antipsychotic clozapine. J. Neurosci. Res. 2018, 96, 1294–1310. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Salehpour, F.; Hamblin, M.R. Photobiomodulation for Parkinson’s Disease in Animal Models: A Systematic Review. Biomolecules 2020, 10, 610. [Google Scholar] [CrossRef]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef]
- Tran, T.V.; Shin, E.J.; Nguyen, L.T.T.; Lee, Y.; Kim, D.J.; Jeong, J.H.; Jang, C.G.; Nah, S.Y.; Toriumi, K.; Nabeshima, T.; et al. Protein Kinase Cδ Gene Depletion Protects Against Methamphetamine-Induced Impairments in Recognition Memory and ERK(1/2) Signaling via Upregulation of Glutathione Peroxidase-1 Gene. Mol. Neurobiol. 2018, 55, 4136–4159. [Google Scholar] [CrossRef] [PubMed]
- William, J.H.; Fomberstein, K.M.; Niyogi, S.; Coble, M.M.; Banks, S.; Dinges, D.F. Development of a briefer version of the psychomotor vigilance task. Sleep 2006, 29, A344–A345. [Google Scholar]
- Lind, J.; Enquist, M.; Ghirlanda, S. Animal memory: A review of delayed matching-to-sample data. Behav. Process. 2015, 117, 52–58. [Google Scholar] [CrossRef]
- Gómez-de-Regil, L. Assessment of Executive Function in Patients with Traumatic Brain Injury with the Wisconsin Card-Sorting Test. Brain Sci. 2020, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.; Moser, T. Considering optogenetic stimulation for cochlear implants. Heart Res. 2015, 322, 224–234. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, F.; Wei, Y.; Chen, W.R.; Chen, Q.; Xing, D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid. Redox Signal 2014, 20, 733–746. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar] [CrossRef]
- Noël, J.; Zimmermann, K.; Busserolles, J.; Deval, E.; Alloui, A.; Diochot, S.; Guy, N.; Borsotto, M.; Reeh, P.; Eschalier, A.; et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. Embo J. 2009, 28, 1308–1318. [Google Scholar] [CrossRef]
- Barrett, J.N.; Rincon, S.; Singh, J.; Matthewman, C.; Pasos, J.; Barrett, E.F.; Rajguru, S.M. Pulsed infrared releases Ca(2+) from the endoplasmic reticulum of cultured spiral ganglion neurons. J. Neurophysiol. 2018, 120, 509–524. [Google Scholar] [CrossRef]
- Suh, E.; Matic, A.I.; Otting, M.; Walsh, J.T., Jr.; Richter, C.-P. Optical stimulation in mice lacking the TRPV1 channel. In Proceedings of the Conference on Photons and Neurons, San Jose, CA, USA, 25–26 January 2009. [Google Scholar]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef] [PubMed]
- Plaksin, M.; Kimmel, E.; Shoham, S. Thermal transients excite neurons through universal intramembrane mechano-electrical effects. Phys. Rev. X 2018, 8, 011043. [Google Scholar]
- Liu, Q.; Frerck, M.J.; Holman, H.A.; Jorgensen, E.M.; Rabbitt, R.D. Exciting cell membranes with a blustering heat shock. Biophys. J. 2014, 106, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh-Ansari, M.J.; Ansari, M.A.; Zakeri, M.; Haghjoo, M. Influence of radiant exposure and repetition rate in infrared neural stimulation with near-infrared lasers. Lasers Med. Sci. 2019, 34, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Beier, H.T.; Tolstykh, G.P.; Musick, J.D.; Thomas, R.J.; Ibey, B.L. Plasma membrane nanoporation as a possible mechanism behind infrared excitation of cells. J. Neural Eng. 2014, 11, 066006. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Cell biology: Power games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef]
- Waypa, G.B.; Smith, K.A.; Schumacker, P.T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Asp. Med. 2016, 47–48, 76–89. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Yang, Y.; Tucker, D.; Lu, Y.; Xin, N.; Zhang, G.; Yang, L.; Li, J.; Du, X.; et al. Low-Level Laser Irradiation Improves Depression-Like Behaviors in Mice. Mol. Neurobiol. 2017, 54, 4551–4559. [Google Scholar] [CrossRef]
- Tian, F.; Hase, S.N.; Gonzalez-Lima, F.; Liu, H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 2016, 48, 343–349. [Google Scholar] [CrossRef]
| IR Category | Wavelength (μm) | Frequency (THz) |
|---|---|---|
| NIR | 0.75–2.5 | 120–400 |
| MIR | 2.5–25 | 12–120 |
| FIR | 25–1000 | 0.3–12 |
| Neural Modulatory Methods | Applications | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Electrical stimulation | Treating disorders of the nervous, cardiovascular, and sensory systems | Mature technology and wide applicability | Poor spatial specificity, susceptibility to environmental electrical interference, and risk of tissue trauma | [15,16,17] |
| Magnetic stimulation | Noninvasive treatment of central and peripheral nervous system disorders | Safe, noninvasive, and highly tissue penetrability | Inaccurate spatial locations and individual variations | [18,19] |
| Optogenetics | Precise modulation of neuronal subtypes at temporal and spatial scales | Precise modulation with high temporal and spatial resolution | Requires invasive surgery with fibre implantation | [20,21,22] |
| Visual stimulation | 40 Hz flicker stimulation reduces Aβ and phosphorylated tau levels in AD transgenic mice; 3000 lux of bright light has antinociceptive effects on mice | Noninvasive and less adverse reactions | Cannot target specific brain regions, and the mechanism remains unclear | [23,24] |
| Experimental Model | Exposure Conditions | Effects | Reference |
|---|---|---|---|
| Healthy elderly women | 627 nm, 70 mW/cm2, 2 min/d, 4 d/week, 4 weeks | Blood and vasomotor behaviors of the basilar and middle cerebral arteries were promoted | [45] |
| TBI mice | 800 nm, 60 J/cm2 60–80 min | Memory function was restored and inflammation was reduced | [46] |
| Reserpine-induced depression rats | 804 nm 80, 200 and 400 mW, continuous wave 6 min/d, 7 d | After 80 mW IR exposure, the behavior of depressed rats improved, and the EEG activity returned to normal | [47] |
| MPTP-induced Parkinson’s disease mice | 810 nm, 160 μW | Number and motor activity of dopaminergic nerves increased | [48] |
| Chronic mild stress-induced Wistar rats | 810 nm, 100 Hz, 20% duty ratio, 120 J/cm2, 3 weeks | Forced swimming performance improved | [49] |
| TBI patients | 810 nm and 980 nm, 10–15 weeks | Neurological function improved, and cerebral inflammation decreased | [50] |
| Human subjects | 810 nm, 100 mW/cm2 20 min | Function active task-related brain regions were suppressed | [51] |
| AD patients | 1060–1080 nm, 23.1 mW/cm2, 8 weeks | Cognitive function and sleep quality improved, anxiety symptoms decreased | [52] |
| Wistar rats | 1064 nm, 30 mW 1 h/d, 5 d | The activity of the brain network involved in the spatial memory task was more efficient | [53] |
| Human subjects | 1064 nm, 250 mW/cm2, 2 weeks | Cognitive, emotional and executive functions in humans were improved | [54,55] |
| CD1 female mice | 1072 nm 6 min/d, 10 d | Performance in 3D spatial navigation task improved | [56] |
| APP/PS1/Tau transgenic mouse brain slices | 5.00, 6.17 and 7.19 μm, 35–4 5 mJ/cm2 | Aβ fibrils were dissociated | [57] |
| 5 × FAD mice | 8–10 μm, 1 W/cm2, 5 months | EGF and BDNF levels increased significantly | [58] |
| MA-induced mental disorder model | 5–20 μm 14 d, 40 min/d | Memory function was restored | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Wang, H.; Peng, R. Advances in the Regulation of Neural Function by Infrared Light. Int. J. Mol. Sci. 2024, 25, 928. https://doi.org/10.3390/ijms25020928
Song L, Wang H, Peng R. Advances in the Regulation of Neural Function by Infrared Light. International Journal of Molecular Sciences. 2024; 25(2):928. https://doi.org/10.3390/ijms25020928
Chicago/Turabian StyleSong, Lequan, Hui Wang, and Ruiyun Peng. 2024. "Advances in the Regulation of Neural Function by Infrared Light" International Journal of Molecular Sciences 25, no. 2: 928. https://doi.org/10.3390/ijms25020928
APA StyleSong, L., Wang, H., & Peng, R. (2024). Advances in the Regulation of Neural Function by Infrared Light. International Journal of Molecular Sciences, 25(2), 928. https://doi.org/10.3390/ijms25020928





