Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides
Abstract
1. Introduction
2. Results and Discussion
2.1. Melting Points Evaluated by the DSC
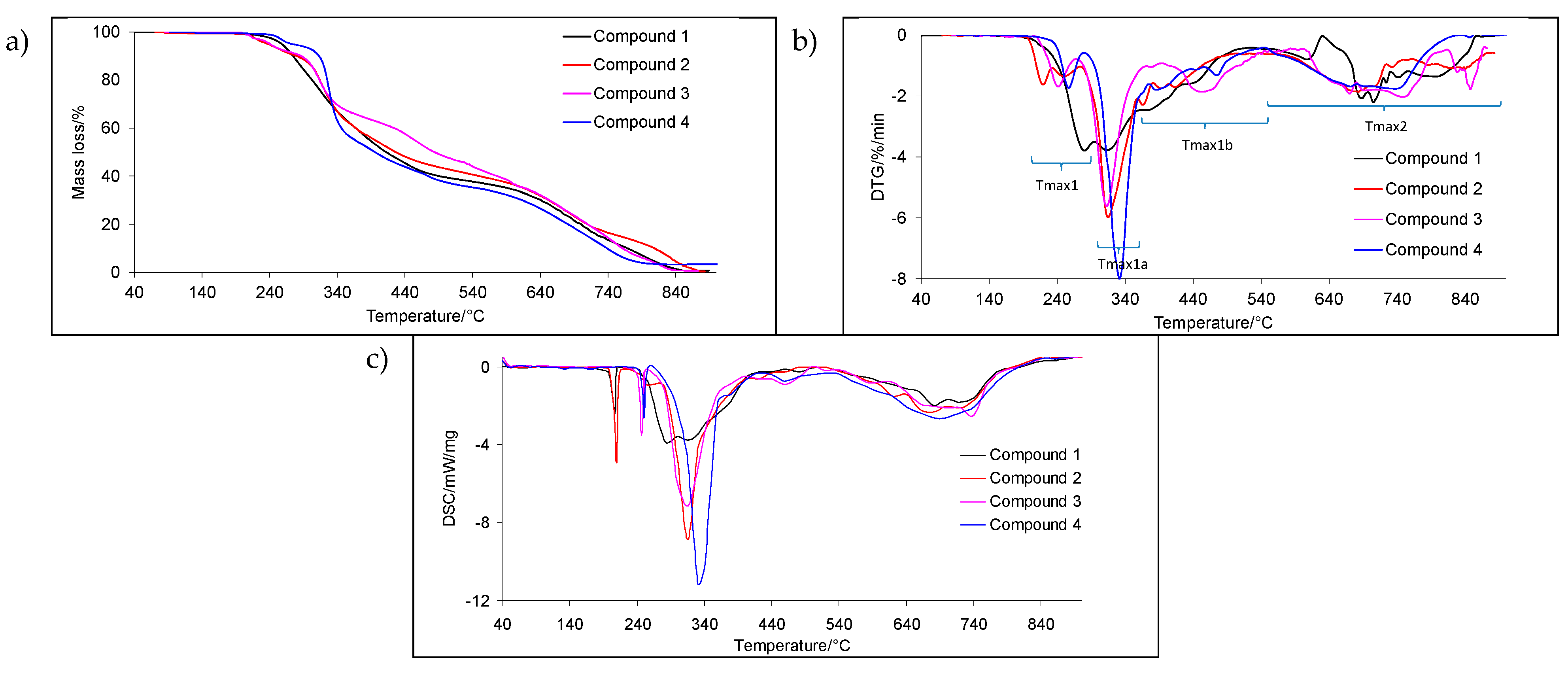
2.2. TG/DTG Analyses (Inert Conditions)
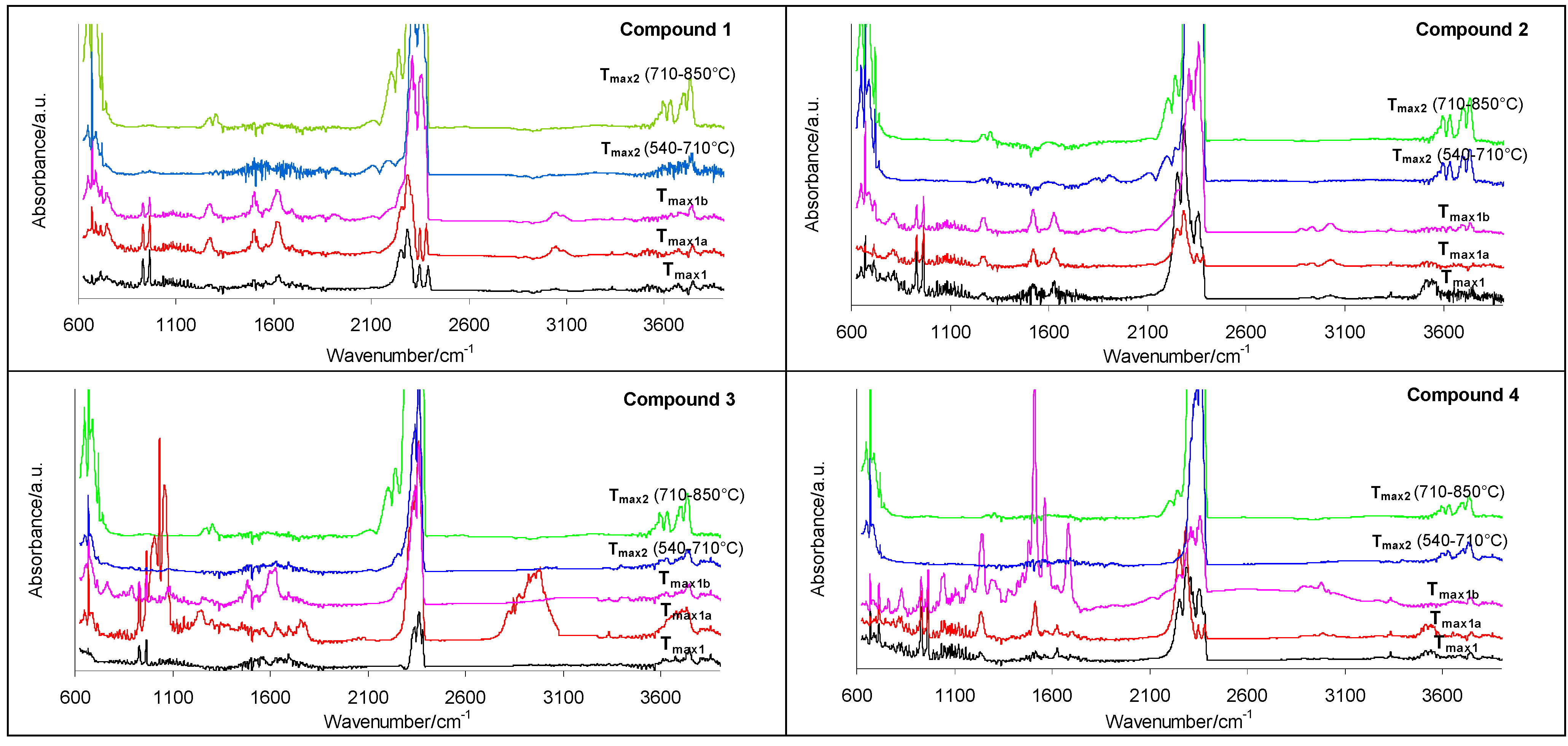
2.3. Decomposition Course of the Tested Compounds Evaluated with the Use of Simultaneous TG/FTIR/QMS Method (Inert Conditions)
2.4. TG/DTG Analyses (Oxidative Conditions)
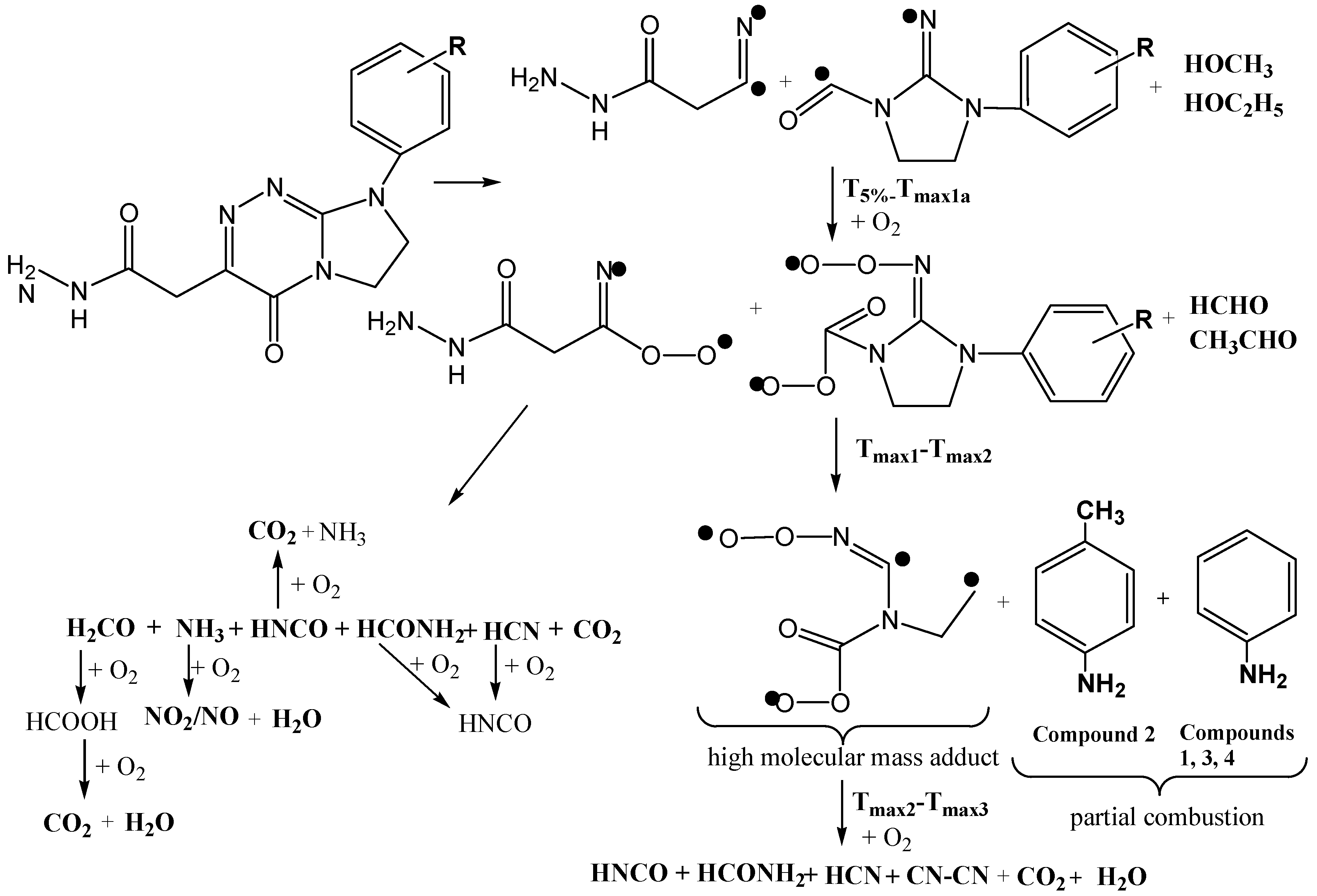
2.5. Decomposition Course of the Tested Compounds Evaluated with the Use of Simultaneous TG/FTIR/QMS Method (Oxidative Conditions)
3. Materials and Methods
3.1. Differential Scanning Calorimetry (DSC)
3.2. Simultaneous Thermogravimetric Analysis Coupled on-Line with FTIR and QMS Analysers (TG/DTG/FTIR/QMS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sztanke, M.; Sztanke, K. Biologically important hydrazide-containing fused azaisocytosines as antioxidant agents. Redox Rep. 2017, 22, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sirohi, T.S.; Singh, H.; Yadav, R.; Roy, R.K.; Chaudhary, A.; Pandeya, S.N. 1,2,4-triazine analogs as novel class of therapeutic agents. Mini Rev. Med. Chem. 2014, 14, 168–207. [Google Scholar] [CrossRef] [PubMed]
- Zálešák, F.; Slouka, J.; Stýskala, J. General synthesis of 1-aryl-6-azaisocytosines and their utilization for the preparation of related condensed 1,2,4-triazines. Molecules 2019, 24, 3558. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; Andrews, S.P.; Doré, A.S.; Hollenstein, K.; Hurrell, E.; Langmead, C.J.; Mason, J.S.; Ng, I.W.; Tehan, B.; Zhukov, A.; et al. Discovery of 1,2,4-triazine derivatives as adenosine A2A antagonists using structure based drug design. J. Med. Chem. 2012, 55, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Mahindra, A.; Jenkins, L.; Marsango, S.; Huggett, M.; Huggett, M.; Robinson, L.; Gillespie, J.; Rajamanickam, M.; Morrison, A.; McElroy, S.; et al. Investigating the Structure-Activity Relationship of 1,2,4-Triazine G-Protein-Coupled Receptor 84 (GPR84) Antagonists. J. Med. Chem. 2022, 65, 11270–11290. [Google Scholar] [CrossRef] [PubMed]
- Moura Ramos, J.J.; Diogo, H.P. Calorimetric (DSC) and dielectric (TSDC) investigation on the thermal behavior and molecular mobility in the supercooled liquid and glassy states of bifonazole and lamotrigine. J. Therm. Anal. Calorim. 2021, 145, 3077–3085. [Google Scholar] [CrossRef]
- Fasihi, Z.; Zakeri-Milani, P.; Nokhodchi, A.; Akbari, J. Thermodynamic approaches for the prediction of oral drug absorption. J. Therm. Anal. Calorim. 2017, 130, 1371–1382. [Google Scholar] [CrossRef]
- Attia, A.K.; Souaya, E.R.; Soliman, E.A. Thermal analysis investigation of dapoxetine and vardenafil hydrochlorides using molecular orbital calculations. Adv. Pharm. Bull. 2015, 5, 523–529. [Google Scholar] [CrossRef][Green Version]
- Epishina, M.A.; Kulikov, A.S.; Fershtat, L.L. Revisiting the synthesis of functionally substituted 1,4-dihydrobenzo[e][1,2,4]triazines. Molecules 2022, 27, 2575. [Google Scholar] [CrossRef]
- Worzakowska, M.; Sztanke, M.; Sztanke, K. Decomposition mechanism of annelated triazinones bearing the para-nitrophenyl group. J. Anal. Appl. Pyrolysis 2020, 149, 104856. [Google Scholar] [CrossRef]
- Ostasz, A.; Łyszczek, R.; Sztanke, K.; Sztanke, M. TG-DSC and TG-FTIR Studies of Annelated Triazinylacetic Acid Ethyl Esters—Potential Anticancer Agents. Molecules 2023, 28, 1735. [Google Scholar] [CrossRef] [PubMed]
- Worzakowska, M.; Sztanke, M.; Sztanke, K. Pyrolysis and oxidative decomposition mechanism of trifluoromethylated fused triazinones. J. Anal. Appl. Pyrolysis 2021, 157, 105226. [Google Scholar] [CrossRef]
- Yar, M.; Sidra, L.R.; Pontiki, E.; Mushtaq, N.; Ashraf, M.; Nasar, R.; Khan, I.U.; Mahmood, N.; Naqvi, S.A.R.; Khan, Z.A.; et al. Synthesis, in vitro lipoxygenase inhibition, docking study and thermal stability analyses of novel indole derivatives. Non-isothermal kinetic study of potent LOX inhibitor N’-(diphenylmethylene)-2-(1H-indole-3-yl)acetohydrazide. J. Iran. Chem. Soc. 2014, 11, 369–378. [Google Scholar] [CrossRef]
- Emara, E.M.; El-Sayed, W.A.; Khalaf-Allah, A.S.A.; Alminderej, F.M.; Abdel-Monem, Y.K.; Abd-Rabou, A.A. Spectral studies, thermal investigations, and anticancer activity of some divalent metal complexes derived from 2-(4-bromophenylamino)acetohydrazide ligand. Appl. Organomet. Chem. 2022, 36, e6657. [Google Scholar] [CrossRef]
- Cai, Y.-H. Synthesis, morphology and theoretical calculations of 1H-benzotriazole-acetohydrazide. J. Chem. Pharm. Res. 2014, 6, 673–678. [Google Scholar]
- Abdel-Monem, Y.K.; Emam, S.M.; Okda, H.M.Y. Solid state thermal decomposition synthesis of CuO nanoparticles from coordinated pyrazolopyridine as novel precursors. J. Mater. Sci. Mater. Electron. 2017, 28, 2923–2934. [Google Scholar] [CrossRef]
- Wesolowski, M.; Leyk, E. Coupled and Simultaneous Thermal Analysis Techniques in the Study of Pharmaceuticals. Pharmaceutics 2023, 15, 1596. [Google Scholar] [CrossRef]
- Bondarchuk, S.V. On prediction of melting points without computer simulation: A focus on energetic molecular crystals. FirePhysChem. 2022, 2, 160–167. [Google Scholar] [CrossRef]
- Liu, Z. Review and prospect of thermal analysis technology applied to study thermal properties of energetic materials. FirePhysChem. 2021, 1, 129–138. [Google Scholar] [CrossRef]
- Hogge, J.W.; Long, E.A.; Christian, M.L.; Fankhauser, A.D.; Quist, N.L.; Rice, D.M.; Wilding, W.V.; Knotts, T.A. Melting point, enthalpy of fusion, and heat capacity measurements of several polyfunctional, industrially important compounds by differential scanning calorimetry. J. Chem. Eng. Data 2018, 63, 2500–2511. [Google Scholar] [CrossRef]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. On the Determination of the Enthalpy of Fusion ofα-Crystalline Isotactic Polypropylene Using Differential Scanning Calorimetry, X-Ray Diffraction, and Fourier-Transform Infrared Spectroscopy: An Old Story Revisited. Adv. Eng. Mater. 2020, 22, 1900796. [Google Scholar] [CrossRef]
- NIST Chemistry Webbook. NIST Standard Reference Data. 2011. Available online: https://webbook.nist.gov (accessed on 2 November 2023).
- Koltsov, I. Thermal stability of polymeric carbon nitride (PCN)-Al2O3-ZrO2 nanocomposites used in photocatalysis. J. Therm. Anal. Calorim. 2022, 147, 7675–7682. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J. Hazard. Mater. 2010, 173, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, J.; Szala, M. Thermally enhanced FTIR spectroscopy applied to study of explosives stability. Measurement 2021, 184, 110000. [Google Scholar] [CrossRef]
- Zhou, J.G.; Gao, P.; Dong, C.G.; Yang, Y.P. TG-FTIR analysis of nitrogen conversion during straw pyrolysis: A model compound study. J. Fuel Chem. Technol. 2015, 43, 1427–1432. [Google Scholar] [CrossRef]
- Zhai, P.; Shi, C.; Zhao, S.; Liu, W.; Wang, W.; Yao, L. Thermal decomposition of ammonium perchlorate-based molecular perovskite from TG-DSC-FTIR-MS and ab initio molecular dynamics. RSC Adv. 2021, 11, 16388–16395. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Che, L.; Zheng, J.; Huang, G.; Wu, X.; Chen, P.; Zhang, L.; Hu, Q. Synthesis and thermal degradation property study of N-vinylpyrrolidone and acrylamide copolymer. RSC Adv. 2014, 4, 33269–33278. [Google Scholar] [CrossRef]
- Sivabalan, R.; Talawar, M.B.; Senthilkumar, N.; Kavitha, B.; Asthana, S.N. Studies on azotetrazolate based high nitrogen content high energy materials potential additives for rocket propellants. J. Therm. Anal. Calorim. 2004, 78, 781–792. [Google Scholar] [CrossRef]
- Rishwana, S.S.; Mahendran, A.; Vijayakumar, C.T. Studies on structurally different benzoxazines based on diphenols and diamines: Kinetics of thermal degradation and TG-FTIR studies. Thermochim. Acta 2015, 618, 74–87. [Google Scholar] [CrossRef]
- Tudorachi, N.; Mustata, F.; Bicu, I. Thermal decomposition of some Diels-Alder adducts of resin acids. Study of kinetics process. J. Anal. Appl. Pyrolysis 2012, 98, 106–114. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C62533&Mask=200#Mass-Spec (accessed on 3 November 2023).
- Garrigues, J.M.; Perez-Ponce, A.; Garrigues, S.; Guardia, M. Direct determination of ethanol and methanol in liquid samples by means of vapor phase-Fourier transform infrared spectroscopy. Vib. Spectrosc. 1997, 15, 219–228. [Google Scholar] [CrossRef]
- Liu, Z.; Luan, Y.; Sun, X. Thermogravimetry-Infrared spectroscopy analysis of the pyrolysis of willow leaves, stems and branchers. Adv. Mater. Sci. Eng. 2015, 2015, 303212. [Google Scholar] [CrossRef]
- Fasina, O.; Littlefield, B. TG-FTIR of pecan shells thermal decomposition. Fuel Proc. Tech. 2012, 102, 61–66. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Beigi, A.A.M.; Heydari, R.; Khatibi, M. Thermal stability and decomposition kinetic studies of acyclovir and zidovudine drug compounds. AAPS PharmSciTech 2013, 14, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.I.; Gomes, E.C.; Soares, C.D.; Oliveira, M.A. Thermal behavior study and decomposition kinetics of amiodarone hydrochloride under isothermal conditions. Drug Dev. Ind. Pharm. 2011, 37, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Drummond, L.J. High temperature oxidation of ammonia. Combust. Sci. Technol. 1972, 5, 175–182. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modelling study on the high-temperature oxidation of ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Holden, S.R.; Zhang, Z.; Gao, J.; Wu, J.; Zhang, D. An experimental observation of the thermal effects and NO emissions during dissociation and oxidation of ammonia in the presence of a bundle of thermocouples in a vertical flow reactor. Adv. Chem. Eng. Sci. 2023, 13, 250–264. [Google Scholar] [CrossRef]
- Cullis, C.F.; Newitt, E.J. The gaseous oxidation of aliphatic alcohols. I. Ethyl alcohol: The products formed in the early stages. Proc. R. Soc. Lond. A 1956, 237, 530–542. [Google Scholar]
- Taylor, P.; Shanbhag, S.; Dellinger, B. The high-temperature pyrolysis and oxidation of methanol and ethanol: Experimental results and comparison with vehicle emission tests. J. Fuels Lubr. 1994, 103, 982–992. [Google Scholar]
- Dunphy, M.P.; Simmie, J.M. High-temperature oxidation of ethanol. Part 1.—Ignition delays in shock waves. J. Chem. Soc. Faraday Trans. 1991, 87, 1691–1696. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Langford, D.H.; Matchan, M.J.; Walker, R.W.; Yorke, D.A. The high-temperature oxidation of aldehydes. Symp. (Int.) Combust. 1971, 13, 251–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Cai, H.; He, S.; Shan, O.; Zhao, H.; Chen, Y.; Wang, B. Catalyst-free aerobic oxidation of aldehydes into acids in water under mild conditions. Green Chem. 2017, 19, 5708–5713. [Google Scholar] [CrossRef]
- Pelucchi, M.; Namysl, S.; Ranzi, E.; Frassoldati, A.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An experimental and kinetic modelling study of n-C4-C6 aldehydes oxidation in a jet-stirred reactor. Proc. Combust. Inst. 2019, 37, 389–397. [Google Scholar] [CrossRef]
- Jin, H.; Yuan, W.; Li, W.; Yang, J.; Zhou, Z.; Zhao, L.; Li, Y.; Qi, F. Combustion chemistry of aromatic hydrocarbons. Prog. Energy Combust. Sci. 2023, 96, 101076. [Google Scholar] [CrossRef]
- Burgoyne, J.H. The combustion of aromatic and alicyclic hydrocarbons. V. The products of combustion of benzene and its monoalkyl derivatives. Proc. R. Soc. Lond. A 1940, 175, 539–563. [Google Scholar]
- Madarasz, J.; Varga, P.P.; Pokol, G. Evolved gas analyses (TG/DTA-MS and TG-FTIR) on dehydration and pyrolysis of magnesium nitrate hexahydrate in air and nitrogen. J. Anal. Appl. Pyrolysis 2007, 79, 475–478. [Google Scholar] [CrossRef]
- Schuster, J.; Zmrhalova, Z.; Pelikan, V.; Matyas, R. Thermal decomposition mechanism of cuprous thiocyanate in the presence of synthetic air and nitrogen gas. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C460195&Mask=200#Mass-Spec (accessed on 3 November 2023).
- Dagaut, P.; Glarborg, P.; Aluzeta, M.U. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A. Mechanism and modeling of hydrogen cyanide oxidation in a flow reactor. Combust. Flame 1994, 99, 475–483. [Google Scholar] [CrossRef]
- Higashihara, T.; Saito, K.; Murakami, I. Oxidation of hydrogen cyanide in shock waves. Formation of nitrogen monoxide. J. Phys. Chem. 1983, 87, 3707–3712. [Google Scholar] [CrossRef]
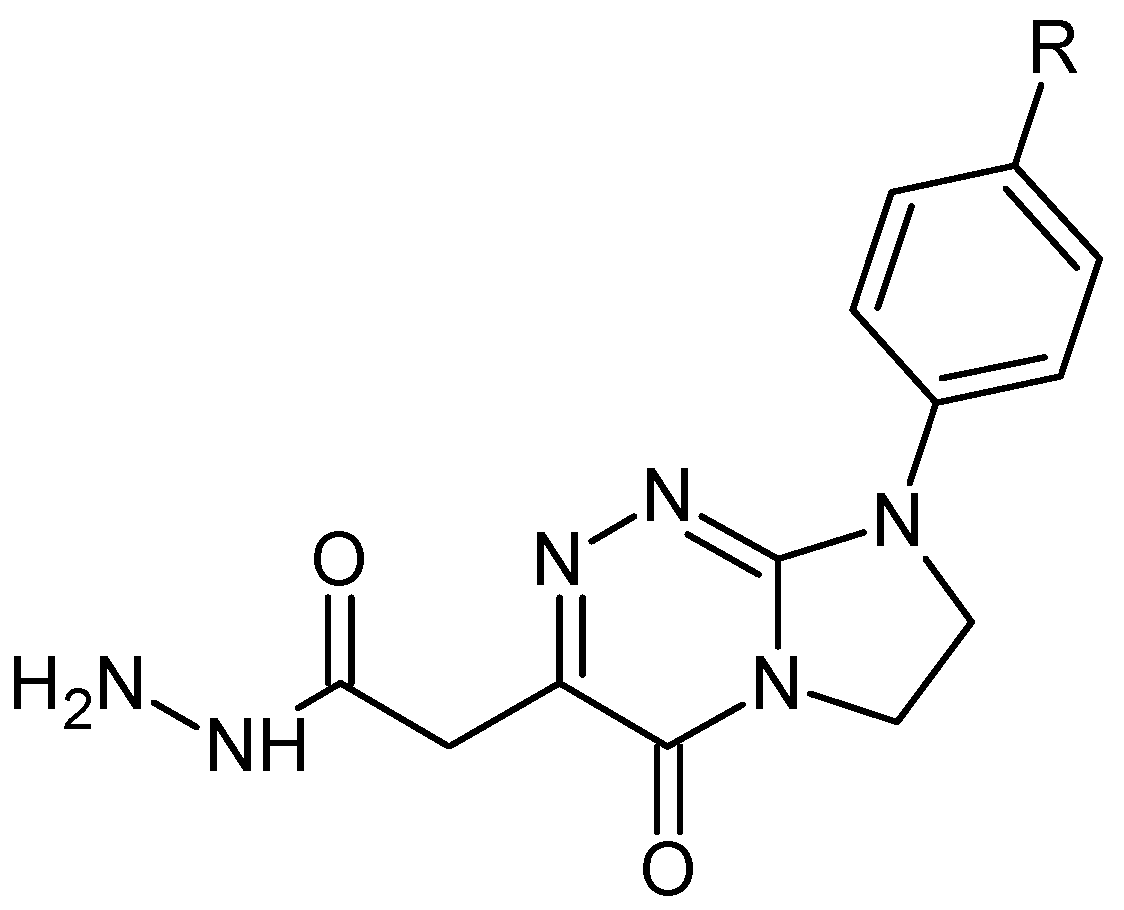
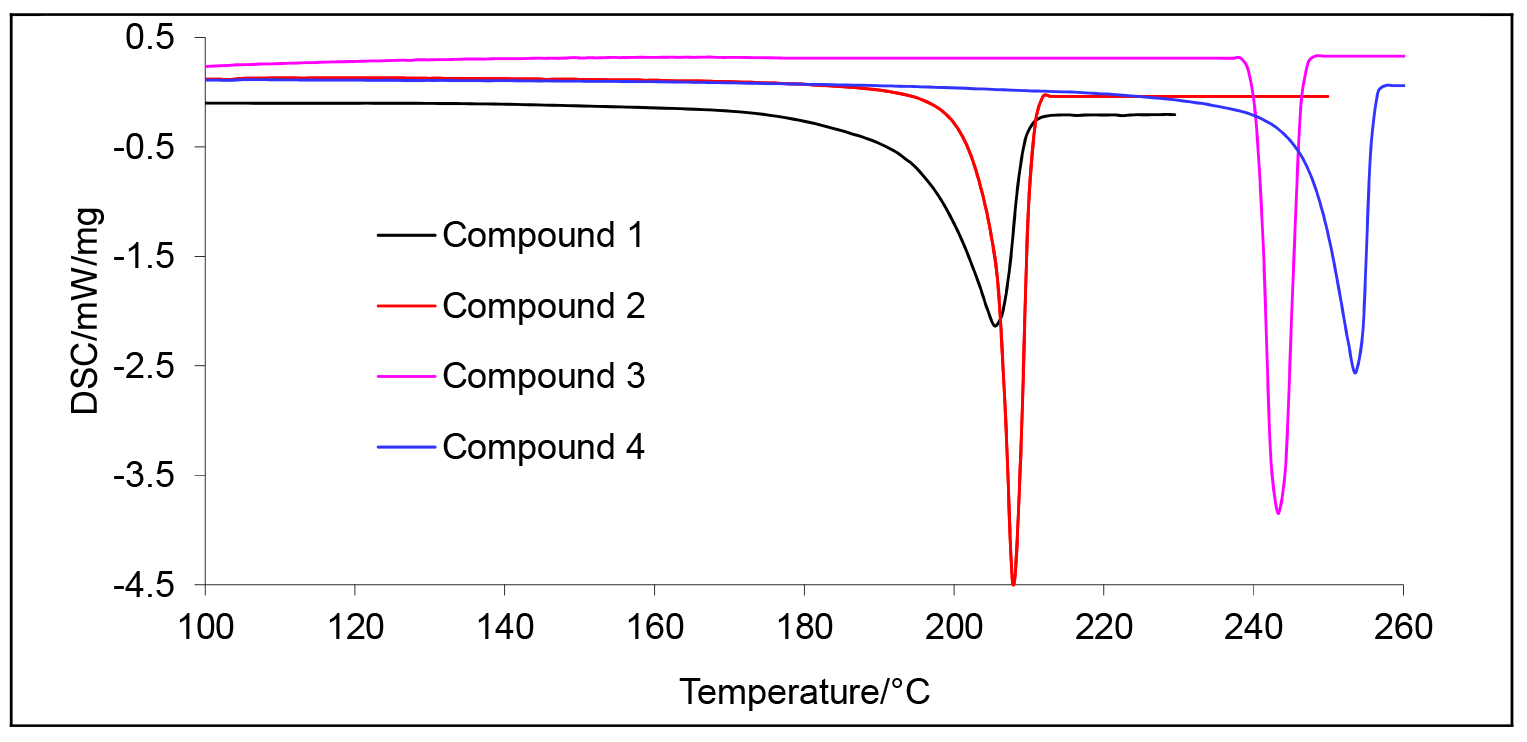
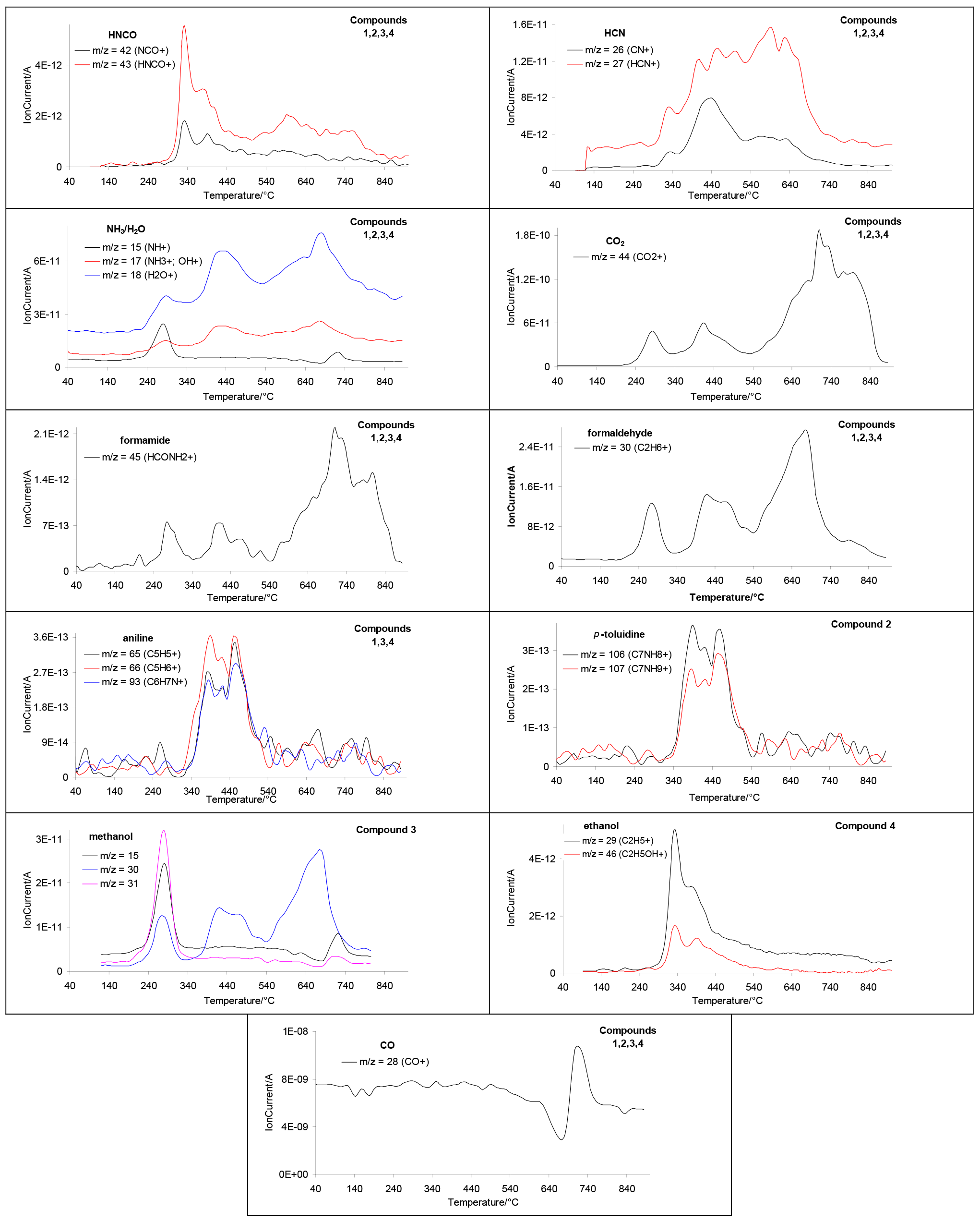
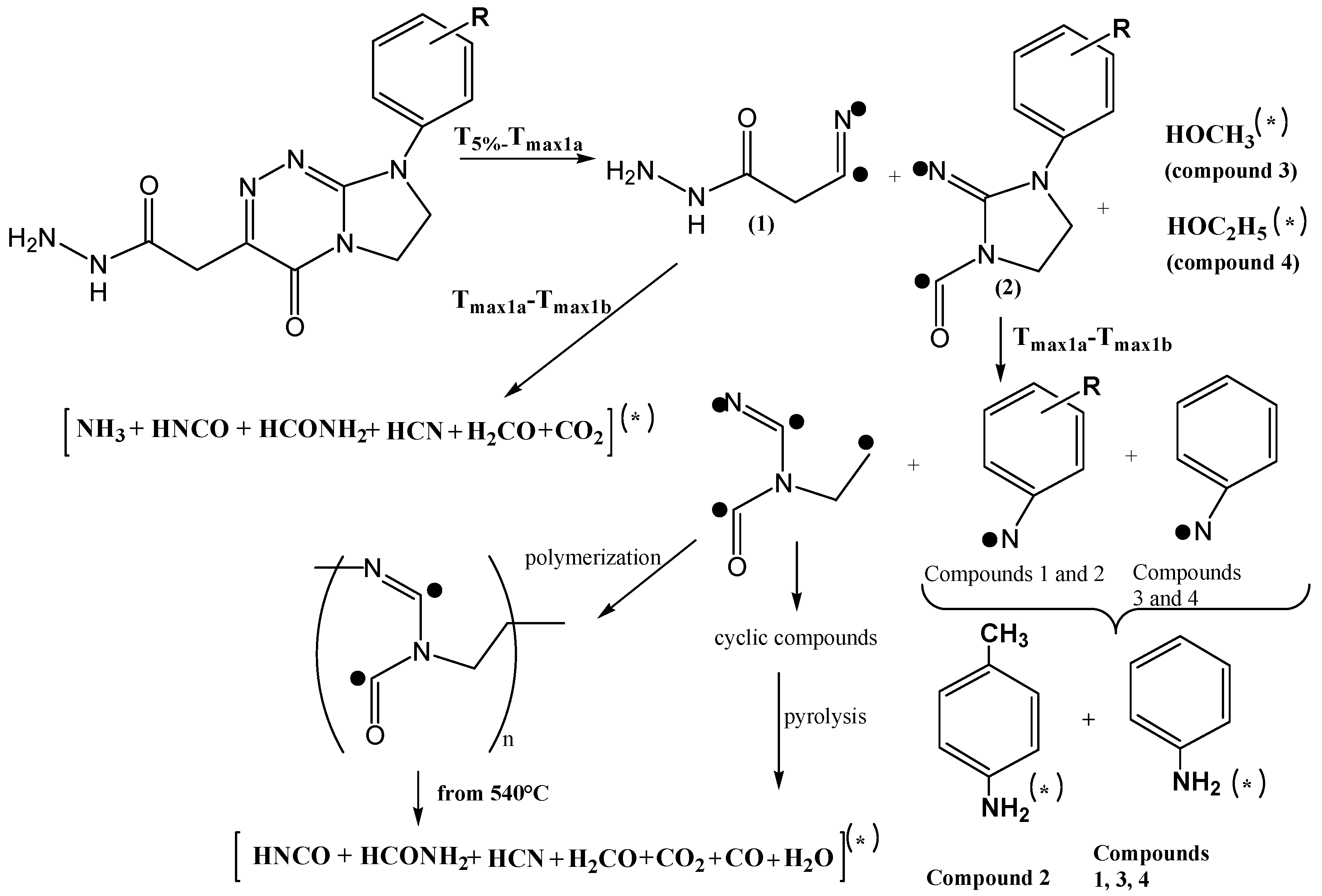

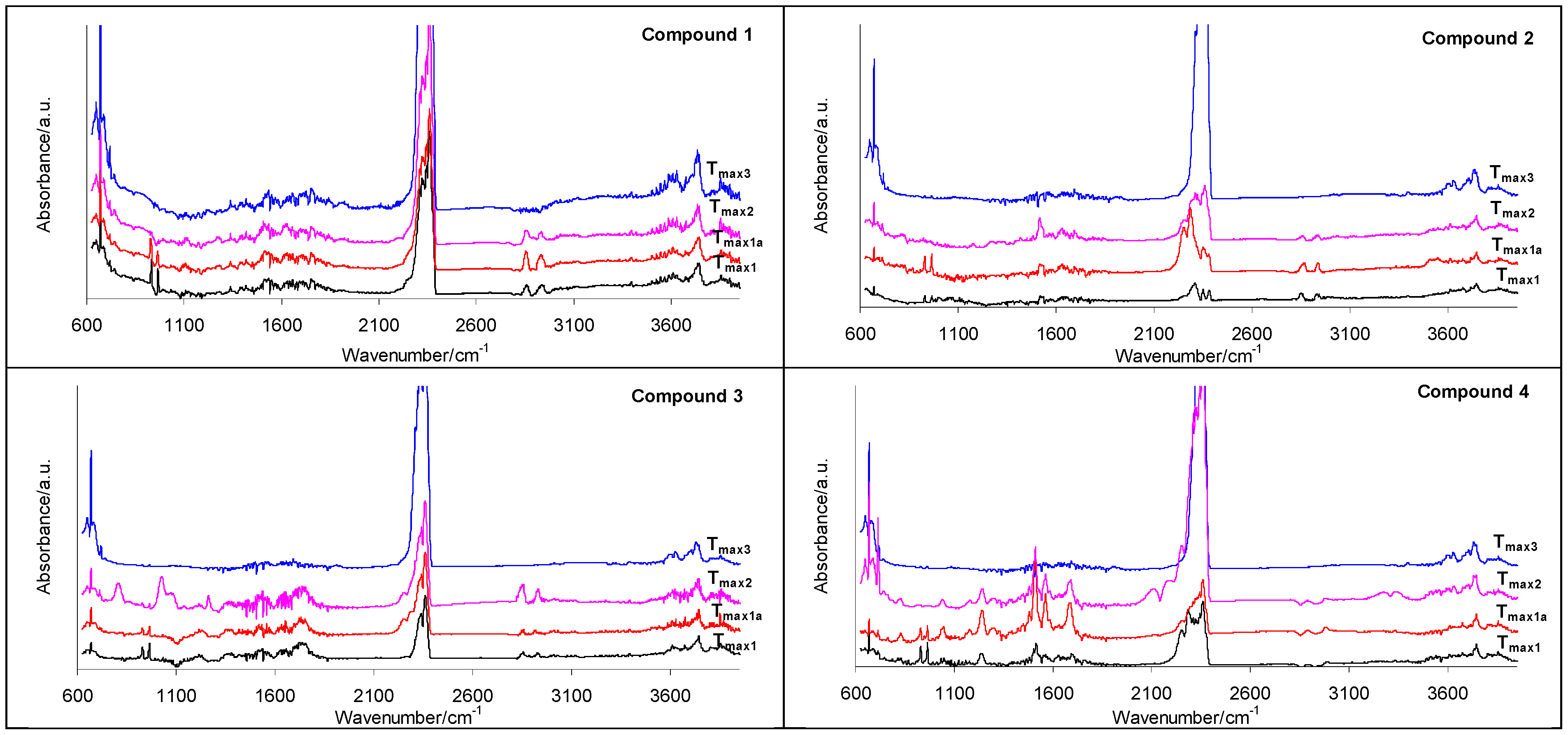
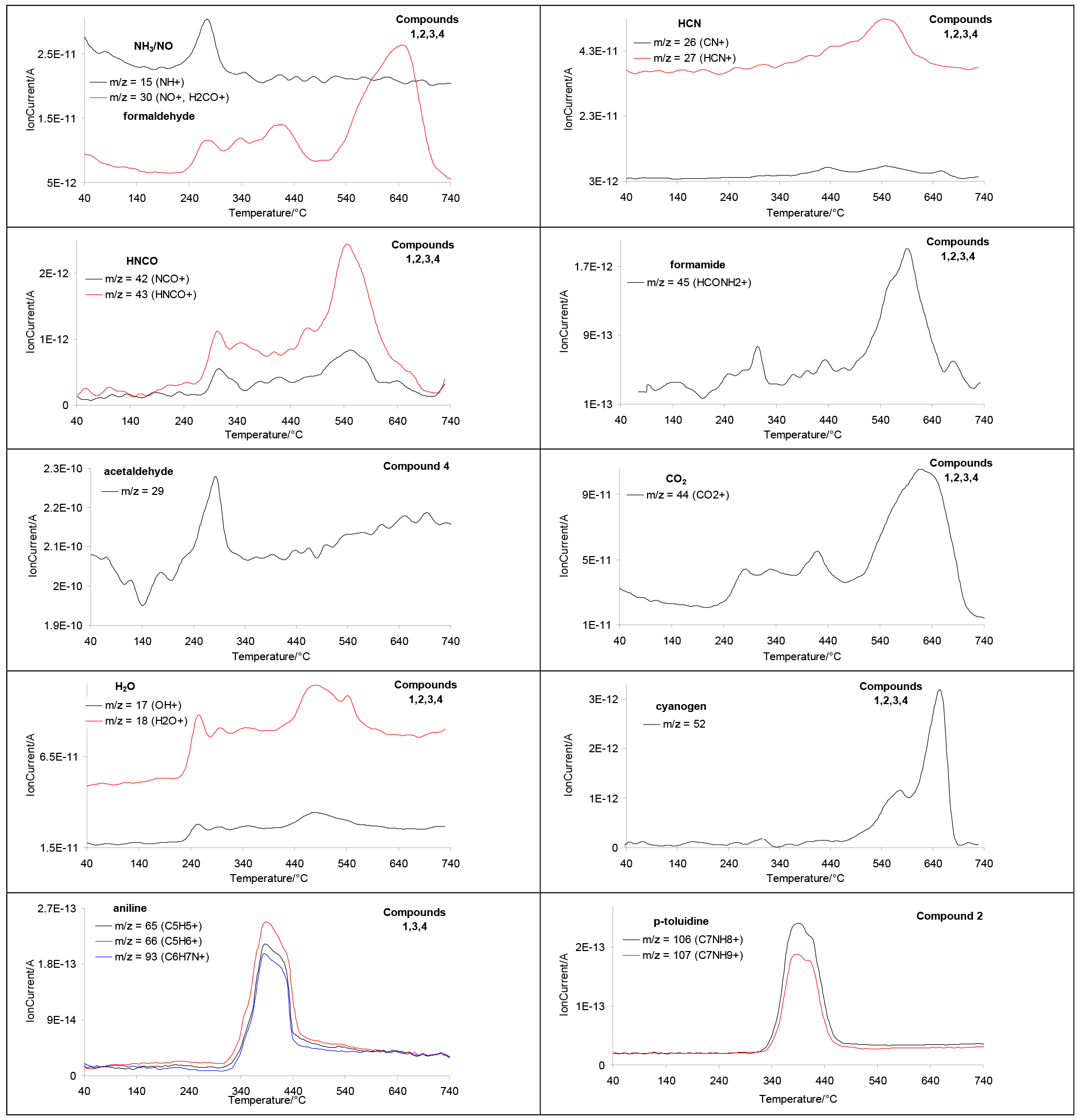
| Compound | M (g/mol) | Tonset (°C) | Tmax (°C) | ΔH (J/g) |
|---|---|---|---|---|
| 1 | 286 | 195 | 205 | 209.4 |
| 2 | 300 | 206 | 208 | 275.3 |
| 3 | 316 | 241 | 243 | 266.5 |
| 4 | 330 | 247 | 254 | 221.9 |
| Compound | Decomposition Process | |||||||
|---|---|---|---|---|---|---|---|---|
| First Decomposition Stage | Second Decomposition Stage | |||||||
| T5% (°C) | Tmax1 (°C) | Tmax1a (°C) | Tmax1b (°C) | Δm1 (%) | Tmax2 (°C) | Δm2 (%) | rm (%) | |
| 1 | 260 | 279 | 314 | 379/444 | 61.4 | 689/709/744/794 | 37.8 | 0.8 |
| 2 | 238 | 221/253 | 316 | 368/415 | 59.5 | 686/710/736/826 | 40.5 | 0.0 |
| 3 | 240 | 245 | 316 | 385/461 | 63.4 | 673/758/835/848 | 35.9 | 0.7 |
| 4 | 272 | 257 | 332 | 392/477 | 65.0 | 672/742/852 | 31.8 | 3.2 |
| Compound | Decomposition Process | |||||||
|---|---|---|---|---|---|---|---|---|
| First Decomposition Stage | Second Decomposition Stage | |||||||
| Δm1a (T5%–Tmax1a) (%) | Δm1b (Tmax1a–Tmax1b) (%) | Δm1a + Δm1b (%) | Δm2 (%) (540–850 °C) | |||||
| Δm1exp | Δm1theor | Δm1exp | Δm1theor | Δm1exp | Δm1theor | Δm1exp | Δm1theor | |
| 1 | 37.0 | 34.6 | 24.4 | 32.1 | 61.4 | 66.7 | 37.8 | 33.3 |
| 2 | 36.5 | 33.0 | 23.0 | 31.6 | 59.5 | 64.6 | 40.5 | 35.4 |
| 3 | 34.5 | 31.3 | 28.9 | 37.6 | 63.4 | 68.9 | 35.9 | 31.1 |
| 4 | 44.0 | 30.0 | 21.0 | 40.3 | 65.0 | 70.3 | 31.8 | 29.7 |
| Compound | Decomposition Process | |||||||
|---|---|---|---|---|---|---|---|---|
| First Decomposition Stage | Second Decomposition Stage | Third Decomposition Stage | ||||||
| T5% (°C) | Tmax1 (°C) | Tmax1a (°C) | Δm1 (%) | Tmax2 (°C) | Δm2 (%) | Tmax3 (°C) | Δm3 (%) | |
| 1 | 250 | 211/276 | 327/364 | 46.7 | 416 | 11.1 | 559/631 | 42.2 |
| 2 | 230 | 218/241 | 304/339 | 35.9 | 401/433 | 13.6 | 586/657 | 50.5 |
| 3 | 230 | 186/244 | 301/361 | 27.4 | 426/466 | 21.0 | 506/606/691 | 51.6 |
| 4 | 254 | 249 | 309/336/389 | 40.4 | 459 | 9.7 | 549/662 | 49.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worzakowska, M.; Sztanke, K.; Sztanke, M. Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides. Int. J. Mol. Sci. 2024, 25, 813. https://doi.org/10.3390/ijms25020813
Worzakowska M, Sztanke K, Sztanke M. Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides. International Journal of Molecular Sciences. 2024; 25(2):813. https://doi.org/10.3390/ijms25020813
Chicago/Turabian StyleWorzakowska, Marta, Krzysztof Sztanke, and Małgorzata Sztanke. 2024. "Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides" International Journal of Molecular Sciences 25, no. 2: 813. https://doi.org/10.3390/ijms25020813
APA StyleWorzakowska, M., Sztanke, K., & Sztanke, M. (2024). Application of Simultaneous and Coupled Thermal Analysis Techniques in Studies on the Melting Process, Course of Pyrolysis and Oxidative Decomposition of Fused Triazinylacetohydrazides. International Journal of Molecular Sciences, 25(2), 813. https://doi.org/10.3390/ijms25020813







