Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
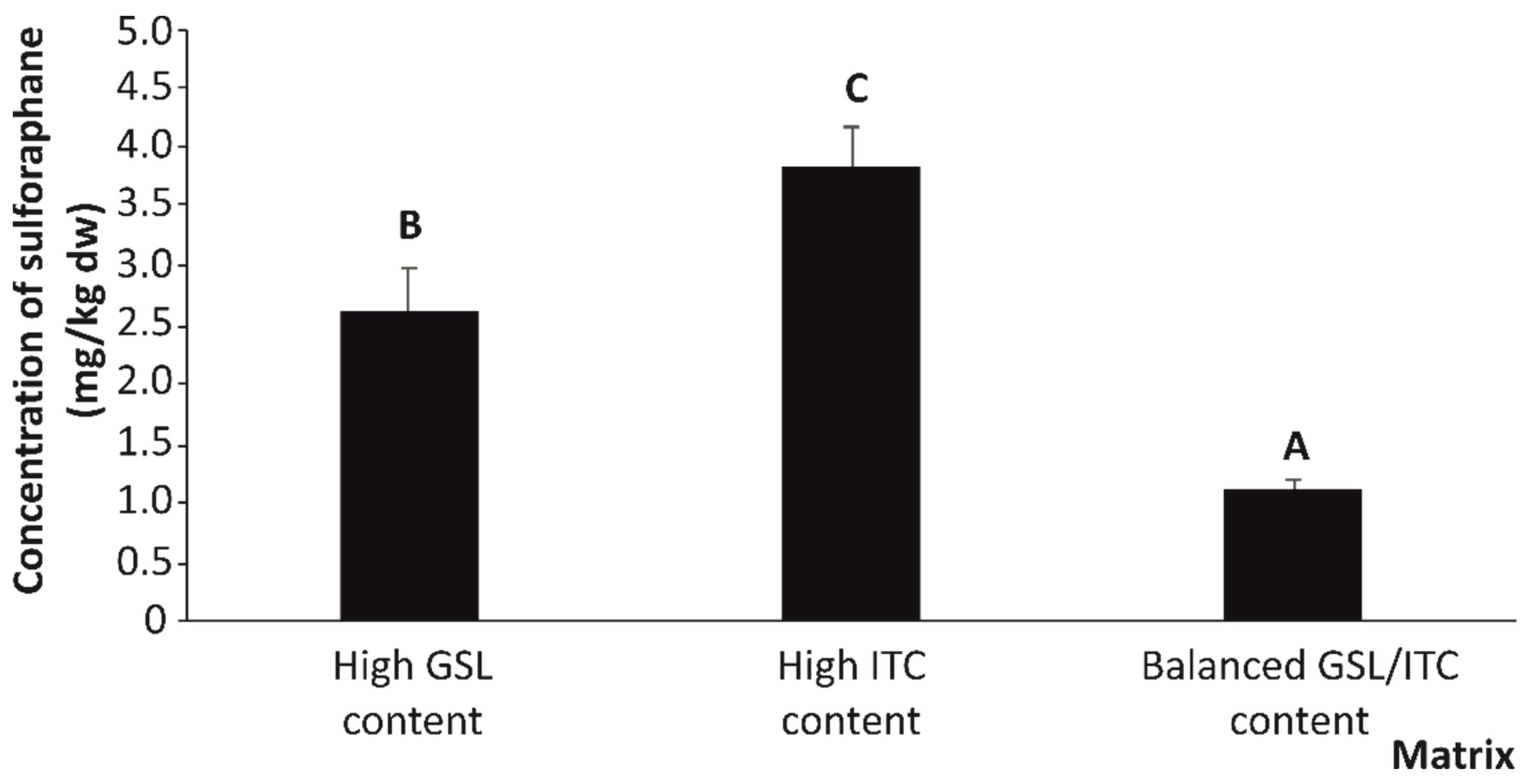
2.1. Quantitative Organosulfur Compound Content of Stabilized Broccoli Stalks
2.2. Effect of Gastrointestinal Digestion on the Bioaccessibility of Organosulfur Compounds
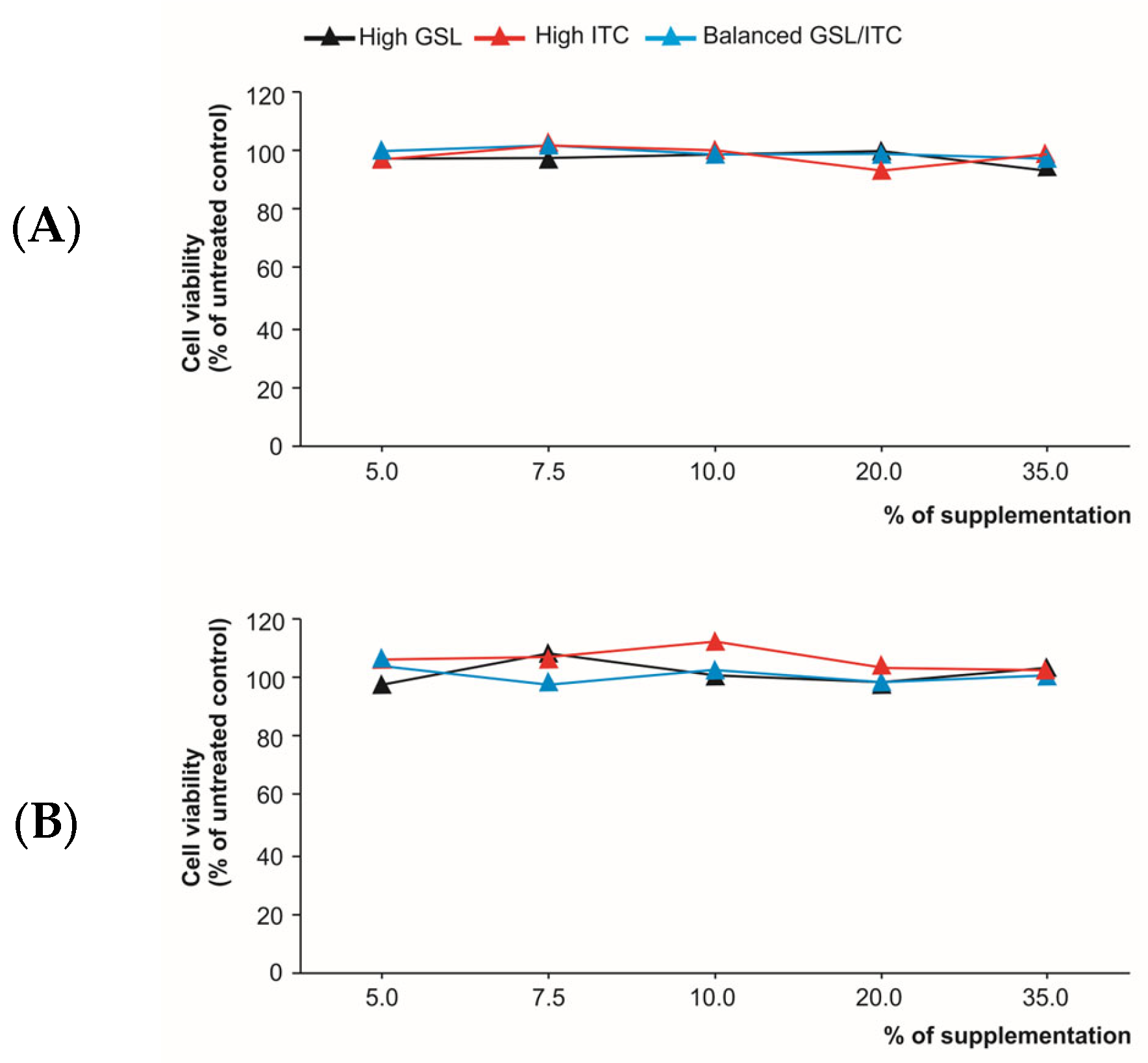
2.3. Cytotoxicity of the Analytical Extracts and Digested Products in Intestinal Epithelium Cells
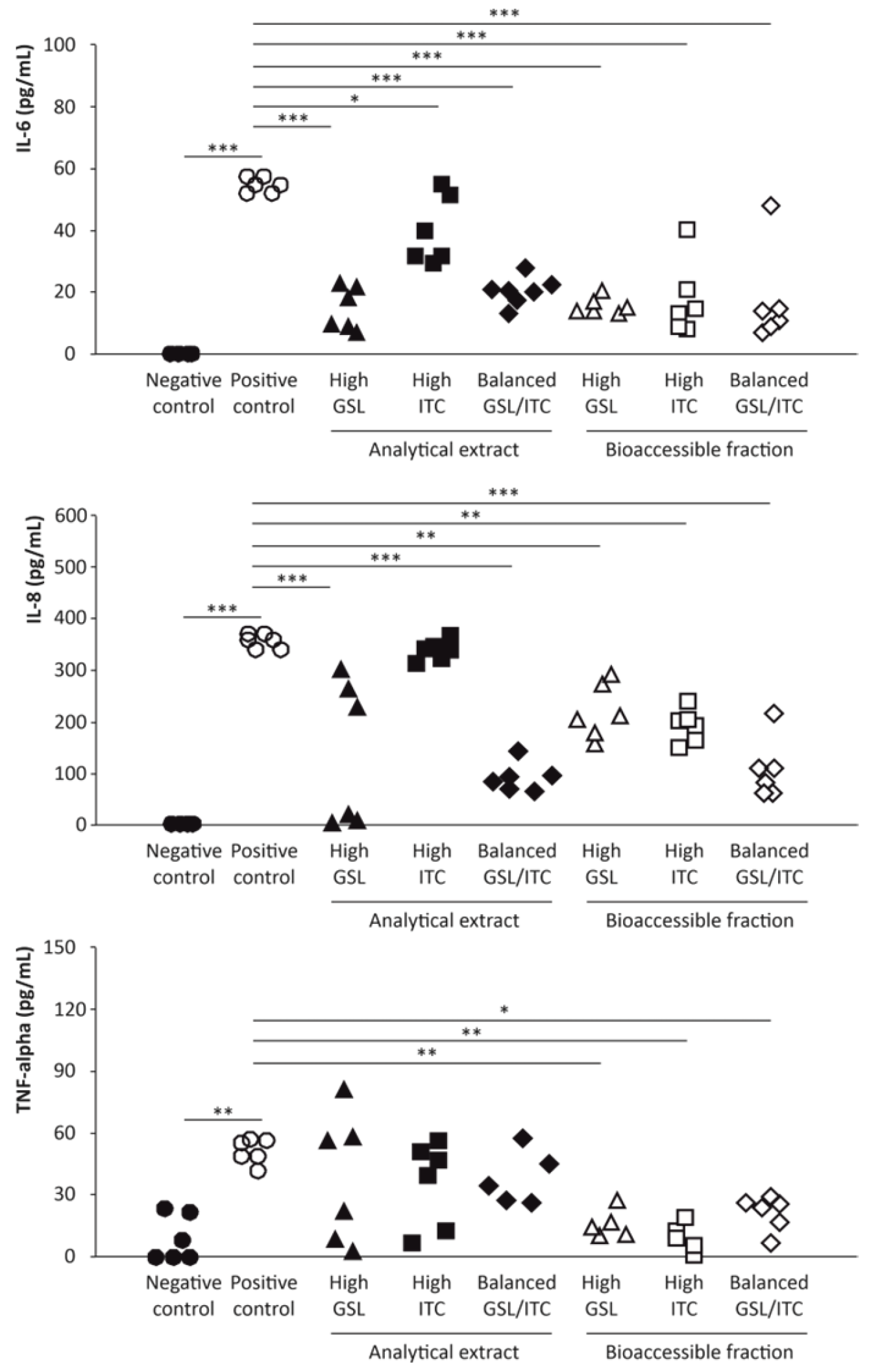
2.4. Anti-Inflammatory Activity of Broccoli Stalks’ Analytical Extracts and Bioaccessible Fractions
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Collection and Processing
4.3. Extraction of Glucosinolates and Breakdown Products from Raw Materials
4.4. Simulated In Vitro Gastrointestinal Digestion
4.5. HPLC-PAD-ESI-MSn Analysis of Glucosinolates
4.6. UHPLC-ESI-QqQ-MS/MS Analysis of Isothiocyanate and Indole Derivatives
4.7. Cell Line, Culture Conditions, and Development of a Monolayer Intestinal Barrier
4.8. Trypan Blue-Based Viability Assay
4.9. Assessment of Inflammatory Modulators in the Monolayer Intestinal Barrier Model
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Simulated Fluids | Constituent Z (mmol/L) | |||||
|---|---|---|---|---|---|---|
| KCl | KH2PO4 | NaHCO3 | NaCl | MgCl2(H2O)6 | (NH4)2CO3 | |
| Simulated gastric fluid (SGF; pH 3) | 6.90 | 0.90 | 25.00 | 47.20 | 0.10 | 0.50 |
| Pepsin enzyme (EC 3.4.23.1) | 2000 U/mL | |||||
| Simulated Intestinal fluid (SIF; pH 8) | 6.80 | 0.80 | 85.00 | 38.40 | 0.13 | --- |
| Pancreatin (EC 232-468-9) | 100 U/mL of trypsin activity 64 U/mL of lipase activity | |||||
| Pancreatic lipase (EC 3.1.1.3) | 2000 U/mL of pancreatic lipase | |||||
| Frozen porcine bile salts | 10 mM | |||||
| Glucosinolate (Abbreviation) | Glucosinolate Type | Retention Time (min) | Molecular Ion (m/z [M-H]−) | Product Ions * (m/z MS2[M-H]−) | Ionization Mode |
|---|---|---|---|---|---|
| Glucoiberin (GI) | Aliphatic | 5.3 | 422 | 259, 97 | Negative |
| Glucoraphanin (GR) | Aliphatic | 6.0 | 436 | 372, 259, 97 | Negative |
| Hydroxy-glucobrassicin (HGB) | Indolic | 16.2 | 463 | 285, 241, 97 | Negative |
| Glucoerucin (GE) | Aliphatic | 18.9 | 420 | 259, 97 | Negative |
| Glucobrassicin (GB) | Indolic | 20.5 | 447 | 404, 259, 97 | Negative |
| Gluconasturtiin (PE) | Aromatic | 23.0 | 422 | 259, 97 | Negative |
| Methoxy-glucobrassicin (MGB) | Indolic | 25.6 | 477 | 259, 97 | Negative |
| Neo-Glucobrassicin (NGB) | Indolic | 27.8 | 477 | 446, 259, 97 | Negative |
| Isothiocyanate (Abbreviation) | Retention Time (min) | MRM Quantitative Transition (m/z [M-H]−) | MRM Quantitative Transition (m/z MS2[M-H]−) | Ionization |
|---|---|---|---|---|
| Erucin (E) | 0.82 | 141.0 > 59.0 | 161.0 > 70.0 | Positive |
| Iberin (IB) | 1.32 | 164.0 > 105.0 | N.d. | Positive |
| Indole-3-Carbinol (I3C) | 1.50 | 130.0 > 77.0 | 247.1 > 130.1 | Positive |
| Sulforaphane (SFN) | 1.56 | 178.0 > 114.0 | 178 > 71.0 | Positive |
| 3,4-diindolylmethane (DIM) | 2.92 | 130.0 > 77.0 | 247.1 > 130.1 | Positive |
References
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Oliván, C.M.C.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed “Gut/Feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef]
- Ye, Y.; Pang, Z.; Chen, W.; Ju, S.; Zhou, C. The Epidemiology and Risk Factors of Inflammatory Bowel Disease. Int. J. Clin. Exp. Med. 2015, 8, 22529–22542. [Google Scholar] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-Wide Association Defines More than 30 Distinct Susceptibility Loci for Crohn’s Disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef]
- Rogler, G.; Vavricka, S. Exposome in IBD: Recent Insights in Environmental Factors That Influence the Onset and Course of IBD. Inflamm. Bowel Dis. 2015, 21, 400–408. [Google Scholar] [CrossRef]
- Lee, J.Y.; Zhao, L.; Youn, H.S.; Weatherill, A.R.; Tapping, R.; Feng, L.; Lee, W.H.; Fitzgerald, K.A.; Hwang, D.H. Saturated Fatty Acid Activates but Polyunsaturated Fatty Acid Inhibits Toll-like Receptor 2 Dimerized with Toll-like Receptor 6 or 1. J. Biol. Chem. 2004, 279, 16971–16979. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of Dietary Fiber on Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Wei, L.Y.; Zhang, J.K.; Zheng, L.; Chen, Y. The Functional Role of Sulforaphane in Intestinal Inflammation: A Review. Food Funct. 2022, 13, 514–529. [Google Scholar] [CrossRef]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-Ols, Anthocyanins, and Inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Moreno, D.A. Novel Varieties of Broccoli for Optimal Bioactive Components under Saline Stress. J. Sci. Food Agric. 2011, 91, 1638–1647. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A.; et al. Systematic Review on the Metabolic Interest of Glucosinolates and Their Bioactive Derivatives for Human Health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Polat, U. The Effects on Metabolism of Glucosinolates and Their Hydrolysis Products. J. Biol. Env. Sci. 2010, 4, 39–42. [Google Scholar]
- Almushayti, A.Y.; Brandt, K.; Carroll, M.A.; Scotter, M.J. Current Analytical Methods for Determination of Glucosinolates in Vegetables and Human Tissues. J. Chromatogr. A 2021, 1643, 462060. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. Engl. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, Á. The Intake of Broccoli Sprouts Modulates the Inflammatory and Vascular Prostanoids but Not the Oxidative Stress-Related Isoprostanes in Healthy Humans. Food Chem. 2015, 173, 1187–1194. [Google Scholar] [CrossRef]
- Queiroz, M.; Oppolzer, D.; Gouvinhas, I.; Silva, A.M.; Barros, A.I.R.N.A.; Domínguez-Perles, R. New Grape Stems’ Isolated Phenolic Compounds Modulate Reactive Oxygen Species, Glutathione, and Lipid Peroxidation in Vitro: Combined Formulations with Vitamins C and E. Fitoterapia 2017, 120, 146–157. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived by-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Garcia-Viguera, C.; Moreno, D.A. Bioactives from Broccoli By-Products. Food Eng. Ingred. 2010, 35, C383–C392. [Google Scholar]
- Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Waking Up from Four Decades’ Long Dream of Valorizing Agro-Food Byproducts: Toward Practical Applications of the Gained Knowledge. J. Agric. Food Chem. 2018, 66, 3069–3073. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Moreno, D.A.; Periago, P.M.; García-Viguera, C.; Domínguez-Perles, R. A New Food Ingredient Rich in Bioaccessible (Poly)Phenols (and Glucosinolates) Obtained from Stabilized Broccoli Stalks. Foods 2022, 11, 1734. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. In Vitro Evidence on Bioaccessibility of Flavonols and Cinnamoyl Derivatives of Cruciferous Sprouts. Nutrients 2021, 13, 4140. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
- Abercrombie, J.M.; Farnham, M.W.; Rushing, J.W. Genetic Combining Ability of Glucoraphanin Level and Other Horticultural Traits of Broccoli. Euphytica 2005, 143, 145–151. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Huang, F.C. The Interleukins Orchestrate Mucosal Immune Responses to Salmonella Infection in the Intestine. Cells 2021, 10, 3492. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase Inhibiting, Antioxidant, and Anti-Inflammatory Activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Coskun, M. Intestinal Epithelium in Inflammatory Bowel Disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr 2016, 3, 24. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of Industrial Broccoli Discards (Brassica Oleracea Var. Italica) for Their Glucosinolate, Polyphenol and Flavonoid Contents Using UPLC MS/MS and Spectrophotometric Methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Angelino, D.; Jeffery, E. Glucosinolate Hydrolysis and Bioavailability of Resulting Isothiocyanates: Focus on Glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and Inter-Conversion of Sulforaphane and Erucin in Human Subjects Consuming Broccoli Sprouts or Broccoli Supplement in a Cross-over Study Design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-ΚB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Royce, S.G.; Licciardi, P.V.; Beh, R.C.; Bourke, J.E.; Donovan, C.; Hung, A.; Khurana, I.; Liang, J.J.; Maxwell, S.; Mazarakis, N.; et al. Sulforaphane Prevents and Reverses Allergic Airways Disease in Mice via Anti-Inflammatory, Antioxidant, and Epigenetic Mechanisms. Cel. Mol. Life Sci. 2022, 79, 1–22. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Shimoyama, M.; Fujii, A.; Sato, J.; Kadena, K.; Ozaki, K.; Hosaka, K. The Anti-Inflammatory Effects of Iberin on TNF-α-Stimulated Human Oral Epithelial Cells: In Vitro Research. Biomedicines 2022, 10, 3155. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Moreno, D.A.; Garcia-Viguera, C.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M. Selecting Cultivars of Broccoli for Optimal Bioactive Components: The Influence of Saline Stress. Acta Hortic. 2012, 939, 159–164. [Google Scholar] [CrossRef]
- Vo, Q.V.; Trenerry, C.; Rochfort, S.; Wadeson, J.; Leyton, C.; Hughes, A.B. Synthesis and Anti-Inflammatory Activity of Indole Glucosinolates. Bioorg Med. Chem. 2014, 22, 856–864. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a Natural Chemical Arsenal: More to Tell than the Myrosinase Story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Cámara-Martos, F.; Obregón-Cano, S.; Mesa-Plata, O.; Cartea-González, M.E.; de Haro-Bailón, A. Quantification and in Vitro Bioaccessibility of Glucosinolates and Trace Elements in Brassicaceae Leafy Vegetables. Food Chem. 2021, 339, 127860. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Hegarty, L.M.; Jones, G.R.; Bain, C.C. Macrophages in Intestinal Homeostasis and Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 538–553. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sarvan, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane Formation and Bioaccessibility Are More Affected by Steaming Time than Meal Composition during in Vitro Digestion of Broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef]
- Yen, G.C.; Wei, Q.K. Myrosinase Activity and Total Glucosinolate Content of Cruciferous Vegetables, and Some Properties of Cabbage Myrosinase in Taiwan. J. Sci. Food Agric. 1993, 61, 471–475. [Google Scholar] [CrossRef]
- Krishnan, J. Effects of Saturation and Enzyme Limitation in Feedforward Adaptive Signal Transduction. IET Syst. Biol. 2011, 5, 208–219. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251. [Google Scholar] [CrossRef]
- Pal, S.; Badireenath Konkimalla, V. Hormetic Potential of Sulforaphane (SFN) in Switching Cells’ Fate Towards Survival or Death. Mini Rev. Med. Chem. 2016, 16, 980–995. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane Protects Cells against Lipopolysaccharide-Stimulated Inflammation in Murine Macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Martínez-Sánchez, M.A.; García-Peñarrubia, P.; Martinez-Esparza, M.; Ramos-Molina, B.; Moreno, D.A. Analysis of the Anti-Inflammatory Potential of Brassica Bioactive Compounds in a Human Macrophage-like Cell Model Derived from HL-60 Cells. Biomed. Pharmacother. 2022, 149, 112804. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Baenas, N.; Suárez-Martínez, C.; García-Viguera, C.; Moreno, D.A. Bioavailability and New Biomarkers of Cruciferous Sprouts Consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting Sprouts of Brassicaceae for Optimum Phytochemical Composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Medina, S.; Moreno, D.Á.; García-Viguera, C.; Ferreres, F.; Gil-Izquierdo, Á. A New Ultra-Rapid UHPLC/MS/MS Method for Assessing Glucoraphanin and Sulforaphane Bioavailability in Human Urine. Food Chem. 2014, 143, 132–138. [Google Scholar] [CrossRef]
- Acosta-Otálvaro, E.; Domínguez-Perles, R.; Mazo-Rivas, J.C.; García-Viguera, C. Bioavailability and Radical Scavenging Power of Phenolic Compounds of Cocoa and Coffee Mixtures. Food Sci. Technol. Int. 2022, 28, 514–523. [Google Scholar] [CrossRef]

| Analyte | High GSL Content | High ITC Content | GSL/ITC Balanced Content | LSD (p < 0.05) | p-Value X |
|---|---|---|---|---|---|
| Glucosinolates | |||||
| Glucoiberin (GI) | 1347.44 b | 484.83 a | 241.46 a | 452.05 | *** |
| Glucoraphanin (GR) | 7065.18 b | 2423.20 a | 2021.38 a | 3578.07 | * |
| Glucoerucin (GE) | 1108.12 b | 235.75 a | 134.40 a | 595.70 | ** |
| Hydroxyglucobrassicin (HGB) | 59.86 a | 107.90 b | 66.95 a | 8.08 | *** |
| Glucobrassicin (GB) | 271.35 a | 111.54 a | 193.26 a | 172.28 | N.s. |
| Methoxyglucobrassicin (MGB) | 386.05 ab | 432.97 b | 279.09 a | 94.70 | * |
| Neoglucobrassicin (NGB) | 385.30 b | 98.84 a | 497.12 b | 233.76 | ** |
| Gluconasturtiin (PE) | 1673.18 b | 513.68 a | 559.63 a | 1020.15 | ** |
| Isothiocyanates and indoles | |||||
| Sulforaphane (SFN) | 2.72 a | 4.77 a | 12.31 b | 0.31 | *** |
| Indole-3-carbinol (I3C) | 0.16 a | 0.72 b | 0.92 b | 1.67 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Pérez, A.; Sánchez-Bravo, P.; Medina, S.; Domínguez-Perles, R.; García-Viguera, C. Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 800. https://doi.org/10.3390/ijms25020800
Costa-Pérez A, Sánchez-Bravo P, Medina S, Domínguez-Perles R, García-Viguera C. Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2024; 25(2):800. https://doi.org/10.3390/ijms25020800
Chicago/Turabian StyleCosta-Pérez, Antonio, Paola Sánchez-Bravo, Sonia Medina, Raúl Domínguez-Perles, and Cristina García-Viguera. 2024. "Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease" International Journal of Molecular Sciences 25, no. 2: 800. https://doi.org/10.3390/ijms25020800
APA StyleCosta-Pérez, A., Sánchez-Bravo, P., Medina, S., Domínguez-Perles, R., & García-Viguera, C. (2024). Bioaccessible Organosulfur Compounds in Broccoli Stalks Modulate the Inflammatory Mediators Involved in Inflammatory Bowel Disease. International Journal of Molecular Sciences, 25(2), 800. https://doi.org/10.3390/ijms25020800








