Abstract
The implantation of good-quality embryos to the receptive endometrium is essential for successful live birth through in vitro fertilization (IVF). The higher the quality of embryos, the higher the live birth rate per cycle, and so efforts have been made to obtain as many high-quality embryos as possible after fertilization. In addition to an effective controlled ovarian stimulation process to obtain high-quality embryos, the composition of the embryo culture medium in direct contact with embryos in vitro is also important. During embryonic development, under the control of female sex hormones, the fallopian tubes and endometrium create a microenvironment that supplies the nutrients and substances necessary for embryos at each stage. During this process, the development of the embryo is finely regulated by signaling molecules, such as growth factors and cytokines secreted from the epithelial cells of the fallopian tube and uterine endometrium. The development of embryo culture media has continued since the first successful human birth through IVF in 1978. However, there are still limitations to mimicking a microenvironment similar to the reproductive organs of women suitable for embryo development in vitro. Efforts have been made to overcome the harsh in vitro culture environment and obtain high-quality embryos by adding various supplements, such as antioxidants and growth factors, to the embryo culture medium. Recently, there has been an increase in the number of studies on the effect of supplementation in different clinical situations such as old age, recurrent implantation failure (RIF), and unexplained infertility; in addition, anticipation of the potential benefits from individuation is rising. This article reviews the effects of representative supplements in culture media on embryo development.
1. Introduction
Since the birth of the first baby conceived through in vitro fertilization (IVF) in the late 1970s, IVF has continuously developed as an important field of assisted reproductive technology (ART), providing numerous infertile couples with the opportunity to bear children. Among its pivotal components of IVF, the quality of embryos plays a crucial role in determining the success of IVF procedures. The transfer of good-quality embryos has been shown to result in higher rates of clinical pregnancy and live birth [1]. The transfer of poor-quality embryos was associated with an increase in the miscarriage rate, which ultimately led to a decrease in the live birth rate [2,3].
Embryo culture media constitute a vital aspect of the IVF process, providing a controlled environment for embryo development outside the maternal uterus [4]. In vitro cultured embryos often show delayed or abnormal development during the cell division phase, suggesting a lack of essential factors that are naturally present within the female reproductive tract. Researchers have investigated supplementing culture media with various bioactive molecules due to the increased prevalence of infertility stemming from factors like advanced age, recurrent failure (RIF), and unexplained infertility [5,6,7,8,9].
This review aims to summarize the effects of various supplements in culture media on embryo development by comprehensively reviewing the existing literature. The study population, experimental methodologies, and outcomes from in vitro and in vivo studies were examined, focusing on the impact of supplements on various aspects of embryo development, including embryo quality, blastocyst formation, implantation potential, and pregnancy outcomes. Through a thorough evaluation of existing evidence, this review endeavors to consolidate our understanding of the potential advantages of incorporating these supplements into embryo culture media. Ultimately, a comprehensive assessment of their effects will aid in optimizing the culture conditions for improving the outcomes of IVF procedures, offering new avenues to enhance the success rates and overall reproductive health of individuals undergoing ART.
2. Effect of Supplements in IVF Culture Media on Embryonic Development
Since the first human IVF baby was born on 25 July 1978, there have been great advancements in clinical human embryo culture conditions [10,11]. Culture conditions that affect embryos cultured in vitro are crucial factors related to pregnancy outcomes [12,13,14,15,16]. Among air quality, culture medium, incubator type, temperature, and pH, the composition of the culture medium holds paramount importance, as it is in direct contact with the embryo and engages with it via paracrine and autocrine mechanisms [17].
Research on the impact of embryo culture media on embryos in ART cycles has investigated parameters such as embryo quality, pregnancy outcomes, and neonatal outcomes. In a comparative study of two culture media, GⅢ (Vitrolife, Gothenburg, Sweden) media exhibited higher quality and more viable embryos, and correlated with heightened rates of implantation and pregnancy success in frozen–thawed cycles compared to G1.2-G2.2 (Vitrolife, Gothenburg, Sweden) media [18]. Similarly, significant differences were observed between the G5 culture medium and HTF culture medium in implantation rates, clinical pregnancy rate, and birth weight [19]. A UK study reported the impact of eight different culture media types—Cook sequential, Irvine Single-step, LifeGlobal Single-step, Sage sequential, and Vitrolife sequential, among others—on the live birth rate [20]. Conversely, there are studies indicating no difference in miscarriage rate, live birth rate, and birth weights among different culture media [21,22]. Efforts have been made to enhance culture media for in vitro embryos, recognizing the pivotal role of medium composition compared to the in vivo environment’s trophic support. Therefore, four different culture media most commonly used for embryo development, namely, G-TL (Vitrolife, Gothenburg, Sweden), 1-Step (Origio, Måløv, Denmark), Global-Total (LifeGlobal, Guilford, CT, USA), and CSC (Irvine Scientific, Santa Ana, CA, USA), were investigated with a focus on their composition in terms of components critical for embryo growth (Table 1) [23,24,25,26,27,28,29,30]. The primary energy substrates for preimplantation embryos include pyruvate, lactate, and glucose, which were present in all four media, albeit at varying concentrations. Amino acid compositions and ionic electrolyte levels also varied significantly among the four media [31,32]. The amino acid and electrolyte levels should be maintained in the embryo culture media for synthesis of protein, cell signaling, metabolism, cell balance, pH and membrane stability [33,34].

Table 1.
Components of single-step media for human embryo culture and their concentrations.
Signal pathways regulate transcription factors that the embryo encounters, potentially altering its development competence [35,36,37,38,39,40]. Supplements in embryo culture media include hormones, growth hormones, growth factors, and cytokines. In this review, we selected the most frequently studied supplementations for each class of compound for investigation. Hence, we have chosen four molecules—melatonin, GM-CSF, IGF-Ⅰ, and LIF—and conducted a review regarding their molecular signal pathways (Figure 1) and potential impact on pre-implantation embryo development and, if available, pregnancy outcomes.
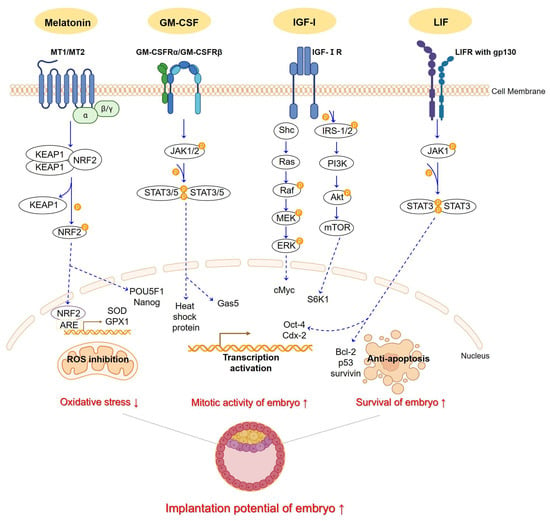
Figure 1.
An illustrative schematic depicts how melatonin, GM-CSF, IGF-1, and LIF pathways function in embryo culture media, showing their expected effects. Melatonin binding to MT1,2 receptors trigger the KEAP1/NRF2 complex formation. Protein kinases aid NRF2 dissociation, enabling its translocation. NRF2, post-translocation, binds ARE, initiating transcription of SOD and GPX1 and activating POU5F1 and Nanog. GM-CSF binds to its receptors, GM-CSFRα/β. Upon binding, the receptors undergo autophosphorylation, activating the JAK1,2/STAT3,5 pathway. Once STATs are activated, they translocate and form dimer to suppress target genes, including heat shock protein and Gas5. IGF-Ⅰ interacts with its receptors, IGF-ⅠR. Upon binding, the receptor autophosphorylates and recruits IRS-1/2 and Shc. This activates the PI3K/Akt/mTOR pathway and S6K1 gene. Simultaneously, Shc recruitments activate the Ras/Raf/MEK/ERK pathway and cMyc gene. LIF binds to its receptor, LIFR, along with gp130. Upon binding, it promotes receptor heterodimerization, triggering the activation of the JAK1,2/STAT3 pathway. This activation results in the regulation of apoptosis-related genes, such as Bcl-2, p53, and survivin. It also activates the crucial transcription genes, Oct-4 and Cdx-2. These supplementations ultimately have the potential to reduce oxidative stress in the embryo and elevate mitotic activity and survival, thus presenting promise for successful implantation. The dotted line indicates translocation in each pathway. This Figure was created using PPT Drawing Toolkits-BIOLOGY Bundle from Motifolio, Inc, and created with BioRender.com.
2.1. N-Acetyl-5-Methoxytryptamine (Melatonin)
Melatonin, derived from tryptophan and originating in the pineal gland, exhibits a circadian rhythm and is primarily secreted during the dark hours of the night [41,42]. It is predominantly synthesized within the mitochondria, and can easily pass through the cell membrane due to its amphiphilic nature [43,44]. Melatonin acts as an activator of antioxidants and regulator of inflammation, effectively scavenging free radicals in organisms [45,46,47]. Melatonin could increase the activity of other antioxidant enzymes, such as glutathione and superoxide dismutase (SOD), and is an inhibitor of pro-oxidant enzymes. In mammals, the melatonin receptor functions as a G protein-coupled protein (GPCR) with two known types: Mt1 and Mt2 [48]. The elevated expression of proteins (NRF2 and KEAP1) after melatonin treatment offers evidence that melatonin effectively protects IVF-derived embryos from oxidative stress through the Nrf2/ARE signaling pathway [49]. Melatonin exhibits antioxidant properties by regulating the expression of NFE2L2, SOD1, GPX1, and GPX4 genes. Melatonin also activates essential genes, such as ErbB1, ErbB4, GJA1, POU5F1, Nanog, and vascular endothelial growth factor (VEGF) and VEGF type 1 receptor (VEGF-R1), which are involved in embryo implantation, blastocyst growth, and angiogenesis (Figure 1) [50,51,52].
During embryo development, oxygen is used in three pathways to produce adenosine triphosphate, fulfilling energy needs and converting some of it into reactive oxygen species (ROS) [53,54]. ROS, including the superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH), can accumulate inside gametes and embryos due to oocyte activation, cleavage, transcription regulation, and hatching to implantation and exogenous factors during ART [55,56,57,58,59,60,61,62]. Optimal ROS levels sustain cellular metabolism and embryonic development, while excessive ROS may adversely impact embryo morphokinetics, gene expression, and survival [63,64]. Cells have antioxidant mechanisms to defend against these ROS [65]. There are two categories of antioxidant systems: enzymes like SOD, catalase, and glutathione peroxidase (GSH); and non-enzyme types like vitamins, polyphenols, carotenoids, and minerals. During the in vivo preimplantation stage of embryo development, embryos protect themselves with oxygen scavengers that are present in the reproductive tract, such as vitamins, pyruvate, GSH, SOD, and cysteamine [66]. However, embryos cultured in vitro rely solely on their intrinsic antioxidant pathways to rescue ROS without support from the maternal environment. Therefore, although there are still pros and cons, the need for research to reduce ROS by adding antioxidants to the embryo culture medium has emerged.
Among the studies investigating the effects of melatonin, half were retrospective randomized controlled trial (RCT)s on humans and the rest were studies on animals (Table 2). Most recently, a human clinical study using 10−7 M of melatonin in the embryo culture medium investigated the blastocyst development rate and gene expression [67]. The addition of melatonin to the embryo culture medium improved the rate of high-quality day 3 embryos (29.6% (Melatonin) vs. 19.5% (Control), p = 0.0123) in patients with repeated poor-quality-embryos, although no significant difference was observed in the clinical pregnancy rate. In addition, melatonin enhanced the blastocyst development rate (42.25% (Melatonin) vs. 26.38% (Control), p < 0.05) in the patients with frozen–thawed cycles. The ROS levels did not differ significantly between the two groups, but only the CAT gene exhibited a significant increase in the cultured blastocysts among the anti-apoptosis and antioxidant genes (1.68 ± 0.43 (Melatonin) vs. 1.00 ± 0.09 (Control), p < 0.05). This research indicates that melatonin supplementation could benefit repeated poor-quality embryos as well as frozen–warmed embryos in terms of the preimplantation embryo development rate and quality. It suggests that further research is needed using a large-scale sample and focusing on implantation outcomes.
Another human study investigated the embryonic development rate, implantation rate, and live birth rate based on the presence or absence of melatonin supplementations; this study used 140 patients who underwent at least one failed repeated IVF/ICSI cycle [68]. The melatonin group showed a significant increase in fertilization rate (87.7% (Melatonin) vs. 83.6% (Control), p < 0.01), cleavage development rate (94.1% (Melatonin) vs. 90.5% (Control), p < 0.01), high-quality embryo rate (58.3% (Melatonin) vs. 43.8% (Control), p < 0.0001), and high-quality blastocyst development rate (43.4% (Melatonin) vs. 22.9% (Control), p < 0.0001). When the vitrified/warmed blastocysts were transferred, the implantation rate (65.6% (Melatonin) vs. 9.7% (Control), p < 0.0001) and the clinical pregnancy rate (40.0% (Melatonin) vs. 11.7% (Control), p < 0.0001) were significantly higher in the melatonin group. Notably, the melatonin group included two women who delivered two healthy individual newborns. This demonstrates that melatonin-containing embryo culture media could increase both preimplantation embryo development and clinical outcomes; this finding particularly stands out with regard to the implantation rate and live births in patients with repeated failed IVF/ICSI cycles.
Another RCT was carried out with polycystic ovary syndrome (PCOS) patients with in vitro maturation (IVM)-IVF cycles; in this RCT, 10 μmol/L of melatonin was added to the culture medium [69]. PCOS patients showed significantly higher implantation rates in the melatonin-supplemented group both when using non-stimulation protocols (22.6% (Melatonin) vs. 11.6% (Control), p < 0.05) and human chorionic gonadotropin (hCG) priming protocols (26.4% (Melatonin) vs. 12.7% (Control), p < 0.05). However, there were no differences in embryo development competency and ongoing pregnancy rate. This study showed that melatonin could bring beneficial effects to PCOS patients’ IVM and implantation competency.
The most recent study with in vivo-derived and in vitro-derived porcine embryo examined the effects of the sequential addition of melatonin to the IVM, IVF, and culture medium by development competence [70]. Melatonin accelerated embryo development (p < 0.05), showing a significant increase in the cleavage development rate (p < 0.001) while indicating no notable difference in the blastocyst rate. Additionally, intracellular glutathione levels, (~40% increase, p < 0.03) showed a significant increase, while the reactive oxygen species level showed a significant decrease (~26% decrease, p < 0.01) in the melatonin group. Ultimately, this study shows that exogenous melatonin addition could increase the cleavage development rate but not the blastocyst development rate or its cell number. In addition, the study suggests that melatonin supplements in culture media function like antioxidants; this finding was supported by a decrease in the intracellular levels of oxygen free radicals and deoxyribo nucleic acid (DNA) damage in in vitro porcine embryos.
The animal study analyzed the developmental rate and gene expression using 10−7 M melatonin, investigating its impact on in vitro vitrified/warmed bovine embryos [71]. The melatonin group exhibited a higher cleavage development rate (87.78 ± 1.02% (Melatonin) vs. 82.50 ± 1.12% (Control), p < 0.05) and blastocyst development rate at day 7 (38.33 ± 2.21% (Melatonin) vs. 26.67 ± 1.05% (Control), p < 0.05) compared to the control group. Furthermore, melatonin improved the re-expansion of vitrified/warmed blastocysts and development-related gene expression levels. In summary, this finding indicates that melatonin enhances the progression of in vitro embryo development and the cryotolerance of blastocysts. In addition, vitrified/warmed blastocysts demonstrated increased re-expansion rates, potentially mediated by the regulation of gene expression, including DNMT3A, OCC, CDH1, and AOP3.
Using 10−7 M of melatonin, the same author investigated the effects of melatonin on embryo development competence and its impact on conception and birth outcomes [72]. There was a significant difference in the cleavage development rate (92.0 ± 5.68% (Melatonin) vs. 89.3 ± 4.17% (Control), p < 0.05) and the blastocyst development rate (80.3 ± 4.57% (Melatonin) vs. 64.6 ± 6.04% (Control), p < 0.05) in the melatonin group. Using a recipient mouse with transferred embryos, the implantation rate (95.0 ± 3.42% (Melatonin) vs. 67.8 ± 5.03% (Control), p < 0.01), litter size (4.1 ± 0.37 pups/litter (Melatonin) vs. 2.7 ± 0.42 pups/litter (Control), p < 0.05), and the survival rate (96.8 ± 2.15% (Melatonin) vs. 81.2 ± 4.36 (Control), p < 0.05) were increased in the melatonin group while the body weight of the offspring was comparable between the two groups. Additionally, gene expressions associated with antioxidant and apoptotic factors were regulated differently in embryos at each developmental stages in the two groups. The author concluded that a higher pregnancy rate could be linked to the improved blastocyst following melatonin treatment, as the melatonin group showed an increase in the cell number of blastocysts and decreases in the apoptotic rate. Similarly, improved pregnancy quality in recipients led to the birth of heal their offspring, resulting in increased postnatal survival.
Overall, current studies support the finding that melatonin may enhance preimplantation embryonic development [67,68,70,71,72] and the success rate of implantation [68,72], as indicated by human clinical studies and animal research. Furthermore, some studies indicate that melatonin could result in a reduction in ROS during embryo development, and this may lead to a decrease in DNA damage [70,72,73]. While human studies have shown consistent results, the dosage of melatonin varied within the same species, highlighting the need for further research to standardize its dosage before clinical application. Additionally, the exact mechanism by which melatonin affects embryos in in vitro culture remains unclear. Since melatonin is not a major essential factor for pregnancy, there is a greater need to determine whether its addition to culture media has any negative impact on fetus and perinatal outcomes. Therefore, further investigation of the downstream signaling pathways is necessary to gain a precise understanding of how melatonin influences embryo development. Additionally, melatonin operates in conjunction with other molecules rather than in isolation, potentially forming intricate interactions. Thus, a comprehensive approach encompassing diverse perspectives is imperative for its thorough research and application. Melatonin shows promise as a beneficial addition to embryo culture media, particularly when substantiated by studies featuring a larger sample size, multicenter involvement, and comprehensive long-term follow-ups, including birth outcomes.

Table 2.
Effects of melatonin in culture media on embryonic development and pregnancy outcomes.
Table 2.
Effects of melatonin in culture media on embryonic development and pregnancy outcomes.
| Year | Model | Study Type | Dose of Melatonin | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | Human (unexplained infertility) | RCT | 10−7 M | COC | - | - | ↑ day 3 | - | - | [67] |
| Day 3 embryo after vitrified/warmed | - | ↑ | ↑ day 5 | - | - | |||||
| 2022 | Human (RIF) | RCT | 10−9 M | COC or oocyte | ↑ | ↑ | ↑, ↑ day 3, day 5 | ↑ | ↑ | [68] |
| 2013 | Human (PCOS) | RCT | 10−6 M | Immature COC surrounded by compact cumulus cells | → | - | → day 3 | ↑ | - | [69] |
| 2022 | Porcine | animal | 10−9 M | Presumed zygote | ↑ | → | - | - | - | [70] |
| 2014 | Bovine | animal | 10−7 M | Zygote | ↑ | ↑ | - | - | - | [71] |
| 2013 | Murine | animal | 10−7 M | Zygote | ↑ | ↑ | - | - | ↑ | [72] |
-: not analyzed; →: non-significance; ↑: significantly increased.
2.2. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
GM-CSF, also known as CSF2, is the most well-studied cytokine among the CSF group of cytokines. GM-CSF has specific receptors that are comprised a cytokine-specific α-chain (GM-Rα) and b-chain (GM-Rβ) [74,75]. The main target cells of GM-CSF are immune cells, myeloid cells, dendritic cells, lymphocytes, and macrophages identified by its receptors [76]. GM-CSF has mediated cell survival, differentiation, adhesion, growth, and even immune regulation. In mice, GM-CSF k/o mice showed decreases in litter size and litter weights and increased fetal death [77]. GM-CSF is synthesized in the epithelial cells of the female reproductive tract and plays a crucial role by altering its activity during pregnancy [78,79,80,81,82]. GM-CSF appears in its highest levels from conception to early implantation and then gradually declines; then, it is produced by chorionic villi cells and decidual cells until labor [83,84,85]. Unlike most growth factors and cytokines, GM-CSF cannot be produced by the embryo. Rather, as confirmed in both human and mouse models, its receptor synthesis occurs during the preimplantation stage of embryo development [86,87]. Several studies have shown that GM-CSF has positive effects on embryo development, including blastocyst quality, the survival rate, and the expression of genes which are associated with apoptosis [86,88,89]. In other species, mouse embryos cultured with GM-CSF showed evidence of reduced apoptosis [11,90]. There are already commercial embryo culture media supplemented with GM-CSF, namely, EmbryoGen® (Origio, Måløv, Denmark) and BlastGen™(Origio, Måløv, Denmark).
The binding of GM-CSF to its receptor initiates the activation of janus kinase (JAK) 2 and JAK1 kinases, facilitated by the transphosphorylation of these kinases following receptor subunit oligomerization. Activation of JAK kinases leads to tyrosine phosphorylation, creating docking sites for two members of the signal transducers and activators of transcription (STAT) family. GM-CSF functions through the JAK1,2/STAT3,5 pathway, inhibiting the expression of heat shock protein (HSP) and apoptosis pathway genes Cbl, Hspa5, Hsp90aa1, Hsp90ab1, and Gas5, thereby facilitating the growth and survival of embryos (Figure 1) [89,91]
Of the seven studies involving GM-CSF, six studies were human studies (Table 3). One of these was a human retrospective study of the effect of a dose of 0.6 ng/mL of GM-CSF [92]. The objective of this study was to investigate the effect of relatively low concentrations of GM-CSF on human embryo quality and the clinical outcomes with fresh embryo transfer cycles. This study retrospectively grouped patients by age, based on the use of GM-CSF, and compared them accordingly. Regardless of the patient’s age, there were no significant differences in the cleavage development rate, blastocyst development rate, blastocyst morphology grade, and the clinical outcomes. However, the group with patients aged over 38 years old showed a significant increase in the cleavage development rate (99.4% (GM-CSF) vs. 97.8% (Control), p = 0.027) and the blastocyst development rate (45.7% (GM-CSF) vs. 34.9% (Control), p = 0.028). There were no comparable data on pregnancy outcomes and infant characteristics. This result demonstrates that 0.6 ng/mL of GM-CSF has no beneficial effect on pregnancy outcomes but has an advantage in patients older than 38 years in terms of increasing the opportunity for embryos.
A study utilizing commercial embryo culture media containing GM-CSF evaluated embryo quality and clinical outcomes among patients meeting inclusion criteria indicative of poor prognoses [93]. The researchers adjusted for age, body mass index, and fertilization procedure for the results. There was no significant difference in the cleavage development rate between the two groups, but there was a significant decrease in blastocyst development rate (OR 0.70, p = 0.02) and embryo grade (OR 0.35, p = 0.009) in GM-CSF group. After embryo transfer, there was no statistical difference between the two groups in live birth, and the fetal heart rate. In summary, it showed the negative effects of GM-CSF media on the blastocyst development rate and the embryo grade in patients with poor prognoses, while comparable results were observed in clinical outcomes.
Another RCT conducted an explorative secondary analysis based on Ziebe [94] to investigate the implantation potential of poor-quality embryos [95]. The main finding that GM-CSF could not increase the implantation rate for embryos, which are morphologically poor grade on day 3. According to the morphological quality on the day of transfer, there were no statistical differences in total ongoing pregnancy at week 12 and total live birth rate between subjects with GM-CSF and without GM-CSF in top-/intermediate-/poor-grade embryo groups. The researchers concluded that GM-CSF in human embryo culture did not alter clinical results, including ongoing pregnancy rate, live birth rate, and pregnancy loss, in morphological poor-quality embryos. There is a limitation to this study: it shows only favorable effects in top-grade embryo quality and not in the quality of poor-grade embryos.
The earliest human study using GM-CSF containing commercial culture media was carried out with patients who previously failed an ART attempt [96]. Although there was no statistical difference between the two groups in the frequency of the clinical pregnancy rate, the GM-CSF group showed significant increases in the implantation rate at 7 weeks (20.4% (GM-CSF) vs. 11.6% (Control), p < 0.05) and 12 weeks (17.4% (GM-CSF) vs. 9.1% (Control), p < 0.001). This research demonstrated the positive effects of GM-CSF on the implantation rate of patients who underwent failed IVF/ICSI cycles; however, this study was conducted over a short period of six months, and patient characteristics are lacking.
One of the major studies in which GM-CSF containing culture media could be produced using commercial culture media was conducted with patients from 14 fertility clinics [94]. A total of 1332 patients were mainly divided into two groups: EmbryoAssist medium, with 2 mg/mL human serum albumin (HSA) or 5 mg/mL HSA, and supplementation, with or without 2 ng/mL GM-CSF. There was no statistical difference in the top-quality rate of day-3 embryos. The results showed that supplementation of GM-CSF with low HSA to embryo culture media produced significant increases in the ongoing implantation rate (23.0% (GM-CSF) vs. 19.7% (Control), p = 0.02) and the live birth rate (28.9% (GM-CSF) vs. 24.1% (Control), p = 0.03). The researchers also showed exploratory data for subgroups who had previous miscarriages. These data showed efficacious results in the ongoing implantation rate (23.2% (GM-CSF) vs. 16.5% (Control), p = 0.003) and the live birth rate (29.6% (GM-CSF) vs. 23.1% (Control), p = 0.02) when using GM-CSF. This study indicated that GM-CSF had positive effects on the ongoing implantation rate and the live birth rate and that these effects were further enhanced in patients who underwent a previous miscarriage. This study holds significance in demonstrating GM-CSF stability in embryo culture media. Although exploratory subgroup study requires further investigation, this analysis has shown promise that GM-CSF may function positively in particular patients.
Another study determined the developmental potential of early embryos depending on the presence or absence of GM-CSF in embryo culture in a mouse model [97]. The blastomere was mechanically isolated to minimize the specificity of each embryo and was assigned to different groups. The 4-cell rate (59.33% (GM-CSF) vs. 53.57% (Control) p < 0.05) showed a significant increase in the GM-CSF group, although no significant difference in blastocyst development rate, blastocyst size (93.89 ± 4.93 µm (GM-CSF) vs. 81 ± 3.02 µm (Control), p < 0.05), and cell number (52.8 ± 1.79 (GM-CSF) vs. 44.7 ± 2.84 (Control), p < 0.05) were demonstrated in the GM-CSF group.
Based on a multicenter study targeting patients with a history of previous miscarriage, which noted an increase in the ongoing pregnancy rate and live birth rate [94], subsequent RCTs conducted on infertility patient groups have shown either a negative effect [93] or a non-significant effect with GM-CSF [92,95]. There were no consistent research findings demonstrating a positive effect consistent enough to suggest routine use of GM-CSF in specific patient groups. Despite the availability of commercial media containing cytokines with determined optimal concentrations, the widespread adoption of routine application has not ensued. Among the seven RCTs reviewed, three studies indicated a significant increase when GM-CSF was added to the embryo culture medium, while the remaining four studies showed either non-significant or negative effects. This suggests uncertainty regarding the overall positive impact of GM-CSF on a broader scale.

Table 3.
Effects of GM-CSF in culture media on embryonic development and pregnancy outcomes.
Table 3.
Effects of GM-CSF in culture media on embryonic development and pregnancy outcomes.
| Year | Model | Study Type | Dose of GM-CSF | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | Human (infertility) | Retrospective study | 0.6 ng/mL | After fertilization | →,↑ | →,↑ | → | → | - | [92] |
| 2020 | Human (RIF) | RCT | 2 ng/mL (EmbryoGen, BlastGen) | COC or oocyte | → | ↓ | ↓ | - | → | [93] |
| 2020 | Human (infertility) | Explorative secondary RCT | 2 ng/mL | COC or oocyte | - | - | - | - | → | [95] |
| 2014 | Human (RIF) | RCT | 2 ng/mL (EmbryoGen) | COC or oocyte | - | - | → | ↑ | - | [96] |
| 2013 | Human (unexplained infertility) | Multicenter RCT | 2 ng/mL | COC or oocyte | → | - | → day3 | ↑ | ↑ | [94] |
| → | → | → day3 | ↑ | ↑ | ||||||
| 2008 | Murine | Animal | 2 ng/mL | Isolated blastomere from 2-cell stage | ↑ | → | - | - | - | [97] |
-: not analyzed; →: non-significance; ↑: significantly increased; ↓: significantly decreased.
2.2.1. Insulin-like Growth Factor 1 (IGF-Ⅰ)
The human reproductive tract produces numerous growth factors, and the preimplantation embryo expresses those receptors to maintain the maternal–fetal interface. Therefore, clinical reports have been produced concerning the effects of controlled ovarian stimulation using different types of growth factors to increase the success rate IVF [98,99,100,101]. Among various types of growth hormones, the insulin-like growth factor (IGF) family includes peptide hormones consisting of two ligands (IGF-Ⅰ, IGF-Ⅱ), their receptors, and IGF-binding proteins [102,103]. IGF-Ⅰ comprises a small amino acid chain and is linked to its receptor, IGF-ⅠR. Various mammalian species expressed IGF-Ⅰ and IGF-Ⅱ ligands, along with their respective receptors, at the mRNA or protein level in early embryos [6,104,105,106,107]. Additionally, IGF-Ⅰ is produced by the female reproductive tract, the fallopian tube, follicle fluid, and uterine fluid [108,109].
The IGFs are involved in a variety of roles promoting cell growth, proliferation, differentiation, and anti-apoptosis by acting as endocrine, paracrine, and autocrine factors [110,111,112]. IGF plays pivotal roles in various functions, including follicle growth and subsequent embryonic development [113,114,115,116,117]. There have been previous studies utilizing animal models to investigate the effects of IGF in preimplantation embryo culture media; these studies demonstrated an increase in blastocyst formation rate, cell number, glucose uptake, and a decrease in apoptosis [118,119,120]. Additionally, a postnatal experiment in mice indicated a significant reduction in body weight in IGF-Ⅰ(-/-) mutant mice compared to wild-type mice [121].
Growth hormone (GH) and insulin-like growth factor (IGF)—which is closely related to GH—are found in various reproductive tissues, indicating that the GH/IGF axis may directly influence the development. GH and IGF have the ability to modulate key signal transduction pathways involved in cell division and hormone production, such as the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), Jak/STAT, and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways. When IGF interacts with the insulin receptor, it triggers the recruitment and phosphorylation of either insulin receptor substrate-1 or -2 (IRS-1 or -2). Although IGF-ⅠR and the insulin receptor use the same pathway through IRS-1 and PI3K, IGF-ⅠR acts dependently on IRS-1 and PI3K [122,123]. ERK1/2 enhances cellular mitogenic signals and can be activated by PLC/PKC through increased intracellular calcium and indirectly through PKA. These pathways have downstream associations with S6K1, GSK3, and GLUT4/8, which play a role in apoptosis, glucose homeostasis, glycogen synthase, and glucose transport (Figure 1) [124,125,126,127,128].
The studies related to IGF-Ⅰ were divided into two subcategories: those using a mixture of IGF-Ⅰ with other substances and those using IGF-Ⅰ alone (Table 4). There was a foundational study conducted using IGF-Ⅰ in culture media that are related to the study of human embryos [107], based on which, the effects of IGF-Ⅰ during human preimplantation embryo development were investigated [129]. After fertilization, day 2 embryos of good morphology were cultured with or without 13 ng/mL IGF-Ⅰ up to day 6. Embryos in the IGF-Ⅰ group showed a significantly increased rate of blastocyst formation (74% (IGF-Ⅰ) vs. 49% (Control), p < 0.05). In addition, these embryos showed decreased numbers of apoptotic nucleus cells in the IGF-Ⅰ group (16.3 ± 2.9% (IGF-Ⅰ) vs. 8.7 ± 1.5% (Control), p < 0.01). However, there was no difference in total cell number between the two groups. The study should have taken into consideration that patient characteristics may not have been randomly distributed and that IGF- I treatment was initiated after day 2. Regardless, this study demonstrated the possibility that IGF-Ⅰ could reduce apoptosis, and outlined the appropriate dose for human embryo culture.
Among the three experiments that examined the impact of using IGF- I exclusively in embryo culture media, the most recent paper examined the effects of IGF-Ⅰ on cat embryo development [130]. This study contained three experimental designs, the last of which experiment was excluded in this review, as it analyzed the effects of IGF-Ⅱ. Using different fertilization techniques, the cleavage development rate was studied. Briefly, one study showed 20 ng/mL of IGF-Ⅰ did not produce a statistically significant change in the cleavage development rate compared to the control group, regardless of fertilization method, though one of the designs showed a significantly higher morula rate (87.0% (IGF-Ⅰ) vs. 52.2% (Control), p < 0.05) and blastocyst development rate (43.5% (IGF-Ⅰ) vs. 13.0% (Control), p < 0.05) in single-culture group through IVF. In addition, in a group with combined GM-CSF and IGF-Ⅰ, a significant increase only in the morula rate (81.0% (Combination) vs. 52.2% (Control), p < 0.05) of the single-culture group was demonstrated, but this did not translate into an increase in the blastocyst development rate. Collectively, the first experiment showed positive effects, the second experiment showed non-significant effects, and the last experiment showed non-significant effects with IGF-Ⅰ. The researchers concluded that the maturation rate increased with IGF-Ⅰ supplementation in the IVM medium; however, caution is required in interpreting this finding as it was inferred from the cleavage development rate.
Another study analyzed the impact of IGF-Ⅰ on the development of mouse embryos [131]. In brief, this study confirmed that regardless of the culture media volume, the presence of 10 ng/mL of IGF-Ⅰ had no impact on the cleavage development rate but notably increased the blastocyst development rate (p < 0.05). In addition, this study showed the autocrine role of IGF-Ⅰ by treating embryos with IGF-ⅠR-neutralizing antibodies and demonstrated increased phosphorylation of Akt in blastocysts after a 10 min treatment with 100 ng/mL of IGF-Ⅰ.

Table 4.
Effects of IGF-Ⅰ in culture media on embryonic development and pregnancy outcomes.
Table 4.
Effects of IGF-Ⅰ in culture media on embryonic development and pregnancy outcomes.
| Year | Model | Study Type | Dose of IGF-Ⅰ | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Implantation Rate | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2000 | Human (infertility) | Prospective study | 13 ng/mL | Day 2 embryos of good morphology | → | ↑ | - | [129] |
| 2021 | Cat | Animal | 10 ng/mL, 20 ng/mL (GM-CSF 2 ng/mL) | COC | → | →, ↑ (20 ng/mL) | - | [130] |
| 20 ng/mL (IGF-Ⅱ 20 ng/mL) | COC | → | → | - | ||||
| 20 ng/mL | Oocyte | → | - | - | ||||
| → | - | - | ||||||
| 2019 | Yak-cattle crossbred | Animal | 100 ng/mL (EGF 10 ng/mL, L-cysteine 0.6 mM) | COC | - | ↑ | - | [132] |
| 2013 | Murine | Animal | 10 ng/mL | Zygote | → | ↑ | - | [131] |
| 2013 | Bovine | Animal | 10 ng/mL (EGF 10 ng/mL) | Zygote | → | ↑ | - | [133] |
| 2010 | Bovine | Animal | 500,000 ng/mL (IGF-Ⅱ 10 µg/mL, bFGF 25 µg/mL, TGF- β1 1 µg/mL, GM-CSF 1 µg/mL, LIF µg/mL) | Zygote | - | ↑ | - | [134] |
| 2003 | Bovine | Animal | 50 ng/mL | Zygote | → | ↑ | - | [120] |
-: not analyzed; →: non-significance; ↑: significantly increased.
Finally, a study determined the effects of IGF-Ⅰ and epidermal growth factor (EGF) in bovine embryo culture medium, respectively; however, we specifically emphasized the role of IGF-Ⅰ, as the combination outcomes were identical to the outcomes when each substance was used individually [120]. In the experimental results for IGF-Ⅰ, this study showed significant differences in the blastocyst development rate (56.1% (IGF-Ⅰ) vs. 43.4% (Control), p < 0.01), the total cell number (162 ± 6.83 (IGF-Ⅰ) vs. 141 ± 6.59 (Control), p < 0.05), and the apoptosis rate (2.1 ± 0.28 (IGF-Ⅰ) vs. 3.3 ± 0.42 (Control), p < 0.05) between the two groups. This study provided support for the positive effects of IGF-Ⅰ on in vitro bovine blastocyst development, particularly in reducing apoptosis.
2.2.2. Insulin-like Growth Factor 1 (IGF-Ⅰ) Combined with Other Supplements
There are three studies that investigated the results of treating embryo culture media with a combination of IGF-Ⅰ and other substances such as EGF, GM-CSF, IGF-Ⅱ, etc.
The substances of IGF-Ⅰ, cysteine, and EGF treated with yak–cattle crossbred embryos before fertilization [132]. All the parameters showed positive results in the maturation rate (84.44 ± 0.02 (Combination) vs. 66.50 ± 0.04 (Control), p < 0.05), the cleavage development rate (80.45 ± 0.12 (Combination) vs. 64.77 ± 0.10 (Control), p < 0.05), and the blastocyst development rate (38.67 ± 0.06 (Combination) vs. 21.16 ± 0.08 (Control), p < 0.05) with the combination group compared to the control group. In addition, with treated IGF-Ⅰ alone, there was an increase in the blastocyst development rate (30.06 ± 0.05 (IGF-Ⅰ) vs. 21.16 ± 0.08 (Control), p < 0.05), but the cleavage development rate did not show a statistical difference. The researchers concluded that the combination of IGF-Ⅰ, cysteine, and EGF could enhance maturation rate and embryo development.
In another study, using bovine embryos, the effects of the combination of IGF-Ⅰ and EGF on embryo culture media were investigated [133]. There was a non-significant difference in the cleavage development rate between the groups, while the blastocyst development rate was significantly increased in IGF-Ⅰ with the EGF group (23.5% (Combination) vs. 17.1% (Control), p < 0.05) and the EGF-alone group. In addition, the apoptosis rate in blastocysts showed statically decreased data in the IGF-Ⅰ with EGF (10.5 ± 0.7 (Combination) vs. 21.9 ± 1.6 (Control), p < 0.05) group and EGF-alone group compared to the control group, respectively.
There is another study on the impact of a combination of various growth factors and cytokines on bovine embryo culture medium [134]. The culture medium contained six kinds of supplements: IGF-Ⅰ, IGF-Ⅱ, transforming growth factor beta (TGF-β), GM-CSF, basic fibroblast growth factor (bFGF), and LIF. The researchers obtained positive results regarding the effects of supplements on the blastocyst development rate (45% (Combination) vs. 24% (Control without serum), p < 0.05) and the blastocyst cell number (154 ± 15 (Combination) vs. 125 ± 11 (Control without serum), p < 0.05). Combination groups showed a significant increase in hatched blastocyst development rate, compared to the control, with 10% fetal calf serum (72% (Combination) vs. 48% (Control with serum), p < 0.05). This study demonstrated that growth factors and cytokines could act synergistically to promote embryo development competency, especially in blastocysts; nevertheless, accurate data on the viability and quality of embryos may require additional assessment.
Overall, the lack of implantation data in IGF-Ⅰ studies limits the precise analysis of the effects when IGF-Ⅰ is added to embryo culture media. Despite the addition of combination supplementations, all studies consistently show a non-significant result in the cleavage development rate [120,129,130,133]. However, most of the research results, including the study that solely added IGF-Ⅰ, showed an increase in the blastocyst development rate [120,129,130,132,133]. There are limited data on the effects of adding IGF-Ⅰ to embryo culture media in terms of obtained clinical outcomes, both in animal models and in human models. Most of the studies examining the effects of IGF-Ⅰ supplementation involve its application in combination with other supplementations rather than alone. Further research is needed to investigate the effects of adding IGF-Ⅰ alone to embryo culture media. The routine application of IGF-Ⅰ in embryo culture media would require human clinic data and studies that examine pregnancy outcomes to support its effectiveness.
2.2.3. Leukemia Inhibitory Factor (LIF)
Leukemia inhibitory factor (LIF), an interleukin 6 class cytokine, is expressed in the trophectoderm and regulates various cellular functions by binding to specific receptors, LIFR, and glycoprotein 130 [135]. These receptors have two forms: membrane-bound and soluble [136]. LIF contributes to cellular growth, proliferation, and survival through cell signaling pathways.
In the various cell types, LIF could promote JAK/STAT, PI3K/PKB, and ERK/MAPK pathways [137,138,139,140,141]. Within a mouse model study, the LIF signaling mechanism in uterine tissue and trophoblast cells occurs through the activation of the JAK1/STAT3 pathway [142,143]. LIF promotes receptor heterodimerization, leading to the activation of a JAK/STAT pathway. JAK1 phosphorylates tyrosine residues, providing binding sites for STAT3. Multiple studies have shown that STAT3 stimulates various downstream genes related to proliferation, angiogenesis, differentiation, cell cycle progression, and apoptosis, such as c-Myc, VEGF, HIF1a, Rac1, Notch1, p53, and Bcl-2 (Figure 1) [144,145,146,147,148,149,150,151,152,153,154,155].
It has been observed that human oocytes, cleavage, and blastocysts express LIFR and gp130 in mRNA and protein levels [156]. These receptors, expressed in the endometrium and upregulated during the luteal phase coinciding with implantation, correlate with studies associating LIF with the implantation process [157,158,159,160]. In a mouse study, gp130 k/o and LIFR k/o types of embryos could not survive during post-implantation development [161]. Indeed, LIF has been extensively studied and found to play a crucial role, particularly during the process of implantation in pregnancy in various species [162,163]. Another study showed that supplements of LIF could increase the cell number in mouse blastocysts and in gene expression of Oct-4 and Cdx22, related to ICM and trophectoderm, respectively [164]. Through various human and animal studies, it has been demonstrated that LIF plays a crucial role in not only implantation but also embryo development [165,166]. There is a study that higher concentration of LIF in embryo culture media is associated with a higher pregnancy rate when blastocysts are transferred [167]. Recently, the expression of LIF in follicular fluid and ovarian stromal cells has also been associated with young women with poor ovarian response during ovarian stimulation cycle [168].
Six studies have analyzed the effects on embryonic development of adding LIF to embryo culture media (Table 5). Among them, three studies focused on human models, while the remaining three studies investigated animal models. Furthermore, four studies specifically investigated the effects of adding LIF alone to the culture medium, while the remaining two studies assessed the use of a combination of LIF with other supplements.
A RCT with human frozen–thawed cleavage was conducted in early 2000 to evaluate the effects of LIF [169]. It used two kinds of base culture media, human tubal fluid medium, and M3 medium. The results showed no significant difference in cleavage development rate with the addition of LIF. However, there was a significant increase in both morula rates using HTF medium for the base medium (23.2% (LIF) vs. 6.9% (Control-1), p < 0.05) (23.1% (LIF) vs. 19.7% (Control-2), NS) and the blastocyst development rate (11.0%, 12.8% (LIF) vs. 0% (Control), p > 0.05). The researchers concluded that LIF has the potential to affect early embryo development, but further, larger-scale multicenter studies are needed to confirm these findings.
Another study was performed to assess blastocyst development, total cell number, hatching rate, and LIF and its receptor expression in preimplantation buffalo embryos [170]. The group of LIF supplements demonstrated a positive influence on blastocyst development. There was no significant difference in the cleavage development rate and the morula rate. In terms of blastocyst development parameters, the expanding blastocyst development rate (16.57 ± 1.13 (LIF) vs. 9.80 ± 1.19 (Control), p < 0.05) and total cell number (81.5 ± 2.93 (LIF) vs. 70.5 ± 2.2 (Control), p < 0.05) were significantly increased in the LIF group. Additionally, the expression of LIFR mRNA and protein was confirmed from the oocyte to blastocyst stages, while the expression of LIF ligand mRNA and protein was observed from the 8 cell stage to the blastocyst stage.

Table 5.
Effects of LIF in culture media on embryonic development and pregnancy outcomes.
Table 5.
Effects of LIF in culture media on embryonic development and pregnancy outcomes.
| Year | Model | Study Type | Dose of LIF | Timing of Intervention | Cleavage Development Rate | Blastocyst Development Rate | Embryo Grade | Implantation Rate | Live Birth Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | Human (infertility) | RCT | 5 ng/mL (GM-CSF 2 ng/mL, HB-EGF 5 ng/mL) | After ICSI | → | ↑ | →, ↑ day 3, day 5/6 | ↑, → Fresh, FER | ↑ | [171] |
| 2000 | Human (infertility) | RCT | 1000 IU/mL | Cleavage after vitrified-thawed | → | ↑ | - | - | - | [169] |
| 2021 | Bovine | Animal | 20 ng/mL (FGF2 40 ng/mL, IGF-Ⅰ 20 ng/mL) | Putative zygote | → | ↑ | - | - | - | [172] |
| 2013 | Buffalo | Animal | 100 ng/mL | Presumptive zygote | → | ↑ | - | - | - | [170] |
| 2008 | Murine | Animal | 1500 IU/mL | Isolated blastomere from 2-cell stage | ↑ | → | - | - | - | [97] |
-: not analyzed; →: non-significance; ↑: significantly increased.
In the previously mentioned paper on GM-CSF, the researchers also investigated the impact of supplementing the embryo culture medium with LIF on mouse embryo development [97]. Using isolated blastomeres from mouse 2 cell embryos, LIF showed a significant increase in cleavage development rate (61.10% (LIF) vs. 52.4% (Control), p < 0.001) after 48 h of culture. There was no significant difference in the blastocyst development rate between the two groups, but the total cell number (53.4 ± 1.82 (LIF) vs. 44.7 ± 2.84 (Control), p < 0.05) was higher in the LIF group.
2.2.4. Leukemia Inhibitory Factor (LIF) Combined with Other Supplements
Two studies used LIF with other supplementations such as GM-CSF, HB-EGF, FGF2, and IGF-Ⅰ. An RCT was conducted to investigate the effects of culture media integrated with LIF, GM-CSF, and heparin-binding epidermal growth factor-like growth factor (HB-EGF) on embryonic development and pregnancy outcomes with a human ICSI cycle [171]. The incorporation of cytokines and growth factors could have directly targeted potential effects in hatching or implantation, leading to improved clinical outcomes for the recurrent implantation failure (RIF) and pregnancy-loss groups. The cytokines and growth factors demonstrated potential effects on clinical outcomes in terms of ongoing pregnancy rate (47% (Combination) vs. 36% (Control), p = 0.012) and live birth rate (45% (Combination) vs. 33% (Control), p = 0.0062). In addition, embryologic characteristics and blastocyst development rate (68% (Combination) vs. 61% (Control), p = 0.0001), showed a good quality of blastocyst development rate according to Istanbul guidelines (48% (Combination) vs. 36% (Control), p = 0.0001), and implanted fresh embryo rate (38% (Combination) vs. 30% (Control), p = 0.023) showed a statistical increase in the combination group while cleavage development rate was statistically non-significant. This study showed that the integration of LIF, GM-CSF, and HB-EGF could enhance embryonic development and pregnancy outcomes with no adverse consequences.
The remaining studies all focused on the effects of supplementation in mammalian embryos, primarily observing the development of embryos without pregnancy outcomes. In 2021, the effects of LIF, IGF-Ⅰ, and fibroblast growth factor 2 (FGF2) on bovine embryo development were studied [172]. Although there was no significant difference in cleavage development rate between the supplementation group and control group, the supplementation group exhibited an increase in blastocyst development rate (46.2 ± 1.3 (Combination) vs. 32.9 ± 1.3 (Control), p < 0.05). However, there were no statistical differences in total blastocyst cell number. After the blastocyst was frozen–thawed, the hatching rate (40.4% ± 0.04 (Combination) vs. 19.8% ± 0.04 (Control), p < 0.05) and apoptosis index (6.70% ± 1.32 (Combination) vs. 18.06% ± 1.62 (Control), p < 0.05) was statistically significantly different between the two groups. This study suggests that LIF supplementations have positive influences on blastocyst development rate and its quality.
These two studies both showed an increase in blastocyst development rate although they used different combinations of supplementations and models. Both studies selected supplementations based on other research that demonstrated their positive effects [134,173].
In conclusion, LIF supplementation of embryo culture media showed developmental potential in blastocysts. Only one human RCT has demonstrated positive effects in implantation and birth outcomes, although they used a mixture of supplementations [171]. All of the studies showed an increase in the blastocyst development rate using LIF or a LIF combination [169,170,171,172], except for one study, which showed no significant effect [97]. While the diverse roles and significant importance of LIF in embryo development and the pregnancy process have been well established, there have been relatively few studies on the supplementation of LIF in embryo culture media. Therefore, there may need to be more studies including pregnancy outcomes and human RCT.
3. Conclusions
In this review, we summarized the effects on embryonic development of several representative supplements added to culture media. Melatonin, GM-CSF, IGF-Ⅰ, and LIF all showed positive effects on embryo-related outcomes such as blastocyst development rate and the grade of the embryo. Melatonin, GM-CSF, IGF-Ⅰ, and LIF seem to have a generally positive effect on pregnancy-related outcomes such as the implantation rate and the live birth rate. Studies have been recently published on the effect of supplements in different clinical situations, such as old age, RIF, and unexplained infertility. In the case of melatonin and GM-CSF, many clinical studies have been conducted on humans, but for the rest of the supplements, only preclinical studies on murine and bovine models have been conducted. The results of human studies should be built on with future clinical trials. In addition, studies to find the optimal dose and timing of intervention should be continued, as each study used different doses of supplements and timings of intervention. Supplementation strategies in culture media have shown promise in enhancing blastocyst development rates and improving certain aspects of ART outcomes. However, the translation of these benefits into increased live birth rates requires further investigation. Robust clinical studies encompassing a wider range of substances and participant profiles are essential to unraveling the true potential of supplementation in culture media. Ultimately, understanding the molecular mechanisms behind these effects will enable us to use an embryo culture medium containing personalized supplements tailored to the individual circumstances of infertility patients, bringing renewed hope to those on the journey to parenthood.
Author Contributions
Conceptualization, S.-W.K. and S.-Y.K.; writing—original draft preparation, J.-W.C., H.-S.K., M.-J.K. and S.-A.K.; writing—review and editing, S.-W.K. and J.-Y.H.; visualization, J.-W.C.; supervision, H.K.; project administration, S.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C1010293 and 2021R1F1A1060218).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IVF | in vitro fertilization |
| ART | assisted reproductive technology |
| Nrf2/ARE | nuclear factor erythroid 2-related factor 2/antioxidant-responsive element |
| VEGF | vascular endothelial growth factor |
| ATP | adenosine triphosphate |
| RCT | randomized clinical trial |
| hCG | human chorionic gonadotropin |
| DNA | deoxyribo nucleic acid |
| PCOS | polycystic ovary syndrome |
| IVM | in vitro maturation |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HSP | heat shock protein |
| GH | growth hormone |
| IGF | insulin-like growth factor |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| IRS | 1insulin receptor substrate |
| EGF | epidermal growth factor |
| bFGF | basic fibroblast growth factor |
| LIF | leukemia inhibitory factor |
| JAK | Janus family tyrosine kinase |
| STAT | signal transducers and activators of transcription |
| PI3K | phosphoinositide 3-kinase |
| Akt | protein kinase B |
| ROS | reactive oxygen species |
| GPCR | G protein-coupled receptor |
| O2− | superoxide anion |
| H2O2 | hydrogen peroxide |
| •OH | hydroxyl radical |
| SOD | superoxide dismutase |
| GSH | glutathione peroxidase |
| Th1 | Type 1 helper T cells |
| Th2 | Type 2 helper T cells |
| HSA | human serum albumin |
| HB-EGF | heparin-binding epidermal growth factor-like growth factor |
| RIF | recurrent implantation failure |
| FGF2 | fibroblast growth factor 2 |
References
- Fauque, P.; Leandri, R.; Merlet, F.; Juillard, J.C.; Epelboin, S.; Guibert, J.; Jouannet, P.; Patrat, C. Pregnancy outcome and live birth after IVF and ICSI according to embryo quality. J. Assist. Reprod. Genet. 2007, 24, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, Y.; Li, M.; Chen, L.; Liu, P.; Qiao, J. Does IVF cleavage stage embryo quality affect pregnancy complications and neonatal outcomes in singleton gestations after double embryo transfers? J. Assist. Reprod. Genet. 2014, 31, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Oron, G.; Son, W.Y.; Buckett, W.; Tulandi, T.; Holzer, H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: A pilot study. Hum. Reprod. 2014, 29, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Chen, Y.; Shu, Y.; Cheng, Y.; Qiao, J.; Behr, B.; Pera, R.A.; Hsueh, A.J. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS ONE 2012, 7, e49328. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Barlow, D.H.; Sargent, I.L. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum. Reprod. 1998, 13, 1645–1652. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, M.; Hao, F.; Zhao, R.; Teng, X.; He, B.; Zhu, C.; Chen, Z.; Li, K. Effect of hyaluronic acid-enriched transfer medium on frozen-thawed embryo transfer outcomes in RIF patients: A single-centre retrospective study. Front. Endocrinol. 2023, 14, 1170727. [Google Scholar] [CrossRef]
- Rock, J.; Menkin, M.F. In Vitro Fertilization and Cleavage of Human Ovarian Eggs. Science 1944, 100, 105–107. [Google Scholar] [CrossRef]
- Behr, B.; Wang, H. Effects of culture conditions on IVF outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115 (Suppl. S1), S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod. Biomed. Online 2016, 32, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E. Decisions for the IVF laboratory: Comparative analysis of embryo culture incubators. Reprod. Biomed. Online 2014, 28, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.A.; Albert, C.; Marcos, J.; Larreategui, Z.; Bori, L.; Meseguer, M. A propensity score-based, comparative study assessing humid and dry time-lapse incubation, with single-step medium, on embryo development and clinical outcomes. Hum. Reprod. 2022, 37, 1980–1993. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Emad, M.; Gad, M.A.; Sabry, M.; Kasem, H.; Mahmoud, M.; Bedaiwy, M.A. Comparing 36.5 degrees C with 37 degrees C for human embryo culture: A prospective randomized controlled trial. Reprod. Biomed. Online 2018, 36, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hentemann, M.; Mousavi, K.; Bertheussen, K. Differential pH in embryo culture. Fertil. Steril. 2011, 95, 1291–1294. [Google Scholar] [CrossRef]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef]
- Balaban, B.; Urman, B. Comparison of two sequential media for culturing cleavage-stage embryos and blastocysts: Embryo characteristics and clinical outcome. Reprod. Biomed. Online 2005, 10, 485–491. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.; van Montfoort, A.P.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef]
- Castillo, C.M.; Harper, J.; Roberts, S.A.; O’Neill, H.C.; Johnstone, E.D.; Brison, D.R. The impact of selected embryo culture conditions on ART treatment cycle outcomes: A UK national study. Hum. Reprod. Open 2020, 2020, hoz031. [Google Scholar] [CrossRef]
- Stimpfel, M.; Bacer-Kermavner, L.; Jancar, N.; Vrtacnik-Bokal, E. The influence of the type of embryo culture media on the outcome of IVF/ICSI cycles. Taiwan. J. Obstet. Gynecol. 2020, 59, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, B.; Boada, M.; Rodriguez, I.; Coroleu, B.; Barri, P.N.; Veiga, A. Does culture medium influence offspring birth weight? Fertil. Steril. 2013, 100, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Baumann, N.A.; Oglesbee, D. Composition of single-step media used for human embryo culture. Fertil. Steril. 2017, 107, 1055–1060.e1051. [Google Scholar] [CrossRef] [PubMed]
- Gruber, I.; Klein, M. Embryo culture media for human IVF: Which possibilities exist? J. Turk. Ger. Gynecol. Assoc. 2011, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Finn-Sell, S.; Cheong, Y.C.; Brook, N.; Eckert, J.J.; Macklon, N.S.; Houghton, F.D. Amino acid composition of human uterine fluid: Association with age, lifestyle and gynaecological pathology. Hum. Reprod. 2015, 30, 917–924. [Google Scholar] [CrossRef]
- Baltz, J.M. Osmoregulation and cell volume regulation in the preimplantation embryo. Curr. Top. Dev. Biol. 2001, 52, 55–106. [Google Scholar] [CrossRef]
- Devreker, F.; Hardy, K.; Van den Bergh, M.; Vannin, A.S.; Emiliani, S.; Englert, Y. Amino acids promote human blastocyst development in vitro. Hum. Reprod. 2001, 16, 749–756. [Google Scholar] [CrossRef]
- Tarahomi, M.; Vaz, F.M.; van Straalen, J.P.; Schrauwen, F.A.P.; van Wely, M.; Hamer, G.; Repping, S.; Mastenbroek, S. The composition of human preimplantation embryo culture media and their stability during storage and culture. Hum. Reprod. 2019, 34, 1450–1461. [Google Scholar] [CrossRef]
- Rodriguez-Alonso, B.; Maillo, V.; Acuna, O.S.; Lopez-Ubeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Aviles, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681. [Google Scholar] [CrossRef]
- Mitchell, M.; Cashman, K.S.; Gardner, D.K.; Thompson, J.G.; Lane, M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol. Reprod. 2009, 80, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.R.; Baumann, N.A.; Matern, D.; Moyer, T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766.e759. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Krieg, T.; Ulber, R.; Holtmann, D. Growth medium and electrolyte-How to combine the different requirements on the reaction solution in bioelectrochemical systems using Cupriavidus necator. Eng. Life Sci. 2017, 17, 781–791. [Google Scholar] [CrossRef]
- Salazar, A.; Keusgen, M.; von Hagen, J. Amino acids in the cultivation of mammalian cells. Amino Acids 2016, 48, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.S.; Mann, M.R.; Tremblay, K.D.; Bartolomei, M.S.; Schultz, R.M. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol. Reprod. 2000, 62, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Coutifaris, C.; Sapienza, C.; Mainigi, M. Global DNA methylation levels are altered by modifiable clinical manipulations in assisted reproductive technologies. Clin. Epigenetics 2017, 9, 14. [Google Scholar] [CrossRef]
- Market-Velker, B.A.; Fernandes, A.D.; Mann, M.R. Side-by-side comparison of five commercial media systems in a mouse model: Suboptimal in vitro culture interferes with imprint maintenance. Biol. Reprod. 2010, 83, 938–950. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, Z.; Miao, K.; Yu, Y.; Sui, L.; Tian, J.; An, L. Dynamic integrated analysis of DNA methylation and gene expression profiles in in vivo and in vitro fertilized mouse post-implantation extraembryonic and placental tissues. Mol. Hum. Reprod. 2016, 22, 485–498. [Google Scholar] [CrossRef]
- Reis e Silva, A.R.; Bruno, C.; Fleurot, R.; Daniel, N.; Archilla, C.; Peynot, N.; Lucci, C.M.; Beaujean, N.; Duranthon, V. Alteration of DNA demethylation dynamics by in vitro culture conditions in rabbit pre-implantation embryos. Epigenetics 2012, 7, 440–446. [Google Scholar] [CrossRef][Green Version]
- Perrimon, N.; Pitsouli, C.; Shilo, B.Z. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb. Perspect. Biol. 2012, 4, a005975. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Yamashita, M.; Tojo, C.; Kondo, M.; Morita, T.; Wakamura, T. Can tryptophan supplement intake at breakfast enhance melatonin secretion at night? J. Physiol. Anthropol. 2017, 36, 20. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Cheung, B.; Tang, J.; Rheinstadter, M.C. The organization of melatonin in lipid membranes. Biochim. Biophys. Acta 2015, 1848, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Nikmard, F.; Hosseini, E.; Bakhtiyari, M.; Ashrafi, M.; Amidi, F.; Aflatoonian, R. The boosting effects of melatonin on the expression of related genes to oocyte maturation and antioxidant pathways: A polycystic ovary syndrome- mouse model. J. Ovarian Res. 2022, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamura, E.K.; Cecon, E.; Monteiro, A.W.; Silva, C.L.; Markus, R.P. Melatonin inhibits LPS-induced NO production in rat endothelial cells. J. Pineal Res. 2009, 46, 268–274. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Jones, A.J.; Rajnarayanan, R.V.; Dubocovich, M.L. Pharmacological Actions of Carbamate Insecticides at Mammalian Melatonin Receptors. J. Pharmacol. Exp. Ther. 2021, 376, 306–321. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Qasim, M.; Ahn, C.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef]
- Mehaisen, G.M.; Saeed, A.M.; Gad, A.; Abass, A.O.; Arafa, M.; El-Sayed, A. Antioxidant Capacity of Melatonin on Preimplantation Development of Fresh and Vitrified Rabbit Embryos: Morphological and Molecular Aspects. PLoS ONE 2015, 10, e0139814. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; Gao, C.; He, C.; Fu, Y.; Ji, P.; Li, Y.; Li, N.; Liu, G. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 2014, 56, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Chernyshov, V.; Kryukova, E.; Tsetlin, V.; Komisarenko, S.; Skok, M. Mitochondria express alpha7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: Study on isolated mitochondria. PLoS ONE 2012, 7, e31361. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Partridge, R.J.; Houghton, F.D.; Cox, C.I.; Leese, H.J. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J. Reprod. Fertil. 1996, 106, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D.; Thompson, J.G.; Kennedy, C.J.; Leese, H.J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 1996, 44, 476–485. [Google Scholar] [CrossRef]
- Morado, S.; Cetica, P.; Beconi, M.; Thompson, J.G.; Dalvit, G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction 2013, 145, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.M.; Johnson, M.H. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991, 113, 551–560. [Google Scholar] [CrossRef]
- Thomas, M.; Jain, S.; Kumar, G.P.; Laloraya, M. A programmed oxyradical burst causes hatching of mouse blastocysts. J. Cell Sci. 1997, 110 Pt 14, 1597–1602. [Google Scholar] [CrossRef]
- Ahelik, A.; Mandar, R.; Korrovits, P.; Karits, P.; Talving, E.; Rosenstein, K.; Jaagura, M.; Salumets, A.; Kullisaar, T. Systemic oxidative stress could predict assisted reproductive technique outcome. J. Assist. Reprod. Genet. 2015, 32, 699–704. [Google Scholar] [CrossRef]
- Ali, A.A.; Bilodeau, J.F.; Sirard, M.A. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003, 59, 939–949. [Google Scholar] [CrossRef]
- Swain, J.E.; Cabrera, L.; Xu, X.; Smith, G.D. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod. Biomed. Online 2012, 24, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Karagenc, L.; Sertkaya, Z.; Ciray, N.; Ulug, U.; Bahceci, M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod. Biomed. Online 2004, 9, 409–417. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, S.; Li, Y.; Ghebre, Y.T.; Cooke, J.P. Optimal ROS Signaling Is Critical for Nuclear Reprogramming. Cell Rep. 2016, 15, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.C.; Tatone, C.; Delle Monache, S.; Marci, R.; Caserta, D.; Colonna, R.; Amicarelli, F. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 2003, 9, 639–643. [Google Scholar] [CrossRef]
- Bao, Z.; Li, G.; Wang, R.; Xue, S.; Zeng, Y.; Deng, S. Melatonin Improves Quality of Repeated-Poor and Frozen-Thawed Embryos in Human, a Prospective Clinical Trial. Front. Endocrinol. 2022, 13, 853999. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, K.; Zhang, C.; Chen, B.; Zou, H.; Zou, W.; Xue, R.; Ji, D.; Yu, Z.; Rao, B.; et al. Effect of melatonin on the clinical outcome of patients with repeated cycles after failed cycles of in vitro fertilization and intracytoplasmic sperm injection. Zygote 2022, 30, 471–479. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, E.A.; Kim, H.J.; Choi, W.Y.; Cho, J.H.; Lee, W.S.; Cha, K.Y.; Kim, Y.S.; Lee, D.R.; Yoon, T.K. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online 2013, 26, 22–29. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhou, Y.; Tan, D.; Zhu, S.; Dai, Y.; Liu, G. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: Implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014, 9, e93641. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, X.; Zhang, L.; Tan, D.; Reiter, R.J.; Liu, G. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J. Pineal Res. 2013, 55, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- McClure, B.J.; Hercus, T.R.; Cambareri, B.A.; Woodcock, J.M.; Bagley, C.J.; Howlett, G.J.; Lopez, A.F. Molecular assembly of the ternary granulocyte-macrophage colony-stimulating factor receptor complex. Blood 2003, 101, 1308–1315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peralta, O.A.; Bucher, D.; Fernandez, A.; Berland, M.; Strobel, P.; Ramirez, A.; Ratto, M.H.; Concha, I. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances cumulus cell expansion in bovine oocytes. Reprod. Biol. Endocrinol. 2013, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Nicola, N. Guidebook to Cytokines and Their Receptors, 1st ed.; Guidebook series; Oxford University Press: Oxford, NY, USA, 1994; pp. xv, 261p; Illustrations (some color). [Google Scholar]
- Robertson, S.A.; Roberts, C.T.; Farr, K.L.; Dunn, A.R.; Seamark, R.F. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol. Reprod. 1999, 60, 251–261. [Google Scholar] [CrossRef]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol. Reprod. 1992, 46, 1069–1079. [Google Scholar] [CrossRef]
- Zhao, Y.; Chegini, N. The expression of granulocyte macrophage-colony stimulating factor (GM-CSF) and receptors in human endometrium. Am. J. Reprod. Immunol. 1999, 42, 303–311. [Google Scholar] [CrossRef]
- Zhao, Y.; Chegini, N. Human fallopian tube expresses granulocyte-macrophage colony stimulating factor (GM-CSF) and GM-CSF alpha and beta receptors and contain immunoreactive GM-CSF protein. J. Clin. Endocrinol. Metab. 1994, 79, 662–665. [Google Scholar] [CrossRef]
- Giacomini, G.; Tabibzadeh, S.S.; Satyaswaroop, P.G.; Bonsi, L.; Vitale, L.; Bagnara, G.P.; Strippoli, P.; Jasonni, V.M. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum. Reprod. 1995, 10, 3259–3263. [Google Scholar] [CrossRef]
- Robertson, S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Crainie, M.; Guilbert, L.; Wegmann, T.G. Expression of novel cytokine transcripts in the murine placenta. Biol. Reprod. 1990, 43, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol. Reprod. 1996, 54, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Jokhi, P.P.; King, A.; Loke, Y.W. Production of granulocyte-macrophage colony-stimulating factor by human trophoblast cells and by decidual large granular lymphocytes. Hum. Reprod. 1994, 9, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol. Reprod. 2002, 67, 1817–1823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertson, S.A.; Sjoblom, C.; Jasper, M.J.; Norman, R.J.; Seamark, R.F. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol. Reprod. 2001, 64, 1206–1215. [Google Scholar] [CrossRef]
- Sjoblom, C.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 1999, 14, 3069–3076. [Google Scholar] [CrossRef]
- Chin, P.Y.; Macpherson, A.M.; Thompson, J.G.; Lane, M.; Robertson, S.A. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF). Hum. Reprod. 2009, 24, 2997–3009. [Google Scholar] [CrossRef]
- Desai, N.; Kattal, N.; AbdelHafez, F.F.; Szeptycki-Lawson, J.; Goldfarb, J. Granulocyte-macrophage colony stimulating factor (GM-CSF) and co-culture can affect post-thaw development and apoptosis in cryopreserved embryos. J. Assist. Reprod. Genet. 2007, 24, 215–222. [Google Scholar] [CrossRef][Green Version]
- Martinez-Moczygemba, M.; Huston, D.P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J. Allergy Clin. Immunol. 2003, 112, 653–665, quiz 666. [Google Scholar]
- Chu, D.; Fu, L.; Zhou, W.; Li, Y. Relationship between granulocyte-macrophage colony-stimulating factor, embryo quality, and pregnancy outcomes in women of different ages in fresh transfer cycles: A retrospective study. J. Obstet. Gynaecol. 2020, 40, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.D.; Barry, M.F.; Dunstan, E.V.; Yuen, S.M.; Cameron, L.P.; Knight, E.J.; Norman, R.J.; Hull, M.L. The BlastGen study: A randomized controlled trial of blastocyst media supplemented with granulocyte-macrophage colony-stimulating factor. Reprod. Biomed. Online 2020, 40, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ziebe, S.; Loft, A.; Povlsen, B.B.; Erb, K.; Agerholm, I.; Aasted, M.; Gabrielsen, A.; Hnida, C.; Zobel, D.P.; Munding, B.; et al. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil. Steril. 2013, 99, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Wallberg, K.A.; Munding, B.; Ziebe, S.; Robertson, S.A. GM-CSF does not rescue poor-quality embryos: Secondary analysis of a randomized controlled trial. Arch. Gynecol. Obstet. 2020, 301, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Tevkin, S.; Lokshin, V.; Shishimorova, M.; Polumiskov, V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecol. Endocrinol. 2014, 30 (Suppl. S1), 9–12. [Google Scholar] [CrossRef] [PubMed]
- Sheikholslami, B.; Salehnia, M.; Valojerdi, M.R.; Ramezanzadeh, M. Developmental potential of isolated blastomeres from early mouse embryos in the presence and absence of LIF and GM-CSF. J. Assist. Reprod. Genet. 2008, 25, 7–12. [Google Scholar] [CrossRef][Green Version]
- Bassiouny, Y.A.; Dakhly, D.M.R.; Bayoumi, Y.A.; Hashish, N.M. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil. Steril. 2016, 105, 697–702. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Bassiouny, Y.A.; Bayoumi, Y.A.; Hassan, M.A.; Gouda, H.M.; Hassan, A.A. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: Randomized control trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 161–165. [Google Scholar] [CrossRef]
- Altmae, S.; Mendoza-Tesarik, R.; Mendoza, C.; Mendoza, N.; Cucinelli, F.; Tesarik, J. Effect of Growth Hormone on Uterine Receptivity in Women With Repeated Implantation Failure in an Oocyte Donation Program: A Randomized Controlled Trial. J. Endocr. Soc. 2018, 2, 96–105. [Google Scholar] [CrossRef]
- Hazout, A.; Junca, A.; Menezo, Y.; Demouzon, J.; Cohen-Bacrie, P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod. Biomed. Online 2009, 18, 664–670. [Google Scholar] [CrossRef]
- Martin-Estal, I.; de la Garza, R.G.; Castilla-Cortazar, I. Intrauterine Growth Retardation (IUGR) as a Novel Condition of Insulin-Like Growth Factor-1 (IGF-1) Deficiency. Rev. Physiol. Biochem. Pharmacol. 2016, 170, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 activates its receptor. Elife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, B.; Hillensjo, T.; Nilsson, A.; Tornell, J.; Billig, H. Expression of insulin-like growth factor-I (IGF-I) in the rat fallopian tube: Possible autocrine and paracrine action of fallopian tube-derived IGF-I on the fallopian tube and on the preimplantation embryo. Endocrinology 1993, 133, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Smotrich, D.B.; Stillman, R.J.; Widra, E.A.; Gindoff, P.R.; Kaplan, P.; Graubert, M.; Johnson, K.E. Immunocytochemical localization of growth factors and their receptors in human pre-embryos and Fallopian tubes. Hum. Reprod. 1996, 11, 184–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lighten, A.D.; Hardy, K.; Winston, R.M.; Moore, G.E. Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol. Reprod. Dev. 1997, 47, 134–139. [Google Scholar] [CrossRef]
- Lighten, A.D.; Moore, G.E.; Winston, R.M.; Hardy, K. Routine addition of human insulin-like growth factor-I ligand could benefit clinical in-vitro fertilization culture. Hum. Reprod. 1998, 13, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, T.L.; Chegini, N. Immunohistochemical localization of insulin-like growth factor (IGF-I), IGF-I receptor, and IGF binding proteins 1-4 in human fallopian tube at various reproductive stages. Biol. Reprod. 1994, 50, 281–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oosterhuis, G.J.; Vermes, I.; Lambalk, C.B.; Michgelsen, H.W.; Schoemaker, J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum. Reprod. 1998, 13, 285–289. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Harvey, M.B.; Kaye, P.L. IGF-2 stimulates growth and metabolism of early mouse embryos. Mech. Dev. 1992, 38, 169–173. [Google Scholar] [CrossRef]
- Adashi, E.Y.; Resnick, C.E.; D’Ercole, A.J.; Svoboda, M.E.; Van Wyk, J.J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr. Rev. 1985, 6, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.J.; Flint, D.J.; Patel, K. Insulin-like growth factor axis during embryonic development. Reproduction 2001, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Godin, P.A.; Nisolle, M.; Donnez, J. Expression of receptors for insulin-like growth factor-I and transforming growth factor-beta in human follicles. Mol. Hum. Reprod. 2000, 6, 137–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Powell-Braxton, L.; Hollingshead, P.; Warburton, C.; Dowd, M.; Pitts-Meek, S.; Dalton, D.; Gillett, N.; Stewart, T.A. IGF-I is required for normal embryonic growth in mice. Genes. Dev. 1993, 7, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Powell-Braxton, L.; Hollingshead, P.; Giltinan, D.; Pitts-Meek, S.; Stewart, T. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann. N. Y. Acad. Sci. 1993, 692, 300–301. [Google Scholar] [CrossRef]
- White, V.; Jawerbaum, A.; Mazzucco, M.B.; Gauster, M.; Desoye, G.; Hiden, U. IGF2 stimulates fetal growth in a sex- and organ-dependent manner. Pediatr. Res. 2018, 83, 183–189. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol. Reprod. 1997, 56, 229–237. [Google Scholar] [CrossRef]
- Pantaleon, M.; Jericho, H.; Rabnott, G.; Kaye, P.L. The role of insulin-like growth factor II and its receptor in mouse preimplantation development. Reprod. Fertil. Dev. 2003, 15, 37–45. [Google Scholar] [CrossRef]
- Sirisathien, S.; Brackett, B.G. TUNEL analyses of bovine blastocysts after culture with EGF and IGF-I. Mol. Reprod. Dev. 2003, 65, 51–56. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.E.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Shelton, J.G.; White, E.R.; Ludwig, D.L.; McCubrey, J.A. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia 2006, 20, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front. Endocrinol. 2019, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chandrakanthan, V.; Day, M.L.; O’Neill, C. Direct evidence for the action of phosphatidylinositol (3,4,5)-trisphosphate-mediated signal transduction in the 2-cell mouse embryo. Biol. Reprod. 2007, 77, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Adrian, C.; Herington, P.E.L. Signal Transduction Mechanisms Underlying Growth Hormone Receptor Action. Open Endocrinol. J. 2012, 6, 13–21. [Google Scholar]
- Shiota, M.; Sugai, N.; Tamura, M.; Yamaguchi, R.; Fukushima, N.; Miyano, T.; Miyazaki, H. Correlation of mitogen-activated protein kinase activities with cell survival and apoptosis in porcine granulosa cells. Zoolog Sci. 2003, 20, 193–201. [Google Scholar] [CrossRef][Green Version]
- Becker, M.A.; Ibrahim, Y.H.; Cui, X.; Lee, A.V.; Yee, D. The IGF pathway regulates ERalpha through a S6K1-dependent mechanism in breast cancer cells. Mol. Endocrinol. 2011, 25, 516–528. [Google Scholar] [CrossRef]
- Spanos, S.; Becker, D.L.; Winston, R.M.; Hardy, K. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol. Reprod. 2000, 63, 1413–1420. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, L.; Kozhevnikova, V.; Brusentsev, E.; Jansch, S.; Amstislavsky, S.; Jewgenow, K. IGF-I Medium Supplementation Improves Singly Cultured Cat Oocyte Maturation and Embryo Development In Vitro. Animals 2021, 11, 1909. [Google Scholar] [CrossRef]
- Green, C.J.; Day, M.L. Insulin-like growth factor 1 acts as an autocrine factor to improve early embryogenesis in vitro. Int. J. Dev. Biol. 2013, 57, 837–844. [Google Scholar] [CrossRef]
- Yang, R.-f.; Xiong, X.-r.; Zi, X.-d. Effect of cysteine, insulin-like growth factor-1 and epidermis growth factor during in vitro oocyte maturation and in vitro culture of yak-cattle crossbred embryos. J. Appl. Anim. Res. 2019, 47, 463–466. [Google Scholar] [CrossRef]
- Ahumada, C.J.; Salvador, I.; Cebrian-Serrano, A.; Lopera, R.; Silvestre, M.A. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality. Animal 2013, 7, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Tainturier, D.; Pena, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef]
- Berishaj, M.; Gao, S.P.; Ahmed, S.; Leslie, K.; Al-Ahmadie, H.; Gerald, W.L.; Bornmann, W.; Bromberg, J.F. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007, 9, R32. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.; Naeth, G.; Heinrich, P.C.; Muller-Newen, G. Novel inhibitors for murine and human leukemia inhibitory factor based on fused soluble receptors. J. Biol. Chem. 2008, 283, 5985–5995. [Google Scholar] [CrossRef] [PubMed]
- Su, R.W.; Jia, B.; Ni, H.; Lei, W.; Yue, S.L.; Feng, X.H.; Deng, W.B.; Liu, J.L.; Zhao, Z.A.; Wang, T.S.; et al. Junctional adhesion molecule 2 mediates the interaction between hatched blastocyst and luminal epithelium: Induction by progesterone and LIF. PLoS ONE 2012, 7, e34325. [Google Scholar] [CrossRef]
- Frezzato, F.; Raggi, F.; Martini, V.; Severin, F.; Trimarco, V.; Visentin, A.; Scomazzon, E.; Accordi, B.; Bresolin, S.; Piazza, F.; et al. HSP70/HSF1 axis, regulated via a PI3K/AKT pathway, is a druggable target in chronic lymphocytic leukemia. Int. J. Cancer 2019, 145, 3089–3100. [Google Scholar] [CrossRef]
- Paling, N.R.; Wheadon, H.; Bone, H.K.; Welham, M.J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004, 279, 48063–48070. [Google Scholar] [CrossRef]
- Boeuf, H.; Merienne, K.; Jacquot, S.; Duval, D.; Zeniou, M.; Hauss, C.; Reinhardt, B.; Huss-Garcia, Y.; Dierich, A.; Frank, D.A.; et al. The ribosomal S6 kinases, cAMP-responsive element-binding, and STAT3 proteins are regulated by different leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J. Biol. Chem. 2001, 276, 46204–46211. [Google Scholar] [CrossRef]
- Burdon, T.; Stracey, C.; Chambers, I.; Nichols, J.; Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 1999, 210, 30–43. [Google Scholar] [CrossRef]
- Cheng, J.G.; Chen, J.R.; Hernandez, L.; Alvord, W.G.; Stewart, C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA 2001, 98, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Takahashi, M.; Carpino, N.; Jou, S.T.; Chao, J.R.; Tanaka, S.; Shigeyoshi, Y.; Parganas, E.; Ihle, J.N. Leukemia inhibitory factor regulates trophoblast giant cell differentiation via Janus kinase 1-signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway. Mol. Endocrinol. 2008, 22, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.; Oxford, J.T.; Jorcyk, C.L. Emerging Perspectives on Leukemia Inhibitory Factor and its Receptor in Cancer. Front. Oncol. 2021, 11, 693724. [Google Scholar] [CrossRef] [PubMed]
- Auernhammer, C.J.; Melmed, S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr. Rev. 2000, 21, 313–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lafdil, F.; Kong, X.; Gao, B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 2011, 7, 536–550. [Google Scholar] [CrossRef]
- Liu, L.; McBride, K.M.; Reich, N.C. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc. Natl. Acad. Sci. USA 2005, 102, 8150–8155. [Google Scholar] [CrossRef]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Kujawski, M.; Kortylewski, M.; Lee, H.; Herrmann, A.; Kay, H.; Yu, H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Investig. 2008, 118, 3367–3377. [Google Scholar] [CrossRef]
- Basseres, D.S.; Baldwin, A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef]
- Li, H.; Lee, J.; He, C.; Zou, M.H.; Xie, Z. Suppression of the mTORC1/STAT3/Notch1 pathway by activated AMPK prevents hepatic insulin resistance induced by excess amino acids. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E197–E209. [Google Scholar] [CrossRef]
- Teng, T.S.; Lin, B.; Manser, E.; Ng, D.C.; Cao, X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J. Cell Sci. 2009, 122, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Ma, Y.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D.; et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, Z.G.; Tian, X.Q.; Sun, D.F.; Liang, Q.C.; Zhang, Y.J.; Lu, R.; Chen, Y.X.; Fang, J.Y. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008, 10, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Malaise, M.; Reinhardt, B.; Kedinger, C.; Boeuf, H. A p38 inhibitor allows to dissociate differentiation and apoptotic processes triggered upon LIF withdrawal in mouse embryonic stem cells. Cell Death Differ. 2004, 11, 331–341. [Google Scholar] [CrossRef]
- van Eijk, M.J.; Mandelbaum, J.; Salat-Baroux, J.; Belaisch-Allart, J.; Plachot, M.; Junca, A.M.; Mummery, C.L. Expression of leukaemia inhibitory factor receptor subunits LIFR beta and gp130 in human oocytes and preimplantation embryos. Mol. Hum. Reprod. 1996, 2, 355–360. [Google Scholar] [CrossRef]
- Margioula-Siarkou, C.; Prapas, Y.; Petousis, S.; Milias, S.; Ravanos, K.; Kalogiannidis, I.; Mavromatidis, G.; Haitoglou, C.; Prapas, N.; Rousso, D. LIF and LIF-R expression in the endometrium of fertile and infertile women: A prospective observational case-control study. Mol. Med. Rep. 2016, 13, 4721–4728. [Google Scholar] [CrossRef]
- Serafini, P.; Rocha, A.M.; Osorio, C.T.; da Silva, I.; Motta, E.L.; Baracat, E.C. Endometrial leukemia inhibitory factor as a predictor of pregnancy after in vitro fertilization. Int. J. Gynaecol. Obstet. 2008, 102, 23–27. [Google Scholar] [CrossRef]
- Hambartsoumian, E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am. J. Reprod. Immunol. 1998, 39, 137–143. [Google Scholar] [CrossRef]
- Steck, T.; Giess, R.; Suetterlin, M.W.; Bolland, M.; Wiest, S.; Poehls, U.G.; Dietl, J. Leukaemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transfer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 69–73. [Google Scholar] [CrossRef]
- Nichols, J.; Chambers, I.; Taga, T.; Smith, A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 2001, 128, 2333–2339. [Google Scholar] [CrossRef]
- Kimber, S.J. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Ledee-Bataille, N.; Lapree-Delage, G.; Taupin, J.L.; Dubanchet, S.; Frydman, R.; Chaouat, G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum. Reprod. 2002, 17, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Movaghar, B.; Abkenari, S.A.; Nazari, H.; Bakhtiyari, M. Leukemia Inhibitory Factor Enhanced the Developmental and Implantation Compatibility of Mouse Embryos in Co-culture with Human Endometrial Epithelial Cells. Reprod. Dev. Med. 2021, 5, 199–205. [Google Scholar] [CrossRef]
- Mitchell, M.H.; Swanson, R.J.; Oehninger, S. In vivo effect of leukemia inhibitory factor (LIF) and an anti-LIF polyclonal antibody on murine embryo and fetal development following exposure at the time of transcervical blastocyst transfer. Biol. Reprod. 2002, 67, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Q.; Cao, Y.J.; Duan, E.K. Effects of leukaemia inhibitory factor on embryo implantation in the mouse. Cytokine 2000, 12, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, R.; Li, X.; Dai, H.; Han, X.; Wang, X.; Yang, A. LIF in embryo culture medium is a predictive marker for clinical pregnancy following IVF-ET of patients with fallopian tube obstruction. J. Reprod. Immunol. 2020, 141, 103164. [Google Scholar] [CrossRef]
- Liu, Y.L.; Lee, C.I.; Liu, C.H.; Cheng, E.H.; Yang, S.F.; Tsai, H.Y.; Lee, M.S.; Lee, T.H. Association between Leukemia Inhibitory Factor Gene Polymorphism and Clinical Outcomes among Young Women with Poor Ovarian Response to Assisted Reproductive Technology. J. Clin. Med. 2023, 12, 796. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tsai, H.D.; Chang, C.C.; Hsu, L.W.; Chang, S.C.; Lo, H.Y. Prolonged culture of human cryopreserved embryos with recombinant human leukemia inhibitory factor. J. Assist. Reprod. Genet. 2000, 17, 131–134. [Google Scholar] [CrossRef]
- Eswari, S.; Sai Kumar, G.; Sharma, G.T. Expression of mRNA encoding leukaemia inhibitory factor (LIF) and its receptor (LIFRbeta) in buffalo preimplantation embryos produced in vitro: Markers of successful embryo implantation. Zygote 2013, 21, 203–213. [Google Scholar] [CrossRef]
- Fawzy, M.; Emad, M.; Elsuity, M.A.; Mahran, A.; Abdelrahman, M.Y.; Fetih, A.N.; Abdelghafar, H.; Sabry, M.; Nour, M.; Rasheed, S.M. Cytokines hold promise for human embryo culture in vitro: Results of a randomized clinical trial. Fertil. Steril. 2019, 112, 849–857 e841. [Google Scholar] [CrossRef]
- Stoecklein, K.S.; Ortega, M.S.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 2021, 16, e0243727. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).