5-HT2A Receptor Knockout Mice Show Sex-Dependent Differences following Acute Noribogaine Administration
Abstract
1. Introduction
2. Results
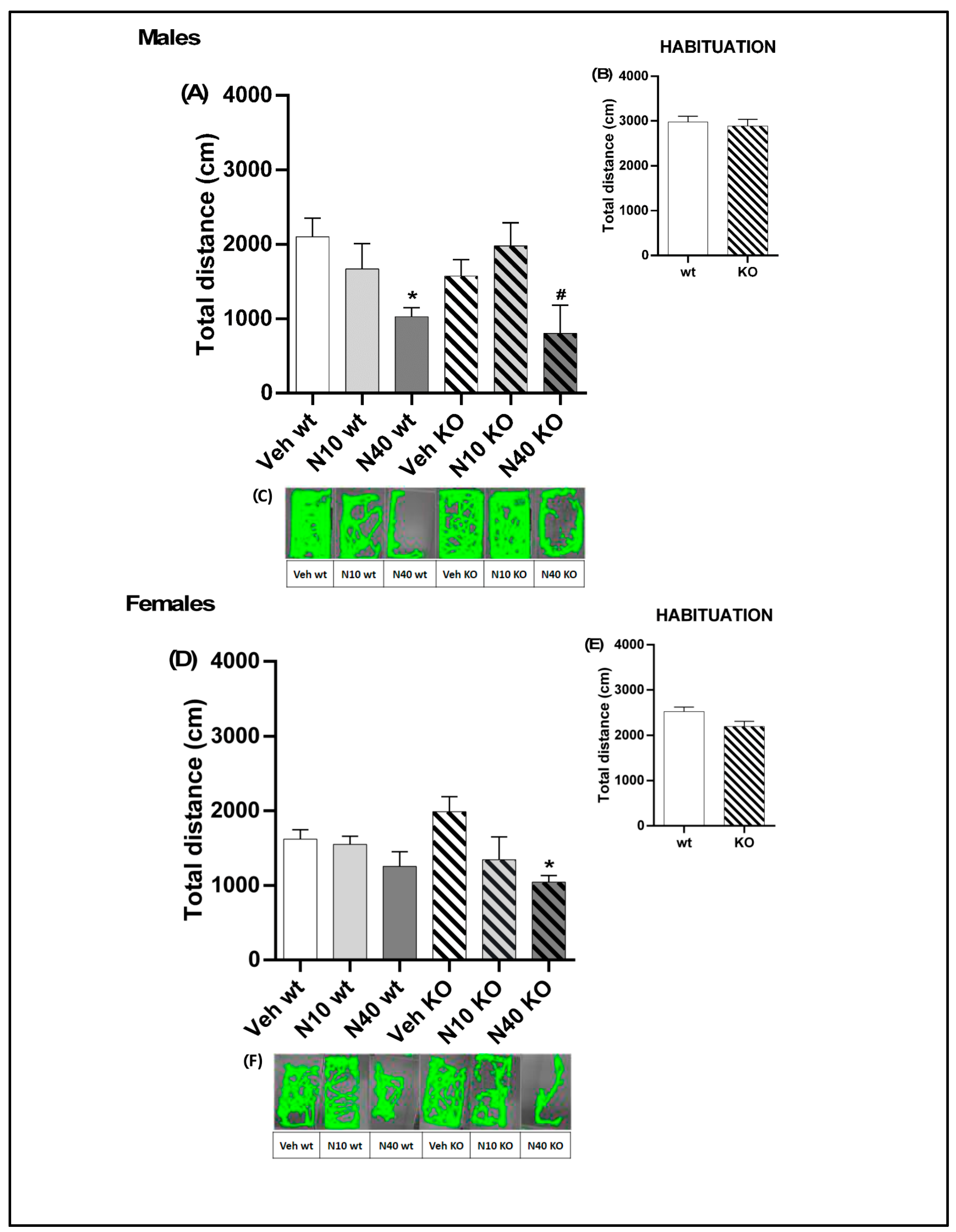
2.1. A Single Administration of Noribogaine Produced Differential Effects on Locomotion in Male vs. Female Mice
2.2. 5-HT2A Receptor Deficiency Alters the Gene Expression Profile Induced by Noribogaine in mPFC in a Sex-Dependent Manner
2.3. 5-HT2A Receptor Plays a Role in the NMDA Current Density of Pyramidal mPFC Neurons in Male WT Mice Following Single Administration of Noribogaine
3. Discussion
The Neuroprotective and Neuroplastic Potential of Noribogaine at mPFC Pyramidal Neurons: The Contribution of NMDA and 5-HT2A Receptors to Rapidly Promoting Plasticity
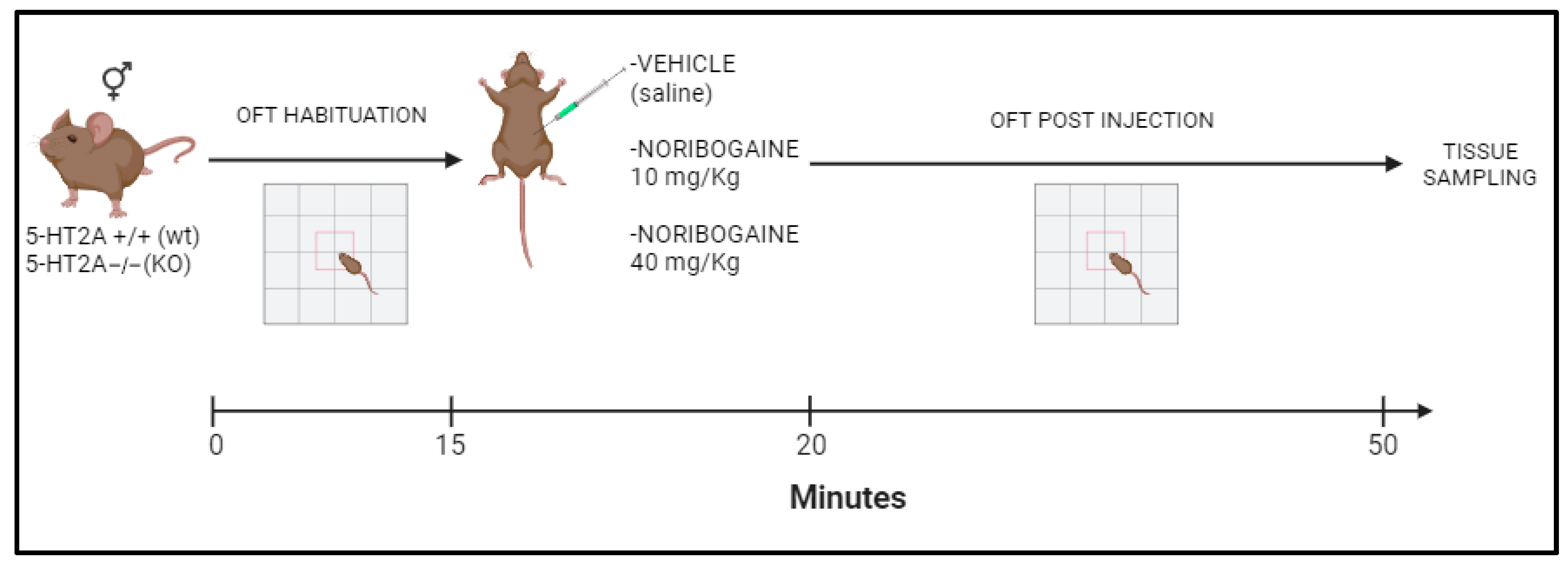
4. Material and Methods
4.1. Animals
4.2. Drugs
4.3. Behavioral Test
Locomotor Activity
4.4. Real-Time qPCR
4.5. Single-Cell Electrophysiological Recordings in Slices
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Sanacora, G.; Woolley, J.; Heinzerling, K.; Dunlop, B.W.; Brown, R.T.; Kakar, R.; Hassman, M.; Trivedi, R.P.; Robison, R.; et al. Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial. JAMA 2023, 330, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Ot’alora, G.M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedo, S.; Balliett, B.; et al. MAPP2 Study Collaborator Group. MDMA-assisted therapy for moderate to severe PTSD: A randomized, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’Donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, S.; Ameli, R.; Sinaii, N.; Berger, A.; Agrawal, M. Psilocybin-assisted therapy improves psycho-social-spiritual well-being in cancer patients. J. Affect. Disord. 2023, 323, 592–597. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef]
- Miner, L.A.H.; Backstrom, J.R.; Sanders-Bush, E.; Sesack, S.R. Ultrastructural localization of serotonin 2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 2003, 116, 107–117. [Google Scholar] [CrossRef]
- Weisstaub, N.V.; Zhou, M.; Lira, A.; Lambe, E.; González-Maeso, J.; Hornung, J.P.; Sibille, E.; Underwood, M.; Itohara, S.; Dauer, W.T.; et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 2006, 313, 536–540. [Google Scholar] [CrossRef]
- Weber, E.T.; Andrade, R. Htr2a Gene and 5-HT2A Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front. Neurosci. 2010, 4, 36. [Google Scholar] [CrossRef]
- Lavaud, C.; Massiot, G. The Iboga Alkaloids. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2017; Volume 105, pp. 89–136. [Google Scholar] [CrossRef]
- González, B.; Fagundez, C.; Peixoto de Abreu Lima, A.; Suescun, L.; Sellanes, D.; Seoane, G.A.; Carrera, I. Efficient Access to the Iboga Skeleton: Optimized Procedure to Obtain Voacangine from Voacanga africana Root Bark. ACS Omega 2021, 6, 16755–16762. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.N.; Favela, D.; Zhang, G.; Olson, D.E. The iboga enigma: The chemistry and neuropharmacology of iboga alkaloids and related analogs. Nat. Prod. Rep. 2021, 38, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Pablo, J.; Mash, D.C. Cytochrome P4502D6 catalyzes the O-demethylation of the psychoactive alkaloid ibogaine to 12-hydroxyibogamine. Drug Metab. Dispos. 1998, 26, 764–768. [Google Scholar] [PubMed]
- Rodríguez, P.; Urbanavicius, J.; Prieto, J.P.; Fabius, S.; Reyes, A.L.; Havel, V.; Sames, D.; Scorza, C.; Carrera, I. A Single Administration of the Atypical Psychedelic Ibogaine or Its Metabolite Noribogaine Induces an Antidepressant-Like Effect in Rats. ACS Chem. Neurosci. 2020, 11, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Alper, K.R.; Lotsof, H.S.; Frenken, G.M.; Luciano, D.J.; Bastiaans, J. Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict. 1999, 8, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Mash, D.C.; Kovera, C.A.; Pablo, J.; Tyndale, R.F.; Ervin, F.D.; Williams, I.C.; Singleton, E.G.; Mayor, M. Ibogaine: Complex Pharmacokinetics, Concerns for Safety, and Preliminary Efficacy Measures. Ann. N. Y. Acad. Sci. 2000, 914, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Schenberg, E.E.; de Castro Comis, M.A.; Chaves, B.R.; da Silveira, D.X. Treating drug dependence with the aid of ibogaine: A retrospective study. J. Psychopharmacol. 2014, 28, 993–1000. [Google Scholar] [CrossRef]
- Mash, D.C.; Duque, L.; Page, B.; Allen-Ferdinand, K. Ibogaine Detoxification Transitions Opioid and Cocaine Abusers Between Dependence and Abstinence: Clinical Observations and Treatment Outcomes. Front. Pharmacol. 2018, 9, 529. [Google Scholar] [CrossRef]
- Köck, P.; Frölich, K.; Walter, M.; Lang, U.; Dürsteler, K.M. A systematic literature review of clinical trials and therapeutic applications of ibogaine. J. Subst. Abuse Treat. 2021, 138, 108717. [Google Scholar] [CrossRef]
- Wasko, M.J.; Witt-Enderby, P.A.; Surratt, C.K. DARK Classics in Chemical Neuroscience: Ibogaine. ACS Chem. Neurosci. 2018, 9, 2475–2483. [Google Scholar] [CrossRef]
- Mash, D.C.; Staley, J.K.; Pablo, J.P.; Holohean, A.M.; Hackman, J.C.; Davidoff, R.A. Properties of ibogaine and its principal metabolite (12-hydroxyibogamine) at the MK-801 binding site of the NMDA receptor complex. Neurosci. Lett. 1995, 192, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.T.; Skolnick, P.; Bertha, C.M.; Bandarage, U.K.; Kuehne, M.E.; Popik, P. Structurally modified ibogaine analogs exhibit differing affinities for NMDA receptors. Eur. J. Pharmacol. 1996, 309, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.K.; Ouyang, Q.; Pablo, J.; Hearn, W.L.; Flynn, D.D.; Rothman, R.B.; Rice, K.C.; Mash, D.C. Pharmacological screen for activities of 12-hydroxyibogamine: A primary metabolite of the indole alkaloid ibogaine. Psychopharmacology 1996, 127, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Cape, G.; Tunnicliff, D.; Lockhart, M.; Lam, F.; Hung, N.; Hung, C.T.; Harland, S.; Devane, J.; Crockett, R.S.; et al. Ascending Single-Dose, Double-Blind, Placebo-Controlled Safety Study of Noribogaine in Opioid-Dependent Patients. Clin. Pharmacol. Drug Dev. 2016, 5, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.W.; Yourish, H.B.; Ionescu, D.F.; Haggarty, S.J. Classics in Chemical Neuroscience: Ketamine. ACS Chem. Neurosci. 2017, 8, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alvarado, R.B.; Madariaga-Mazón, A.; Ortega, A.; Martinez-Mayorga, K. DARK Classics in Chemical Neuroscience: Salvinorin A. ACS Chem. Neurosci. 2020, 11, 3979–3992. [Google Scholar] [CrossRef] [PubMed]
- Helsley, S.; Fiorella, D.; Rabin, R.A.; Winter, J.C. Behavioral and biochemical evidence for a nonessential 5-HT2A component of the ibogaine-induced discriminative stimulus. Pharmacol. Biochem. Behav. 1998, 59, 419–425. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Prieto, J.P.; Rodríguez, P.; Cavelli, M.; Benedetto, L.; Mondino, A.; Pazos, M.; Seoane, G.; Carrera, I.; Scorza, C.; et al. Ibogaine Acute Administration in Rats Promotes Wakefulness, Long-Lasting REM Sleep Suppression, and a Distinctive. Motor Profile. Front. Pharmacol. 2018, 9, 374. [Google Scholar] [CrossRef]
- González, J.; Cavelli, M.; Castro-Zaballa, S.; Mondino, A.; Tort, A.B.; Rubido, N.; Carrera, I.; Torterolo, P. EEG Gamma Band Alterations and REM-like Traits Underpin the Acute Effect of the Atypical Psychedelic Ibogaine in the Rat. ACS Pharmacol. Transl. Sci. 2021, 4, 517–525. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Pearl, S.M.; Hough, L.B.; Boyd, D.L.; Glick, S.D. Sex differences in ibogaine antagonism of morphine-induced locomotor activity and in ibogaine brain levels and metabolism. Pharmacol. Biochem. Behav. 1997, 57, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Tatalović, N.; Vidonja Uzelac, T.; Mijović, M.; Koželj, G.; Nikolić-Kokić, A.; Oreščanin Dušić, Z.; Bresjanac, M.; Blagojević, D. Ibogaine Has Sex-Specific Plasma Bioavailability, Histopathological and Redox/Antioxidant Effects in Rat Liver and Kidneys: A Study on Females. Life 2021, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Glick, S.D.; Pearl, S.M.; Cai, J.; Maisonneuve, I.M. Ibogaine-like effects of noribogaine in rats. Brain Res. 1996, 713, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Maillet, E.L.; Milon, N.; Heghinian, M.D.; Fishback, J.; Schürer, S.C.; Garamszegi, N.; Mash, D.C. Noribogaine is a G-protein biased κ-opioid receptor agonist. Neuropharmacology 2015, 99, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.; Sandin, J.; Terenius, L.; Ogren, S.O. Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J. Pharmacol. Exp. Ther. 2000, 295, 1031–1042. [Google Scholar] [PubMed]
- Yuen, E.Y.; Jiang, Q.; Chen, P.; Feng, J.; Yan, Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J. Biol. Chem. 2008, 283, 17194–17204. [Google Scholar] [CrossRef]
- Jaggar, M.; Banerjee, T.; Weisstaub, N.; Gingrich, J.A.; Vaidya, V.A. 5-HT2A receptor loss does not alter acute fluoxetine-induced anxiety and exhibit sex-dependent regulation of cortical immediate early gene expression. Neuronal Signal. 2019, 3, NS20180205. [Google Scholar] [CrossRef]
- González, B.; Rivero-Echeto, C.; Muñiz, J.A.; Cadet, J.L.; García-Rill, E.; Urbano, F.J.; Bisagno, V. Methamphetamine blunts Ca2+ currents and excitatory synaptic transmission through D1/5 receptor-mediated mechanisms in the mouse medial prefrontal cortex. Addict. Biol. 2016, 21, 589–602. [Google Scholar] [CrossRef]
- Myme, C.I.O.; Sugino, K.; Turrigiano, G.G.; Nelson, S.B. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J. Neurophysiol. 2003, 90, 771–779. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Carroll, N.K.; Brown, M.; MacMillan, C.; Silenieks, L.B. Low Doses of Psilocybin and Ketamine Enhance Motivation and Attention in Poor Performing Rats: Evidence for an Antidepressant Property. Front. Pharmacol. 2021, 12, 640241. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.H.; Nedivi, E. Activity-regulated genes as mediators of neural circuit plasticity. Prog. Neurobiol. 2011, 94, 223–237. [Google Scholar] [CrossRef]
- Veyrac, A.; Besnard, A.; Caboche, J.; Davis, S.; Laroche, S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog. Mol. Biol. Transl. Sci. 2014, 122, 89–129. [Google Scholar] [CrossRef]
- Spiegel, I.; Mardinly, A.R.; Gabel, H.W.; Bazinet, J.E.; Couch, C.H.; Tzeng, C.P.; Harmin, D.A.; Greenberg, M.E. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 2014, 157, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Maya-Vetencourt, J.F.; Tiraboschi, E.; Greco, D.; Restani, L.; Cerri, C.; Auvinen, P.; Maffei, L.; Castrén, E. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J. Physiol. 2012, 590, 4777–4787. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W., Jr. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Aznar, S.; Hervig, M.E.S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci. Biobehav. Rev. 2016, 64, 63–82. [Google Scholar] [CrossRef]
- DeBattista, C. Executive dysfunction in major depressive disorder. Expert Rev. Neurother. 2005, 5, 79–83. [Google Scholar] [CrossRef]
- Qesseveur, G.; Petit, A.C.; Nguyen, H.T.; Dahan, L.; Colle, R.; Rotenberg, S.; Seif, I.; Robert, P.; David, D.; Guilloux, J.P.; et al. Genetic dysfunction of serotonin 2A receptor hampers response to antidepressant drugs: A translational approach. Neuropharmacology 2015, 105, 142–153. [Google Scholar] [CrossRef]
- Popik, P.; Layer, R.T.; Fossom, L.H.; Benveniste, M.; Geter-Douglass, B.; Witkin, J.M.; Skolnick, P. NMDA antagonist properties of the putative antiaddictive drug, ibogaine. J. Pharmacol. Exp. Ther. 1995, 275, 753–760. [Google Scholar] [PubMed]
- Chen, K.; Kokate, T.G.; Donevan, S.D.; Carroll, F.I.; Rogawski, M.A. Ibogaine block of the NMDA receptor: In vitro and in vivo studies. Neuropharmacology 1996, 35, 423–451. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Rantamäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-C gamma signaling pathways in mouse brain. Neuropsychopharmacology 2007, 32, 2152–2162. [Google Scholar] [CrossRef]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Marton, S.; González, B.; Rodríguez-Bottero, S.; Miquel, E.; Martínez-Palma, L.; Pazos, M.; Prieto, J.P.; Rodríguez, P.; Sames, D.; Seoane, G.; et al. Ibogaine Administration Modifies GDNF and BDNF Expression in Brain Regions Involved in Mesocorticolimbic and Nigral Dopaminergic Circuits. Front. Pharmacol. 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Pollina, E.A.; Gilliam, D.T.; Landau, A.T.; Lin, C.; Pajarillo, N.; Davis, C.P.; Harmin, D.A.; Yap, E.L.; Vogel, I.R.; Griffith, E.C.; et al. A NPAS4-NuA4 complex couples synaptic activity to DNA repair. Nature 2023, 614, 732–741. [Google Scholar] [CrossRef]
- Petit, A.C.; Quesseveur, G.; Gressier, F.; Colle, R.; David, D.J.; Gardier, A.M.; Ferreri, F.; Lépine, J.P.; Falissard, B.; Verstuyft, C.; et al. Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 76–82. [Google Scholar] [CrossRef]
- Covington HE 3rd Vialou, V.; Nestler, E.J. From synapse to nucleus: Novel targets for treating depression. Neuropharmacology 2010, 58, 683–693. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–245. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Diazgranados, N.; Ibrahim, L.; Brutsche, N.E.; Newberg, A.; Kronstein, P.; Khalife, S.; Kammerer, W.A.; Quezado, Z.; Luckenbaugh, D.A.; Salvadore, G.; et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 2010, 67, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, W.; Szopa, A.; Serefko, A.; Wyska, E.; Świąder, K.; Dudka, J.; Wlaź, P.; Poleszak, E. Influence of the selective antagonist of the NR2B subunit of the NMDA receptor, traxoprodil, on the antidepressant-like activity of desipramine, paroxetine, milnacipran, and bupropion in mice. J. Neural Transm. 2017, 124, 387–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bisagno, V.; Raineri, M.; Peskin, V.; Wikinski, S.I.; Uchitel, O.D.; Llinás, R.R.; Urbano, F.J. Effects of T-type calcium channel blockers on cocaine-induced hyperlocomotion and thalamocortical GABAergic abnormalities in mice. Psychopharmacology 2010, 212, 205–214. [Google Scholar] [CrossRef] [PubMed]

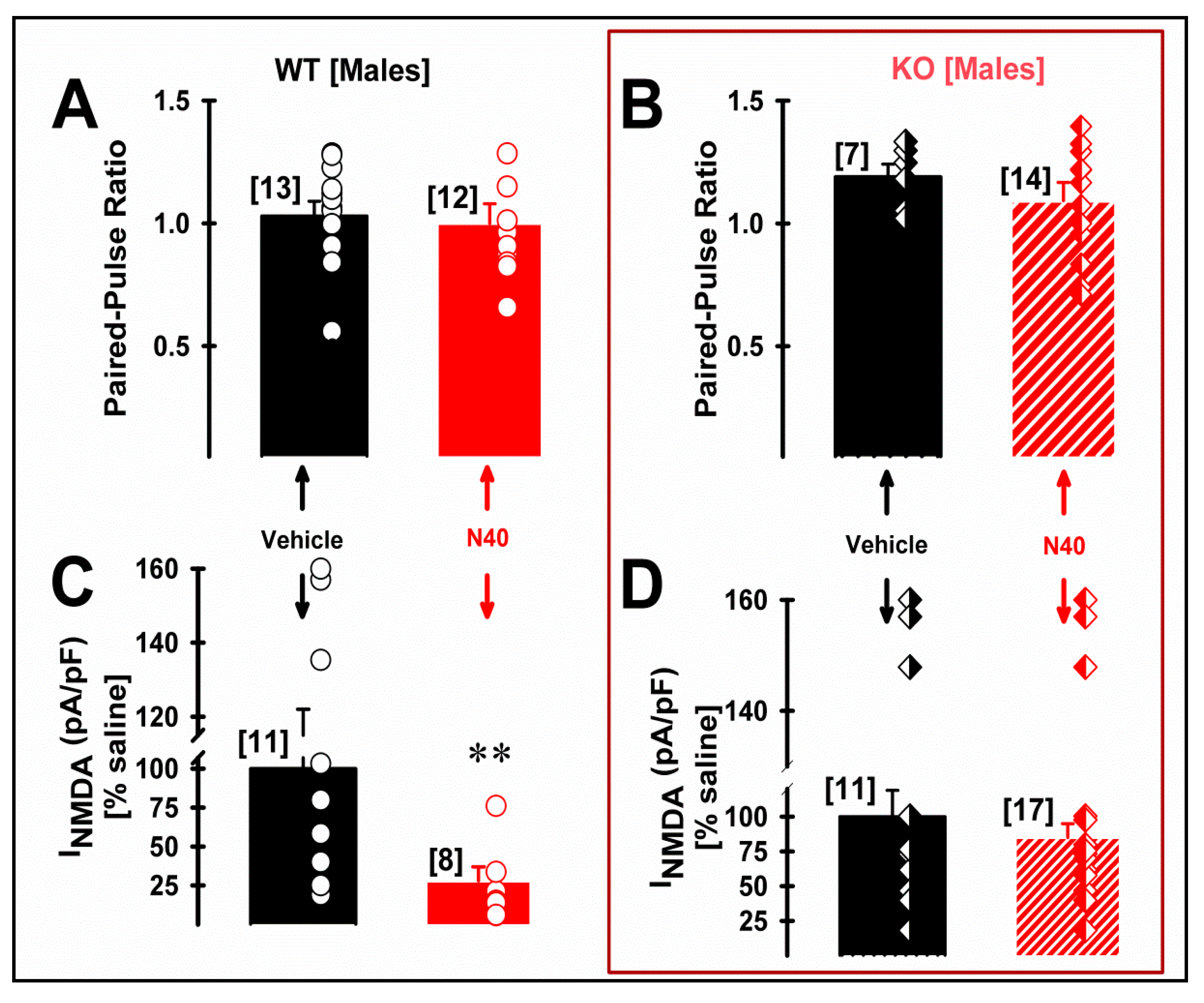

| Paired-Pulse Ratio (PPR) | INMDA (pA/pF) | NMDA/AMPA Ratio | ||||
|---|---|---|---|---|---|---|
| Wildtype | 5HT2A Knockout | Wildtype | 5HT2A Knockout | Wildtype | 5HT2A Knockout | |
| Vehicle | 1.03 ± 0.06 (13) | 1.19 ± 0.05 (7) | 6.02 ± 1.37 (11) | 2.35 ± 0.45 (11) ** | 0.75 ± 0.04 (10) | 0.55 ± 0.04 (10) && |
| Noribo 40 | 1.00 ± 0.08 (12) | 1.09 ± 0.07 (14) | 1.48 ± 0.56 (8) $ | 1.95 ± 0.22 (17) | 0.56 ± 0.03 (8) # | 0.60 ± 0.04 (10) |
| Paired-Pulse Ratio (PPR) | INMDA (pA/pF) | NMDA/AMPA Ratio | ||||
|---|---|---|---|---|---|---|
| Wildtype | 5HT2A Knockout | Wildtype | 5HT2A Knockout | Wildtype | 5HT2A Knockout | |
| Vehicle | 1.09 ± 0.03 (12) | 1.09 ± 0.05 (11) | 2.20 ± 0.49 (11) | 2.90 ± 0.45 (11) | 0.49 ± 0.04 (7) | 0.62 ± 0.05 (10) |
| Noribo 40 | 1.10 ± 0.07 (11) | 0.95 ± 0.06 (11) | 1.59 ± 0.38 (11) | 1.87 ± 0.35 (11) | 0.51 ± 0.05 (8) | 0.60 ± 0.11 (7) |
| Gene | Gene Symbol | Primer Forward | Primer Reverse |
|---|---|---|---|
| Beta Actin | Act B | TGACGTTGACATCCGTAAAG | GAGGAGCAATGATCTTGATCT |
| Neuronal PAS Domain Protein 4 | Npas4 | CATCTGGGCCACTCTATGGT | GAGGGACTTGGAGGTGTTGA |
| Early Growth Response 1 | Egr1 | GATGGTGGAGACGAGTTAT | GATTGGTCATGCTCACG |
| Fos Proto-Oncogene, AP-1 Transcription Factor Subunit | cFos | TCCCCAAACTTCGACCATGA | AGTTGGCACTAGAGACGGAC |
| Glutamate Ionotropic Receptor AMPA Type Subunit 1 | GRIA1 | CTGTGAATCAGAACGCCTCA | TCACTTGTCCTCCACTGCTG |
| Glutamate Ionotropic Receptor NMDA Type Subunit 2A | GRIN2A | TTGTCTCTGCCATTGCTGTC | CAAAGAAGGCCCACACTGAT |
| Serotonin Receptor 2A | Htr2a | CGTGTCCATGTTAACCATCC | TCAGGAAGGCTTTGGTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalba, S.; González, B.; Junge, S.; Bernardi, A.; González, J.; Fagúndez, C.; Torterolo, P.; Carrera, I.; Urbano, F.J.; Bisagno, V. 5-HT2A Receptor Knockout Mice Show Sex-Dependent Differences following Acute Noribogaine Administration. Int. J. Mol. Sci. 2024, 25, 687. https://doi.org/10.3390/ijms25020687
Villalba S, González B, Junge S, Bernardi A, González J, Fagúndez C, Torterolo P, Carrera I, Urbano FJ, Bisagno V. 5-HT2A Receptor Knockout Mice Show Sex-Dependent Differences following Acute Noribogaine Administration. International Journal of Molecular Sciences. 2024; 25(2):687. https://doi.org/10.3390/ijms25020687
Chicago/Turabian StyleVillalba, Sofía, Bruno González, Stephanie Junge, Alejandra Bernardi, Joaquín González, Catherine Fagúndez, Pablo Torterolo, Ignacio Carrera, Francisco J. Urbano, and Verónica Bisagno. 2024. "5-HT2A Receptor Knockout Mice Show Sex-Dependent Differences following Acute Noribogaine Administration" International Journal of Molecular Sciences 25, no. 2: 687. https://doi.org/10.3390/ijms25020687
APA StyleVillalba, S., González, B., Junge, S., Bernardi, A., González, J., Fagúndez, C., Torterolo, P., Carrera, I., Urbano, F. J., & Bisagno, V. (2024). 5-HT2A Receptor Knockout Mice Show Sex-Dependent Differences following Acute Noribogaine Administration. International Journal of Molecular Sciences, 25(2), 687. https://doi.org/10.3390/ijms25020687







