Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport
Abstract
1. Introduction
1.1. Physical and Chemical Method of Hydrogen Storage
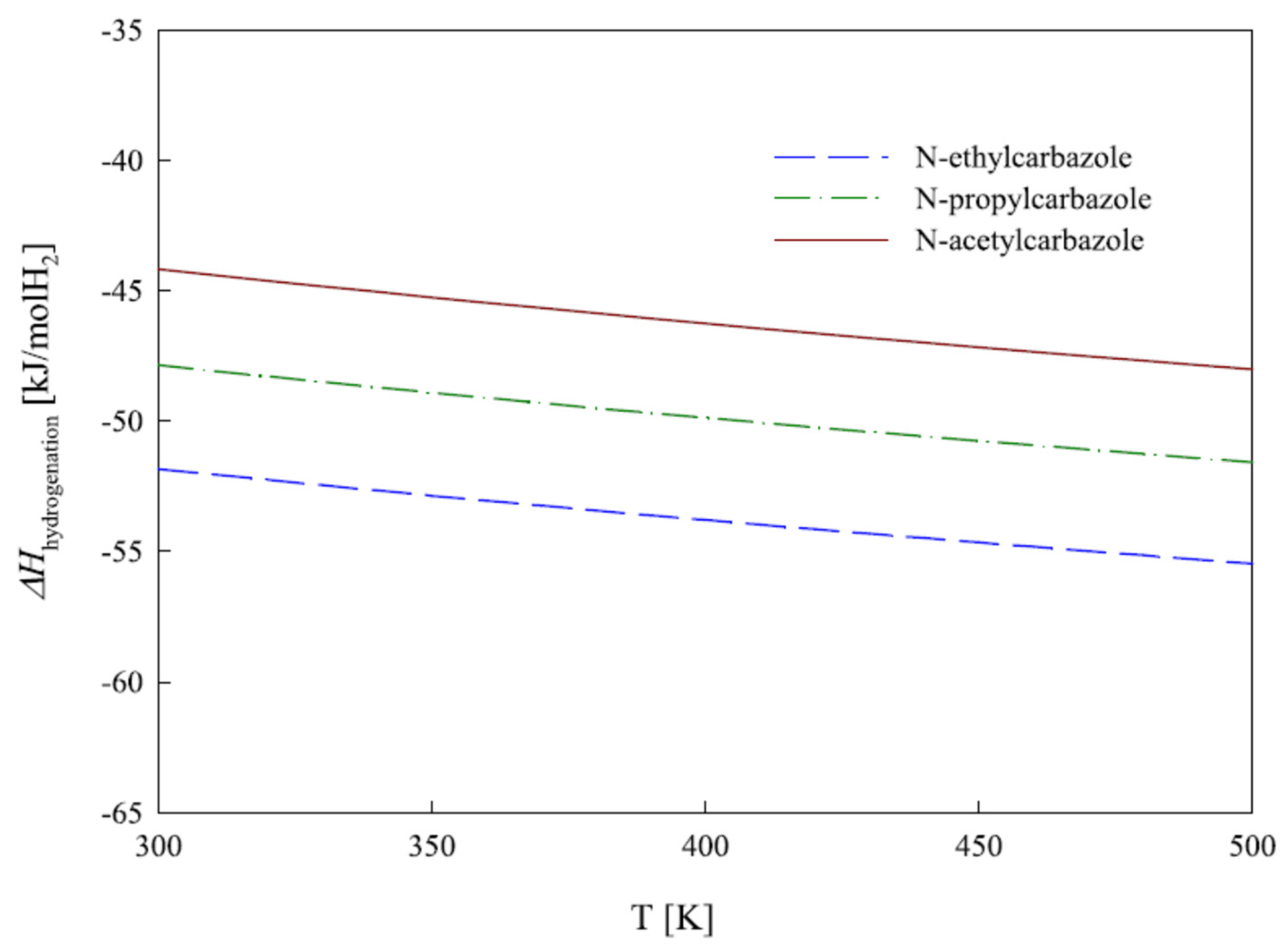
1.2. Thermodynamics of Hydrogen Storage
- = free energy of the reaction;
- = enthalpy of the reaction;
- = entropy of the reaction;
- T = temperature.
1.3. Techniques of H Storage
1.4. The Risks Linked with the LOHC Systems
1.5. Kinetics of Hydrogen Storage
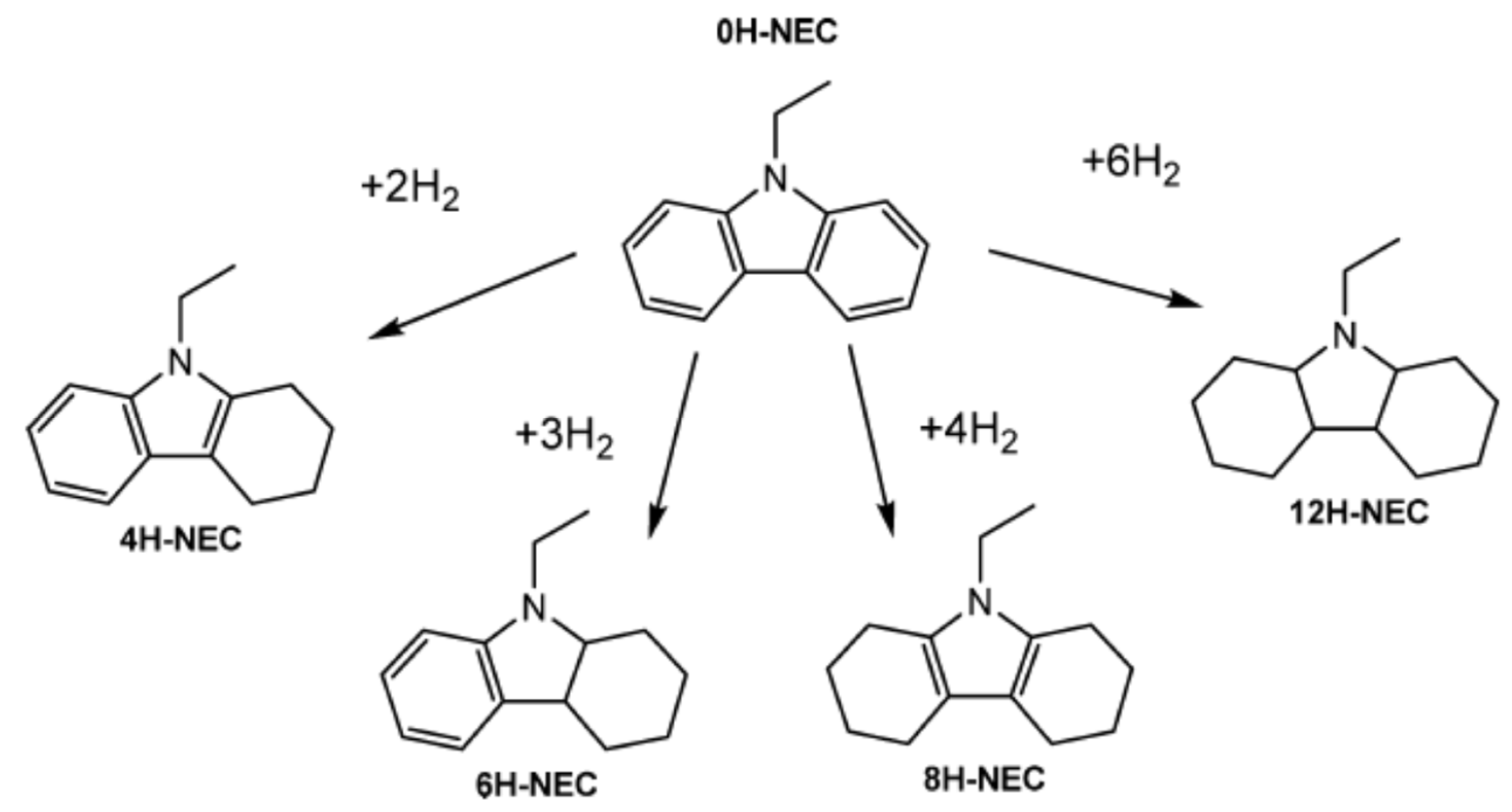
1.6. Effect of Melting Points of N-Alkyl Carbazoles
2. LOHC Molecules
2.1. N-Alkylcarbazole
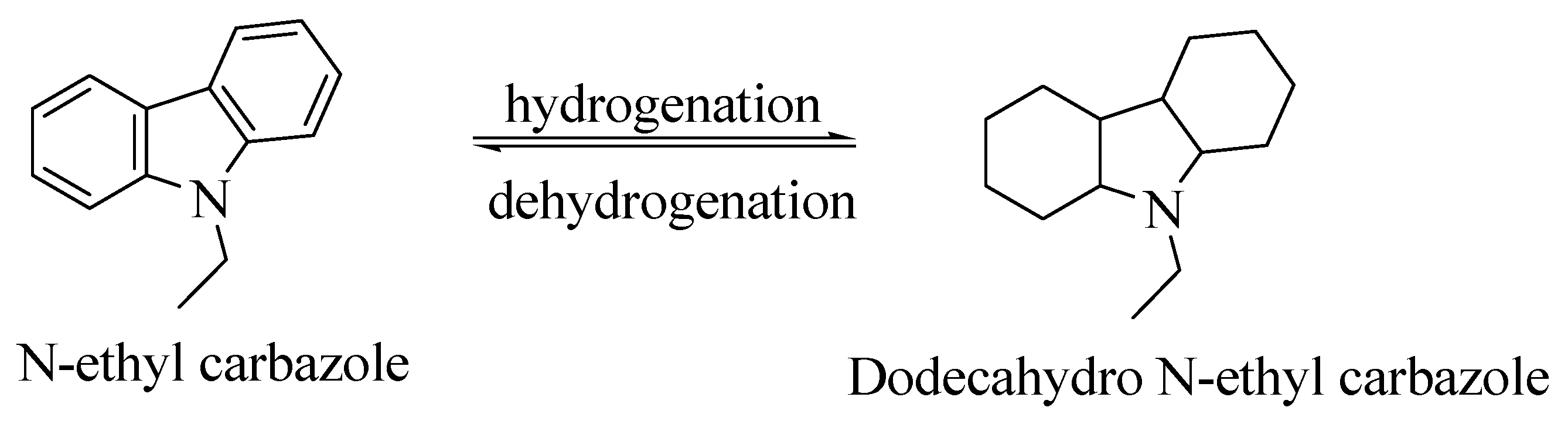
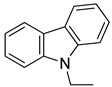
2.1.1. N-Ethyl Carbazole
2.1.2. N-Propyl Carbazole
2.2. 2-Amino Ethanol
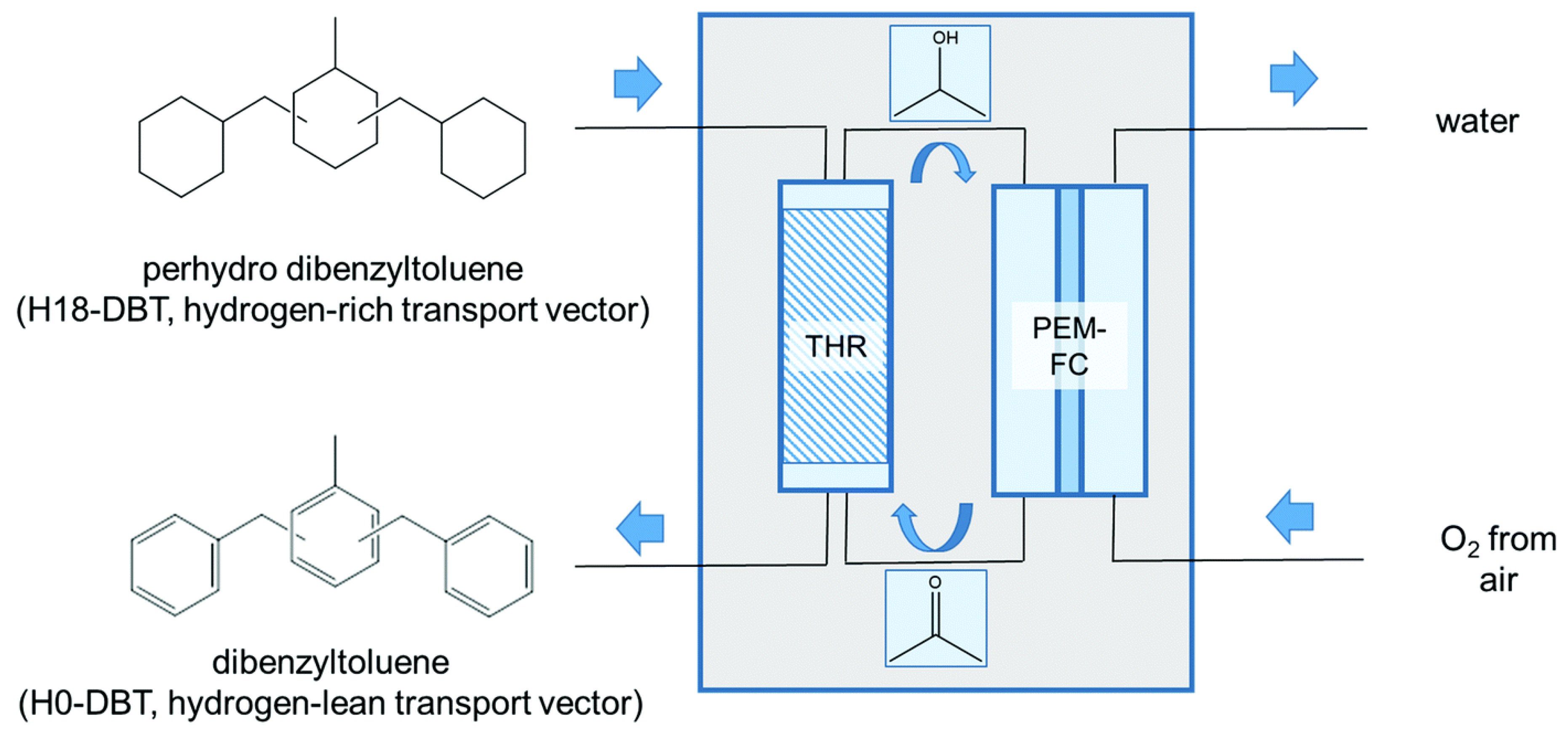
2.3. Dibenzyl Toluene (DBT)
2.4. Cyclohexane
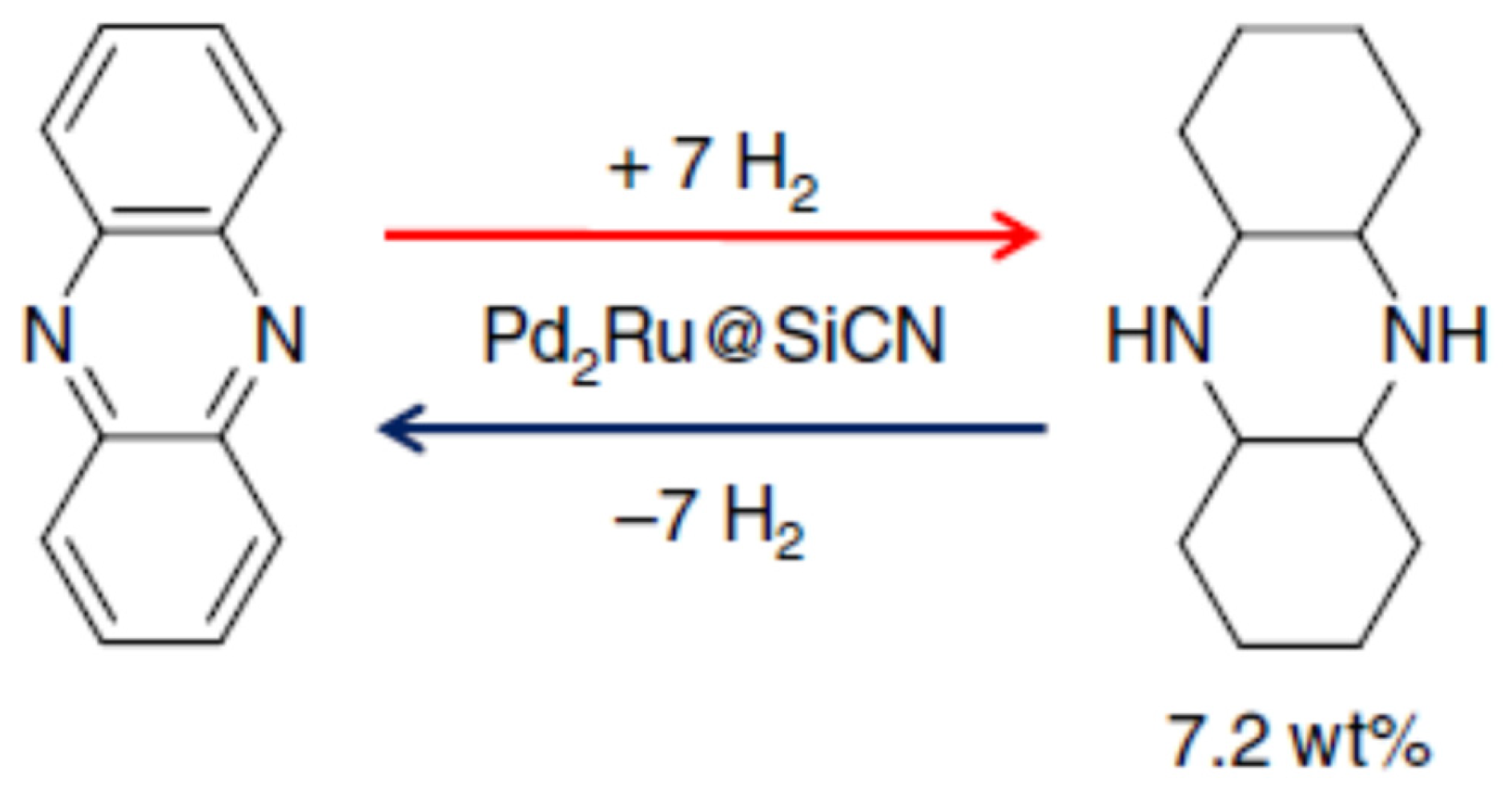
2.5. Phenazine
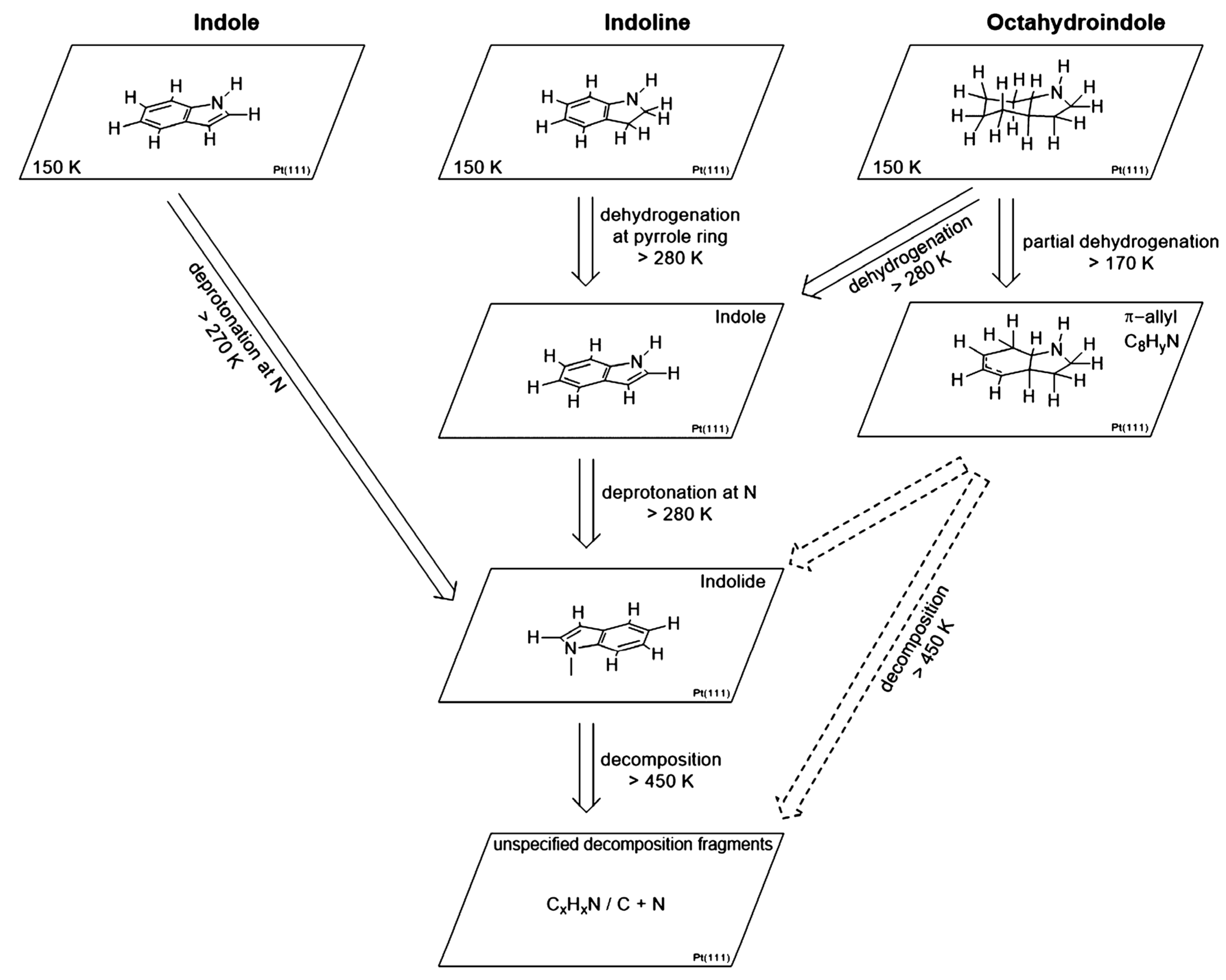
2.6. Indole Derivatives
2.7. Pyridine Derivative
2.8. Pyrrolidine
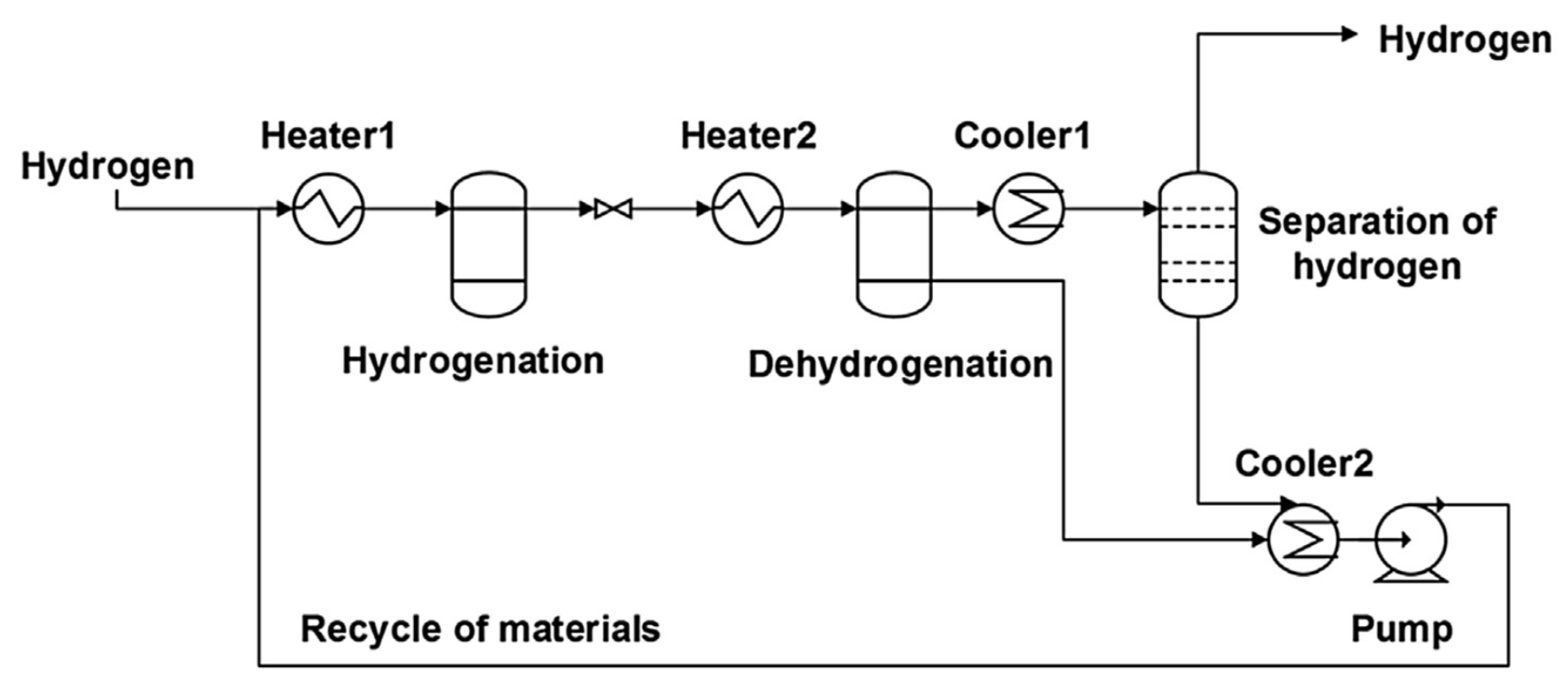
3. Reactors Used for Dehydrogenation
4. Catalysts Used for Dehydrogenation
5. Application
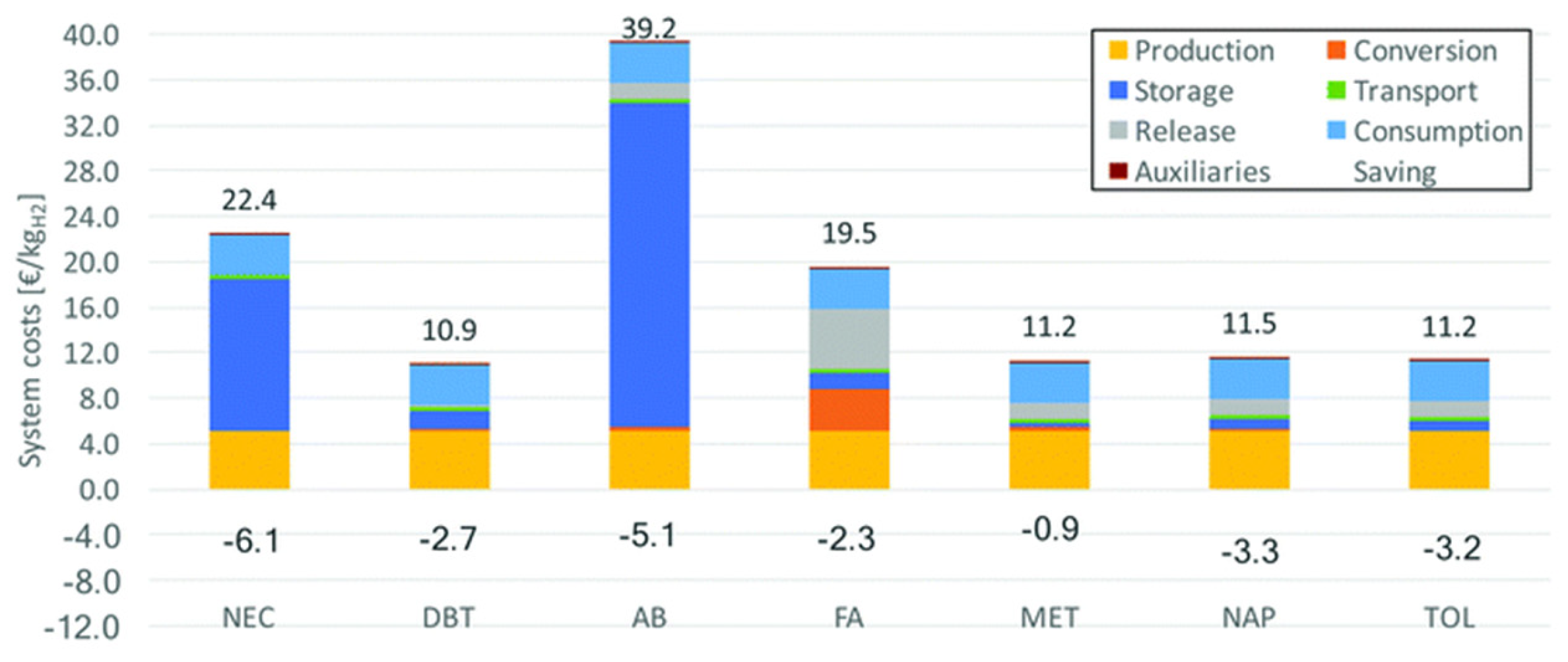
6. Technoeconomic Analysis
6.1. Technical Analysis
- = hydrogen transportation and storage efficiency;
- = useable hydrogen output energy content;
- = useable hydrogen input energy content;
- = energy demand of the storage system;
- = energy of hydrogenation;
- = energy of dehydrogenation.
- = chain efficiency;
- = transport efficiency;
- = energy consumption during transport;
- = energy consumption during electrolysis;
- = energy consumption during storage;
- = energy output of the fuel cell.
6.2. Economic Analysis
- = the cost of design;
- = the cost of hydrogenation reactors;
- = the cost of dehydrogenation reactors;
- STY = space, time, and yield;
- = maximum power rating in kW;
- SF = the scaling factor.
- = moles of the target (A) per mole of the reactant (Ao);
- = the equilibrium conversion;
- = the molar mass;
- = the volume of 1 mole of reactants;
- = reaction time.
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chu, C.; Wu, K.; Luo, B.; Cao, Q.; Zhang, H. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology—A review. Carbon Resour. Convers. 2023, 6, 334–351. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Sreedhar, A.; Noh, J.S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Amende, M.; Kaftan, A.; Bachmann, P.; Brehmer, R.; Preuster, P.; Koch, M.; Wasserscheid, P.; Libuda, J. Regeneration of LOHC dehydrogenation catalysts: In-situ IR spectroscopy on single crystals, model catalysts, and real catalysts from UHV to near ambient pressure. Appl. Surf. Sci. 2016, 360, 671–683. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.S. Highly surface-active Si-doped TiO2/Ti3C2Tx heterostructure for gas sensing and photodegradation of toxic matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Crabtree, R.H. Nitrogen-containing liquid organic hydrogen carriers: Progress and prospects. ACS Sustain. Chem. Eng. 2017, 5, 4491–4498. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, Q.; Xu, J.; Fang, T. Current situation and prospect of hydrogen storage technology with new organic liquid. Int. J. Hydrogen Energy 2014, 39, 17442–17451. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Sievi, G.; Geburtig, D.; Skeledzic, T.; Bösmann, A.; Preuster, P.; Brummel, O.; Waidhas, F.; Montero, M.A.; Khanipour, P.; Katsounaros, I.; et al. Towards an efficient liquid organic hydrogen carrier fuel cell concept. Energy Environ. Sci. 2019, 12, 2305–2314. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef]
- Sultan, O.; Shaw, H. Study of automotive storage of hydrogen using recyclable liquid chemical carriers. NASA Tech. Rep. N 1975, 76, 33642. [Google Scholar]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H.; Wilhelm, F.C.; Abdourazak, A.H. Hydrogen Storage by Reversible Hydrogenation of Pi-Conjugated Substrates. U.S. Patent US7351395B1, 1 April 2008. [Google Scholar]
- Stark, K.; Emelyanenko, V.N.; Zhabina, A.A.; Varfolomeev, M.A.; Verevkin, S.P.; Müller, K.; Arlt, W. Liquid organic hydrogen carriers: Thermophysical and thermochemical studies of carbazole partly and fully hydrogenated derivatives. Ind. Eng. Chem. Res. 2015, 54, 7953–7966. [Google Scholar] [CrossRef]
- Choi, I.Y.; Shin, B.S.; Kwak, S.K.; Kang, K.S.; Yoon, C.W.; Kang, J.W. Thermodynamic efficiencies of hydrogen storage processes using carbazole-based compounds. Int. J. Hydrogen Energy 2016, 41, 9367–9373. [Google Scholar] [CrossRef]
- Suppino, R.S.; Landers, R.; Cobo, A.J.G. Influence of noble metals (Pd, Pt) on the performance of Ru/Al2O3 based catalysts for toluene hydrogenation in liquid phase. Appl. Catal. A Gen. 2016, 525, 41–49. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]
- Parker, D.H.; Pettiette-Hall, C.L.; Li, Y.; McIver, R.T.; Hemminger, J.C. Kinetic study of the initial stages of dehydrogenation of cyclohexane on the platinum (111) surface. J. Phys. Chem. 1992, 96, 1888–1894. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, L.; Xu, L.; Wan, C.; An, Y.; Ye, M. Recent developments of effective catalysts for hydrogen storage technology using N-Ethylcarbazole. Catalysts 2020, 10, 648. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment of compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.K. Dynamics of cryogenic hydrogen storage in insulated pressure vessels for automotive applications. Int. J. Hydrogen Energy 2008, 33, 4622–4633. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hydrogen storage in liquid organic heterocycles. Energy Environ. Sci. 2008, 1, 134–138. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, B.S.; Hashem, M.; Harris, K.D.M.; Msayib, K.J.; Xu, M.; Budd, P.M.; Chaukura, N.; Book, D.; Tedds, S.; Walton, A.; et al. Triptycene-based polymers of intrinsic microporosity: Organic materials that can be tailored for gas adsorption. Macromolecules 2010, 43, 5287–5294. [Google Scholar] [CrossRef]
- Cacciola, G.; Giordano, N.; Restuccia, G. Cyclohexane as a liquid phase carrier in hydrogen storage and transport. Int. J. Hydrogen Energy 1984, 9, 411–419. [Google Scholar] [CrossRef]
- Cui, Y.; Kwok, S.; Bucholtz, A.; Davis, B.; Whitney, R.A.; Jessop, P.G. The effect of substitution on the utility of piperidines and octahydroindoles for reversible hydrogen storage. New J. Chem. 2008, 32, 1027–1037. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen safety: An obstacle that must be overcome on the road towards future hydrogen economy. Int. J. Hydrogen Energy 2024, 51, 1055–1078. [Google Scholar] [CrossRef]
- Müller, K.; Völkl, J.; Arlt, W. Thermodynamic evaluation of potential organic hydrogen carriers. Energy Technol. 2013, 1, 20–24. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Ouyang, M. Efficiency analysis of novel Liquid Organic Hydrogen Carrier technology and comparison with high pressure storage pathway. Int. J. Hydrogen Energy 2016, 41, 18062–18071. [Google Scholar] [CrossRef]
- Cooper, A.C.; Campbell, K.M.; Pez, G.P. An integrated hydrogen storage and delivery approach using organic liquid-phase carriers. In Proceedings of the 16th World Hydrogen Energy Conference 2006, WHEC 2006, Lyon, France, 13–16 June 2006; Volume 3, pp. 2164–2175. [Google Scholar]
- Adametz, P.; Müller, K.; Arlt, W. Efficiency of low-temperature adsorptive hydrogen storage systems. Int. J. Hydrogen Energy 2014, 39, 15604–15613. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Energy and Transport Processes. Core Programming Topic at the 2011 AICHE Spring Meeting and 7th Global Congress on Process Safety; American Institute of Chemical Engineers (AIChE): New York, NY, USA, 2011; pp. 336–338. [Google Scholar]
- Tegrotenhuis, W.E.; Humble, P.; Northwest, P. Liquid Organic Hydrogen Carriers (LOHC): An Auspicious Alternative to Liquid Organic Hydrogen Carriers (LOHC): An auspicious al-ternative to conventional hydrogen storage technologies. Ess Schriften Des Forschungszentrums Jülich/Energy Environ. 2015, 78, 189–197. [Google Scholar]
- Wan, C.; An, Y.; Xu, G.; Kong, W. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst. Int. J. Hydrogen Energy 2012, 37, 13092–13096. [Google Scholar] [CrossRef]
- He, T.; Liu, L.; Wu, G.; Chen, P. Covalent triazine framework-supported palladium nanoparticles for catalytic hydrogenation of N-heterocycles. J. Mater. Chem. A Mater. 2015, 3, 16235–16241. [Google Scholar] [CrossRef]
- Teichmann, D.; Stark, K.; Müller, K.; Zöttl, G.; Wasserscheid, P.; Arlt, W. Energy storage in residential and commercial buildings via Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2012, 5, 9044–9054. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen storage using a hot pressure swing reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Bösmann, A.; Brückner, N.; Thöming, J.; Wasserscheid, P.; Stolte, S. Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems—challenges and preliminary results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- Benigni, R. Structure–activity relationship studies of chemical mutagens and carcinogens: Mechanistic investigations and prediction approaches. Chem. Rev. 2005, 105, 1767–1800. [Google Scholar] [CrossRef]
- LaVoie, E.J.; Briggs, G.; Bedenko, V.; Hoffmann, D. Mutagenicity of substituted carbazoles in Salmonella typhimurium. Mutat. Res./Genet. Toxicol. 1982, 101, 141–150. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Empl, M.T.; Lykaki, M.; Thöming, J.; Steinberg, P.; Stolte, S. Hazard assessment of quinaldine-, alkylcarbazole-, benzene-and toluene-based liquid organic hydrogen carrier (LOHCs) systems. Energy Environ. Sci. 2019, 12, 366–383. [Google Scholar] [CrossRef]
- Mehranfar, A.; Izadyar, M. N-Ethylcarbazole-doped fullerene as a potential candidate for hydrogen storage, a kinetics approach. RSC Adv. 2015, 5, 49159–49167. [Google Scholar] [CrossRef]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.; Kaner, R. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef] [PubMed]
- Eblagon, K.M.; Tam, K.; Yu, K.M.K.; Tsang, S.C.E. Comparative study of catalytic hydrogenation of 9-ethylcarbazole for hydrogen storage over noble metal surfaces. J. Phys. Chem. C 2012, 116, 7421–7429. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Rentsch, D.; Friedrichs, O.; Remhof, A.; Zuettel, A.; Ramirez-Cuesta, A.J.; Tsang, S.C. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier. Int. J. Hydrogen Energy 2010, 35, 11609–11621. [Google Scholar] [CrossRef]
- Stark, K.; Keil, P.; Schug, S.; Müller, K.; Wasserscheid, P.; Arlt, W. Melting points of potential liquid organic hydrogen carrier systems consisting of N-alkylcarbazoles. J. Chem. Eng. Data 2016, 61, 1441–1448. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage. Appl. Catal. A Gen. 2012, 419–420, 67–72. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Bai, X. Highly efficient dehydrogenation of dodecahydro-N-ethylcarbazole over Al2O3 supported PdCo bimetallic nanocatalysts prepared by galvanic replacement. Int. J. Hydrogen Energy 2024, 49, 1547–1557. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, H.; Oh, J.E.; Park, B.Y. Recent advances in homogeneous/heterogeneous catalytic hydrogenation and dehydrogenation for potential liquid organic hydrogen carrier (LOHC) systems. Catalysts 2021, 11, 1497. [Google Scholar] [CrossRef]
- Emel’Yanenko, V.N.; Varfolomeev, M.A.; Verevkin, S.P.; Stark, K.; Müller, K.; Müller, M.; Bösmann, A.; Wasserscheid, P.; Arlt, W. Hydrogen storage: Thermochemical studies of N-alkylcarbazoles and their derivatives as a potential liquid organic hydrogen carriers. J. Phys. Chem. C 2015, 119, 26381–26389. [Google Scholar] [CrossRef]
- Dean, D.; Davis, B.; Jessop, P.G. The effect of temperature, catalyst and sterics on the rate of N-heterocycle dehydrogenation for hydrogen storage. New J. Chem. 2011, 35, 417–422. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Mehranfar, A.; Izadyar, M.; Esmaeili, A.A. Hydrogen storage by N-ethylcarbazol as a new liquid organic hydrogen carrier: A DFT study on the mechanism. Int. J. Hydrogen Energy 2015, 40, 5797–5806. [Google Scholar] [CrossRef]
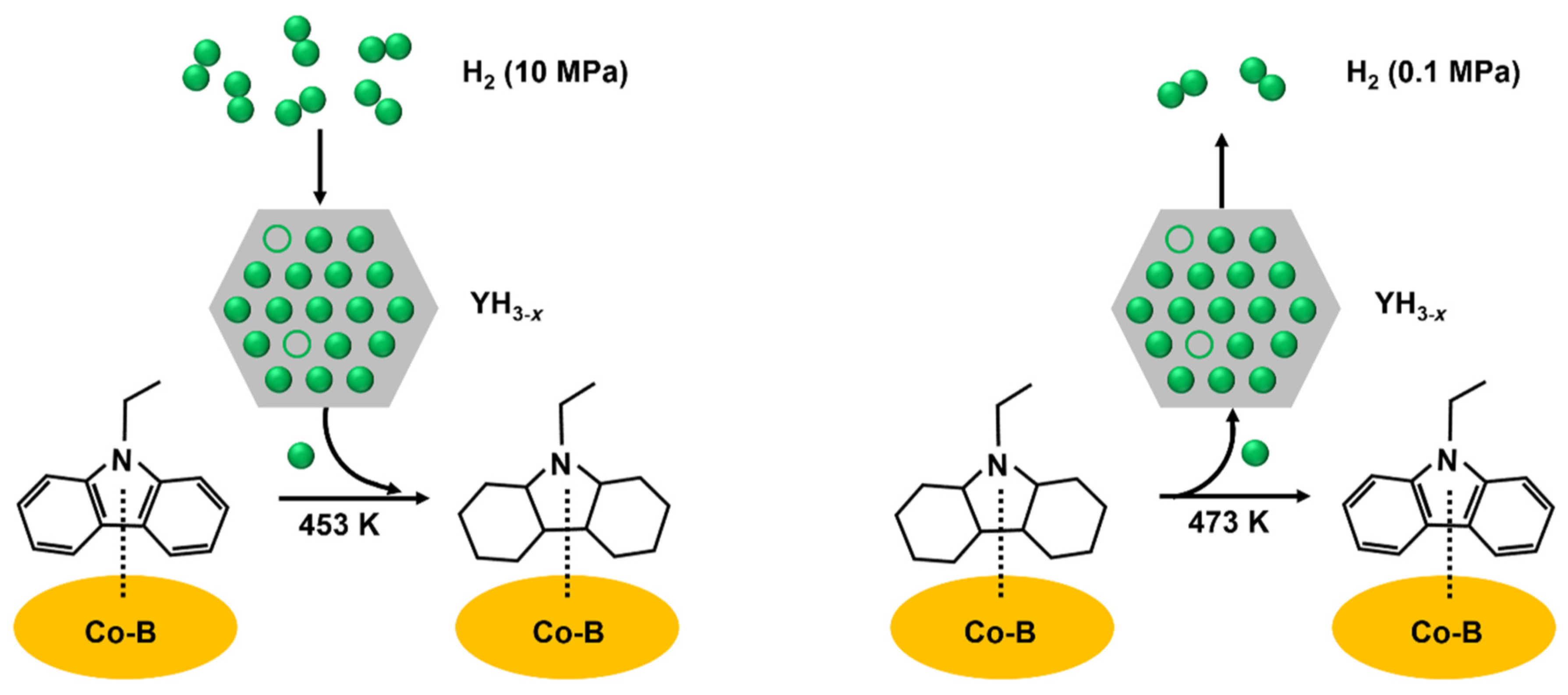
- Wu, Y.; Guo, Y.; Yu, H.; Jiang, X.; Zhang, Y.; Qi, Y.; Fu, K.; Xie, L.; Li, G.; Zheng, J.; et al. Nonstoichiometric yttrium hydride–promoted reversible hydrogen storage in a liquid organic hydrogen carrier. CCS Chem. 2021, 3, 974–984. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Mei, P.; Li, C.; Li, L. Dehydrogenation kinetics study of perhydro-N-ethylcarbazole over a supported Pd catalyst for hydrogen storage application. Int. J. Hydrogen Energy 2016, 41, 8498–8505. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, X.; Bai, X. Catalytic dehydrogenation of liquid organic hydrogen carrier dodecahydro-N-ethylcarbazole over palladium catalysts supported on different supports. Environ. Sci. Pollut. Res. 2020, 27, 36172–36185. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Zhao, H.; Zheng, X.; Wu, L.; Pan, H.; Zhu, J.; Chen, Y.; Lu, J. Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid. J. Catal. 2017, 352, 371–381. [Google Scholar] [CrossRef]
- Li, C.; Yang, M.; Liu, Z.; Zhang, Z.; Zhu, T.; Chen, X.; Dong, Y.; Cheng, H. Ru–Ni/Al2O3 bimetallic catalysts with high catalytic activity for N-propylcarbazole hydrogenation. Catal. Sci. Technol. 2020, 10, 2268–2276. [Google Scholar] [CrossRef]
- Huang, L.; Han, B.; Xi, Y.; Forrey, R.C.; Cheng, H. Influence of charge on the reactivity of supported heterogeneous transition metal catalysts. ACS Catal. 2015, 5, 4592–4597. [Google Scholar] [CrossRef]
- Hu, P.; Fogler, E.; Diskin-Posner, Y.; Iron, M.A.; Milstein, D. A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emelyanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid organic hydrogen carriers: Thermophysical and thermochemical studies of benzyl-and dibenzyl-toluene derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Krieger, C.; Müller, K.; Arlt, W. Coupling of a liquid organic hydrogen carrier system with industrial heat. Chem. Eng. Technol. 2016, 39, 1570–1574. [Google Scholar] [CrossRef]
- Auer, F.; Blaumeiser, D.; Bauer, T.; Bösmann, A.; Szesni, N.; Libuda, J.; Wasserscheid, P. Boosting the activity of hydrogen release from liquid organic hydrogen carrier systems by sulfur-additives to Pt on alumina catalysts. Catal. Sci. Technol. 2019, 9, 3537–3547. [Google Scholar] [CrossRef]
- Jossens, L.W.; Petersen, E.E. Fouling of a platinum reforming catalyst accompanying the dehydrogenation of methyl cyclohexane. J. Catal. 1982, 73, 377–386. [Google Scholar] [CrossRef]
- Ali, A.; Kumar, G.U.; Lee, H.J. Parametric study of the hydrogenation of dibenzyltoluene and its dehydrogenation performance as a liquid organic hydrogen carrier. J. Mech. Sci. Technol. 2020, 34, 3069–3077. [Google Scholar] [CrossRef]
- Gan, L.Y.; Tian, R.Y.; Yang, X.B.; Lu, H.D.; Zhao, Y.J. Catalytic reactivity of CuNi alloys toward H2O and CO dissociation for an efficient water–gas shift: A DFT study. J. Phys. Chem. C 2012, 116, 745–752. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-catalyst high-weight% hydrogen storage in an N-heterocycle synthesized from lignin hydrogenolysis products and ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef]
- Bachmann, P.; Schwarz, M.; Steinhauer, J.; Späth, F.; Düll, F.; Bauer, U.; Silva, T.N.; Mohr, S.; Hohner, C.; Scheuermeyer, M.; et al. Dehydrogenation of the liquid organic hydrogen carrier system indole/indoline/octahydroindole on Pt(111). J. Phys. Chem. C 2018, 122, 4470–4479. [Google Scholar] [CrossRef]
- Schwarz, M.; Bachmann, P.; Silva, T.N.; Mohr, S.; Scheuermeyer, M.; Späth, F.; Bauer, U.; Düll, F.; Steinhauer, J.; Hohner, C.; et al. Surface Reactions of Dicyclohexylmethane on Pt(111). Chem.—A Eur. J. 2017, 23, 14806–14818. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Yang, Z.; Ke, H.; Cheng, H. Catalytic hydrogenation and dehydrogenation of N-ethylindole as a new heteroaromatic liquid organic hydrogen carrier. Int. J. Hydrogen Energy 2015, 40, 10918–10922. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Xu, Z.; Liu, L.; Zhu, T.; Chen, Z.; Dong, Y.; Yang, M. Ce-promoted highly active bifunctional Pd/Al2O3 catalyst for reversible catalytic hydrogenation and dehydrogenation of N-propylcarbazole. Int. J. Hydrogen Energy 2023, 48, 90–100. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Dong, Y.; Mei, P.; Cheng, H. Hydrogen storage and release from a new promising Liquid Organic Hydrogen Storage Carrier (LOHC): 2-methylindole. Int. J. Hydrogen Energy 2016, 41, 16129–16134. [Google Scholar] [CrossRef]
- Bachmann, P.; Steinhauer, J.; Späth, F.; Düll, F.; Bauer, U.; Eschenbacher, R.; Hemauer, F.; Scheuermeyer, M.; Bösmann, A.; Büttner, M.; et al. Dehydrogenation of the liquid organic hydrogen carrier system 2-methylindole/2-methylindoline/2-methyloctahydroindole on Pt (111). J. Chem. Phys. 2019, 151, 144711. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. On H2 supply through liquid organic hydrides—Effect of functional groups. Int. J. Hydrogen Energy 2017, 42, 16214–16224. [Google Scholar] [CrossRef]
- Xie, Y.; Milstein, D. Pd catalyzed, acid accelerated, rechargeable, liquid organic hydrogen carrier system based on methylpyridines/methylpiperidines. ACS Appl. Energy Mater. 2019, 2, 4302–4308. [Google Scholar] [CrossRef]
- Oh, J.; Bathula, H.B.; Park, J.H.; Suh, Y.W. A sustainable mesoporous palladium-alumina catalyst for efficient hydrogen release from N-heterocyclic liquid organic hydrogen carriers. Commun. Chem. 2019, 2, 68. [Google Scholar] [CrossRef]
- Amende, M.; Schernich, S.; Sobota, M.; Nikiforidis, I.; Hieringer, W.; Assenbaum, D.; Gleichweit, C.; Drescher, H.J.; Papp, C.; Steinrück, H.P.; et al. Dehydrogenation mechanism of liquid organic hydrogen carriers: Dodecahydro-N-ethylcarbazole on Pd (111). Chem.—Eur. J. 2013, 19, 10854–10865. [Google Scholar] [CrossRef] [PubMed]
- Brayton, D.F.; Jensen, C.M. Dehydrogenation of pyrrolidine based liquid organic hydrogen carriers by an iridium pincer catalyst, an isothermal kinetic study. Int. J. Hydrogen Energy 2015, 40, 16266–16270. [Google Scholar] [CrossRef]
- Peters, W.; Eypasch, M.; Frank, T.; Schwerdtfeger, J.; Körner, C.; Bösmann, A.; Wasserscheid, P. Efficient hydrogen release from perhydro-N-ethylcarbazole using catalyst-coated metallic structures produced by selective electron beam melting. Energy Environ. Sci. 2015, 8, 641–649. [Google Scholar] [CrossRef]
- Wang, Z.; Tonks, I.; Belli, J.; Jensen, C.M. Dehydrogenation of N-ethyl perhydrocarbazole catalyzed by PCP pincer iridium complexes: Evaluation of a homogenous hydrogen storage system. J. Organomet. Chem. 2009, 694, 2854–2857. [Google Scholar] [CrossRef]
- Sage, V.; Patel, J.; Hazewinkel, P.; Yasin QU, A.; Wang, F.; Yang, Y.; Kozielski, K.; Li, C.E. Recent progress and techno-economic analysis of liquid organic hydrogen carriers for Australian renewable energy export—A critical review. Int. J. Hydrogen Energy 2024, 56, 1419–1434. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Zhao, L.; Smith, K.J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst. Appl. Catal. A Gen. 2009, 362, 155–162. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, B.; Fang, T. A theoretical study on the complete dehydrogenation of methanol on Pd (100) surface. Appl. Surf. Sci. 2016, 364, 613–619. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 2715–2722. [Google Scholar] [CrossRef]
- García, A.; Marín, P.; Ordóñez, S. Hydrogenation of liquid organic hydrogen carriers: Process scale-up, economic analysis and optimization. Int. J. Hydrogen Energy 2024, 52, 1113–1123. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Kirichenko, O.A. Microwave-activated dehydrogenation of perhydro-N-ethylcarbazol over bimetallic Pd-M/TiO2 catalysts as the second stage of hydrogen storage in liquid substrates. Int. J. Hydrogen Energy 2017, 42, 26723–26729. [Google Scholar] [CrossRef]
- Fei, S.; Han, B.; Li, L.; Mei, P.; Zhu, T.; Yang, M.; Cheng, H. A study on the catalytic hydrogenation of N-ethylcarbazole on the mesoporous Pd/MoO3 catalyst. Int. J. Hydrogen Energy 2017, 42, 25942–25950. [Google Scholar] [CrossRef]
- Peters, W.; Seidel, A.; Herzog, S.; Bösmann, A.; Schwieger, W.; Wasserscheid, P. Macrokinetic effects in perhydro-N-ethylcarbazole dehydrogenation and H2 productivity optimization by using egg-shell catalysts. Energy Environ. Sci. 2015, 8, 3013–3021. [Google Scholar] [CrossRef]
- Wang, B.; Yan, T.; Chang, T.; Wei, J.; Zhou, Q.; Yang, S.; Fang, T. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole. Carbon. N. Y. 2017, 122, 9–18. [Google Scholar] [CrossRef]
- López-Corral, I.; Germán, E.; Juan, A.; Volpe, M.A.; Brizuela, G.P. DFT study of hydrogen adsorption on palladium decorated graphene. J. Phys. Chem. C 2011, 115, 4315–4323. [Google Scholar] [CrossRef]
- Wang, B.; Chang, T.Y.; Jiang, Z.; Wei, J.J.; Zhang, Y.H.; Yang, S.; Fang, T. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide. Int. J. Hydrogen Energy 2018, 43, 7317–7325. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Rakrai, W.; Wanno, B. Hydrogen adsorption on graphene sheets doped with group 8B transition metal: A DFT investigation. Vacuum 2017, 139, 101–108. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, L.; Du, L.; An, Y.; Wan, C. Palladium supported on carbon nanotubes as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole. Catalysts 2018, 8, 638. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Tkachenko, O.P.; Kustov, L.M. Mono and Bimetallic Pt–(M)/Al2O3 Catalysts for Dehydrogenation of Perhydro-N-ethylcarbazole as the Second Stage of Hydrogen Storage. Catal. Lett. 2018, 148, 1472–1477. [Google Scholar] [CrossRef]
- Jiang, Z.; Gong, X.; Wang, B.; Wu, Z.; Fang, T. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts. Int. J. Hydrogen Energy 2019, 44, 2951–2959. [Google Scholar] [CrossRef]
- Amende, M.; Gleichweit, C.; Schernich, S.; Höfert, O.; Lorenz, M.P.A.; Zhao, W.; Koch, M.; Obesser, K.; Papp, C.; Wasserscheid, P.; et al. Size and structure effects controlling the stability of the liquid organic hydrogen carrier dodecahydro-N-ethylcarbazole during dehydrogenation over Pt model catalysts. J. Phys. Chem. Lett. 2014, 5, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chang, T.Y.; Jiang, Z.; Wei, J.J.; Fang, T. Component controlled synthesis of bimetallic PdCu nanoparticles supported on reduced graphene oxide for dehydrogenation of dodecahydro-N-ethylcarbazole. Appl. Catal. B 2019, 251, 261–272. [Google Scholar] [CrossRef]
- Alabbadi, A.A.; Obaid, O.A.; AlZahrani, A.A. A comparative economic study of nuclear hydrogen production, storage, and transportation. Int. J. Hydrogen Energy 2024, 54, 849–863. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Eypasch, M.; Schimpe, M.; Kanwar, A.; Hartmann, T.; Herzog, S.; Frank, T.; Hamacher, T. Model-based techno-economic evaluation of an electricity storage system based on Liquid Organic Hydrogen Carriers. Appl. Energy 2017, 185, 320–330. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Chemical engineering design: Principles, practice and economics of plant and process design. Chem. Eng. Des. Princ. Pract. Econ. Plant Process Des. 2021, 1–1027. [Google Scholar]
- Hurskainen, M.; Ihonen, J. Techno-economic feasibility of road transport of hydrogen using liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2020, 45, 32098–32112. [Google Scholar] [CrossRef]
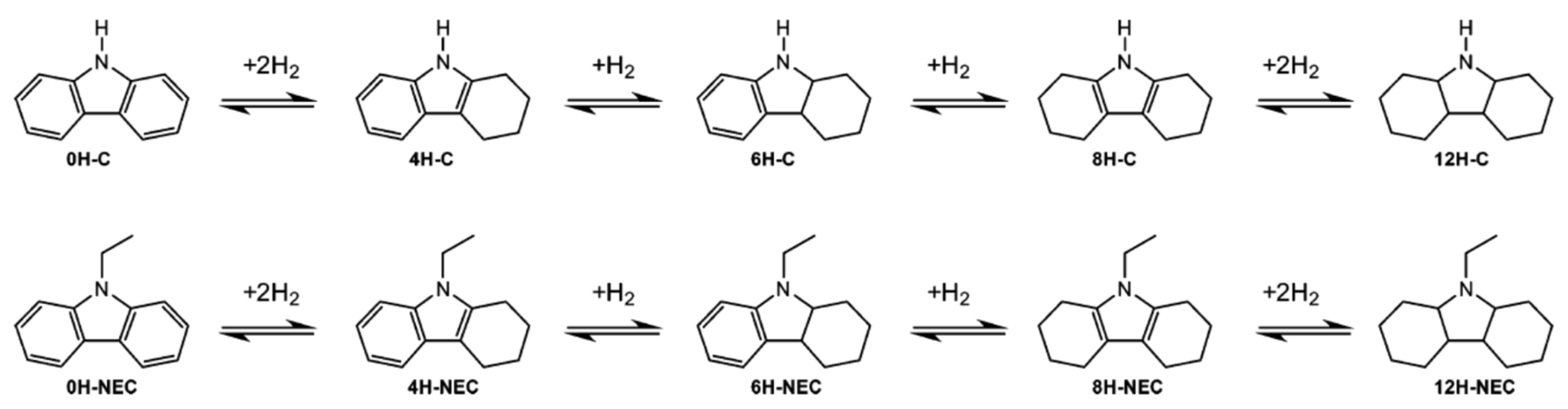

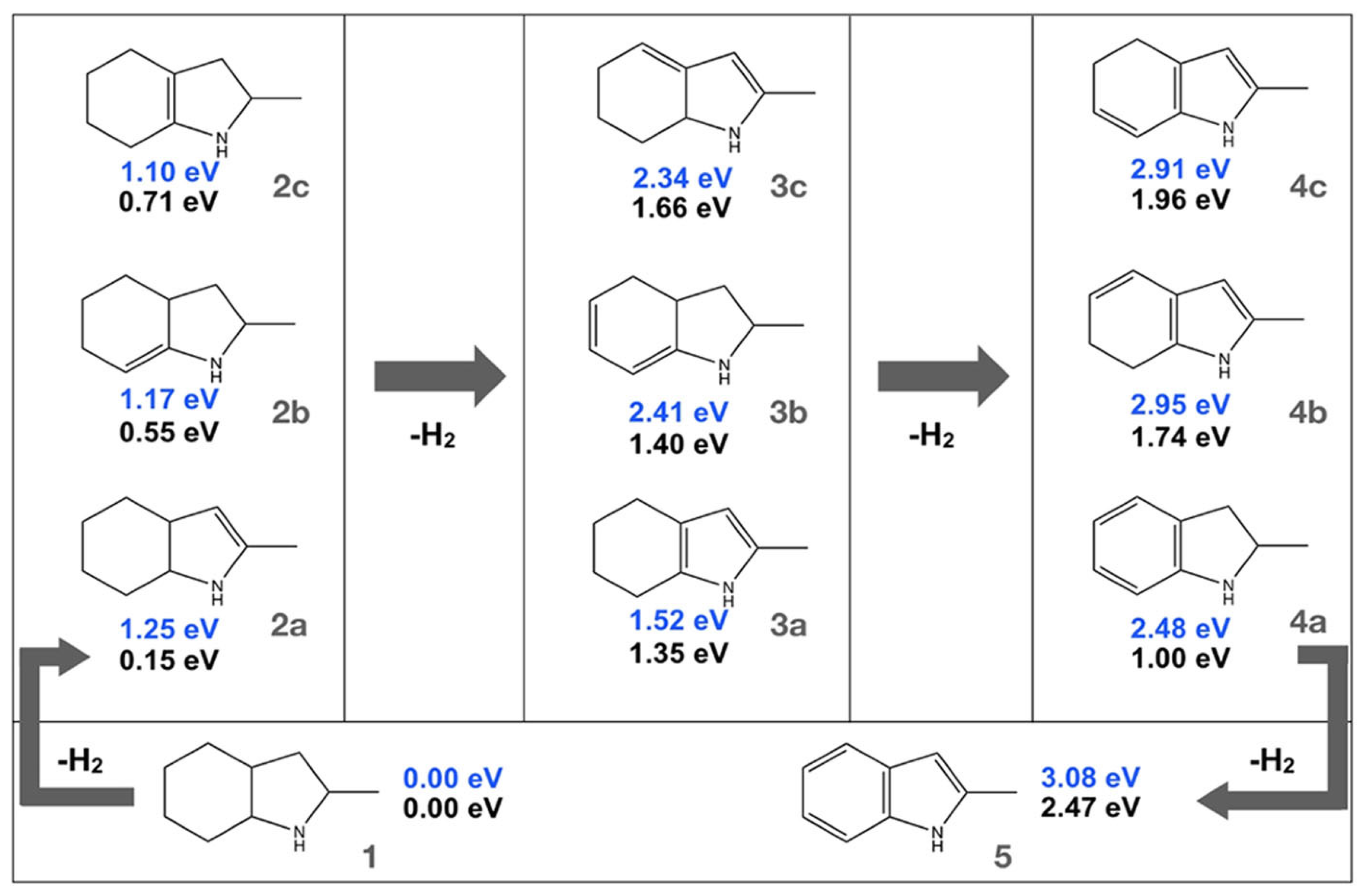
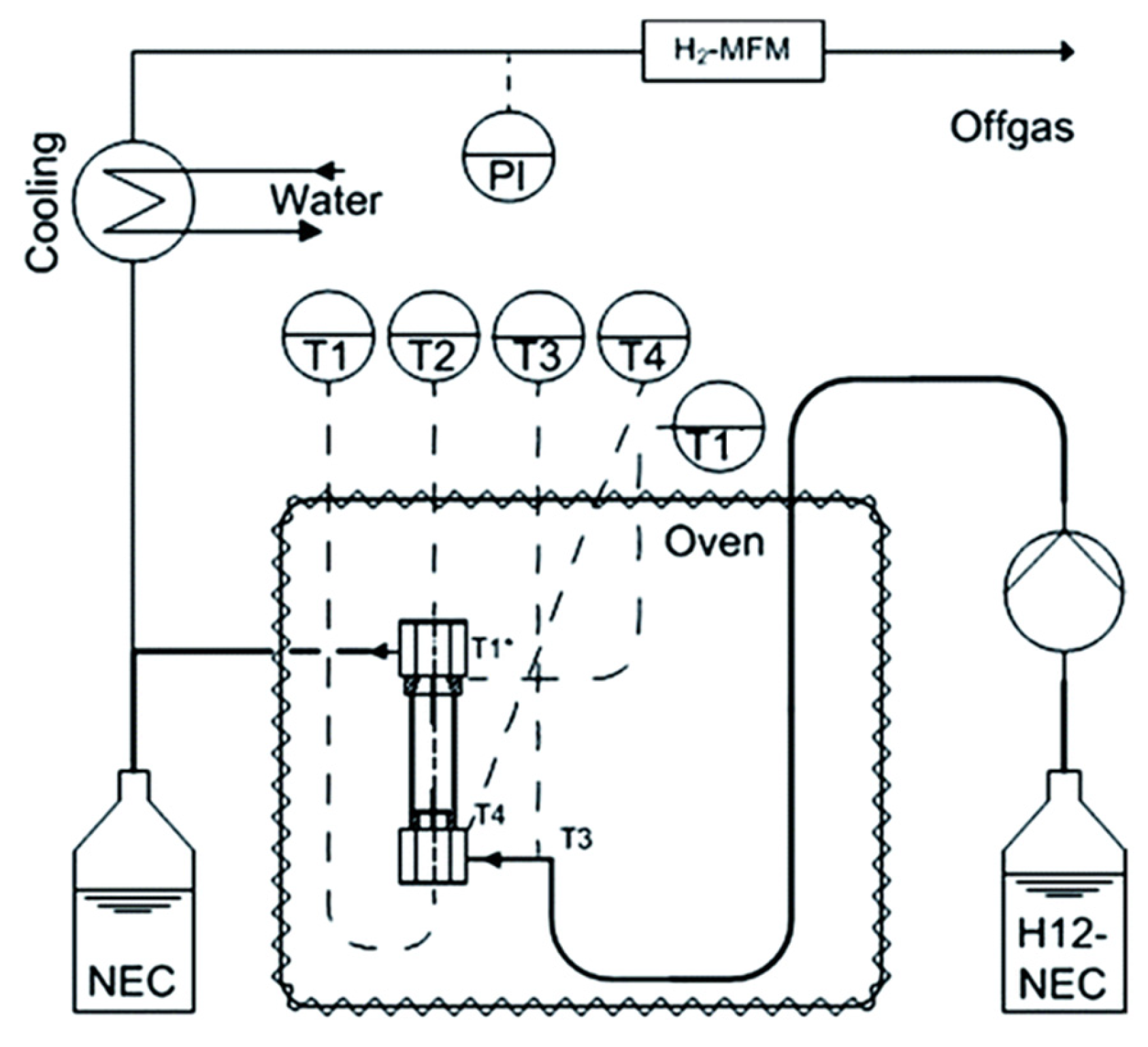
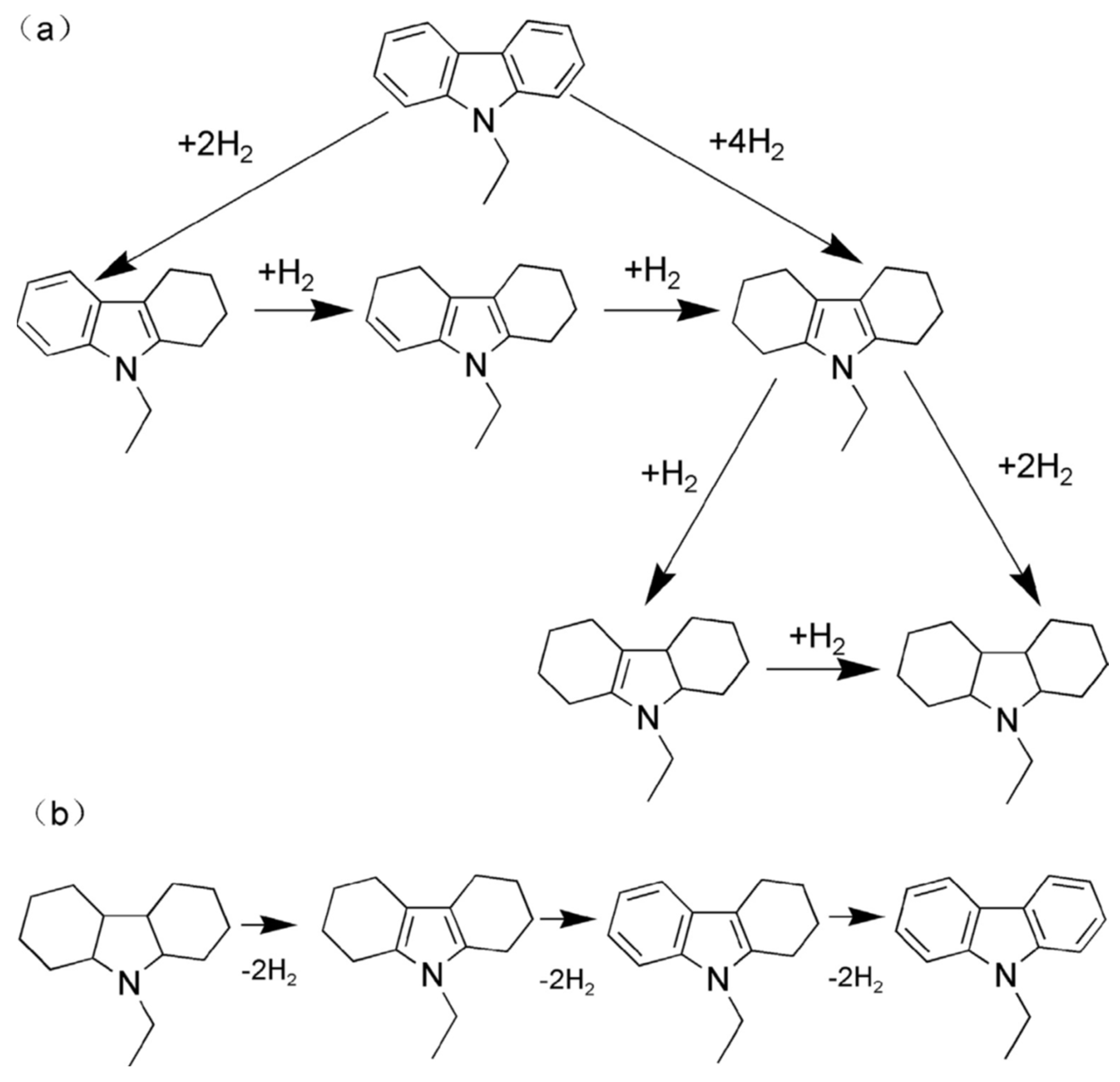
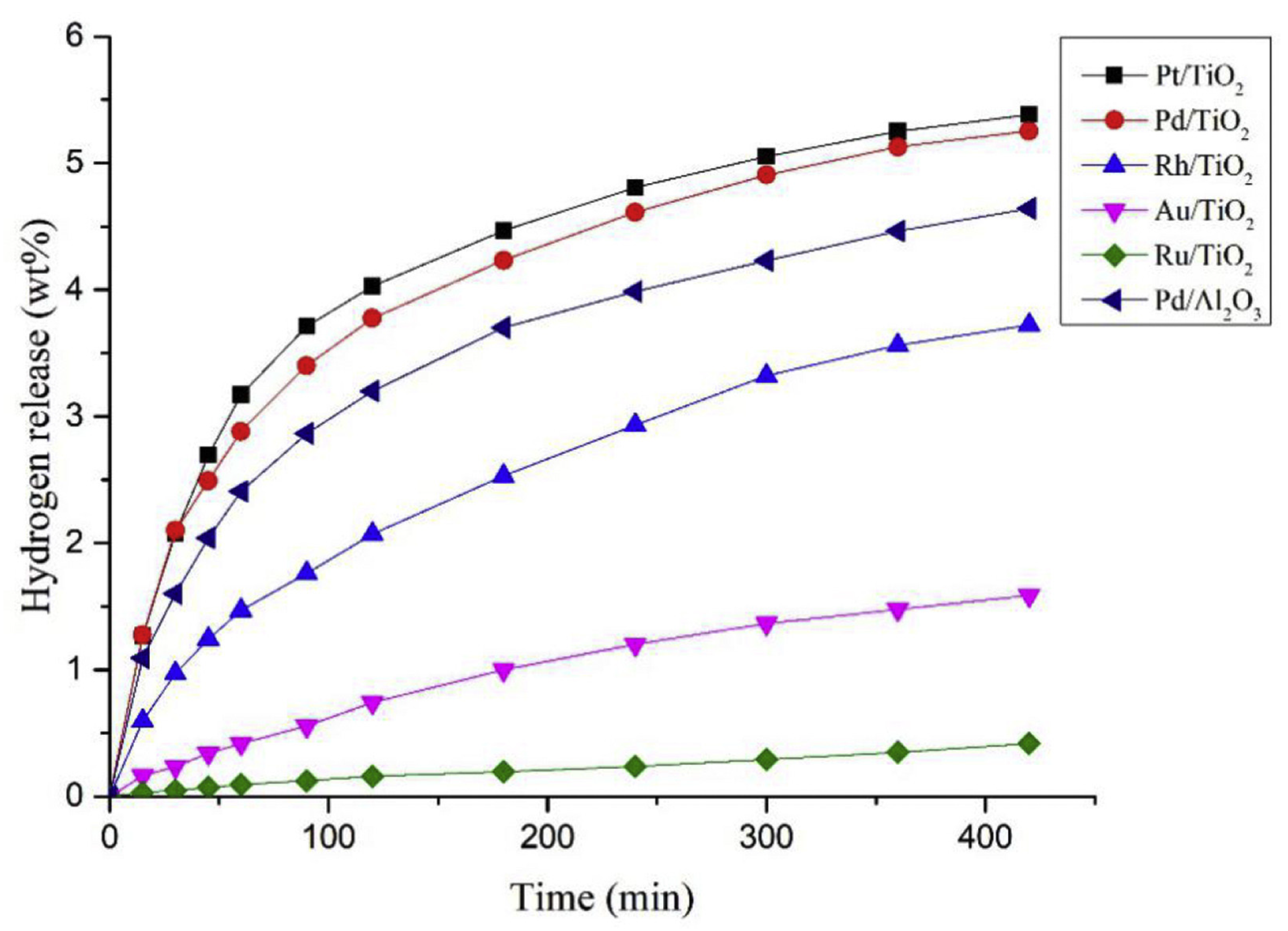


| LOHC | Free Energy of Reaction | Enthalpy of Reaction |
|---|---|---|
 | −100.4 | −136.3 |
 | −83.3 | −118.4 |
 | −32.6 | −68.7 |
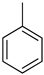 | −31.6 | −68.3 |
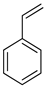 | −43.6 | −79.7 |
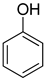 | −25.6 | −63.3 |
 | −20.5 | −66.3 |
 | −19.6 | −66.9 |
 | −40.3 | −74.7 |
 | −40.4 | −74.6 |
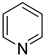 | −29.5 | −63.1 |
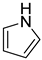 | −22.8 | −55.9 |
 | −18.2 | −53.2 |
| Unsaturated Compound | Saturated Compound | Gravimetric H2 Carrying Capacity (%) |
|---|---|---|
 Naphthalene Naphthalene | 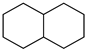 Decaline Decaline | 7.5 |
 Benzene Benzene |  Cyclohexane Cyclohexane | 7.5 |
 Biphenyl Biphenyl |  Bicyclohexyl Bicyclohexyl | 7.5 |
 MLH MLH | 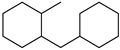 H12-MLH H12-MLH | 7.0 |
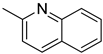 Quinaldine Quinaldine | 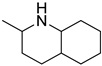 H10-Quinaldine H10-Quinaldine | 7.0 |
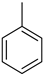 Toluene Toluene | 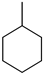 Methyl cyclohexane Methyl cyclohexane | 6.5 |
 Ethyl carbazole Ethyl carbazole | 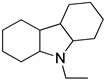 H12-ethyl carbazole H12-ethyl carbazole | 6.0 |
 Methyl thiophene Methyl thiophene |  H4-methyl thiophene H4-methyl thiophene | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-H.; Tran, N.; Lee, H.-J. Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. Int. J. Mol. Sci. 2024, 25, 1359. https://doi.org/10.3390/ijms25021359
Le T-H, Tran N, Lee H-J. Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. International Journal of Molecular Sciences. 2024; 25(2):1359. https://doi.org/10.3390/ijms25021359
Chicago/Turabian StyleLe, Thi-Hoa, Ngo Tran, and Hyun-Jong Lee. 2024. "Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport" International Journal of Molecular Sciences 25, no. 2: 1359. https://doi.org/10.3390/ijms25021359
APA StyleLe, T.-H., Tran, N., & Lee, H.-J. (2024). Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. International Journal of Molecular Sciences, 25(2), 1359. https://doi.org/10.3390/ijms25021359








