Advances in Mass Spectrometry of Gangliosides Expressed in Brain Cancers
Abstract
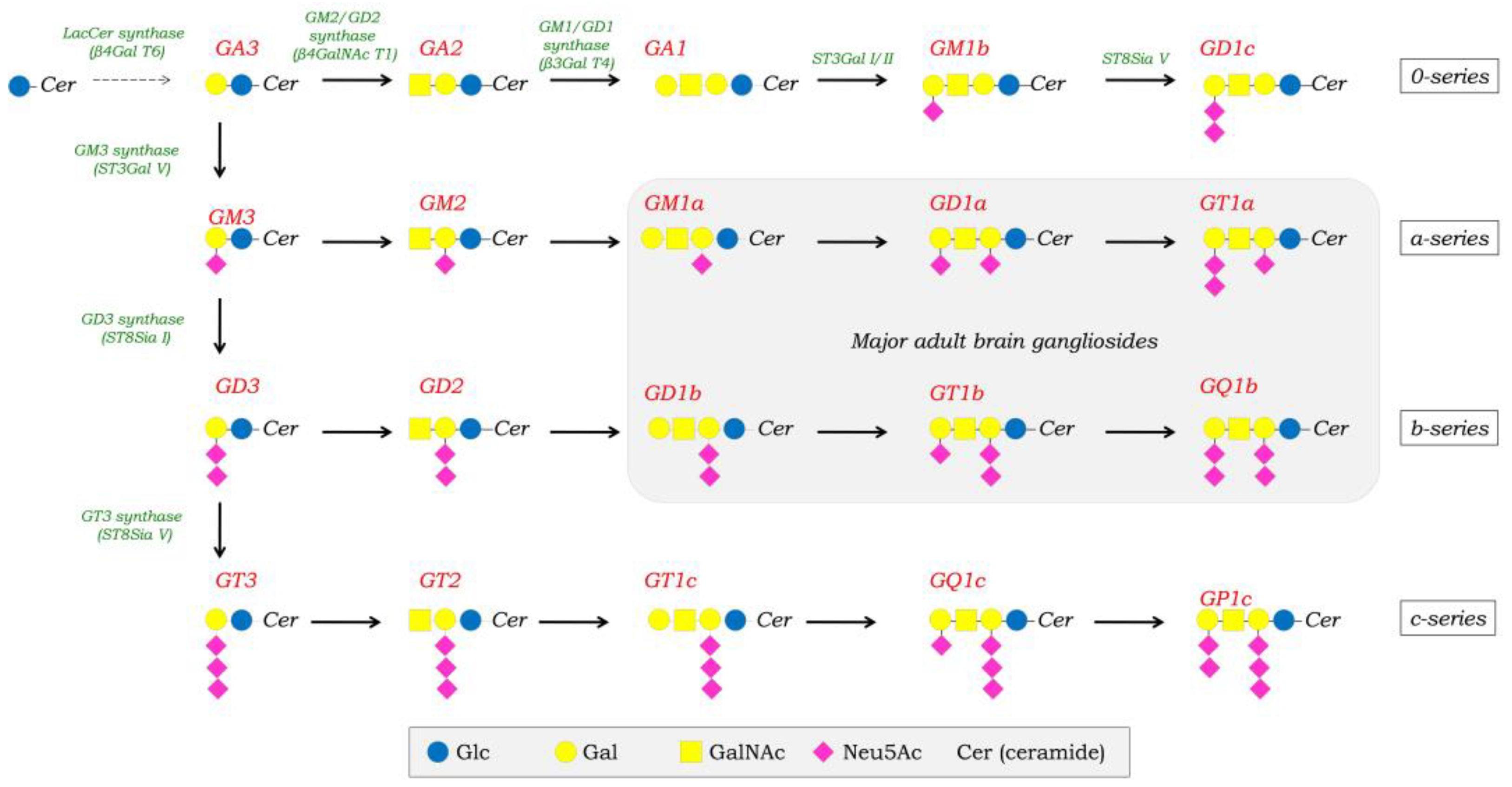
1. Introduction
2. Primary Brain Tumors
2.1. Benign Tumors
2.2. Malignant Tumors
3. Gangliosides in Brain Metastases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schnaar, R.L. Gangliosides of the Vertebrate Nervous System. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef]
- Vasques, J.F.; De Jesus Gonçalves, R.G.; Da Silva-Junior, A.J.; Martins, R.S.; Gubert, F.; Mendez-Otero, R. Gangliosides in Nervous System Development, Regeneration, and Pathologies. Neural Regen. Res. 2023, 18, 81–86. [Google Scholar] [CrossRef]
- Lunghi, G.; Fazzari, M.; Di Biase, E.; Mauri, L.; Chiricozzi, E.; Sonnino, S. The Structure of Gangliosides Hides a Code for Determining Neuronal Functions. FEBS Open Bio 2021, 12, 3193–3200. [Google Scholar] [CrossRef]
- Vilcaes, A.A.; Garbarino-Pico, E.; Torres Demichelis, V.; Daniotti, J.L. Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages. Int. J. Mol. Sci. 2020, 21, 1063. [Google Scholar] [CrossRef]
- Komura, N.; Suzuki, K.G.; Ando, H.; Konishi, M.; Koikeda, M.; Imamura, A.; Chadda, R.; Fujiwara, T.K.; Tsuboi, H.; Sheng, R.; et al. Raft-Based Interactions of Gangliosides with A GPI-Anchored Receptor. Nat. Chem. Biol. 2016, 12, 402–410. [Google Scholar] [CrossRef]
- Kolter, T. Ganglioside Biochemistry. ISRN Biochem. 2012, 2012, 506160. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Sonnino, S.; Mauri, L. GM1 Ganglioside is a Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 868. [Google Scholar] [CrossRef]
- Van Der Haar Àvila, I.; Windhouwer, B.; Van Vliet, S.J. Current State-of-the-Art on Ganglioside-Mediated Immune Modulation in the Tumor Microenvironment. Cancer Metastasis Rev. 2023, 42, 941–958. [Google Scholar] [CrossRef]
- Hakomori Si, S.I. The Glycosynapse. Proc. Natl. Acad. Sci. USA 2002, 99, 225–232. [Google Scholar] [CrossRef]
- Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as Signaling Regulators in Cancer. Int. J. Mol. Sci. 2021, 22, 5076. [Google Scholar] [CrossRef]
- Matsuzaki, K. Aβ-Ganglioside Interactions in the Pathogenesis of Alzheimer’s Disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183233. [Google Scholar] [CrossRef]
- Zuverink, M.; Barbieri, J.T. Protein Toxins that Utilize Gangliosides as Host Receptors. Prog. Mol. Biol. Transl. Sci. 2018, 156, 325–354. [Google Scholar]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef]
- Capitan, F.; Robu, A.C.; Popescu, L.; Flangea, C.; Vukelić, Ž.; Zamfir, A.D. B Subunit Monomers of Cholera Toxin Bind G1 Ganglioside Class as Revealed by Chip-Nanoelectrospray Multistage Mass Spectrometry. J. Carbohydr. Chem. 2015, 34, 388–408. [Google Scholar] [CrossRef]
- Zamfir, A.D. Neurological Analyses: Focus on Gangliosides and Mass Spectrometry. Adv. Exp. Med. Biol. 2014, 806, 153–204. [Google Scholar]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Ledeen, R.; Chowdhury, S. Gangliosides in Neurodegenerative Diseases. Adv. Neurobiol. 2023, 29, 391–418. [Google Scholar]
- Guo, Z. Ganglioside GM1 and the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 9558. [Google Scholar] [CrossRef]
- Ica, R.; Mlinac-Jerkovic, K.; Ilic, K.; Sajko, T.; Munteanu, C.V.A.; Zamfir, A.D.; Kalanj-Bognar, S. Gangliosidome of a Human Hippocampus in Temporal Lobe Epilepsy Resolved by High-Resolution Tandem Mass Spectrometry. Molecules 2022, 27, 4056. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, R.K. Gangliosides: Structure, Isolation, and Analysis. Methods Enzymol. 1982, 83, 139–191. [Google Scholar]
- Lacomba, R.; Salcedo, J.; Alegría, A.; Lagarda, M.J.; Barberá, R.; Matencio, E. Determination of Sialic Acid and Gangliosides in Biological Samples and Dairy Products: A Review. J. Pharm. Biomed. Anal. 2010, 51, 346–357. [Google Scholar] [CrossRef]
- Ogawa-Goto, K.; Abe, T. Gangliosides and Glycosphingolipids of Peripheral Nervous System Myelins—A Minireview. Neurochem. Res. 1998, 23, 305–310. [Google Scholar] [CrossRef]
- Kotani, M.; Kawashima, I.; Ozawa, H.; Ogura, K.; Ishizuka, I.; Terashima, T.; Tai, T. Immunohistochemical Localization of Minor Gangliosides in the Rat Central Nervous System. Glycobiology 1994, 4, 855–865. [Google Scholar] [CrossRef]
- Vajn, K.; Viljetić, B.; Večeslav-Degmečić, I.; Schnaar, R.L.; Heffer, M. Differential Distribution of Major Brain Gangliosides in the Adult Mouse Central Nervous System. PLoS ONE 2013, 8, E75720. [Google Scholar] [CrossRef]
- Kotan, M.; Kawashima, I.; Terashima, H.T.; Tai, T. Immunohistochemical Localization of Major Gangliosides in Rat Cerebellum. Proc. Jpn. Acad. 1992, 68, 109–113. [Google Scholar] [CrossRef][Green Version]
- Mennel, H.D.; Bosslet, K.; Geissel, H.; Bauer, B.L. Immunohistochemically Visualized Localisation of Gangliosides Glac2 (GD3) and Gtri2 (GD2) in Cells of Human Intracranial Tumors. Exp. Toxic Pathol. 2000, 52, 277–285. [Google Scholar] [CrossRef]
- Suzuki, K.G.N.; Ando, H.; Komura, N.; Konishi, M.; Imamura, A.; Ishida, H.; Kiso, M.; Fujiwara, T.K.; Kusumi, A. Revealing the Raft Domain Organization in the Plasma Membrane by Single-Molecule Imaging of Fluorescent Ganglioside Analogs. Methods Enzymol. 2018, 598, 267–282. [Google Scholar]
- Gu, R.X.; Ingólfsson, H.I.; De Vries, A.H.; Marrink, S.J.; Tieleman, D.P. Ganglioside-Lipid and Ganglioside-Protein Interactions Revealed by Coarse-Grained and Atomistic Molecular Dynamics Simulations. J. Phys. Chem. B 2017, 121, 3262–3275. [Google Scholar] [CrossRef]
- Gustafsson, T.; Hua, Y.J.; Dahlgren, M.W.; Livingston, M.; Johansson-Lindbom, B.; Yrlid, U. Direct Interaction Between Cholera Toxin and Dendritic Cells is Required for Oral Adjuvant Activity. Eur. J. Immunol. 2013, 43, 1779–1788. [Google Scholar] [CrossRef]
- Worstell, N.C.; Krishnan, P.; Weatherston, J.D.; Wu, H.-J. Binding Cooperativity Matters: A GM1- Like Ganglioside-Cholera Toxin B Subunit Binding Study Using a Nanocube-Based Lipid Bilayer Array. PLoS ONE 2016, 11, E0153265. [Google Scholar] [CrossRef]
- Hgge, H.; Peter-Katalinić, J.; Reuter, G.; Schauer, R.; Ghidoni, R.; Sonnino, S.; Tettamanti, G. Analysis of Gangliosides Using Fast Atom Bombardment Mass Spectrometry. Chem. Phys. Lipids 1985, 37, 127–141. [Google Scholar]
- Metelmann, W.; Vukelić, Z.; Peter-Katalinić, J. Nanoelectrospray Ionization Time-Of-Flight Mass Spectrometry of Gangliosides from Human Brain Tissue. J. Mass Spectrom. 2001, 36, 21–29. [Google Scholar] [CrossRef]
- Suzuki, A.; Suzuki, M.; Ito, E.; Nitta, T.; Inokuchi, J.I. Mass Spectrometry of Gangliosides. Methods Mol. Biol. 2018, 1804, 207–221. [Google Scholar]
- Zhang, Y.; Wang, J.; Liu, J.; Han, J.; Xiong, S.; Yong, W.; Zhao, Z. Combination of ESI and MALDI Mass Spectrometry for Qualitative, Semi-Quantitative and in Situ Analysis of Gangliosides in Brain. Sci. Rep. 2016, 6, 25289. [Google Scholar] [CrossRef]
- Zamfir, A.; Vukelić, Ž.; Bindila, L.; Peter-Katalinić, J.; Almeida, R.; Sterling, A.; Allen, M. Fully-Automated Chip-Based Nanoelectrospray Tandem Mass Spectrometry of Gangliosides from Human Cerebellum. J. Am. Soc. Mass Spectrom. 2004, 15, 1649–1657. [Google Scholar] [CrossRef]
- Zamfir, A.D.; Lion, N.; Vukelić, Ž.; Bindila, L.; Rossier, J.; Girault, H.H.; Peter-Katalinić, J. Thin Chip Microsprayer System Coupled to Quadrupole Time-of-Flight Mass Spectrometer for Glycoconjugate Analysis. Lab Chip 2005, 5, 298–307. [Google Scholar] [CrossRef]
- Flangea, C.; Serb, A.; Sisu, E.; Zamfir, A.D. Chip-Based Nanoelectrospray Mass Spectrometry of Brain Gangliosides. Biochim. Biophys. Acta 2011, 1811, 513–535. [Google Scholar] [CrossRef]
- Lageveen-Kammeijer, G.S.M.; de Haan, N.; Mohaupt, P.; Wagt, S.; Filius, M.; Nouta, J.; Falck, D.; Wuhrer, M. Highly Sensitive CE-ESI-MS Analysis of N-glycans from Complex Biological Samples. Nat. Commun. 2019, 10, 2137. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, Ž.; Zamfir, A.D.; Bindila, L.; Froesch, M.; Peter-Katalinić, J.; Usuki, S.; Yu, R.K. Screening and Sequencing of Complex Sialylated and Sulfated Glycosphingolipid Mixtures by Negative Ion Electrospray Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2005, 16, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Mcfarland, M.A.; Marshall, A.G.; Hendrickson, C.L.; Nilsson, C.L.; Fredman, P.; Månsson, J.E. Structural Characterization of the GM1 Ganglioside by Infrared Multiphoton Dissociation, Electron Capture Dissociation, and Electron Detachment Dissociation Electrospray Ionization FT-ICR MS/MS. J. Am. Soc. Mass Spectrom. 2005, 16, 752–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ica, R.; Munteanu, C.V.; Vukelić, Ž.; Zamfir, A.D. High-Resolution Mass Spectrometry Reveals a Complex Ganglioside Pattern and Novel Polysialylated Structures Associated with the Human Motor Cortex. Eur. J. Mass Spectrom. 2021, 27, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Ica, R.; Petrut, A.; Vukelić, Ž.; Munteanu, C.V.A.; Petrescu, A.J.; Zamfir, A.D. Gangliosidome of Human Anencephaly: A High Resolution Multistage Mass Spectrometry Study. Biochimie 2019, 163, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Robu, A.C.; Ghiulai, R.M.; Vukelić, Ž.; Clemmer, D.E.; Zamfir, A.D. Electrospray Ionization Ion Mobility Mass Spectrometry of Human Brain Gangliosides. Anal. Chem. 2016, 88, 5166–5178. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Vukelić, Ž.; Clemmer, D.E.; Zamfir, A.D. Ion Mobility Mass Spectrometry Provides Novel Insights into the Expression and Structure of Gangliosides in the Normal Adult Human Hippocampus. Analyst 2018, 143, 5234–5246. [Google Scholar] [CrossRef]
- Zemaitis, K.J.; Izydorczak, A.M.; Thompson, A.C.; Wood, T.D. Streamlined Multimodal DESI and MALDI Mass Spectrometry Imaging on a Singular Dual-Source FT-ICR Mass Spectrometer. Metabolites 2021, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Škrášková, K.; Claude, E.; Jones, E.A.; Towers, M.; Ellis, S.R.; Heeren, R.M. Enhanced Capabilities for Imaging Gangliosides in Murine Brain with Matrix-Assisted Laser Desorption/Ionization and Desorption Electrospray Ionization Mass Spectrometry Coupled to Ion Mobility Separation. Methods 2016, 104, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, J.M.; Li, J.B. Elution, Partial Separation, and Identification of Lipids Directly from Tissue Slices on Planar Chromatography Media by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 8866–8874. [Google Scholar] [CrossRef]
- Huang, F.; Bailey, L.S.; Gao, T.; Jiang, W.; Yu, L.; Bennett, D.A.; Zhao, J.; Basso, K.B.; Guo, Z. Analysis and Comparison of Mouse and Human Brain Gangliosides via Two-Stage Matching of MS/MS Spectra. ACS Omega 2022, 7, 6403–6411. [Google Scholar] [CrossRef]
- Minyan, L.; Bingjia, H.; Zhen, X.; Limin, W.; Yan, C.; Xiangjiao, L.; Shuang, S.; Ning, S. Prenatal Ultrasound Evaluation of Fetal Cutaneous Hemangioma and Related Complications. J. Matern.-Fetal Neonatal Med. 2023, 36, 2157257. [Google Scholar]
- Kawaguchi, K.; Kunimoto, Y.; Inaba, N.; Mikita, C.; Kaminaka, N.; Kanazawa, Y.; Yamamoto, N.; Kakimoto, T.; Suenaga, T.; Takeuchi, H.; et al. Distribution Analysis of Infantile Hemangioma or Capillary Malformation on the Head and Face in Japanese Patients. J. Dermatol. 2019, 46, 849–852. [Google Scholar] [CrossRef]
- Mespreuve, M.; Vanhoenacker, F.; Lemmerling, M. Familial Multiple Cavernous Malformation Syndrome: MR Features in this Uncommon but Silent Threat. J. Belg. Soc. Radiol. 2016, 100, 51. [Google Scholar] [PubMed]
- Dickison, P.; Christou, E.; Wargon, O. A Prospective Study of Infantile Hemangiomas with a Focus on Incidence and Risk Factors. Pediatr. Dermatol. 2011, 28, 663–669. [Google Scholar] [CrossRef]
- Idiculla, P.S.; Gurala, D.; Philipose, J.; Rajdev, K.; Patibandla, P. Cerebral Cavernous Malformations, Developmental Venous Anomaly, and its Coexistence. Eur. Neurol. 2020, 83, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Brown, Z.J.; Baghdadi, A.; Kamel, I.R.; Pawlik, T.M. A Comprehensive Review of Hepatic Hemangioma Management. J. Gastrointest. Surg. 2022, 26, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Moosa, S.; Rajagopal, R. Cavernous Hemangioma of the Conjunctiva. Indian J. Ophthalmol. 2019, 67, 2061. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Polster, S.P. Cavernous Angiomas: Deconstructing a Neurosurgical Disease. J. Neurosurg. 2019, 131, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caton, M.T.; Shenoy, V.S. Cerebral Cavernous Malformations. In Statpearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cavalcanti, D.D.; Kalani, M.Y.; Martirosyan, N.L.; Eales, J.; Spetzler, R.F.; Preul, M.C. Cerebral Cavernous Malformations: From Genes to Proteins to Disease. J. Neurosurg. 2012, 116, 122–132. [Google Scholar] [CrossRef]
- Fischer, A.; Zalvide, J.; Faurobert, E.; Albiges-Rizo, C.; Tournier-Lasserve, E. Cerebral Cavernous Malformations: From CCM Genes to Endothelial Cell Homeostasis. Trends Mol. Med. 2013, 19, 302–308. [Google Scholar] [CrossRef]
- Ledeen, R.; Wu, G. Gangliosides of the Nervous System. Methods Mol. Biol. 2018, 1804, 19–55. [Google Scholar]
- Liu, J.; Zheng, X.; Pang, X.; Li, L.; Wang, J.; Yang, C.; Du, G. Ganglioside GD3 Synthase (GD3S), a Novel Cancer Drug Target. Acta Pharm. Sin. 2018, 8, 713–720. [Google Scholar] [CrossRef]
- Schiopu, C.; Flangea, C.; Capitan, F.; Serb, A.; Vukelić, Ž.; Kalanj-Bognar, S.; Zamfir, A.D. Determination of Ganglioside Composition and Structure in Human Brain Hemangioma by Chip-Based Nanoelectrospray Ionization Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2009, 395, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Mosoarca, C.; Chirita, M.; Udrescu, V.; Dinca, N.; Vukelić, Z.; Allen, M.; Zamfir, A.D. Coupling of Fully Automated Chip-Based Electrospray Ionization to High-Capacity Ion Trap Mass Spectrometer for Ganglioside Analysis. Anal. Biochem. 2008, 378, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Serb, A.; Schiopu, C.; Flangea, C.; Vukelić, Ž.; Sisu, E.; Dinca, N.; Zagrean, L.; Zamfir, A.D. High-Throughput Analysis of Gangliosides in Defined Regions of Fetal Brain by Fully Automated Chip-Based Nanoelectrospray Ionization Multi-Stage Mass Spectrometry. Eur. J. Mass Spectrom. 2009, 15, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Levery, S.B. Glycosphingolipid Structural Analysis and Glycosphingolipidomics. Methods Enzymol. 2005, 405, 300–369. [Google Scholar] [PubMed]
- Sarbu, M.; Dehelean, L.; Munteanu, C.V.; Vukelić, Ž.; Zamfir, A.D. Assessment of Ganglioside Age-Related and Topographic Specificity in Human Brain by Orbitrap Mass Spectrometry. Anal. Biochem. 2017, 521, 40–54. [Google Scholar] [CrossRef]
- Ica, R.; Simulescu, A.; Sarbu, M.; Munteanu, C.V.A.; Vukelić, Ž.; Zamfir, A.D. High Resolution Mass Spectrometry Provides Novel Insights into the Ganglioside Pattern of Brain Cavernous Hemangioma. Anal. Biochem. 2020, 609, 113976. [Google Scholar] [CrossRef]
- Galleguillos, D.; Wang, Q.; Steinberg, N.; Zaidi, A.; Shrivastava, G.; Dhami, K.; Daskhan, G.C.; Schmidt, E.N.; Dworsky-Fried, Z.; Giuliani, F.; et al. Anti-Inflammatory Role of GM1 and Other Gangliosides on Microglia. J. Neuroinflamm. 2022, 19, 9. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, H.; Lee, S.J. Distinct Roles of GT1b And CSF-1 in Microglia Activation in Nerve Injury-Induced Neuropathic Pain. Mol. Pain. 2021, 17, 17448069211020918. [Google Scholar] [CrossRef]
- Netsky, M.G.; Lapresle, J. The First Account of a Meningioma. Bull. Hist. Med. 1956, 30, 465–468. [Google Scholar]
- Salari, N.; Ghasemi, H.; Fatahian, R.; Mansouri, K.; Dokaneheifard, S.; Shiri, M.H.; Hemmati, M.; Mohammadi, M. The Global Prevalence of Primary Central Nervous System Tumors: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2023, 28, 39. [Google Scholar] [CrossRef]
- Decimo, I.; Fumagalli, G.; Berton, V.; Krampera, M.; Bifari, F. Meninges: From Protective Membrane to Stem Cell Niche. Am. J. Stem Cells. 2012, 92–105. [Google Scholar]
- Ostrom, Q.T.; Price, M.; Neff, K.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.R.; Roelcke, U. Meningioma. Curr. Neurol. Neurosci. Rep. 2023, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sofela, A.A.; Mcgavinc, L.; Whitfield, P.C.; Hanemann, C.O. Biomarkers for Differentiating Grade II Meningiomas from Grade I: A Systematic Review. Br. J. Neurosurg. 2021, 35, 696–702. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, Therapeutic, and Prognostic Implications of the 2021 World Health Organization Classification of Tumors of the Central Nervous System. Cancer 2021, 128, 47–58. [Google Scholar] [CrossRef]
- Abbritti, R.V.; Polito, F.; Cucinotta, M.; Lo Giudice, C.; Caffo, M.; Tomasello, C.; Germanò, A.; Aguennouz, M. Meningiomas and Proteomics: Focus on New Potential Biomarkers and Molecular Pathways. Cancer Genom. Proteom. 2016, 13, 369–380. [Google Scholar]
- Fahlström, A.; Dwivedi, S.; Drummond, K. Multiple Meningiomas: Epidemiology, Management, and Outcomes. Neurooncol. Adv. 2023, 5, 3–48. [Google Scholar] [CrossRef]
- Lamszus, K. Meningioma Pathology, Genetics, and Biology. J. Neuropathol. Exp. Neurol. 2004, 63, 275–286. [Google Scholar] [CrossRef]
- Pawloski, J.A.; Fadel, H.A.; Huang, Y.W.; Lee, I.Y. Genomic Biomarkers of Meningioma: A Focused Review. Int. J. Mol. Sci. 2021, 22, 10222. [Google Scholar] [CrossRef]
- Alruwaili, A.A.; De Jesus, O. Meningioma. In Statpearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nazem, A.A.; Ruzevick, J.; Ferreira, M.J. Advances in Meningioma Genomics, Proteomics, and Epigenetics: Insights into Biomarker Identification and Targeted Therapies. Oncotarget 2020, 11, 4544–4553. [Google Scholar] [CrossRef] [PubMed]
- Barkhoudarian, G.; Whitelegge, J.P.; Kelly, D.F.; Simonian, M. Proteomics Analysis of Brain Meningiomas in Pursuit of Novel Biomarkers of the Aggressive Behavior. J. Proteom. Bioinform. 2016, 9, 53–57. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System Tumours: A Practical Update on What Neurosurgeons Need to Know-A Minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Zhang, J.; Han, L.; Zhao, H. DNA Methylation Meningioma Biomarkers: Attributes and Limitations. Front. Mol. Neurisci. 2023, 16, 1182759. [Google Scholar] [CrossRef]
- Davidsson, P.; Fredman, P.; Collins, V.P.; Von Holst, H.; Miinsson, J.E.; Svennerholm, L. Ganglioside Composition in Human Meningiomas. J. Neurochem. 1989, 53, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Berra, B.; Papi, L.; Bigozzi, U.; Serino, D.; Morichi, R.; Mennonna, P.; Rapelli, S.; Cogliati, T.; Montali, E. Correlation Between Cytogenetic Data and Ganglioside Pattern in Human Meningiomas. Int. J. Cancer 1991, 47, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Radić, B.; Vukelić, Ž.; Bognar, S.K. Serum Gangliosides in Patients with Brain Tumors. Coll. Antropol. 2008, 32, 171–175. [Google Scholar]
- Schiopu, C.; Vukelic, Z.; Capitan, F.; Kalanj-Bognar, S.; Sisu, E.; Zamfir, A.D. Chip-Nanoelectrospray Quadrupole Time-of-Flight Tandem Mass Spectrometry of Meningioma Gangliosides: A Preliminary Study. Electrophoresis 2012, 33, 1778–1786. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A Systematic Nomenclature of Carbohydrate Fragmentation in FAB-MS/MS Spectra of Glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Ann, Q.; Adams, J. Structure Determination of Ceramide and Neutral Glycosphingolipids by Collisional Activation of [M+Li]+ Ions. J. Am. Soc. Mass Spectrom. 1992, 3, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor. Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, L.; Li, M.; Wang, X. Immunological Classification of Gliomas Based on Immunogenomic Profiling. J. Neuroinflamm. 2020, 17, 360. [Google Scholar] [CrossRef]
- Perry, A.; Wesseling, P. Histologic Classification of Gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, F.B.; Al-Dhahir, M.A. Gliomas. In Statpearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fyllingen, E.H.; Bø, L.E.; Reinertsen, I.; Jakola, A.S.; Sagberg, L.M.; Berntsen, E.M.; Salvesen, Ø.; Solheim, O. Survival of Glioblastoma in Relation to Tumor Location: A Statistical Tumor Atlas of a Population-Based Cohort. Acta Neurochir. 2021, 163, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.; Turcan, S. Origin of Gliomas. Semin. Neurol. 2018, 38, 5–10. [Google Scholar]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Sarhan, A. Brain Tumor Classification in Magnetic Resonance Images Using Deep Learning and Wavelet Transform. J. Biomed. Sci. Eng. 2020, 13, 102–112. [Google Scholar] [CrossRef]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and Quality of Life Analysis in Glioblastoma Multiforme with Adjuvant Chemoradiotherapy: A Retrospective Study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Gupta, V. Astrocytoma. In Statpearls; Statpearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Walker, D.G.; Kaye, A.H. Diagnosis and Management of Astrocytomas, Oligodendrogliomas and Mixed Gliomas: A Review. Australas. Radiol. 2001, 45, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.J.; Davies, P.; Herbert, C.; Kurian, K.M. Pre-Diagnostic Blood Biomarkers for Adult Glioma. Front. Oncol. 2023, 13, 1163289. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Butler, H.J.; Board, R.; Brennan, P.M.; Chalmers, A.J.; Dawson, T.; Goodden, J.; Hamilton, W.; Hegarty, M.G.; James, A.; et al. Health Economic Evaluation of a Serum-Based Blood Test for Brain Tumour Diagnosis: Exploration of Two Clinical Scenarios. BMJ Open. 2018, 8, E017593. [Google Scholar] [CrossRef] [PubMed]
- Brandner, S.; Jaunmuktane, Z. IDH Mutant Astrocytoma: Biomarkers for Prognostic Stratification and the Next Frontiers. Neuropathol. Appl. Neurobiol. 2019, 45, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Tesileanu, C.M.S.; Vallentgoed, W.R.; French, P.J.; Van Den Bent, M.J. Molecular Markers Related to Patient Outcome in Patients with IDH-Mutant Astrocytomas Grade 2 to 4: A Systematic Review. Eur. J. Cancer 2022, 175, 214–223. [Google Scholar] [CrossRef]
- Ghorbani, A.; Avery, L.M.; Sohaei, D.; Soosaipillai, A.; Richer, M.; Horbinski, C.; Mccortney, K.; Xu, W.; Diamandis, E.P.; Prassas, I. Discovery of Novel Glioma Serum Biomarkers by Proximity Extension Assay. Clin. Proteom. 2023, 20, 12. [Google Scholar] [CrossRef]
- Pienkowski, T.; Kowalczyk, T.; Kretowski, A.; Ciborowski, M. A Review of Gliomas-Related Proteins. Characteristics of Potential Biomarkers. Am. J. Cancer Res. 2021, 11, 3425–3444. [Google Scholar]
- Abdul Rashid, K.; Ibrahim, K.; Wong, J.H.D.; Mohd Ramli, N. Lipid Alterations in Glioma: A Systematic Review. Metabolites 2022, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Huang, Z.; Wei, B.; Li, M. Comprehensive Metabolomics Study on the Pathogenesis of Anaplastic Astrocytoma via UPLC-Q/TOF-MS. Medicine 2022, 101, E29594. [Google Scholar] [CrossRef] [PubMed]
- Björkblom, B.; Wibom, C.; Eriksson, M.; Bergenheim, A.T.; Sjöberg, R.L.; Jonsson, P.; Brännström, T.; Antti, H.; Sandström, M.; Melin, B. Distinct Metabolic Hallmarks of WHO Classified Adult Glioma Subtypes. Neuro-Oncology 2022, 24, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Semreen, A.M.; El-Huneidi, W.; Bustanji, Y.; Abu-Gharbieh, E.; Alqudah, M.A.Y.; Alhusban, A.; Shara, M.; Abuhelwa, A.Y.; Soares, N.C.; et al. Preclinical and Clinical Applications of Metabolomics and Proteomics in Glioblastoma Research. Int. J. Mol. Sci. 2022, 24, 348. [Google Scholar] [CrossRef] [PubMed]
- Feraco, P.; Bacci, A.; Ferrazza, P.; Van Den Hauwe, L.; Pertile, R.; Girlando, S.; Barbareschi, M.; Gagliardo, C.; Morganti, A.G.; Petralia, B. Magnetic Resonance Imaging Derived Biomarkers of IDH Mutation Status and Overall Survival in Grade III Astrocytomas. Diagnostics 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Fortin Ensign, S.P.; Jenkins, R.B.; Giannini, C.; Sarkaria, J.N.; Galanis, E.; Kizilbash, S.H. Translational Significance of CDKN2A/B Homozygous Deletion in Isocitrate Dehydrogenase-Mutant Astrocytoma. Neuro-Oncology 2023, 25, 28–36. [Google Scholar] [CrossRef]
- Ohba, S.; Kuwahara, K.; Yamada, S.; Abe, M.; Hirose, Y. Correlation between IDH, ATRX, and TERT Promoter Mutations in Glioma. Brain Tumor Pathol. 2020, 37, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Gerges, N.; Korshunov, A.; Sabha, N.; Khuong-Quang, D.A.; Fontebasso, A.M.; Fleming, A.; Hadjadj, D.; Schwartzentruber, J.; Majewski, J.; et al. Frequent ATRX Mutations and Loss of Expression in Adult Diffuse Astrocytic Tumors Carrying IDH1/IDH2 and TP53 Mutations. Acta Neuropathol. 2012, 124, 615–625. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, L.; Su, Y. Aquaporin-4 As A New Potential Molecular Biomarker for Prognosis of Low-Grade Glioma: Comprehensive Analysis Based on Online Platforms. World Neurosurg. 2023, 175, E713–E722. [Google Scholar] [CrossRef]
- Ran, Z.; Yang, J.; Liu, Y.; Chen, X.; Ma, Z.; Wu, S.; Huang, Y.; Song, Y.; Gu, Y.; Zhao, S.; et al. Gliomarker: An Integrated Database for Knowledge Exploration of Diagnostic Biomarkers in Gliomas. Front. Oncol. 2022, 12, 792055. [Google Scholar] [CrossRef]
- Traylor, T.D.; Hogan, E.L. Gangliosides of Human Cerebral Astrocytomas. J. Neurochem. 1980, 34, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.S.; Hogan, E.L.; Koontz, D.A.; Traylor, T.D. Serum Gangliosides in Cerebral Astrocytoma. Ann. Neurol. 1980, 8, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Ladisch, S.; Chang, F.; Li, R.; Cogen, P.; Johnson, D. Detection of Medulloblastoma and Astrocytoma-Associated Ganglioside GD3 in Cerebrospinal Fluid. Cancer Lett. 1997, 120, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F.; et al. Targeting and Killing Glioblastoma with Monoclonal Antibody to O-Acetyl GD2 Ganglioside. Oncotarget 2016, 7, 41172–41185. [Google Scholar] [CrossRef] [PubMed]
- Kasperzyk, J.L.; El-Abbadi, M.M.; Hauser, E.C.; D’Azzo, A.; Platt, F.M.; Seyfried, T.N. N-Butyldeoxygalactonojirimycin Reduces Neonatal Brain Ganglioside Content in a Mouse Model of GM1 Gangliosidosis. J. Neurochem. 2004, 89, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Aixinjueluo, W.; Kasama, T.; Ohkawa, Y.; Yoshihara, M.; Ohmi, Y.; Tajima, O.; Suzumura, A.; Kittaka, D.; Furukawa, K. Disruption of GM2/GD2 Synthase Gene Resulted in Overt Expression of 9-O-Acetyl GD3 Irrespective of Tis21. J. Neurochem. 2008, 105, 1057–1066. [Google Scholar] [CrossRef]
- Wagener, R.; Kobbe, B.; Stoffel, W. Quantification of Gangliosides by Microbore High-Performance Liquid Chromatography. J. Lipid Res. 1996, 37, 1823–1829. [Google Scholar] [CrossRef]
- Zamfir, A.D.; Fabris, D.; Capitan, F.; Munteanu, C.; Vukelić, Ž.; Flangea, C. Profiling and Sequence Analysis of Gangliosides in Human Astrocytoma by High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 7321–7335. [Google Scholar] [CrossRef]
- Bartik, P.; Maglott, A.; Entlicher, G.; Vestweber, D.; Takeda, K.; Martin, S.; Dontenwill, M. Detection of a Hypersialylated Beta1 Integrin Endogenously Expressed in the Human Astrocytoma Cell Line A172. Int. J. Oncol. 2008, 32, 1021–1031. [Google Scholar]
- Best, M.G.; Sol, N.; Zijl, S.; Reijneveld, J.C.; Wesseling, P.; Wurdinger, T. Liquid Biopsies in Patients with Diffuse Glioma. Acta Neuropathol. 2015, 129, 849–865. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Delannoy, P. Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 6145. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, Ž.; Kalanj-Bognar, S.; Froesch, M.; Bîndila, L.; Radić, B.; Allen, M.; Peter-Katalinić, J.; Zamfir, A.D. Human Gliosarcoma-Associated Ganglioside Composition is Complex and Distinctive as Evidenced by High-Performance Mass Spectrometric Determination and Structural Characterization. Glycobiology 2007, 17, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Maira, M.; Roychoudhury, R.; Galan, A.; Brahimi, F.; Gilbert, M.; Cunningham, A.M.; Josephy, S.; Pirvulescu, I.; Moffett, S.; et al. Vaccination with Tumor-Ganglioside Glycomimetics Activates a Selective Immunity that Affords Cancer Therapy. Cell Chem. Biol. 2019, 26, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Berois, N.; Pittini, A.; Osinaga, E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers 2022, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Delannoy, P.; Groux-Degroote, S. O-Acetylated Gangliosides as Targets for Cancer Immunotherapy. Cells 2020, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Santana, V.M.; Barfield, R.C. Anti-GD2 Antibody Therapy for GD2-Expressing Tumors. Curr. Cancer Drug Targ. 2010, 10, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.H.; Furukawa, K.; Furukawa, K. Enhancement of Malignant Properties of Human Glioma Cells by Ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef]
- Sung, C.C.; Pearl, D.K.; Coons, S.W.; Scheithauer, B.W.; Johnson, P.C.; Zheng, M.; Yates, A.J. Correlation of Ganglioside Patterns of Primary Brain Tumors with Survival. Cancer 1995, 75, 851–859. [Google Scholar] [CrossRef]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 Synthase are Key Drivers for Glioblastoma Stem Cells and Tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Fabris, D.; Rožman, M.; Sajko, T.; Vukelić, Ž. Aberrant Ganglioside Composition in Glioblastoma Multiforme and Peritumoral Tissue: A Mass Spectrometry Characterization. Biochimie 2017, 137, 56–68. [Google Scholar] [CrossRef]
- Sarbu, M.; Vukelić, Ž.; Clemmer, D.E.; Zamfir, A.D. Electrospray Ionization Ion Mobility Mass Spectrometry Provides Novel Insights into the Pattern and Activity of Fetal Hippocampus Gangliosides. Biochimie 2017, 139, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Raab, S.; Henderson, L.; Fabris, D.; Vukelić, Ž.; Clemmer, D.E.; Zamfir, A.D. Cerebrospinal Fluid: Profiling and Fragmentation of Gangliosides by Ion Mobility Mass Spectrometry. Biochimie 2020, 170, 36–48. [Google Scholar] [CrossRef]
- Sarbu, M.; Clemmer, D.E.; Zamfir, A.D. Ion Mobility Mass Spectrometry of Human Melanoma Gangliosides. Biochimie 2020, 177, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Petrica, L.; Clemmer, D.E.; Vukelić, Ž.; Zamfir, A.D. Gangliosides of Human Glioblastoma Multiforme: A Comprehensive Mapping and Structural Analysis by Ion Mobility Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Aoyagi, M.; Kasama, T.; Handa, S.; Hirakawa, K.; Taki, T. GT1b in Human Metastatic Brain Tumors: GT1b as a Brain Metastasis-Associated Ganglioside. Biochim. Biophys. Acta 1999, 1437, 93–99. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.C.; Liapis, E.; Harris, B.T.; Perlin, D.S.; Carter, C.L. Mass Spectrometry Imaging Discriminates Glioblastoma Tumor Cell Subpopulations and Different Microvascular Formations Based on their Lipid Profiles. Sci. Rep. 2022, 12, 17069. [Google Scholar] [CrossRef]
- Ermini, L.; Morganti, E.; Post, A.; Yeganeh, B.; Caniggia, I.; Leadley, M.; Faria, C.C.; Rutka, J.T.; Post, M. Imaging Mass Spectrometry Identifies Prognostic Ganglioside Species in Rodent Intracranial Transplants of Glioma and Medulloblastoma. PLoS ONE 2017, 12, E0176254. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Kallee, N.; Bergès, R.; Hein, V.; Cabaret, S.; Garcia, J.; Gros, A.; Tabouret, E.; Tchoghandjian, A.; Colin, C.; Figarella-Branger, D. Deciphering the Action of Neuraminidase in Glioblastoma Models. Int. J. Mol. Sci. 2023, 24, 11645. [Google Scholar] [CrossRef]
- Chahlavi, A.; Rayman, P.; Richmond, A.L.; Biswas, K.; Zhang, R.; Vogelbaum, M.; Tannenbaum, C.; Barnett, G.; Finke, J.H. Glioblastomas Induce T-Lymphocyte Death by Two Distinct Pathways Involving Gangliosides and CD70. Cancer Res. 2005, 65, 5428–5438. [Google Scholar] [CrossRef]
- Morantz, R.A.; Feigin, I.; Ransohoff, J., 3rd. Clinical and Pathological Study of 24 Cases of Gliosarcoma. J. Neurosurg. 1976, 45, 398–408. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Dinapoli, R.P.; Scheithauer, B.W.; Jenkins, R.B.; Wang, C.H.; O’Fallon, J.R.; Farr, G., Jr. Clinical Outcome of Gliosarcoma Compared with Glioblastoma Multiforme: North Central Cancer Treatment Group Results. J. Neurosurg. 1998, 89, 425–430. [Google Scholar] [CrossRef]
- Frandsen, S.; Broholm, H.; Larsen, V.A.; Grunnet, K.; Møller, S.; Poulsen, H.S.; Michaelsen, S.R. Clinical Characteristics of Gliosarcoma and Outcomes from Standardized Treatment Relative to Conventional Glioblastoma. Front. Oncol. 2019, 9, 1425. [Google Scholar] [CrossRef]
- Din, N.U.; Ishtiaq, H.; Rahim, S.; Abdul-Ghafar, J.; Ahmad, Z. Gliosarcoma in Patients under 20 Years of Age. A Clinicopathologic Study of 11 Cases and Detailed Review of the Literature. BMC Pediatr. 2021, 21, 101. [Google Scholar] [CrossRef]
- Otsu, Y.; Matsumoto, Y.; Higaki, K.; Furuta, T.; Moritsubo, M.; Yoshitake, H.; Nagata, Y.; Hashikawa, T.; Sakai, H.; Nakagawa, S.; et al. Gliosarcoma with Unusual Glial Components: Two Case Reports. Neuropathology 2022, 42, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Lee, J.H.; Lee, M.S.; Suh, S.J.; Lee, Y.S.; Kang, D.G. Primary Gliosarcoma with Extracranial Metastasis. Brain Tumor Res. Treat. 2020, 8, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Dardis, C.; Donner, D.; Sanai, N.; Xiu, J.; Mittal, S.; Michelhaugh, S.K.; Pandey, M.; Kesari, S.; Heimberger, A.B.; Gatalica, Z.; et al. Gliosarcoma vs. Glioblastoma: A Retrospective Case Series Using Molecular Profiling. BMC Neurol. 2021, 21, 231. [Google Scholar]
- Shukla, G.S.; Krag, D.N. Selective Delivery of Therapeutic Agents for the Diagnosis and Treatment of Cancer. Expert. Opin. Biol. Ther. 2006, 6, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.W.; Patel, A.J. Review of Current Principles of the Diagnosis and Management of Brain Metastases. Front. Oncol. 2022, 12, 857622. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, J.W.; Park, M.; Kim, J.W.; Kim, M.; Suh, S.H.; Chang, Y.S.; Ahn, S.J.; Lee, J.M. Brain Metastases from Lung Adenocarcinoma may Preferentially Involve the Distal Middle Cerebral Artery Territory and Cerebellum. Front. Oncol. 2020, 10, 1664. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs. Radiosurgery with whole Brain Radiation Therapy on Cognitive Function in Patients with 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Ramos, R.I.; Bustos, M.A.; Wu, J.; Jones, P.; Chang, S.C.; Kiyohara, E.; Tran, K.; Zhang, X.; Stern, S.L.; Izraely, S.; et al. Upregulation of Cell Surface GD3 Ganglioside Phenotype is Associated with Human Melanoma Brain Metastasis. Mol. Oncol. 2020, 8, 1760–1778. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Zhang, P.; Momota, H.; Kato, A.; Hashimoto, N.; Ohmi, Y.; Bhuiyan, R.H.; Farhana, Y.; Natsume, A.; Wakabayashi, T.; et al. Lack of GD3 Synthase (St8sia1) Attenuates Malignant Properties of Gliomas in Genetically Engineered Mouse Model. Cancer Sci. 2021, 112, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Bobowski, M.; Cazet, A.; Steenackers, A.; Delannoy, P. Role of Complex Gangliosides in Cancer Progression. In Carbohydrate Chemistry; Royal Society of Chemistry Books, Burlington House: London, UK, 2012; Volume 37, pp. 1–20. [Google Scholar]
- Vandermeersch, S.; Vanbeselaere, J.; Delannoy, C.P.; Drolez, A.; Mysiorek, C.; Guérardel, Y.; Delannoy, P.; Julien, S. Accumulation of GD1α Ganglioside in MDA-MB-231 Breast Cancer Cells Expressing ST6GalNAc V. Molecules 2015, 20, 6913–6924. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, Y.; Kambe, M.; Ohkawa, Y.; Hamamura, K.; Tajima, O.; Takeuchi, R.; Furukawa, K.; Furukawa, K. Differential Roles of Gangliosides in Malignant Properties of Melanomas. PLoS ONE 2018, 13, E0206881. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki, H.; Nakanishi, Y.; Yagasaki, H.; Masuda, S. Multiple Immunofluorescence Imaging Analysis Reveals Differential Expression of Disialogangliosides GD3 and GD2 in Neuroblastomas. Pediatr. Dev. Pathol. 2021, 25, 141–154. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P. Ganglioside GM3 is Antiangiogenic in Malignant Brain Cancer. J. Oncol. 2010, 2010, 961243. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; Van De Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that Mediate Breast Cancer Metastasis to the Brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- Zamfir, A.D.; Serb, A.; Vukelic, Ž.; Flangea, C.; Schiopu, C.; Fabris, D.; Kalanj-Bognar, S.; Capitan, F.; Sisu, E. Assessment of the Molecular Expression and Structure of Gangliosides in Brain Metastasis of Lung Adenocarcinoma by an Advanced Approach Based on Fully Automated Chip-Nanoelectrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 2145–2159. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Momota, H.; Kato, A.; Hashimoto, N.; Tsuda, Y.; Kotani, N.; Honke, K.; Suzumura, A.; Furukawa, K.; Ohmi, Y.; et al. Ganglioside GD3 Enhances Invasiveness of Gliomas by Forming a Complex with Platelet-Derived Growth Factor Receptor A nd Yes Kinase. J. Biol. Chem. 2015, 290, 16043–16058. [Google Scholar] [CrossRef]
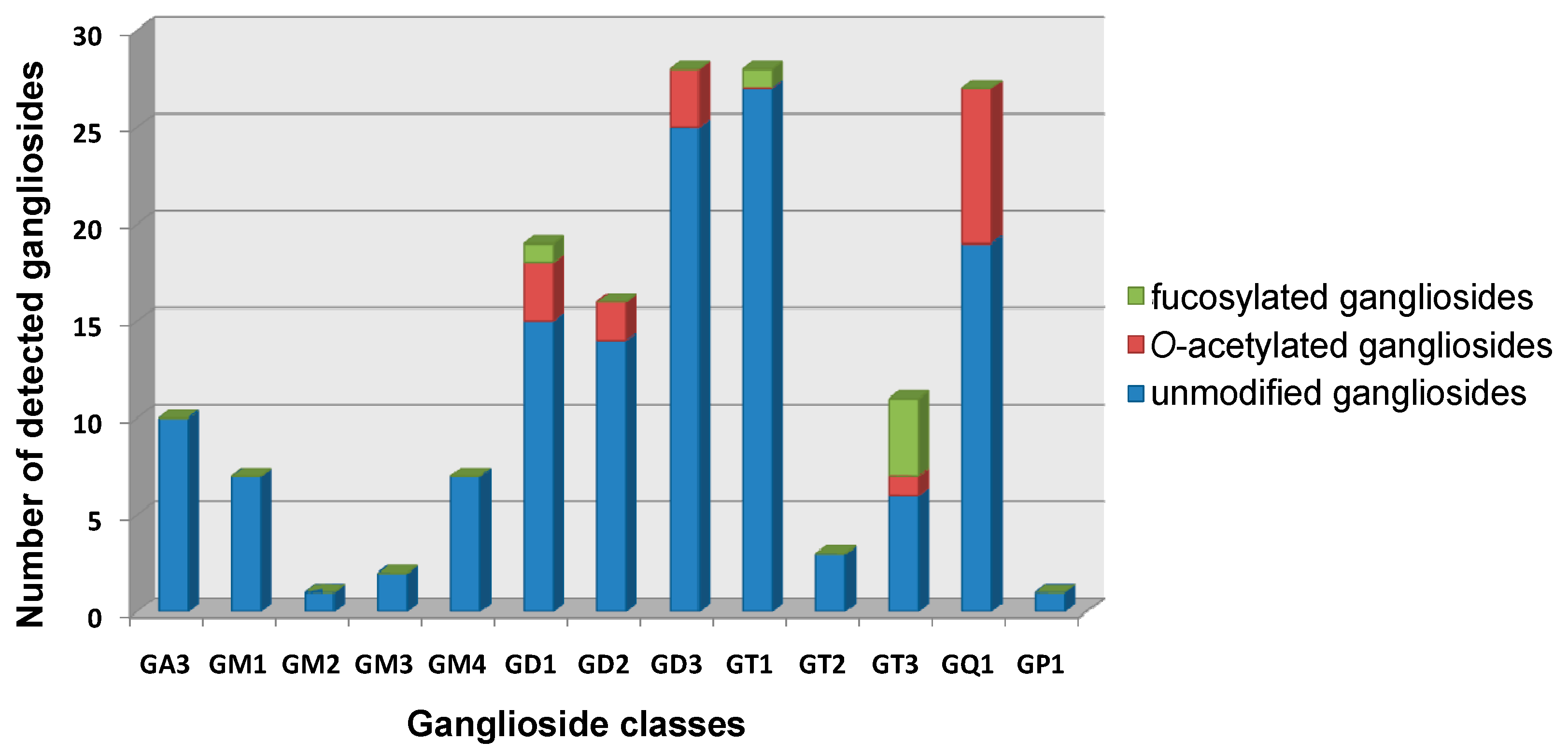
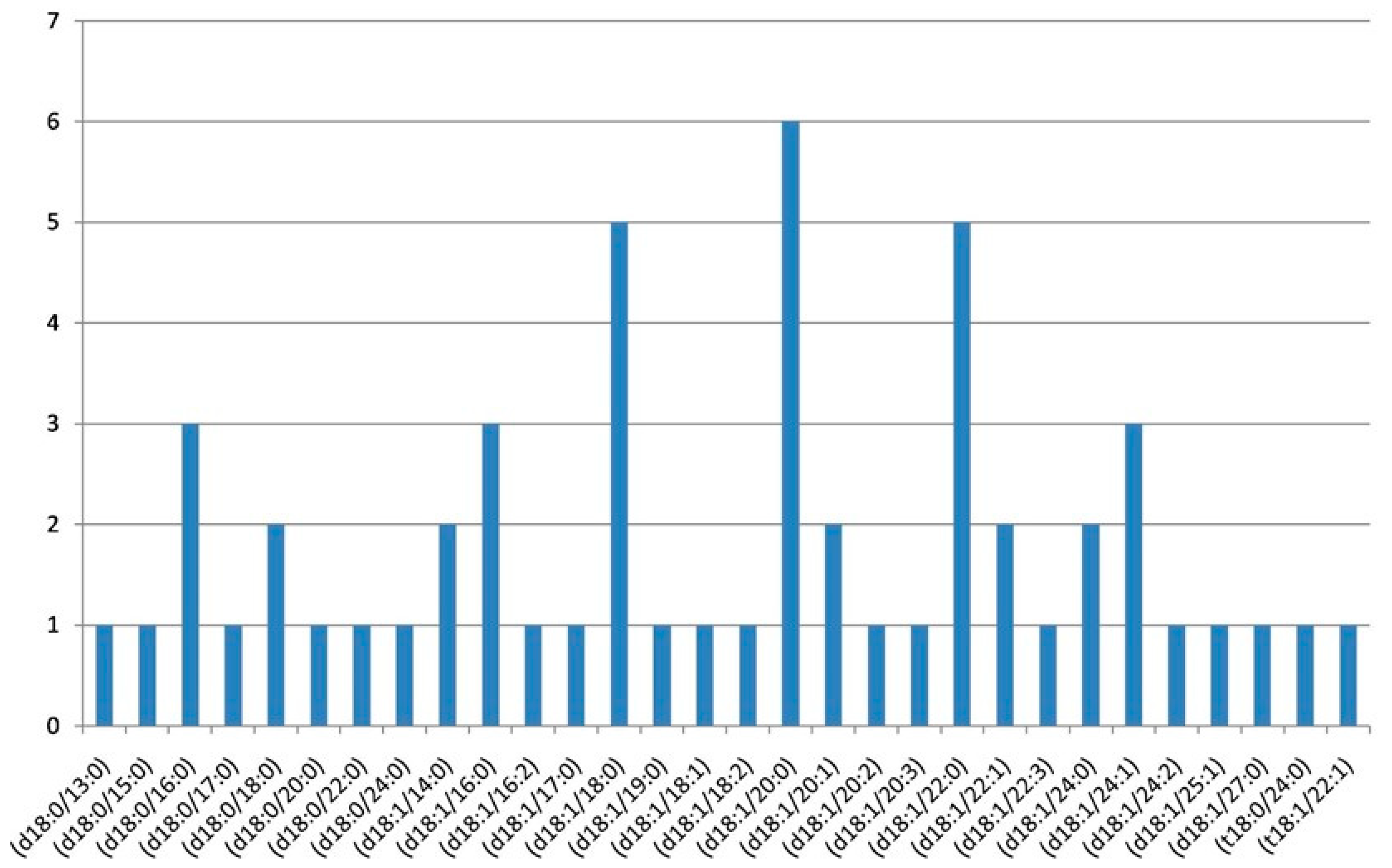
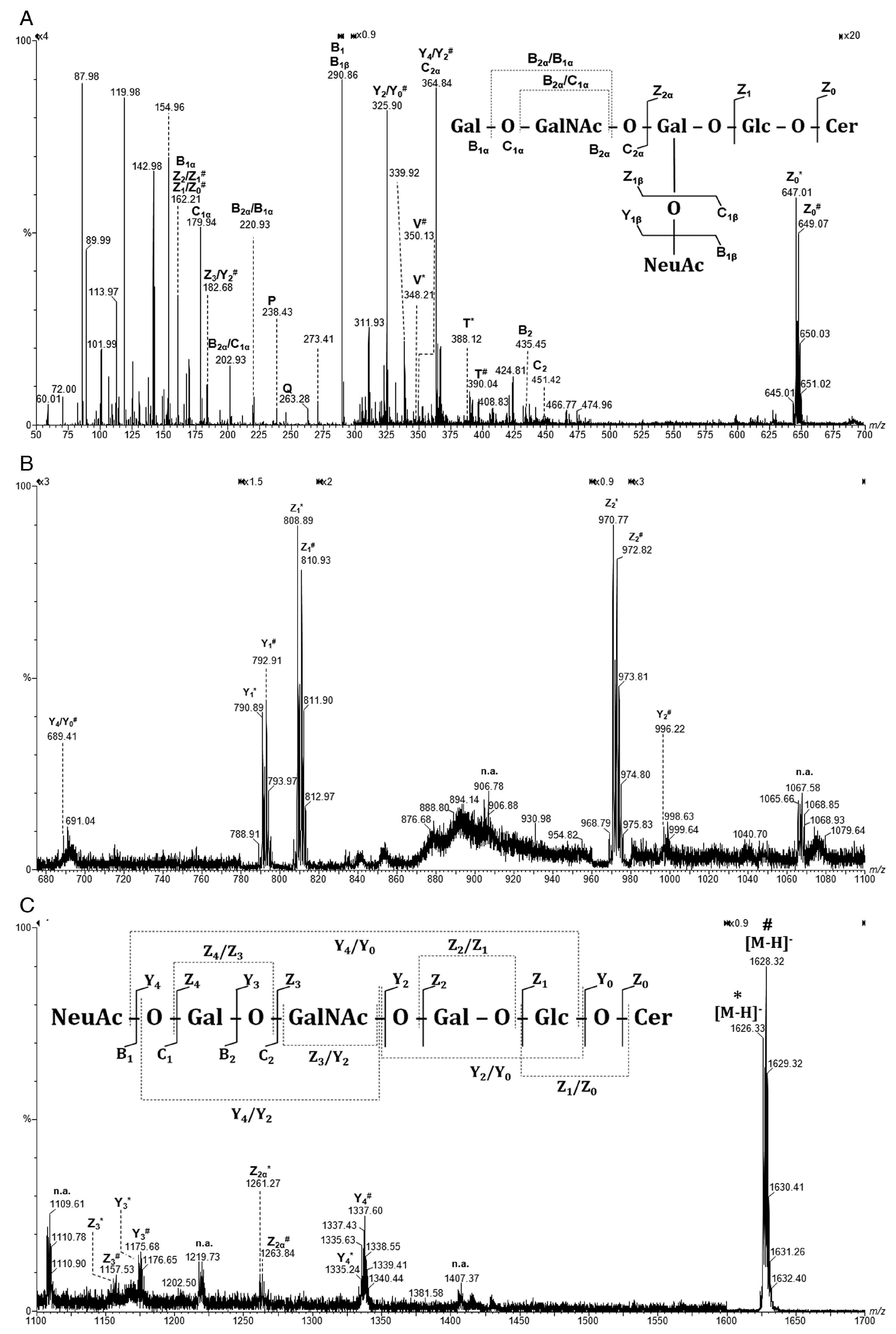
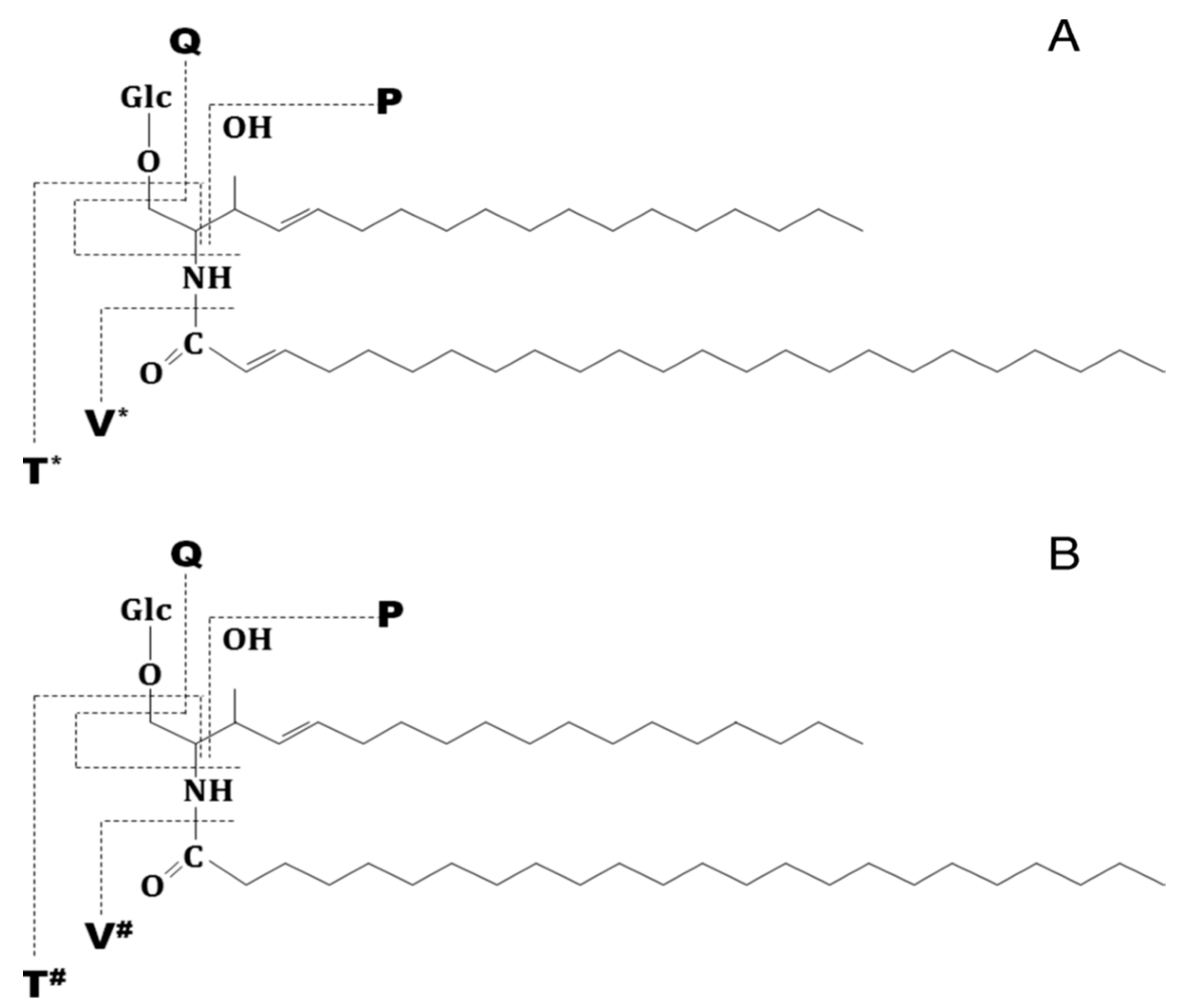
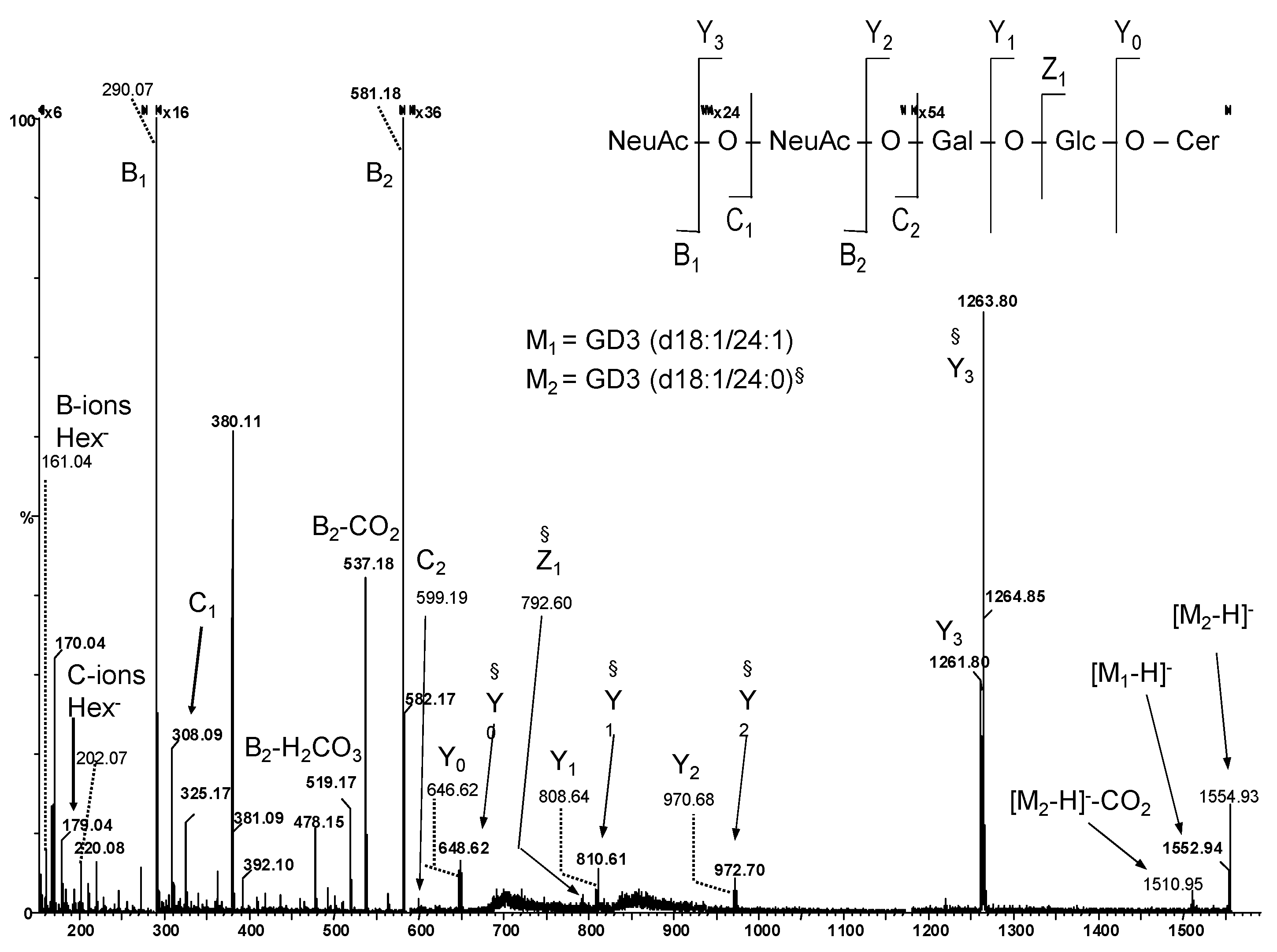

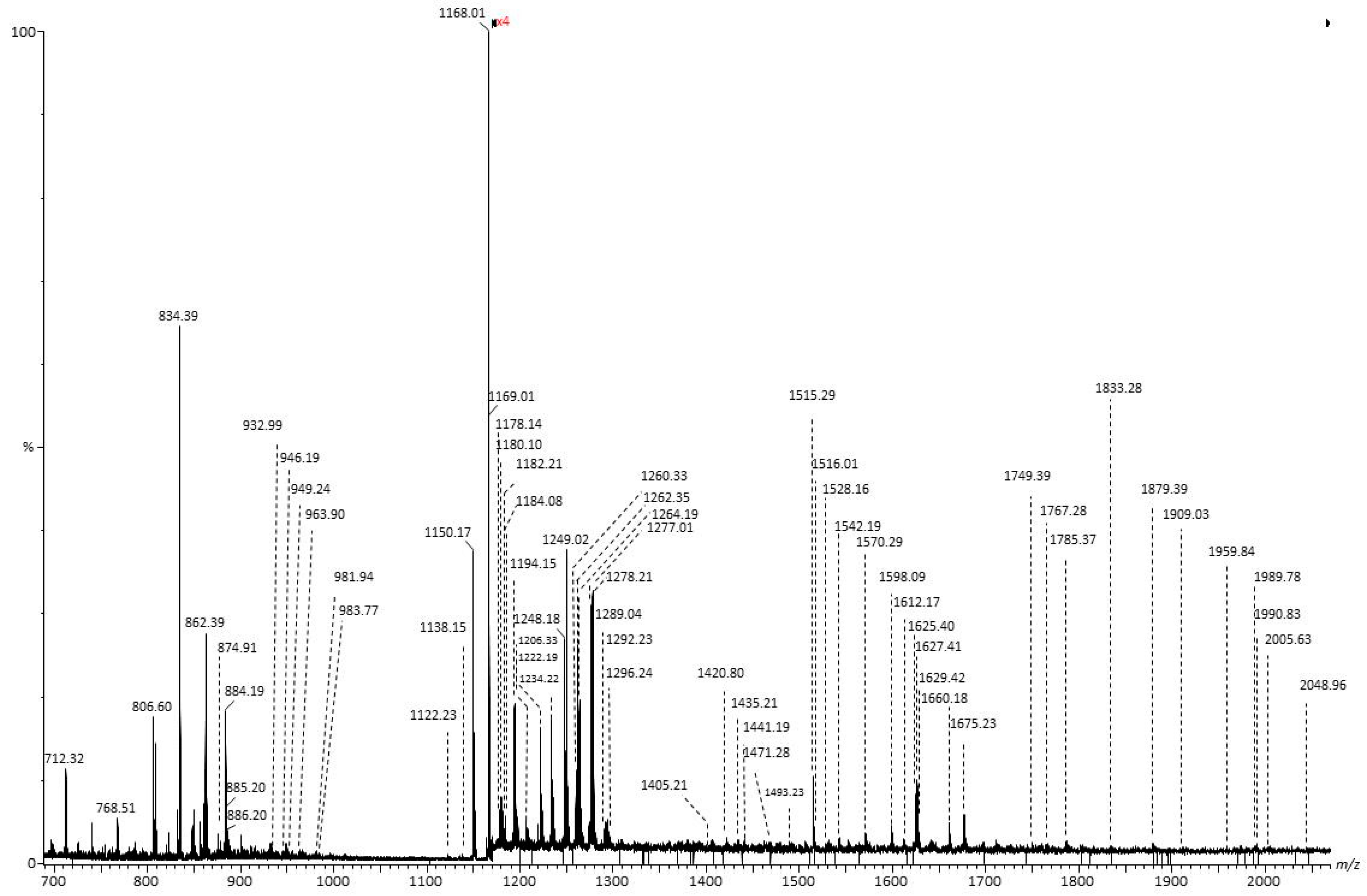

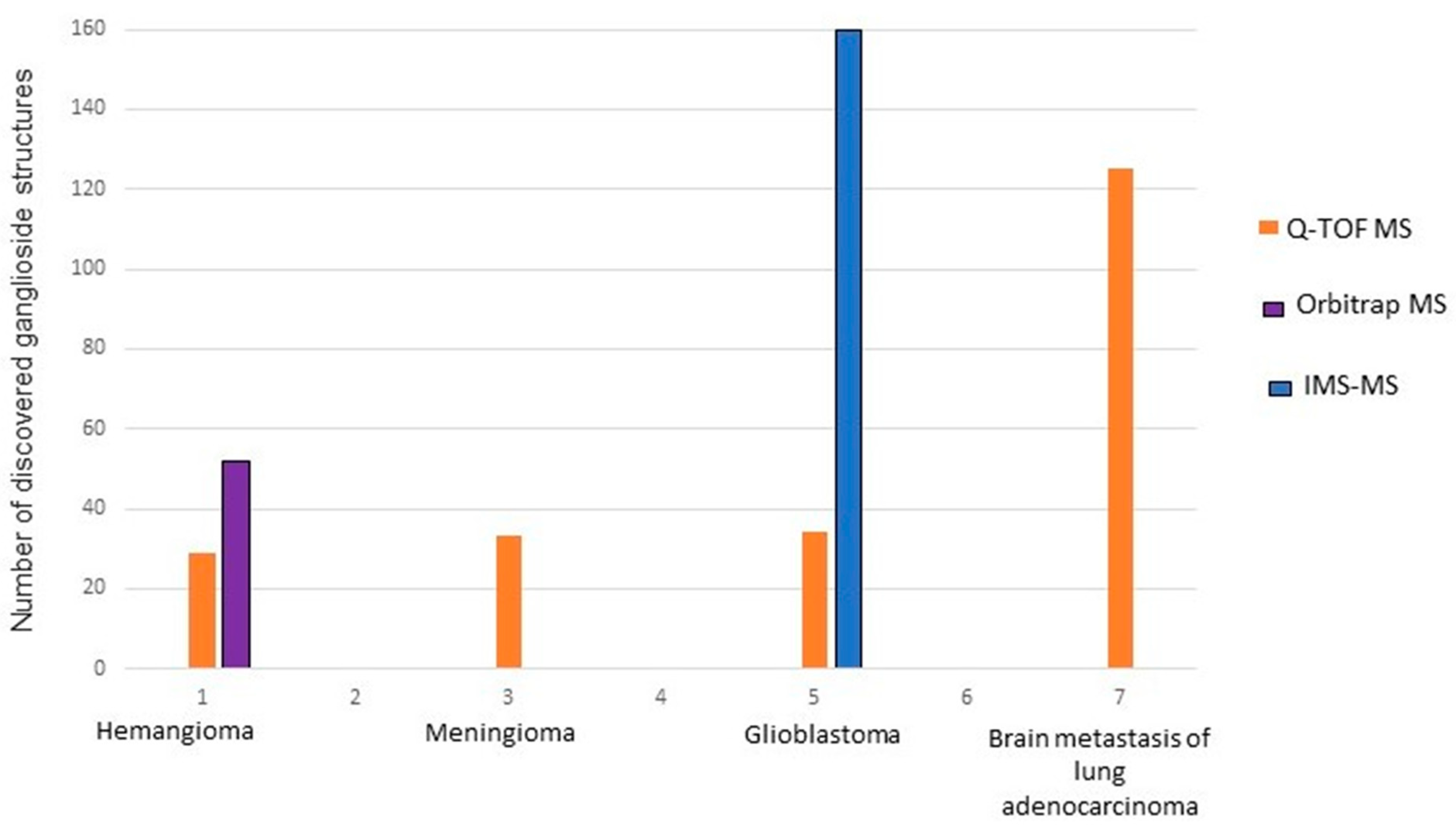
| Nr. crt. | Proposed Structure | m/z theor. | m/z exp. | Molecular Ion | Mass Accuracy (ppm) | Relative Abundance % |
|---|---|---|---|---|---|---|
| 1 | GM3 (d18:0/24:0) | 645.3958 | 645.3930 | [M-4H++2Na+-H2O]2− | 4.3 | 35.03 |
| 2 | GM3 (t18:0/24:0) | 662.3986 | 662.3960 | [M-4H++2Na+]2− | 3.9 | 18.51 |
| 3 | GT1 (d18:1/18:0) | 708.3479 | 708.3472 | [M-3H+]3− | 0.9 | 28.77 |
| 4 | GD3 (d18:0/16:0) | 712.8989 | 712.9012 | [M−2H+-H2O]2− | −3.2 | 30.83 |
| 5 | GD3 (d18:1/16:0) | 720.8964 | 720.8964 | [M-2H+]2− | 0 | 34.05 |
| 6 | GT1 (d18:1/20:0) | 717.6917 | 717.6918 | [M-3H+]3− | −0.1 | 31.83 |
| 7 | GT1 (d18:1/22:0) | 727.0355 | 727.0339 | [M-3H+]3− | 2.2 | 29.41 |
| 8 | GT1 (t18:1/22:1) | 731.6952 | 731.6950 | [M-3H+]3− | 0.2 | 28.77 |
| 9 | GM2 (d18:1/24:0) | 732.9452 | 732.9510 | [M-2H+]2− | −7.9 | 33.55 |
| 10 | GD3 (d18:1/18:1) | 733.9121 | 733.9118 | [M-2H+]2− | 0.4 | 78.73 |
| 11 | GD3 (d18:1/18:0) | 734.9121 | 734.9122 | [M-2H+]2− | −0.1 | 59.21 |
| 12 | O-Ac-GT1 (d18:0/22:0) | 735.7073 | 735.7051 | [M-3H+-H2O]3− | 2.9 | 52.67 |
| 13 | GD3 (d18:1/20:0) | 748.9277 | 748.9276 | [M-2H+]2− | 0.1 | 42.85 |
| 14 | GM1 (d18:1/14:0) | 754.8901 | 754.8914 | [M-3H++Na+]2− | −1.7 | 35.78 |
| 15 | GM1 (d18:0/16:0) | 758.9226 | 758.9269 | [M-2H+]2− | −5.6 | 28.66 |
| 16 | GD3 (d18:1/22:1) | 761.9356 | 761.9365 | [M-2H+]2− | −1.1 | 32.73 |
| 17 | GD3 (d18:1/22:0) | 762.9434 | 762.9432 | [M-2H+]2− | 0.2 | 42.90 |
| 18 | GM1 (d18:1/18:2) | 769.9148 | 769.9210 | [M-2H+]2− | −8.0 | 59.21 |
| 19 | GD3 (d18:1/24:2) | 774.9434 | 774.9432 | [M-2H+]2− | 0.2 | 47.93 |
| 20 | GD3 (d18:1/24:1) | 775.9512 | 775.9512 | [M-2H+]2− | 0.012 | 59.21 |
| 21 | GD3 (d18:1/24:0) | 776.9590 | 776.9592 | [M-2H+]2− | −0.2 | 47.93 |
| 22 | GM1 (d18:0/18:0) | 783.9292 | 783.9283 | [M-3H++Na+]2− | 1.1 | 35.78 |
| 23 | GD2 (d18:0/13:0) | 793.4598 | 793.4618 | [M-2H+-H2O]2− | −2.5 | 51.73 |
| 24 | GM1 (d18:0/18:0) | 794.9202 | 794.9232 | [M-4H++2Na+]2− | −3.7 | 41.82 |
| 25 | GM1 (d18:1/20:0) | 796.9371 | 796.9384 | [M-3H++Na+]2− | −1.6 | 47.12 |
| 26 | GM1 (d18:0/20:0) | 808.9359 | 808.9434 | [M-4H++2Na+]2− | −9.2 | 26.78 |
| 27 | GM1 (d18:0/20:0) | 809.9437 | 809.9441 | [M-4H++2Na+]2− | −0.4 | 30.60 |
| 28 | GQ1 (d18:1/20:0) | 814.7235 | 814.7252 | [M-3H+]3− | −2.0 | 24.76 |
| 29 | GD3 (d18:1/28:0) | 815.9813 | 815.9850 | [M-3H++Na]2− | −4.5 | 29.53 |
| 30 | GD2 (d18:1/18:0) | 827.4464 | 827.4387 | [M-2H+-H2O]2 | 9.3 | 30.19 |
| 31 | GD2 (d18:1/18:0) | 836.4517 | 836.4515 | [M-2H+]2− | 0.2 | 55.21 |
| 32 | GD2 (d18:1/20:0) | 850.4674 | 850.4671 | [M-2H+]2− | 0.3 | 41.82 |
| 33 | Fuc-GM1 (d18:1/18:3) | 863.918 | 863.9180 | [M-4H++2Na+]2− | 0 | 87.86 |
| 34 | GD1 (d18:1/18:0) | 917.4781 | 917.4782 | [M-2H+]2− | −0.1 | 94.38 |
| 35 | GD1 (d18:1/20:0) | 922.4885 | 922.4835 | [M-2H+-H2O]2− | 5.4 | 36.29 |
| 36 | GT3 (d18:1/25:1) | 928.5067 | 928.5061 | [M-2H+]2− | 0.6 | 32.64 |
| 37 | GD1 (d18:1/20:0) | 931.4938 | 931.4938 | [M-2H+]2− | 0 | 100 |
| 38 | GD1 (d18:1/22:0) | 945.5094 | 945.5081 | [M-2H+]2− | 1.3 | 38.41 |
| 39 | O-Ac-GD1 (d18:1/20:1) | 951.4912 | 951.4899 | [M-2H+]2− | 1.3 | 66.04 |
| 40 | GD1 (d18:1/24:1) | 958.5173 | 958.5155 | [M-2H+]2− | 1.8 | 17.46 |
| 41 | Fuc-GT3 (d18:1/20:2) | 965.4888 | 965.4874 | [M−2H+]2− | 1.4 | 12.38 |
| 42 | Fuc GT3 (d18:1/20:1) | 966.4966 | 966.4971 | [M-2H+]2− | −0.5 | 24.90 |
| O-Ac-GD1 (d18:1/22:0) | 966.5068 | 966.4971 | [M-2H+]2− | 10.0 | 12.85 | |
| 43 | Fuc-GD1 (d18:0/18:0) | 991.5149 | 991.5089 | [M-2H+]2− | 6.0 | 12.38 |
| 44 | O-Ac-GM4 (d18:1/16:0) | 1031.6628 | 1031.6579 | [M-H+]− | 4.7 | 79.67 |
| 45 | GT1 (d18:1/14:0) | 1034.995 | 1034.9885 | [M-2H+]2− | 6.2 | 65.37 |
| 46 | GT1 (d18:1/18:0) | 1063.0258 | 1063.0255 | [M-2H+]2− | 0.2 | 59.92 |
| 47 | GT1 (d18:1/20:3) | 1074.0181 | 1074.0160 | [M−2H+]2− | 1.9 | 86.69 |
| GT1 (d18:1/18:0) | 1074.0168 | 1074.0160 | [M-3H++Na+]2− | 0.7 | 86.69 | |
| 48 | GT1 (d18:1/20:0) | 1077.0414 | 1077.0427 | [M-2H+]2− | −1.2 | 62.77 |
| 49 | GT1 (d18:1/22:3) | 1088.0337 | 1088.0338 | [M-2H+]2− | −0.09 | 73.38 |
| GT1 (d18:1/20:0) | 1088.0325 | 1088.0338 | [M-3H++Na+]2− | −1.1 | 73.38 | |
| 50 | GT1 (d18:1/22:0) | 1091.0571 | 1091.0479 | [M-2H+]2− | 8.4 | 54.37 |
| 51 | GT1 (d18:1/24:1) | 1115.0559 | 1115.0584 | [M-3H++Na+]2− | −2.2 | 68.90 |
| 52 | GM3 (d18:0/16:0) | 1151.7052 | 1151.7036 | [M-H+]− | 1.3 | 62.77 |
| 53 | (CH3COO−) GalNAc GT1 (d18:1/16:2) | 1179.049 | 1179.0457 | [M−-H+]2− | 2.7 | 59.92 |
| 54 | GA1 (d18:1/18:0) | 1235.7626 | 1235.7589 | [M-H+ -H2O]− | 2.9 | 49.90 |
| 55 | GM3 (d18:1/27:0) | 1305.8774 | 1305.8889 | [M-H+]− | −8.8 | 28.66 |
| 56 | GM3 (d18:1/27:0) | 1327.859 | 1327.8499 | [M-2H++Na+]− | 6.8 | 78.90 |
| 57 | GD3 (d18:1/16:0) | 1442.801 | 1442.8043 | [M-H+]− | −2.2 | 64.76 |
| 58 | GM1 (d18:1/14:0) | 1488.806 | 1488.8092 | [M-H+]− | −2.1 | 58.46 |
| 59 | GM1 (d18:1/22:0) | 1600.9312 | 1600.9337 | [M-H+]− | −1.5 | 62.07 |
| 60 | GD2 (d18:1/17:0) | 1659.8955 | 1659.8918 | [M-H+]− | 2.2 | 78.90 |
| 61 | GalNAc-GM1 (d18:1/8:1) | 1745.9323 | 1745.9223 | [M-H+]− | 5.7 | 58.08 |
| 62 | GD1 (d18:1/16:0) | 1807.9327 | 1807.9201 | [M-H+]− | 6.9 | 58.45 |
| m/z (Monoisotopic) Experimental | m/z (Monoisotopic) Theoretical | Mass Accuracy (ppm) | Molecular Ion | Proposed Structure |
|---|---|---|---|---|
| 917.47 | 917.48 | 10.90 | [M-2H]2− | GD1, nLD1 or LD1 (d18:1/18:0) |
| 931.33 | 931.34 | 10.74 | [M-2H]2− | GD1, nLD1 or LD1 (d18:1/20:0) |
| 947.33 | 947.34 | 10.55 | [M-H]− | LacCer (d18:0/22:0) |
| 980.19 | 980.21 | 20.40 | [M+Na-2H]− | GM4 (d18:1/14:2) |
| 1019.78 | 1019.80 | 19.60 | [M-2H]2− | GT2 (d18:1/22:2) |
| 1063.31 | 1063.33 | 18.81 | [M-2H]2− | GT1 (d18:1/18:0) or GT1 (d18:0/18:1) |
| 1074.00 | 1074.02 | 18.62 | [M+Na-3H]2− | GT1 (d18:1/18:0) |
| 1098.18 | 1098.20 | 18.21 | [M-2H]2− | GT1 (d18:1/23:0) or GT1 (d18:0/23:1) |
| 1127.42 | 1127.45 | 26.61 | [M+Na-2H]− | GM3 (d18:1/13:2) |
| 1179.71 | 1179.74 | 25.42 | [M-H]− | GM3 (d18:1/18:0) |
| 1224.33 | 1224.36 | 24.50 | [M-H]− | Fuc-GM3 (d18:1/12:0) |
| 1231.01 | 1231.04 | 24.37 | [M+2Na-4H]2− | GQ1 (d18:1/18:0) |
| 1268.66 | 1268.69 | 23.64 | [M-H]− | GM2 (d18:1/10:0) |
| 1382.78 | 1382.82 | 28.92 | [M-H]− | GM2 (d18:1/18:0) |
| 1408.81 | 1408.85 | 28.38 | [M-H]− | GM2 (d18:1/20:1) |
| 1440.74 | 1440.78 | 27.75 | [M-H]− | GD3 (d18:1/16:1) |
| 1470.99 | 1471.03 | 27.19 | [M-H]− | GD3 (d18:1/18:0) |
| 1518.82 | 1518.86 | 26.33 | [M-H]− | GM1 (d18:0/16:0) |
| 1544.78 | 1544.83 | 32.36 | [M-H]− | GM1, nLM1 or LM1 (d18:1/18:0) |
| 1572.80 | 1572.85 | 31.78 | [M-H]− | GM1, nLM1 or LM1 (d18:1/20:0) |
| 1600.86 | 1600.92 | 37.47 | [M-H]− | GM1, nLM1or LM1 (d18:1/22:0) |
| 1700.83 | 1700.89 | 35.27 | [M+2Na-3H]− | GM1 (d18:1/26:0) |
| 1830.33 | 1830.40 | 38.25 | [M-H]− | GT3 (d18:1/23:1) |
| 1835.87 | 1835.94 | 38.12 | [M-H]− | GD1, nLD1or LD1 (d18:1/18:0) |
| 1857.88 | 1857.95 | 37.67 | [M+Na-2H]− | GD1, nLD1 or LD1 (d18:1/18:0) |
| 1885.92 | 1885.99 | 37.11 | [M+Na-2H]− | GD1 (d18:1/20:0) |
| 1916.85 | 1916.92 | 36.51 | [M-H]− | GD1 (d18:1/24:2) |
| 1965.95 | 1966.02 | 35.60 | [M-H]− | GT2 (d18:1/18:1) or GT2 (d18:0/18:2) |
| 1992.46 | 1992.53 | 35.12 | [M-H]− | Hex-HexNAc-nLM1 (d18:1/24:1) |
| 1996.89 | 1996.96 | 35.05 | [M-H]− | GD2–lactone (d18:1/22:2) |
| 2052.52 | 2052.60 | 38.96 | [M-H]− | GT2 (d18:0/24:0) |
| 2098.16 | 2098.24 | 38.13 | [M-H]− | O-Ac-GD1 (d18:1/34:2) |
| 2124.12 | 2124.20 | 37.66 | [M-H]− | GT1 (d18:1/18:2) or GT1 (d18:0/18:3) |
| 2385.13 | 2385.22 | 37.73 | [M+3Na-4H]− | GT1 (18:1/32:2) |
| 2524.14 | 2524.24 | 39.61 | [M+Na-2H]− | GQ1 (d18:1/24:0) |
| 2622.46 | 2622.56 | 38.12 | [M+Na-2H]− | GQ1 (d18:1/31:0) |
| 2827.26 | 2827.37 | 38.91 | [M-H]−(-H2O) | GP1 (d18:1/28:2) |
| m/z (Monoisotopic) Experimental | m/z (Monoisotopic) Theoretical | Mass Accuracy (ppm) | Molecular Ion | Proposed Structure |
|---|---|---|---|---|
| 872.37 | 872.38 | 11.46 | [M-2H]2− | O-Ac-GD2 (d18:1/20:0) |
| 911.30 | 911.31 | 10.97 | [M-2H]2− | GD1, nLD1 or LD1 (d18:1/18:3) |
| 1063.31 | 1063.33 | 18.81 | [M-2H]2− | GT1 (d18:1/18:0) or GT1 (d18:0/18:1) |
| 1175.94 | 1175.96 | 17.00 | [M-H]− | GM3 (d18:1/18:2) |
| 1197.77 | 1197.80 | 25.04 | [M-H]− | GM3 (d18:0/19:0) |
| 1235.78 | 1235.81 | 24.29 | [M-H]− | GM3 (d18:1/22:0) |
| 1306.03 | 1306.06 | 22.97 | [M-H]− | GM3 (d18:1/27:0) |
| 1510.80 | 1510.84 | 26.47 | [M-H]− | O-Ac-GD3 (d18:1/18:1) |
| 1644.27 | 1644.31 | 24.33 | [M-H]− | GM1 (d18:0/25:0) |
| 1662.18 | 1662.22 | 24.06 | [M-H]− | GM1 (d18: 1/29: 2) |
| 1684.78 | 1684.82 | 23.73 | [M-H]− | GD2 (d18:1/19:2) or GD2 (d18:0/19:3) |
| 1847.38 | 1847.42 | 21.65 | [M-H]− | GT3 (d18:0/24:0) |
| 1879.05 | 1879.09 1879.10 | 21.28 26.60 | [M-H]−(-H2O) [M+Na-2H]− | Fuc-GT3 (d18:0/17:0) or O-Ac-GT3 (d18:1/22:2) |
| 2024.17 | 2024.23 | 29.64 | [M+2Na-3H]− | GD1 (d18:0/28:0) |
| 2050.55 | 2050.60 | 24.37 | [M-H]− | GT2 (d18:1/24:0) or GT2 (d18:0/24:1) |
| 2072.15 | 2072.21 | 28.95 | [M-H]− | O-Ac-GD1 (d18:1/32:1) |
| 2076.13 | 2076.19 | 28.90 | [M-H]− | O-Ac-GD1 (d18:0/32:0) |
| 2102.20 | 2102.26 | 28.54 | [M-H]− | O-Ac-GD1 (d18:1/34:0) |
| 2112.02 | 2112.09 | 33.14 | [M-H]− | GT1 (d18:1/17:2) or GT1 (d18:0/17:3) |
| 2123.90 | 2123.97 | 32.95 | [M-H]− | GT1 (d18:1/18:2) |
| 2175.73 | 2175.80 | 32.16 | [M-H]− | GT2 (d18:1/33:0) |
| 2186.52 | 2186.60 | 36.57 | [M-H]− | GT1 (d18:0/22:0) |
| 2237.08 | 2237.16 | 35.76 | [M-H]− | GT1 (d18:1/26:1) |
| 2246.90 | 2246.98 | 35.60 | [M-H]− | Fuc-GT1 (d18:0/16:0) |
| 2287.72 | 2287.80 | 34.96 | [M-H]− | Fuc-GT1 (d18:0/20:0) |
| 2346.00 | 2346.09 | 38.36 | [M-H]-(-H2O) | GQ1 (d18:1/13:1) |
| 2385.13 | 2385.22 | 37.73 | [M+3Na-4H]− | GT1 (18:1/32:2) |
| 2472.12 | 2472.21 | 36.40 | [M-H]− | GQ1 (d18:1/22:1) |
| 2582.46 | 2582.56 | 38.71 | [M-H]−(-H2O) | GQ1 (d18:1/31:0) |
| 2618.41 | 2618.51 | 38.18 | [M+2Na-3H]− | GQ1 (d18:0/29:0) |
| 2642.52 | 2642.62 | 37.83 | [M-H]− | O-Ac-GQ1 (d18:1/31:0) |
| 2673.42 | 2673.52 | 37.41 | [M-H]− | GP2 (d18:1/27:0) |
| 2906.39 | 2906.50 | 37.85 | [M-H]− | Fuc-GP1 (d18:1/23:1) |
| m/z (Monoisotopic) Experimental | m/z (Monoisotopic) Theoretical | Mass Accuracy (ppm) | Molecular Ion | Proposed Structure |
|---|---|---|---|---|
| 917.47 | 917.48 | 10.90 | [M-2H]2− | GD1, nLD1 or LD1 (d18:1/18:0) |
| 931.33 | 931.34 | 10.74 | [M-2H]2− | GD1, nLD1 or LD1 (d18:1/20:0) |
| 947.33 | 947.34 | 10.55 | [M-H]− | LacCer (d18:0/22:0) |
| 980.19 | 980.21 | 20.40 | [M+Na-2H]− | GM4 (d18:1/14:2) |
| 1019.78 | 1019.80 | 19.60 | [M-2H]2− | GT2 (d18:1/22:2) |
| 1063.31 | 1063.33 | 18.81 | [M-2H]2− | GT1 (d18:1/18:0) or GT1 (d18:0/18:1) |
| 1074.00 | 1074.02 | 18.62 | [M+Na-3H]2− | GT1(d18:1/18:0) |
| 1098.18 | 1098.20 | 18.21 | [M-2H]2− | GT1 (d18:1/23:0) or GT1 (d18:0/23:1) |
| 1127.42 | 1127.45 | 26.61 | [M+Na-2H]− | GM3 (d18:1/13:2) |
| 1179.71 | 1179.74 | 25.42 | [M-H]− | GM3 (d18:1/18:0) |
| 1224.33 | 1224.36 | 24.50 | [M-H]− | Fuc-GM3 (d18:1/12:0) |
| 1231.01 | 1231.04 | 24.37 | [M+2Na-4H]2− | GQ1 (d18:1/18:0) |
| 1268.66 | 1268.69 | 23.64 | [M-H]− | GM2 (d18:1/10:0) |
| 1382.78 | 1382.82 | 28.92 | [M-H]− | GM2 (d18:1/18:0) |
| 1408.81 | 1408.85 | 28.38 | [M-H]− | GM2 (d18:1/20:1) |
| 1440.74 | 1440.78 | 27.75 | [M-H]− | GD3 (d18:1/16:1) |
| 1470.99 | 1471.03 | 27.19 | [M-H]− | GD3 (d18:1/18:0) |
| 1518.82 | 1518.86 | 26.33 | [M-H]− | GM1 (d18:0/16:0) |
| 1544.78 | 1544.83 | 32.36 | [M-H]− | GM1, nLM1 or LM1 (d18:1/18:0) |
| 1572.80 | 1572.85 | 31.78 | [M-H]− | GM1, nLM1 or LM1 (d18:1/20:0) |
| 1600.86 | 1600.92 | 37.47 | [M-H]− | GM1,nLM1or LM1 (d18:1/22:0) |
| 1700.83 | 1700.89 | 35.27 | [M+2Na-3H]− | GM1 (d18:1/26:0) |
| 1830.33 | 1830.40 | 38.25 | [M-H]− | GT3 (d18:1/23:1) |
| 1835.87 | 1835.94 | 38.12 | [M-H]− | GD1,nLD1or LD1 (d18:1/18:0) |
| 1857.88 | 1857.95 | 37.67 | [M+Na-2H]− | GD1, nLD1 or LD1 (d18:1/18:0) |
| 1885.92 | 1885.99 | 37.11 | [M+Na-2H]− | GD1 (d18:1/20:0) |
| 1916.85 | 1916.92 | 36.51 | [M-H]− | GD1 (d18:1/24:2) |
| 1965.95 | 1966.02 | 35.60 | [M-H]− | GT2 (d18:1/18:1) or GT2 (d18:0/18:2) |
| 1992.46 | 1992.53 | 35.12 | [M-H]− | Hex-HexNAc-nLM1 (d18:1/24:1) |
| 1996.89 | 1996.96 | 35.05 | [M-H]− | GD2-lactone (d18:1/22:2) |
| 2052.52 | 2052.60 | 38.96 | [M-H]− | GT2 (d18:0/24:0) |
| 2098.16 | 2098.24 | 38.13 | [M-H]− | O-Ac-GD1 (d18:1/34:2) |
| 2124.12 | 2124.20 | 37.66 | [M-H]− | GT1 (d18:1/18:2) or GT1 (d18:0/18:3) |
| 2385.13 | 2385.22 | 37.73 | [M+3Na-4H]− | GT1 (18:1/32:2) |
| 2524.14 | 2524.24 | 39.61 | [M+Na-2H]− | GQ1 (d18:1/24:0) |
| 2622.46 | 2622.56 | 38.12 | [M+Na-2H]− | GQ1 (d18:1/31:0) |
| 2827.26 | 2827.37 | 38.91 | [M-H]−(-H2O) | GP1 (d18:1/28:2) |
| m/z (Monoisotopic) Theoretical | m/z (Monoisotopic) Experimental | Mass Accuracy (ppm) | Molecular Ion | Proposed Structure |
|---|---|---|---|---|
| 875.19 | 874.91 | 33 | [M-H]− | LacCer (d18:1/17:0) |
| 933.31 | 932.99 | 35 | [M-H]− | LacCer (d18:0/21:0) |
| 947.34 | 947.19 | 16 | [M-H]− | LacCer (d18:0/22:0) |
| 949.22 | 949.24 | 21 | [M+2Na-3H]− | LacCer (d18:0/19:0) |
| 964.24 | 963.90 | 35 | [M-H]− | GM4 (d18:0/14:0) |
| 982.19 | 981.94 | 25 | [M+Na-2H]− | GM4 (d18:1/14:1) |
| 984.21 | 983.87 | 34 | [M+Na-2H]− | GM4 (d18:1/14:0) or GM4 (d18:0/14:1) |
| 1122.48 | 1122.23 | 22 | [M-H]− | GA2 (d18:0/20:0) |
| 1138.44 1138.48 | 1138.15 | 25 29 | [M-H]− | Fuc-GM4 (d18:0/16:0) GA2 (t18:0/20:0) |
| 1150.49 1150.40 | 1150.17 | 28 20 | [M-H]− [M-H]− | GA2 (d18:1/21:0) GM3 (d18:1/16:1) |
| 1168.42 | 1168.01 | 35 | [M-H]− | GM3 (t18:0/16:0) |
| 1178.46 | 1178.14 | 27 | [M-H]− | GM3 (d18:1/18:1) |
| 1179.74 | 1180.10 | 30 | [M-H]− | GM3 (d18:1/18:0) |
| 1182.49 | 1182.21 | 24 | [M-H]− | GM3 (d18:0/18:0) |
| 1184.37 | 1184.08 | 24 | [M-H]− | O-Ac-GA1 (d18:1/10:0) |
| 1194.50 | 1194.15 | 29 | [M-H]− | GM3 (d18:1/19:0) or GM3 (d18:0/19:1) |
| 1206.51 1206.64 | 1206.33 | 15 26 | [M-H]− [M-H]− | GM3 (d18:1/20:1) GA2 (d18:0/26:0) |
| 1222.51 1222.55 | 1222.19 | 26 29 | [M-H]− [M-H]− | O-Ac-GM3 (d18:1/18:0) GM3 (d18:0/21:1) or GM3 (d18:1/21:0) |
| 1234.56 1234.52 | 1234.22 | 27 24 | [M-H]− | GM3 (d18:1/22:1) O-Ac-GM3 (d18:1/19:1) |
| 1248.55 1248.59 | 1248.18 | 33 30 | [M-H]− | O-Ac-GM3 (d18:1/20:1) GM3 (d18:1/23:1) |
| 1248.59 | 1249.02 | 34 | [M-H]− | GM3 (d18:1/23:0) |
| 1260.60 | 1260.33 | 21 | [M-H]− | GM3 (d18:1/24:2) |
| 1262.62 | 1262.35 | 21 | [M-H]− | GM3 (d18:1/24:1) |
| 1264.63 | 1264.19 | 35 | [M-H]− | GM3 (d18:1/24:0) |
| 1276.61 1276.64 1276.69 | 1277.01 | 31 29 25 | [M-H]− [M-H]− [M-H]−(-H2O) | O-Ac-GM3 (d18:1/22:0) GM3 (d20:1/23:1) GM3 (d18:0/26:0) |
| 1278.66 1278.61 | 1278.21 | 35 31 | [M-H]− [M-H]− | GM3 (d20:1/23:0) O-Ac-GM3 (d18:1/22:0) or O-Ac-GM3 (d18:0/22:1) |
| 1288.67 1288.67 | 1289. 04 | 29 29 | [M-H]− [M-H]− | GM3 (d18:1/26:2) or GM3 (d18:2/26:1) GM3 (d20:1/24:2) |
| 1292.68 | 1292.23 | 35 | [M-H]− | GM3 (d18:1/26:0) or GM3 (d18:0/26:1) |
| 1296.54 1296.59 1296.61 | 1296.24 | 23 27 28 | [M-H]− [M-H]− [M-H]− | Fuc-GM3 (d18:1/16:1) O-Ac-GA1 (d18:1/18:0) GA1 (d18:0/21:0) or GA1 (d18:0/21:0) |
| 1405.65 | 1405.21 | 31 | [M+Na-2H]− | GM2 (d18:1/18:0) |
| 1420.68 | 1420.80 | 8 | [M-H]− | O-Ac-GM2 (d18:2/18:2) |
| 1435.59 | 1435.21 | 26 | [M+Na-2H]− | GD3 (d18:1/14:1) or GD3 (d18:0/14:2) or GD3 (d18:2/14:0) |
| 1441.66 | 1441.19 | 33 | [M-H]− | GD3 (d18:1/16:1) or GD3 (d18:0/16:2) or GD3 (d18:2/16:0) |
| 1471.73 | 1471.28 | 31 | [M-H]− | GD3 (d18:1/18:0) |
| 1493.71 | 1493.23 | 32 | [M+Na-2H]− | GD3 (d18:1/18:0) |
| 1515.69 1515.74 1515.78 | 1515.29 | 26 30 32 | [M+2Na-3H]− [M-H]− [M-H]− | GD3 (d18:1/18:0) or GD3 (d18:0/18:1) GM1 (d18:1/16:1) or GM1 (d18:0/16:2) or GM1 (d18:2/16:0) O-Ac-GD3 (d18:0/18:0) |
| 1515.71 1515.75 | 1516.01 | 20 17 | [M + Na-2H]− [M-H]− | GD3 (d18:1/20:2) or GD3 (d18:0/20:3) or GD3 (d18:2/20:1) GM1 (d18:2/16:0) or GM1 (d18:1/16:1) or GM1 (d18:0/16:2) |
| 1527.83 | 1528. 16 | 22 | [M-H]− | GD3 (d18:0/22:0) |
| 1541.79 | 1542. 19 | 26 | [M-H]− | GM1 (d18:1/18:2) or GM1 (d18:2/18:1) or GM1 (d18:0/18:3) |
| 1569.78 1569.83 1569.77 | 1570.29 | 32 29 33 | [M+2Na-3H]− [M-H]− [M+Na-2H]− | GD3 (d18:1/22:0) or GD3 (d18:0/22:1) GM1 (d18:1/20:1) or GM1 (d18:0/20:2) or GM1 (d18:2/20:0) GD3 (d18:0/24:2) or GD3 (d18:1/24:1) or GD3 (d18:2/24:0) |
| 1597.88 | 1598.09 | 13 | [M-H]− | GM1 (d18:0/22:2) or GM1 (d18:1/22:1) or GM1 (d18:2/22:0) |
| 1611.77 | 1612.17 | 25 | [M+2Na-3H]− | GM1 (d18:1/20:2) |
| 1625.89 1625.92 | 1625.40 | 30 30 | [M+2Na-3H]− [M-H]− | GD3 (d18:1/26:1) or GD3 (d18:0/26:2) or GD3 (d18:2/26:0) GM1 (d18:1/24:2) |
| 1627.90 1626.93 | 1627.41 | 30 29 | [M+2Na-3H]− [M-H]− | GD3 (d18:0/26:1) or GD3 (d18:1/26:0) GM1 (d18:0/24:2) or GM1 (d18:1/24:1) or GM1 (d18:2/24:0) |
| 1629.92 1628.94 | 1629.42 | 31 29 | [M-H]− [M-H]− | GM1 (d18:0/24:1) or GM1 (d18:1/24:0) di-O-Ac-GM1 (d18:1/18:0) |
| 1659.79 | 1660.18 | 23 | [M+3Na-4H]− | GM1 (d18:1/22:3) or GM1 (d18:0/22:4) or GM1 (d18:2/22:2) |
| 1674.87 | 1675.23 | 21 | [M+Na-2H]− (-H2O) | GD2 (d18:1/18:2) |
| 1748.97 | 1749.39 | 24 | [M+Na-2H]− | GD2 (d18:1/22:1) |
| 1766.97 | 1767.28 | 18 | [M-H]− (-H2O) | GT3 (d18:1/20:1) |
| 1785.07 | 1785.37 | 17 | [M-H]− | O-Ac-GD2 (d18:1/23:0) or O-Ac-GD2 (d18:0/23:1) |
| 1833.81 1833.07 | 1833.28 | 29 11 | [M-H]− [M-H]− | GT3 (d18:0/23:0) O-Ac-GT3 (d18:0/20:0) |
| 1861.12 1861.12 | 1861.24 | 6 6 | [M-H]− [M-H] (-H2O) | O-Ac-GT3-lactone (d18:0/22:0) O-Ac-GT3 (d18:0/22:0) |
| 1879.09 1879.10 1879.99 | 1879.39 | 16 15 32 | [M+Na-2H]− [M-H]−(-H2O) [M-H]− | O-Ac-GT3 (d18:2/22:1) Fuc-GT3 (d18:0/17:0) GT2 (d18:1/12:1) or GT2 (d18:2/12:0) |
| 1909.16 | 1909.03 | 7 | [M-H]− | GD1 (d18:1/22:0) |
| 1960.21 1960.12 | 1959.84 | 19 14 | [M-H]− (-2H2O) [M-H]− | GT2 (d18:0/20:0) GT2 (d18:1/18:3) or GT2 (d18:2/18:2) |
| 1990.17 1990.19 | 1989.78 | 20 21 | [M+Na-2H]− [M-H]− | GT2 (d18:0/18:0) GT2 (d18:0/20:3) or GT2 (d18:1/20:2) or GT2 (d18:2/20:1) |
| 1990.19 | 1990.83 | 32 | [M-H]− | GT2 (d18:1/20:1) or GT2 (d18:0/20:2) or GT2 (d18:2/20:0) |
| 2005.20 2006.19 | 2005.63 | 21 28 | [M-H]− | Fuc-GD1 (d18:1/20:2) O-Ac-GT2 (d18:1/18:1) |
| 2048.23 2048.10 | 2048.80 | 28 34 | [M-H]− [M-H]−(-H2O) | di-O-Ac-GT2 (d18:0/18:0) GT1 (d18:2/14:2) or GT1 (d18:3/14:1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biricioiu, M.R.; Sarbu, M.; Ica, R.; Vukelić, Ž.; Kalanj-Bognar, S.; Zamfir, A.D. Advances in Mass Spectrometry of Gangliosides Expressed in Brain Cancers. Int. J. Mol. Sci. 2024, 25, 1335. https://doi.org/10.3390/ijms25021335
Biricioiu MR, Sarbu M, Ica R, Vukelić Ž, Kalanj-Bognar S, Zamfir AD. Advances in Mass Spectrometry of Gangliosides Expressed in Brain Cancers. International Journal of Molecular Sciences. 2024; 25(2):1335. https://doi.org/10.3390/ijms25021335
Chicago/Turabian StyleBiricioiu, Maria Roxana, Mirela Sarbu, Raluca Ica, Željka Vukelić, Svjetlana Kalanj-Bognar, and Alina D. Zamfir. 2024. "Advances in Mass Spectrometry of Gangliosides Expressed in Brain Cancers" International Journal of Molecular Sciences 25, no. 2: 1335. https://doi.org/10.3390/ijms25021335
APA StyleBiricioiu, M. R., Sarbu, M., Ica, R., Vukelić, Ž., Kalanj-Bognar, S., & Zamfir, A. D. (2024). Advances in Mass Spectrometry of Gangliosides Expressed in Brain Cancers. International Journal of Molecular Sciences, 25(2), 1335. https://doi.org/10.3390/ijms25021335





