Functional Characterization of CsSWEET5a, a Cucumber Hexose Transporter That Mediates the Hexose Supply for Pollen Development and Rescues Male Fertility in Arabidopsis
Abstract
1. Introduction
2. Results
2.1. CsSWEET5a Encodes the Clade II SWEET Protein
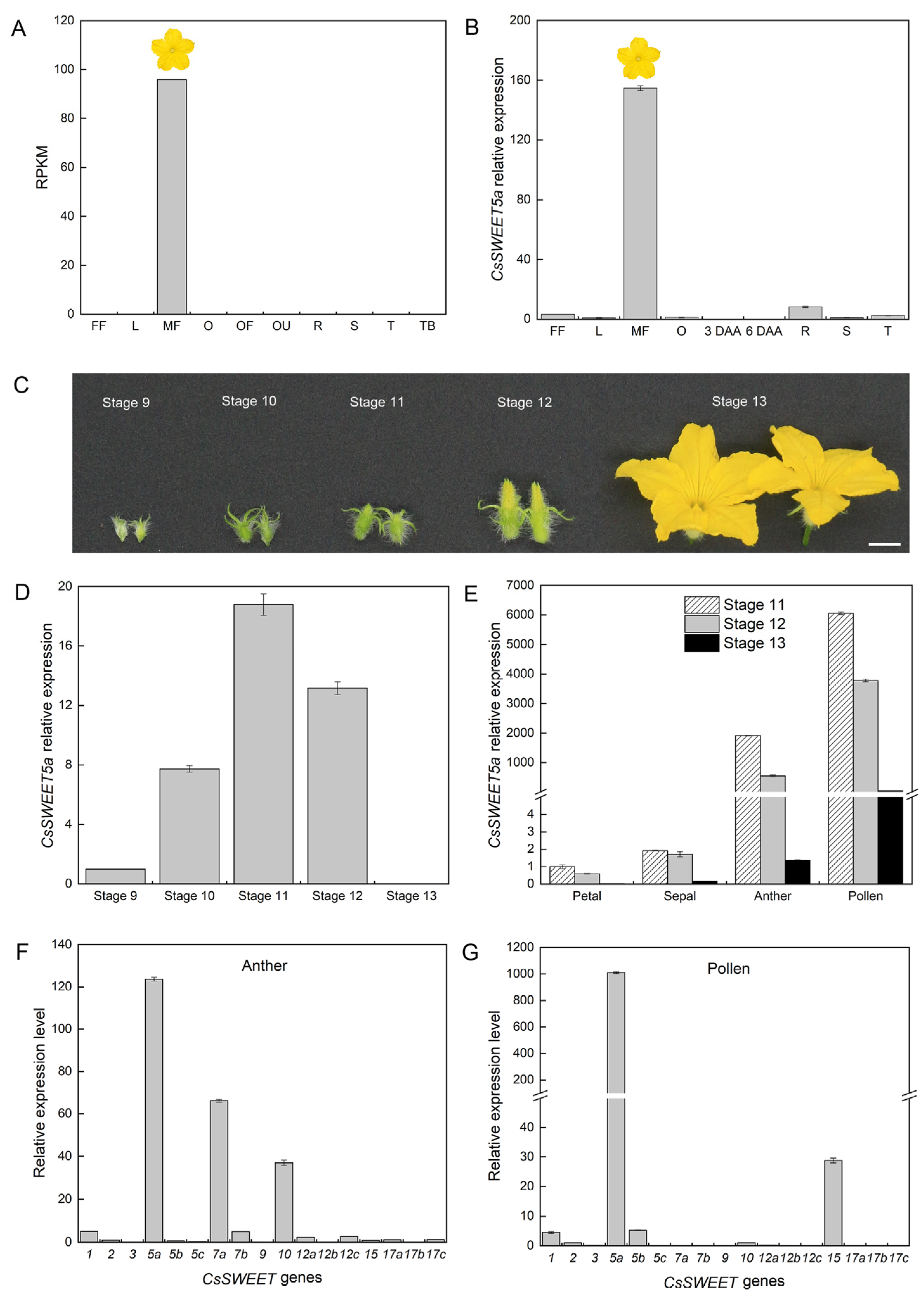
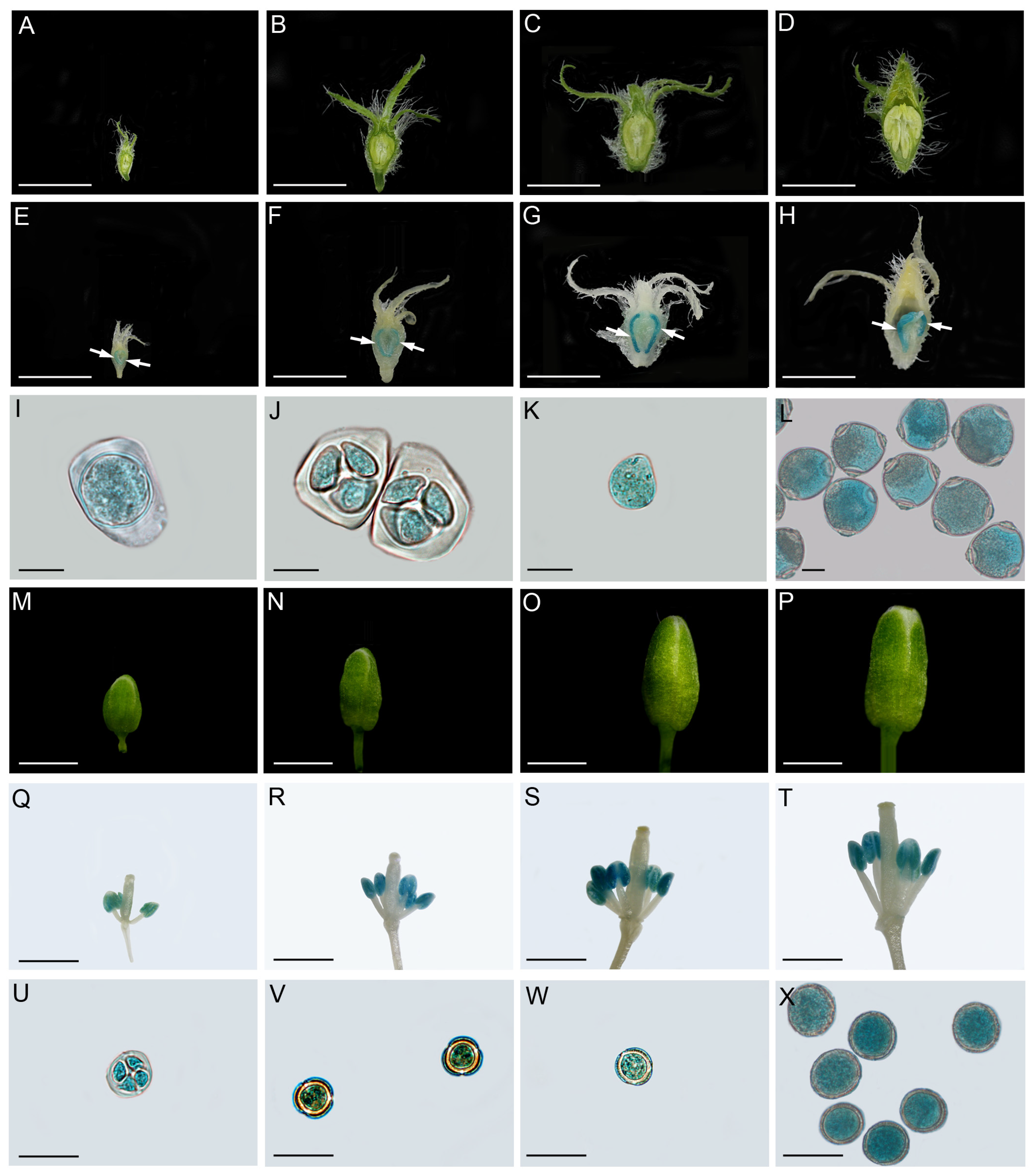
2.2. CsSWEET5a mRNA Accumulates at High Levels in Developing Anthers and Pollen Cells
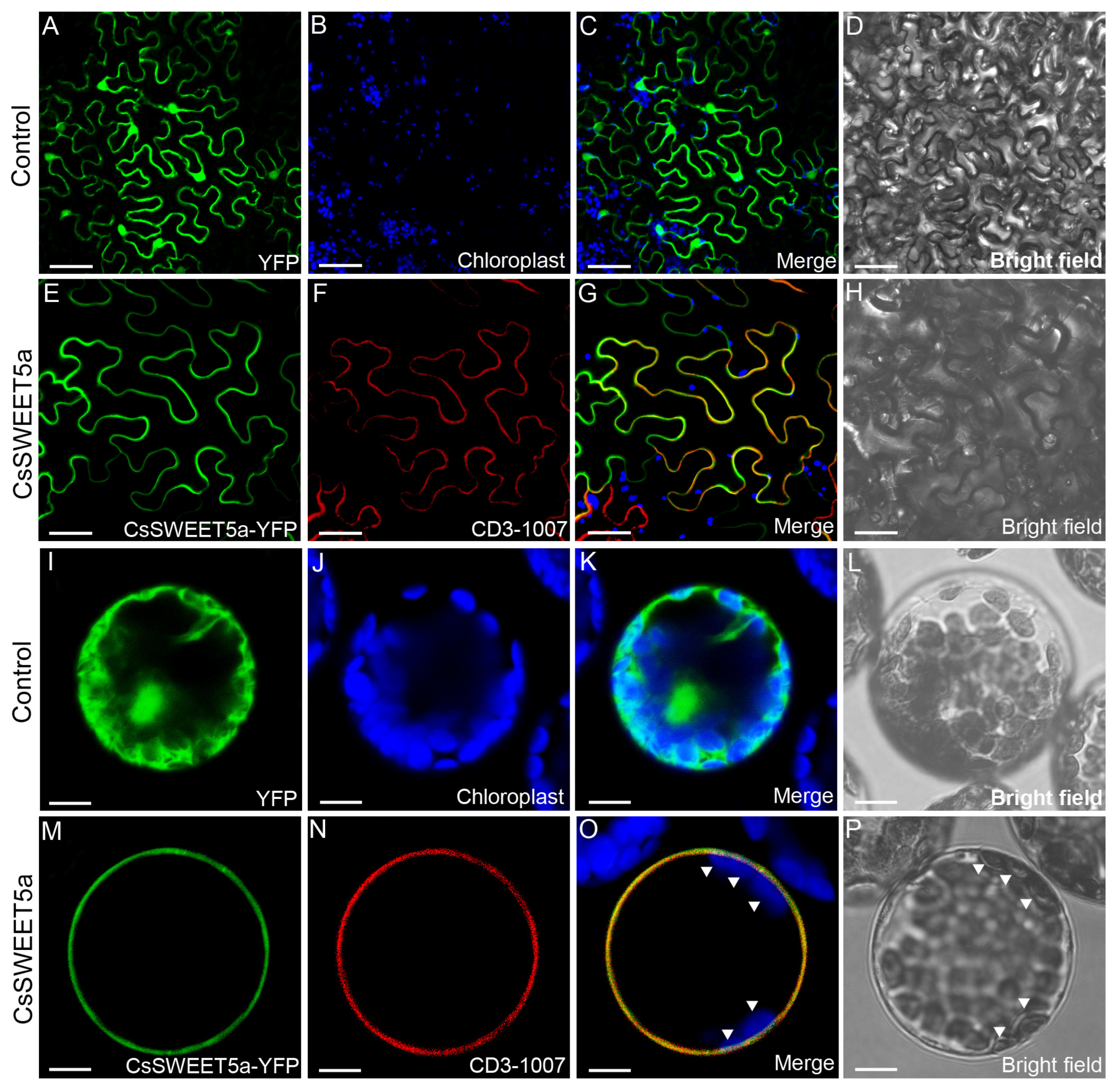
2.3. CsSWEET5a Is a Plasma Membrane Protein
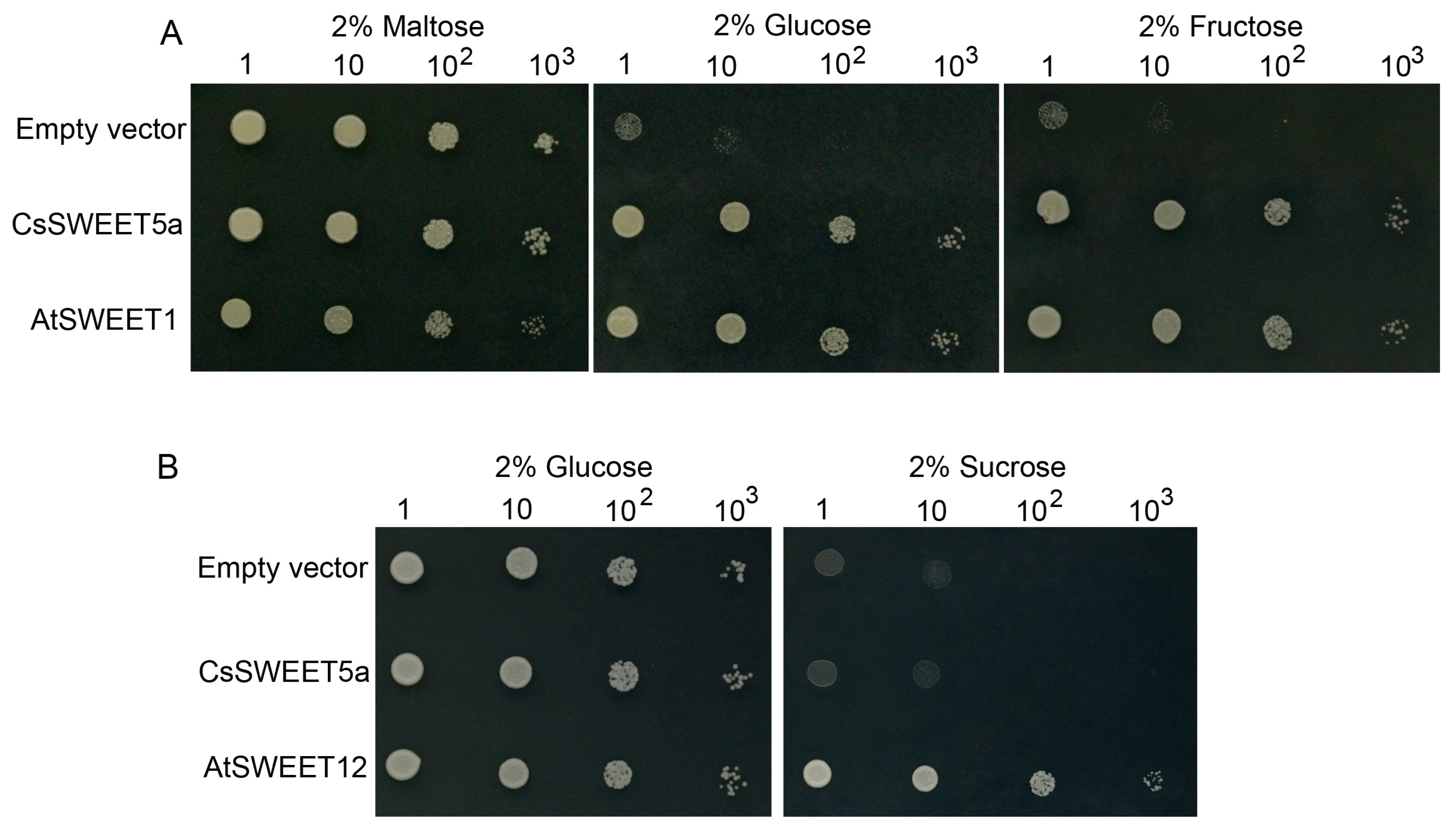
2.4. CsSWEET5a Mediates Glucose and Fructose Transport in Yeast
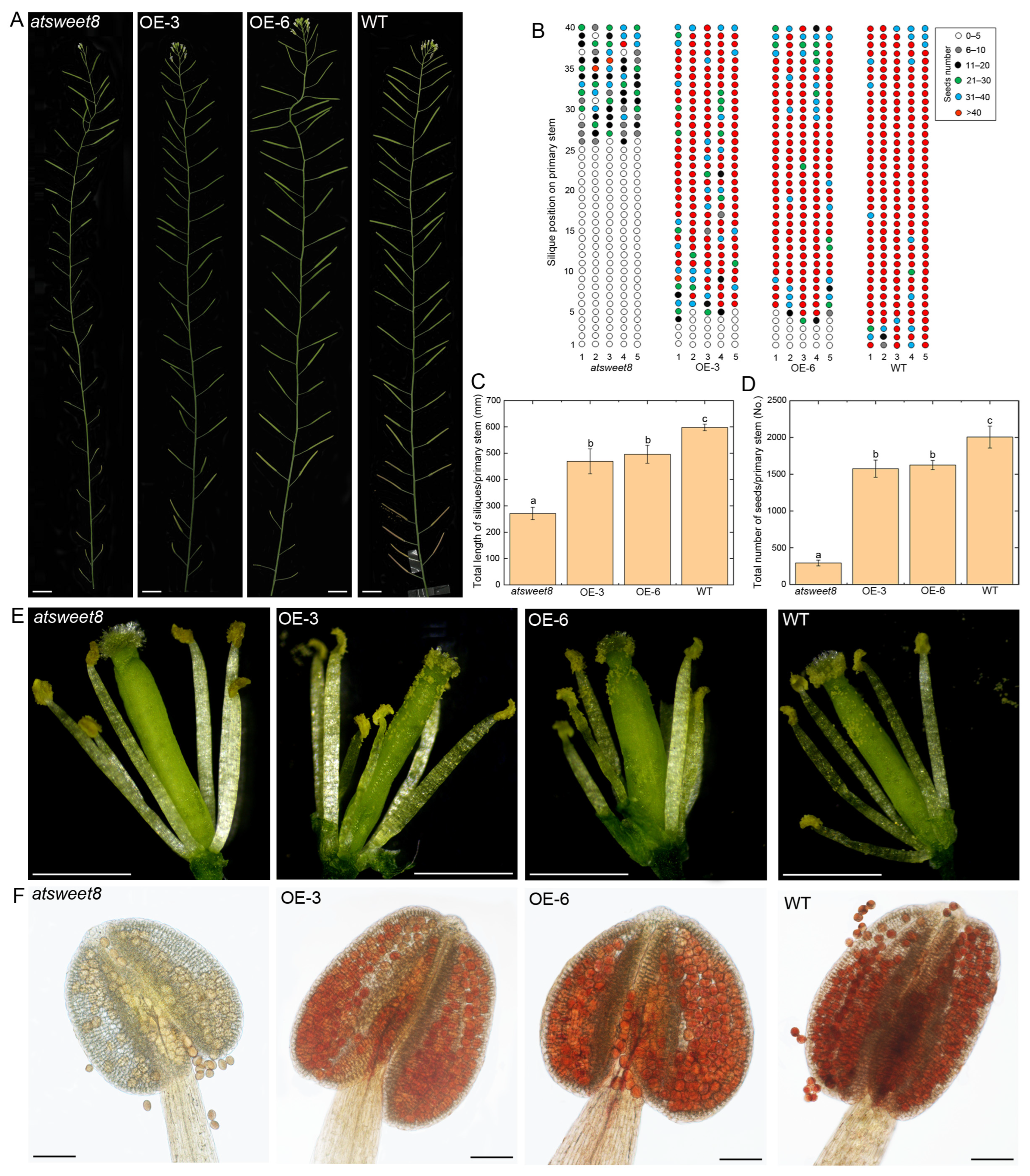
2.5. Overexpression of CsSWEET5a Could Significantly Rescue the Fertility of atsweet8 Mutant Arabidopsis Plants
3. Discussion
3.1. CsSWEET5a Encodes a Plasma Membrane Protein Highly Expressed in Developing Anthers and Pollen Cells
3.2. CsSWEET5a Is a Glucose and Fructose Transporter That Complements the Function of AtSWEET8 in Arabidopsis
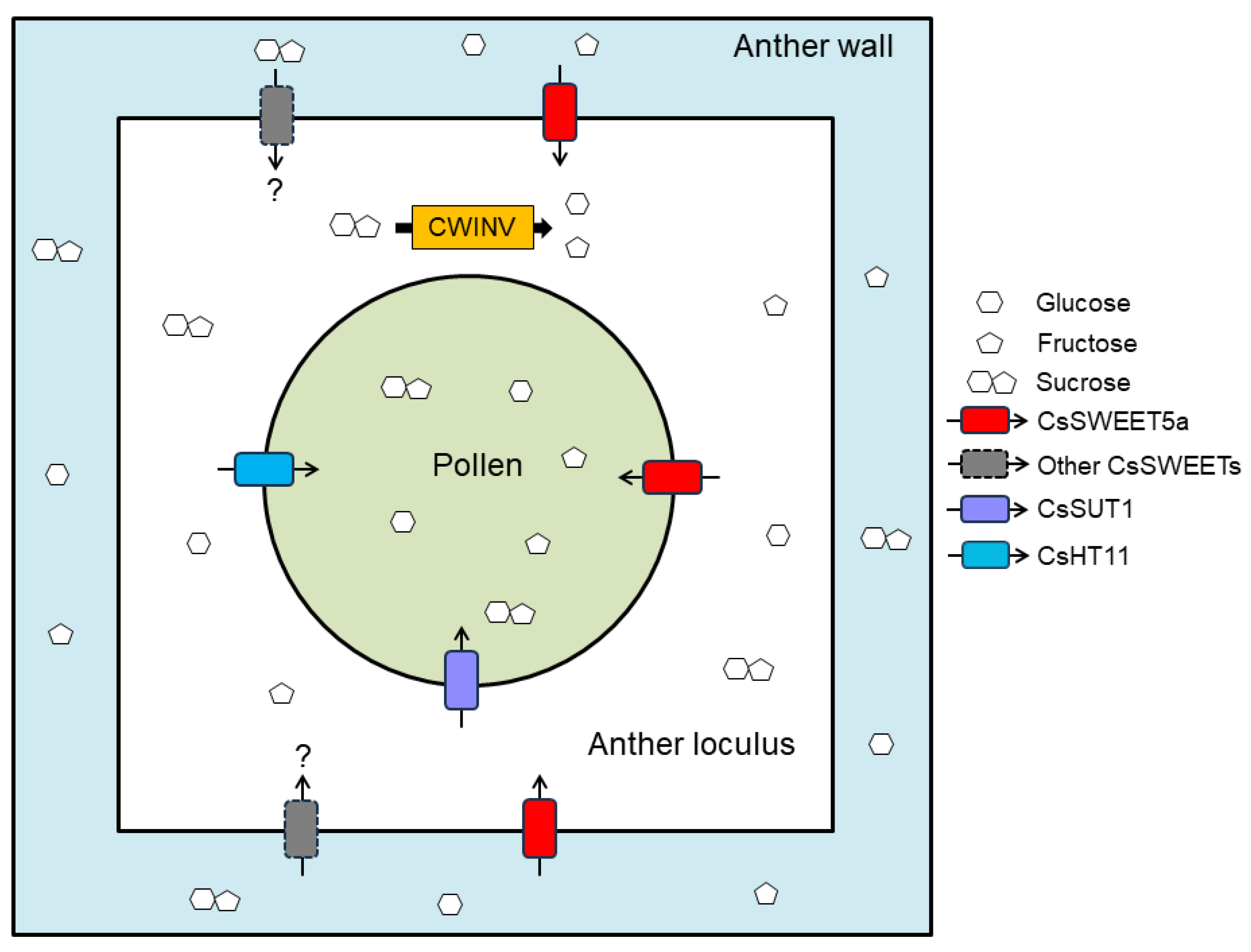
3.3. Hypothetical Model of Sugar Transport from Anther Tissues into Developing Pollen Cells in Cucumbers
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Extraction, cDNA Synthesis, and qRT–PCR
4.3. Cloning, Sequence Alignment, and Phylogenetic Analysis of CsSWEET5a
4.4. Isolation of the Promoter Region of CsSWEET5a and GUS Expression Analysis
4.5. Subcellular Localization of CsSWEET5a
4.6. Complementation Analysis of CsSWEET5a in Yeast
4.7. Ectopic Expression of CsSWEET5a in Arabidopsis
4.8. Characterization of Plant Phenotype and Fertility
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borghi, M.; Fernie, A.R. Floral metabolism of sugars and amino acids: Implications for pollinators’ preferences and seed and fruit set. Plant Physiol. 2017, 175, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, S.; Honys, D. Reproduction multitasking: The male gametophyte. Annu. Rev. Plant Biol. 2021, 72, 581–614. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Godt, D.E.; Guivarc’h, A.; Kahmann, U.; Chriqui, D.; Roitsch, T. Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc. Natl. Acad. Sci. USA 2001, 98, 6522–6527. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ma, S.; Liu, Y.H.; Liao, S.J.; Li, J.; Wu, L.M.; Kartika, D.; Mock, H.P.; Ruan, Y.L. Cell wall invertase and sugar transporters are differentially activated in tomato styles and ovaries during pollination and fertilization. Front. Plant Sci. 2019, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Sui, X.L.; Lucas, W.J.; Li, Y.X.; Feng, S.; Ma, S.; Fan, J.W.; Gao, L.H.; Zhang, Z.X. Down-regulation of the sucrose transporter CsSUT1 causes male sterility by altering carbohydrate supply. Plant Physiol. 2019, 180, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.Y.; Tseng, H.W.; Ho, L.H.; Wang, L.; Chang, T.F.; Lin, A.; Ruan, Y.L.; Neuhaus, H.E.; Guo, W.J. Hexose translocation mediated by SISWEET5b is required for pollen maturation in Solanum lycopersicum. Plant Physiol. 2022, 189, 344–359. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, K.; Walbot, V. Pre-meiotic anther development. Curr. Top. Dev. Biol. 2019, 131, 239–256. [Google Scholar] [PubMed]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Braun, D.M. Phloem loading and unloading of sucrose: What a long, strange trip from source to sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.Y.; Wang, J.; Shukla, D.; Cheung, L.S.; Chen, L.Q. When SWEETs turn tweens: Updates and perspectives. Annu. Rev. Plant Biol. 2022, 73, 379–403. [Google Scholar] [CrossRef]
- Yao, T.S.; Gai, X.T.; Pu, Z.J.; Gao, Y.; Xuan, Y.H. From functional characterization to the application of SWEET sugar transporters in plant resistance breeding. J. Agric. Food Chem. 2022, 70, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.F.; Huang, X.Y.; Zhu, J.; Gao, J.F.; Zhang, H.X.; Yang, Z.N. Ruptured pollen grain1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol. 2008, 147, 852–863. [Google Scholar] [CrossRef]
- Isoda, R.; Palmai, Z.; Yoshinari, A.; Chen, L.Q. SWEET13 transport of sucrose, but not gibberellin, restores male fertility in Arabidopsis sweet13;14. Proc. Natl. Acad. Sci. USA 2022, 119, e2207558119. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.Y.; Zeng, H.Q.; Li, J.K.; Chen, L.Q. Sucrose rather than GA transported by AtSWEET13 and AtSWEET14 supports pollen fitness at late anther development stages. New Phytol. 2022, 236, 525–537. [Google Scholar] [CrossRef]
- Wen, S.Y.; Neuhaus, H.E.; Cheng, J.T.; Bie, Z.L. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Feng, C.Y.; Han, J.X.; Han, X.X.; Jiang, J. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene 2015, 573, 261–272. [Google Scholar] [CrossRef]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Feng, S.; Ma, S.; Sui, X.L.; Zhang, Z.X. Spatiotemporal expression and substrate specificity analysis of the cucumber SWEET gene family. Front. Plant Sci. 2017, 8, 1855. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.H.; Klemens, P.A.W.; Neuhaus, H.E.; Ko, H.Y.; Hsieh, S.Y.; Guo, W.J. SlSWEET1a is involved in glucose import to young leaves in tomato plants. J. Exp. Bot. 2019, 70, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Lin, I.W.; Qu, X.Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.W.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, H.; Yao, X.H.; Sun, L.L.; Sui, X.L. The role of sugar transporter CsSWEET7a in apoplasmic phloem unloading in receptacle and nectary during cucumber anthesis. Front. Plant Sci. 2022, 12, 758526. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, H.; Yao, X.H.; Wang, J.; Feng, S.; Sun, L.L.; Ma, S.; Xu, K.; Chen, L.Q.; Sui, X.L. Hexose transporter CsSWEET7a in cucumber mediates phloem unloading in companion cells for fruit development. Plant Physiol. 2021, 186, 640–654. [Google Scholar] [CrossRef]
- Zhang, X.S.; Feng, C.Y.; Wang, M.N.; Li, T.L.; Liu, X.; Jiang, J. Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits. Hortic. Res. 2021, 8, 186. [Google Scholar] [CrossRef]
- Ren, Y.; Li, M.Y.; Guo, S.G.; Sun, H.H.; Zhao, J.Y.; Zhang, J.; Liu, G.M.; He, H.J.; Tian, S.W.; Yu, Y.T.; et al. Evolutionary gain of oligosaccharide hydrolysis and sugar transport enhanced carbohydrate partitioning in sweet watermelon fruits. Plant Cell 2021, 33, 1554–1573. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.C.; Li, Y.; Chen, L.Q. Hexose transporter SWEET5 confers galactose sensitivity to Arabidopsis pollen germination via a galactokinase. Plant Physiol. 2022, 189, 388–401. [Google Scholar] [CrossRef]
- Klemens, P.A.W.; Patzke, K.; Deitmer, J.; Spinne, L.; Hir, R.L.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Zhang, Y.; Yang, C.; Tian, Z.H.; Li, J.X. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016, 6, 24563. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.P.; Zhang, F.; Song, S.H.; Yu, X.L.; Ren, Y.; Zhao, X.Z.; Liu, H.; Liu, G.M.; Wang, Y.; He, H.J. CsSWEET2, a hexose transporter from cucumber (Cucumis sativus L.), affects sugar metabolism and improves cold tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3886. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Luo, Y.F.; Vu, N.T.Q.; Shen, S.J.; Xia, K.F.; Zhang, M.Y. CRISPR/Cas9-mediated mutation of OsSWEET14 in rice cv. Zhonghua11 confers resistance to Xanthomonas oryzae pv. oryzae without yield penalty. BMC Plant Biol. 2020, 20, 313. [Google Scholar] [CrossRef] [PubMed]
- Breia, R.; Conde, A.; Pimentel, D.; Conde, C.; Fortes, A.M.; Granell, A.; Gerós, H. VvSWEET7 is a mono- and disaccharide transporter up-regulated in response to Botrytis cinerea infection in grape berries. Front. Plant Sci. 2020, 10, 1753. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Figueira-Galán, D.; Manck-Götzenberger, J.; Requena, N. Overexpression of the potato monosaccharide transporter StSWEET7a promotes root colonization by symbiotic and pathogenic fungi by increasing root sink strength. Front. Plant Sci. 2022, 13, 837231. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, J.M.; Kang, S.K.; Kim, S.G.; Park, C.M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 2011, 233, 189–200. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wang, Z.Y.; Yao, F.Z.; Gao, L.H.; Ma, S.; Sui, X.L.; Zhang, Z. Down-regulating CsHT1, a cucumber pollen-specific hexose transporter, inhibits pollen germination, tube growth, and seed development. Plant Physiol. 2015, 168, 635–647. [Google Scholar] [CrossRef]
- Sui, X.L.; Nie, J.; Liu, H.; Lin, T.; Yao, X.H.; Turgeon, R. Complexity untwined: The structure and function of cucumber (Cucumis sativus L.) shoot phloem. Plant J. 2021, 106, 1163–1176. [Google Scholar] [CrossRef]
- Bai, S.L.; Peng, Y.B.; Cui, J.X.; Gu, H.T.; Xu, L.Y.; Li, Y.Q.; Xu, Z.H.; Bai, S.N. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 2004, 220, 230–240. [Google Scholar] [CrossRef]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freide, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Saida, Y.; Yoshimizu, M.; Takanashi, K.; Sosso, D.; Frommer, W.B.; Yazaki, K. Molecular characterization of LjSWEET3, a sugar transporter in nodules of Lotus japonicus. Plant Cell Physiol. 2017, 58, 298–306. [Google Scholar] [PubMed]
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1993, 11, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Huang, W.F.; Yuan, M.; Zhou, F.; Li, X.H.; Lin, Y.J. Overexpression of OsSWEET5 in rice causes growth retardation and precocious senescence. PLoS ONE 2014, 9, e94210. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.B.; Zhang, W.Y.; Hua, B.; Zhu, Z.H.; Zhang, J.J.; Zhang, Z.P.; Miao, M.M. Cucumber STACHYOSE SYNTHASE is regulated by its cis-antisense RNA asCsSTS to balance source-sink carbon partitioning. Plant Cell 2023, 35, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; van Dongen, J.T.; Alfred, S.C.; Mamun, E.A.; Zhao, X.C.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.L.; Sutton, B.G.; Geigenberger, P.; et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 2005, 28, 1534–1551. [Google Scholar] [CrossRef]
- Wen, S.Y.; Bao, T.Y.; Zeng, X.W.; Bie, Z.L.; Cheng, J.T. CsHT11 encodes a pollen-specific hexose transporter and is induced under high level sucrose in pollen tubes of cucumber (Cucumis sativus). Plant Growth Regul. 2020, 90, 237–248. [Google Scholar] [CrossRef]
- Aouali, N.; Laporte, P.; Clément, C. Pectin secretion and distribution in the anther during pollen development in Lilium. Planta 2001, 213, 71–79. [Google Scholar] [CrossRef]
- Spolaore, S.; Trainotti, L.; Casadoro, G. A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium. J. Exp. Bot. 2001, 52, 845–850. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Guo, W.J.; Nagy, R.; Chen, H.Y.; Pfrunder, S.; Yu, Y.C.; Santelia, D.; Frommer, W.B.; Martinoia, E. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014, 164, 777–789. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Tian, J.; Zhang, F.; Song, S.; Cheng, B.; Liu, G.; Liu, H.; Zhao, X.; Wang, Y.; He, H. Functional Characterization of CsSWEET5a, a Cucumber Hexose Transporter That Mediates the Hexose Supply for Pollen Development and Rescues Male Fertility in Arabidopsis. Int. J. Mol. Sci. 2024, 25, 1332. https://doi.org/10.3390/ijms25021332
Hu L, Tian J, Zhang F, Song S, Cheng B, Liu G, Liu H, Zhao X, Wang Y, He H. Functional Characterization of CsSWEET5a, a Cucumber Hexose Transporter That Mediates the Hexose Supply for Pollen Development and Rescues Male Fertility in Arabidopsis. International Journal of Molecular Sciences. 2024; 25(2):1332. https://doi.org/10.3390/ijms25021332
Chicago/Turabian StyleHu, Liping, Jiaxing Tian, Feng Zhang, Shuhui Song, Bing Cheng, Guangmin Liu, Huan Liu, Xuezhi Zhao, Yaqin Wang, and Hongju He. 2024. "Functional Characterization of CsSWEET5a, a Cucumber Hexose Transporter That Mediates the Hexose Supply for Pollen Development and Rescues Male Fertility in Arabidopsis" International Journal of Molecular Sciences 25, no. 2: 1332. https://doi.org/10.3390/ijms25021332
APA StyleHu, L., Tian, J., Zhang, F., Song, S., Cheng, B., Liu, G., Liu, H., Zhao, X., Wang, Y., & He, H. (2024). Functional Characterization of CsSWEET5a, a Cucumber Hexose Transporter That Mediates the Hexose Supply for Pollen Development and Rescues Male Fertility in Arabidopsis. International Journal of Molecular Sciences, 25(2), 1332. https://doi.org/10.3390/ijms25021332






