Trends on Aerogel-Based Biosensors for Medical Applications: An Overview
Abstract
1. Introduction
2. Chemistry of Aerogels and Their Properties
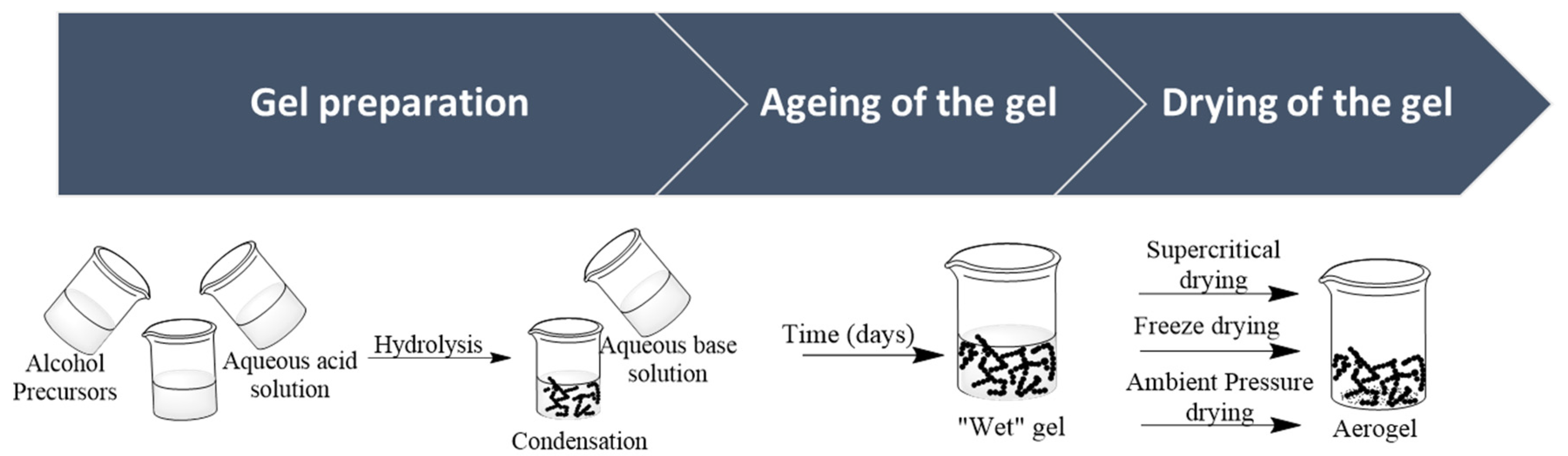
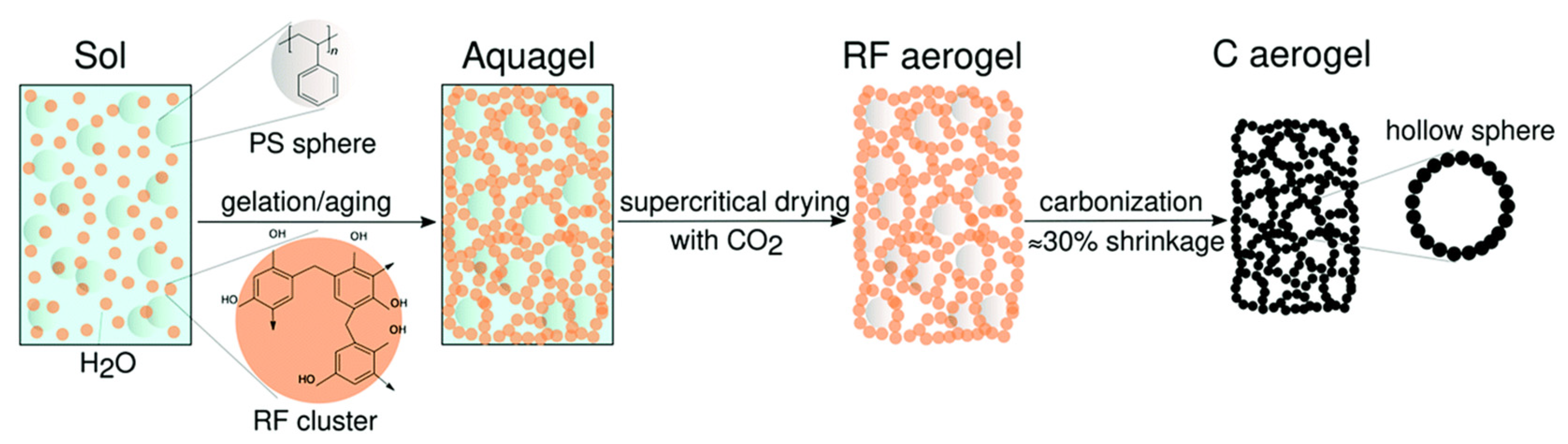
2.1. Aerogels Production Process
2.2. Aerogels Surface Properties
2.2.1. Silica-Based Aerogels
2.2.2. Carbon-Based Aerogels
3. Aerogels-Based Sensors for Biomedical Applications
3.1. Carbon-Based Aerogels
3.2. Silica-Based Aerogels
3.3. Polimeric Aerogels
3.4. Metal-Based Aerogels
3.5. Hybrid Aerogels
4. Recent Advances in Aerogel-Based Biosensors for Sensing Applications
4.1. Enzymes-Based Biosensor
| Aerogel Matrix | System | Target | Detection Technique | Lineal Range | LOD | Reference |
|---|---|---|---|---|---|---|
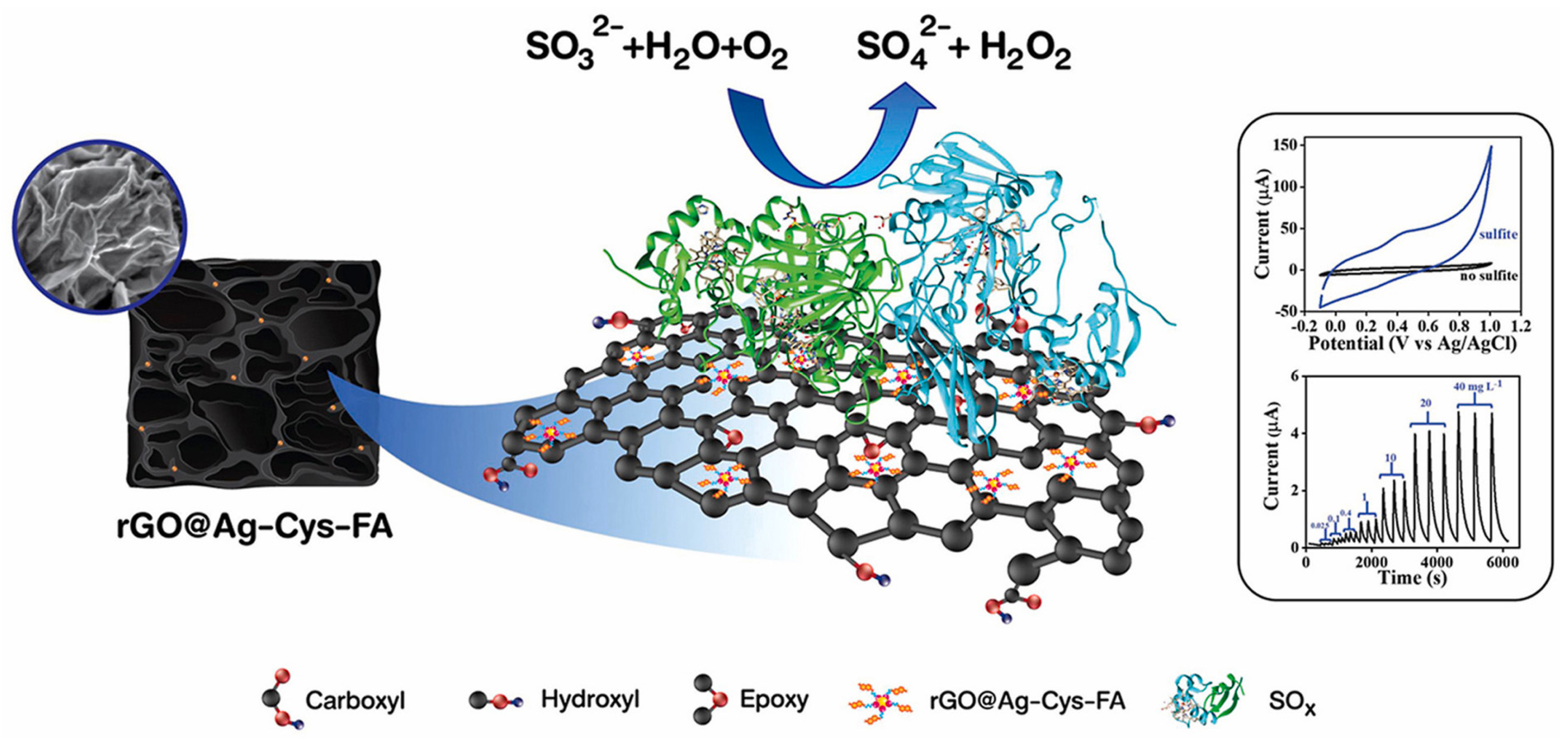
| Silver nanoparticle/graphene | Sulfite oxidase enzyme (SOx) immobilized on silver NP | Sulfite | Electrochemical | 0.025–40 mg/L | 0.007 mg/L | [21] |
| Graphene/Au NP | Cytochrome c (Cyt c) immobilized in 3D graphene aerogel with Au nanoparticles (AuNPs) | H2O2 | Electrochemical | 10–740 μM | 1.1 μM | [115] |
| Graphene | Glucose oxidase immobilized on a graphene aerogel | Glucose | Electrochemical | 1–18 mM | 0.87 mM | [86] |
| UiO-66-NH2 | Glucose oxidase immobilized on UiO-66-NH2 aerogel containing an iron porphyrin | Glucose | Colorimetric | 10–400 μM | 0.3 μM | [116] |
| Poly(vinyl alcohol) (PVA) | multienzyme immobilization | Glucose | Colorimetric | - | 11.4 μM | [117] |
| Au | Au hydrogel nanozyme with glucose oxidase and peroxidase-like activity | Glucose | Colorimetric | 5–80 mM | 1.65 mM | [118] |
4.2. Antibodies-Based Biosensor
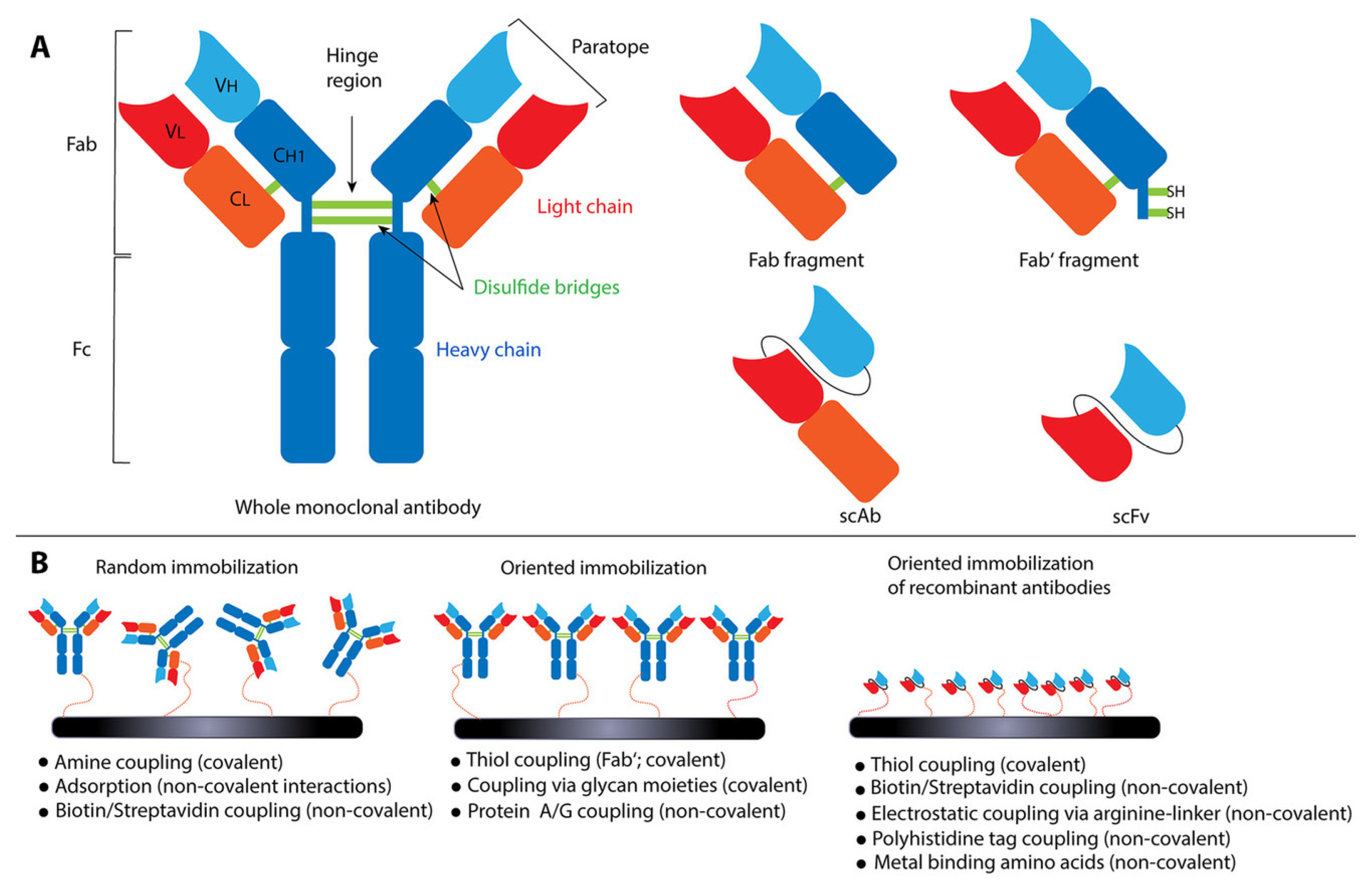
4.3. Aptamer-Based Biosensor
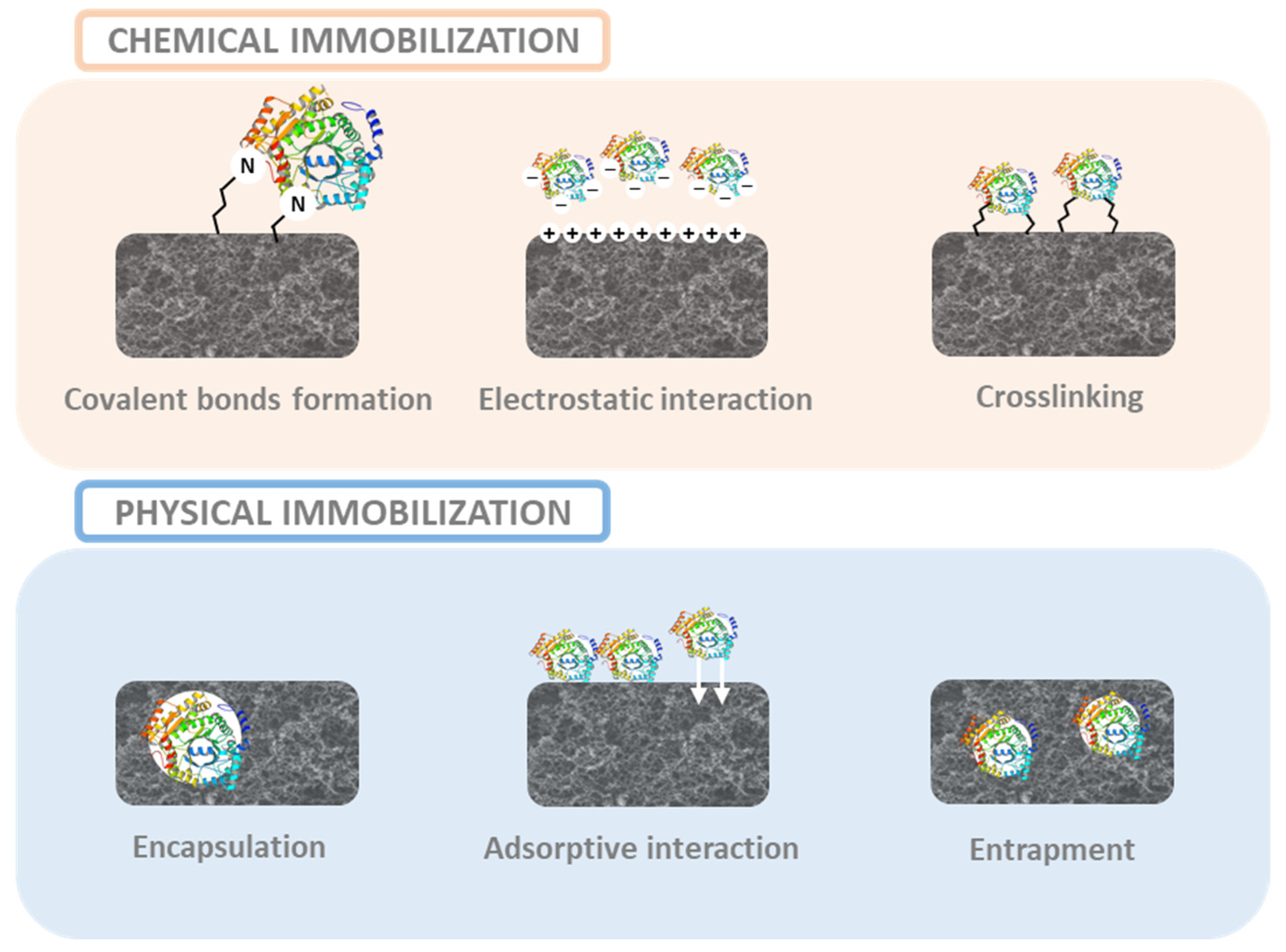
4.4. Aspects of the Bio-Recognition Elements
5. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lamy-Mendes, A.; Pontinha, A.D.R.; Santos, P.; Durães, L. Aerogel Composites Produced from Silica and Recycled Rubber Sols for Thermal Insulation. Materials 2022, 15, 7897. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Moreira, B.B.; Melo, B.L.; Melo-Diogo, D.d.; Correia, I.J.; Alves, P. Silica Aerogel-Polycaprolactone Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10128. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non. Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Merillas, B.; Martín-de León, J.; Villafañe, F.; Rodríguez-Pérez, M.Á. Optical Properties of Polyisocyanurate—Polyurethane Aerogels: Study of the Scattering Mechanisms. Nanomaterials 2022, 12, 1522. [Google Scholar] [CrossRef]
- Montes, S.; Maleki, H. 12—Aerogels and Their Applications. In Colloidal Metal Oxide Nanoparticles; Thomas, S., Tresa Sunny, A., Velayudhan, P., Eds.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2020; pp. 337–399. [Google Scholar] [CrossRef]
- Alves, P.; Dias, D.A.; Pontinha, A.D.R. Silica Aerogel-Rubber Composite: A Sustainable Alternative for Buildings’ Thermal Insulation. Molecules 2022, 27, 7127. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Ali, A.; Petrik, S.; Coufal, R.; Adach, K.; Fijalkowski, M. Aerogels for Biomedical, Energy and Sensing Applications. Gels 2021, 7, 264. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Dolatabadi, J.E.N.; Hasanzadeh, M.; Soleymani, J. Carbon-Based Aerogels for Biomedical Sensing: Advances toward Designing the Ideal Sensor. Adv. Colloid Interface Sci. 2021, 298, 102550. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zheng, Y.; Xu, Y.; Zheng, Z.; Chen, X.; Liu, W. Versatile Aerogels for Sensors. Small 2019, 15, 1902826. [Google Scholar] [CrossRef]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Martins-Lopes, P.; Fernandes, J.R. Biosensors as Diagnostic Tools in Clinical Applications. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188726. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S.; Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, 863196. [Google Scholar] [CrossRef]
- Ali, J.; Najeeb, J.; Asim Ali, M.; Farhan Aslam, M.; Raza, A. Biosensors: Their Fundamentals, Designs, Types and Most Recent Impactful Applications: A Review. J. Biosens. Bioelectron. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Lombardo, C.M.; Neidle, S.; Oliveira-Brett, A.M. Triazole-Linked Phenyl Derivatives: Redox Mechanisms and in Situ Electrochemical Evaluation of Interaction with dsDNA. Bioelectrochemistry 2015, 101, 97–105. [Google Scholar] [CrossRef]
- Zatloukalová, M.; Vavříková, E.; Pontinha, A.D.R.; Coufal, J.; Křen, V.; Fojta, M.; Ulrichová, J.; Oliveira-Brett, A.M.; Vacek, J. Flavonolignan Conjugates as DNA-Binding Ligands and Topoisomerase I Inhibitors: Electrochemical and Electrophoretic Approaches. Electroanalysis 2016, 28, 2866–2874. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Zou, X.; Chen, Y.; Zheng, Z.; Sun, M.; Song, X.; Lin, P.; Tao, J.; Zhao, P. The Sensitive Monitoring of Living Cell-Secreted Dopamine Based on the Electrochemical Biosensor Modified with Nitrogen-Doped Graphene Aerogel/Co3O4 Nanoparticles. Microchem. J. 2022, 183, 107957. [Google Scholar] [CrossRef]
- Prakash, J.; Uppal, S.; Kaushal, A.; Dasgupta, K. Effect of O/N Doping in CNT Aerogel Film on Their Nucleic Acid Hybridization Detection Ability as Electrochemical Impedance Biosensor. Mater. Today Commun. 2022, 32, 103965. [Google Scholar] [CrossRef]
- Sroysee, W.; Kongsawatvoragul, K.; Phattharaphuti, P.; Kullawattanapokin, P.; Jangsan, C.; Tejangkura, W.; Sawangphruk, M. Enzyme-Immobilized 3D Silver Nanoparticle/Graphene Aerogel Composites towards Biosensors. Mater. Chem. Phys. 2022, 277, 125572. [Google Scholar] [CrossRef]
- Pinelli, F.; Piras, C.; Rossi, F. A Perspective on Graphene Based Aerogels and Their Environmental Applications. FlatChem 2022, 36, 100449. [Google Scholar] [CrossRef]
- De Oliveira, S.C.B.; Diculescu, V.C.; Chiorcea Paquim, A.M.; Oliveira-Brett, A.M. Electrochemical Biosensors for DNA-Drug Interactions. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amesterdam, The Netherlands, 2018; pp. 124–139. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Oliveira, S.C.B.; Diculescu, V.C.; Oliveira-Brett, A.M. Chapter Nine—Applications of DNA-Electrochemical Biosensors in Cancer Research. In Past, Present and Future Challenges of Biosensors and Bioanalytical Tools in Analytical Chemistry: A Tribute to Professor Marco Mascini; Palchetti, I., Hansen, P.-D., Barceló, D., Eds.; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 77, pp. 287–336. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Electrochemistry of Chemotherapeutic Alkylating Agents and Their Interaction with DNA. J. Pharm. Biomed. Anal. 2023, 222, 115036. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent Expanded Aerogels. Nature 1931, 127, 741–747. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A Reconsideration on the Definition of the Term Aerogel Based on Current Drying Trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in Carbon Nanostructure-Silica Aerogel Composites: A Review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel Production: Current Status, Research Directions, and Future Opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An Advantageous Technique to Load Drugs into Aerogels: Gas Antisolvent Crystallization inside the Pores. J. Supercrit. Fluids 2017, 120, 310–319. [Google Scholar] [CrossRef]
- Anas, M.; Gönel, A.G.; Bozbag, S.E.; Erkey, C. Thermodynamics of Adsorption of Carbon Dioxide on Various Aerogels. J. CO2 Util. 2017, 21, 82–88. [Google Scholar] [CrossRef]
- Yousefzadeh, H.; Akgün, I.S.; Barim, S.B.; Sari, T.B.; Eris, G.; Uzunlar, E.; Bozbag, S.E.; Erkey, C. Supercritical Fluid Reactive Deposition: A Process Intensification Technique for Synthesis of Nanostructured Materials. Chem. Eng. Process.-Process Intensif. 2022, 176, 108934. [Google Scholar] [CrossRef]
- Barim, S.B.; Raptapoulos, G.; Rommel, S.; Aindow, M.; Paraskevopoulou, P.; Erkey, C. Polyamide Aerogel-Derived n-Doped Carbon Aerogel Decorated with Platinum Nanoparticles as Highly Active and Stable Electrocatalysts for Oxygen Reduction Reaction. Electrochim. Acta 2022, 434, 141251. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel Insulation for Building Applications: A State-of-the-Art Review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Feng, J.; Le, D.; Nguyen, S.T.; Tan Chin Nien, V.; Jewell, D.; Duong, H.M. Silica@cellulose Hybrid Aerogels for Thermal and Acoustic Insulation Applications. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 298–305. [Google Scholar] [CrossRef]
- Strobach, E.; Bhatia, B.; Yang, S.; Zhao, L.; Wang, E.N. High Temperature Annealing for Structural Optimization of Silica Aerogels in Solar Thermal Applications. J. Non. Cryst. Solids 2017, 462, 72–77. [Google Scholar] [CrossRef]
- Alwin, S.; Sahaya Shajan, X. Aerogels: Promising Nanostructured Materials for Energy Conversion and Storage Applications. Mater. Renew. Sustain. Energy 2020, 9, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Y.; Jiang, X.; Zhong, Y.; Chen, Y.; Shen, X. Synthesis of Hydrophobic Silica Aerogel and Its Composite Using Functional Precursor. J. Porous Mater. 2020, 27, 295–301. [Google Scholar] [CrossRef]
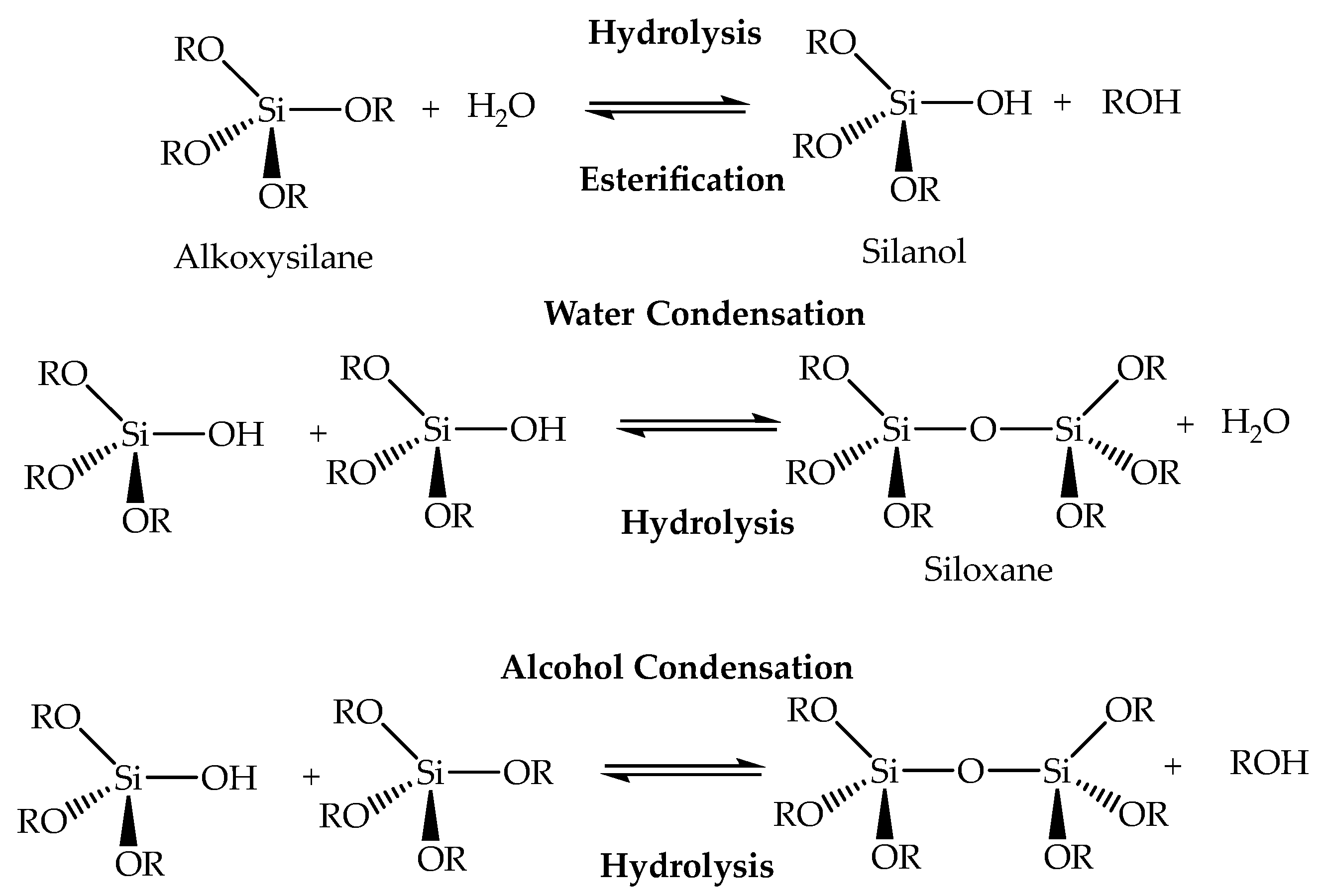
- Schmidt, H. Chemistry of Material Preparation by the Sol-Gel Process. J. Non. Cryst. Solids 1988, 100, 51–64. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Tecnol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Stolarski, M.; Walendziewski, J.; Steininger, M.; Pniak, B. Synthesis and Characteristic of Silica Aerogels. Appl. Catal. A Gen. 1999, 177, 139–148. [Google Scholar] [CrossRef]
- Iler, R.K.; Iler, R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; John Wiley and Sons: Chichester, UK, 1979. [Google Scholar]
- Shea, K.J.; Loy, D.A. Bridged Polysilsesquioxanes. Molecular-Engineered Hybrid Organic—Inorganic Materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, N.; Bhatt, M.; Mishra, N.K.; Chaudhary, P.; Singh, R. Sol-Gel Derived Nanomaterials and It’s Applications: A Review. Res. J. Chem. Sci. 2015, 5, 1–6. [Google Scholar]
- Iswar, S.; Malfait, W.J.; Balog, S.; Winnefeld, F.; Lattuada, M.; Koebel, M.M. Effect of Aging on Silica Aerogel Properties. Microporous Mesoporous Mater. 2017, 241, 293–302. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Venkateswara Rao, A. Effect of Post-Treatment (Gel Aging) on the Properties of Methyltrimethoxysilane Based Silica Aerogels Prepared by Two-Step Sol–Gel Process. J. Sol-Gel Sci. Technol. 2009, 49, 53–59. [Google Scholar] [CrossRef]
- Rao, A.V.; Kalesh, R.R. Organic Surface Modification of TEOS Based Silica Aerogels Synthesized by Co-Precursor and Derivatization Methods. J. Sol-Gel Sci. Technol. 2004, 30, 141–147. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Kim, T.-Y.; Hyun, S.-H. Optimization of Instantaneous Solvent Exchange/Surface Modification Process for Ambient Synthesis of Monolithic Silica Aerogels. J. Colloid Interface Sci. 2008, 322, 224–230. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H. Characteristics of Ambient-Pressure-Dried Aerogels Synthesized via Different Surface Modification Methods. J. Sol-Gel Sci. Technol. 2015, 76, 138–149. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Naguib, H.E.; Park, C.B. Advances in Precursor System for Silica-Based Aerogel Production toward Improved Mechanical Properties, Customized Morphology, and Multifunctionality: A Review. Adv. Colloid Interface Sci. 2020, 276, 102101. [Google Scholar] [CrossRef]
- Merillas, B.; Lamy-Mendes, A.; Villafañe, F.; Durães, L.; Rodríguez-Pérez, M.Á. Silica-Based Aerogel Composites Reinforced with Reticulated Polyurethane Foams: Thermal and Mechanical Properties. Gels 2022, 8, 392. [Google Scholar] [CrossRef]
- Pope, E.J.A.; Mackenzie, J.D. Sol-Gel Processing of Silica: II. The Role of the Catalyst. J. Non. Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Estella, J.; Echeverría, J.C.; Laguna, M.; Garrido, J.J. Silica Xerogels of Tailored Porosity as Support Matrix for Optical Chemical Sensors. Simultaneous Effect of PH, Ethanol: TEOS and Water: TEOS Molar Ratios, and Synthesis Temperature on Gelation Time, and Textural and Structural Properties. J. Non. Cryst. Solids 2007, 353, 286–294. [Google Scholar] [CrossRef]
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Rao, A.V.; Pajonk, G.M.; Bhagat, S.D.; Barboux, P. Comparative Studies on the Surface Chemical Modification of Silica Aerogels Based on Various Organosilane Compounds of the Type RnSiX4−N. J. Non. Cryst. Solids 2004, 350, 216–223. [Google Scholar] [CrossRef]
- Torres, R.B.; Vareda, J.P.; Lamy-Mendes, A.; Durães, L. Effect of Different Silylation Agents on the Properties of Ambient Pressure Dried and Supercritically Dried Vinyl-Modified Silica Aerogels. J. Supercrit. Fluids 2019, 147, 81–89. [Google Scholar] [CrossRef]
- Vrancken, K.C.; Possemiers, K.; Van Der Voort, P.; Vansant, E.F. Surface Modification of Silica Gels with Aminoorganosilanes. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 98, 235–241. [Google Scholar] [CrossRef]
- Fang, G.-Y.; Xu, L.-N.; Cao, Y.-Q.; Wang, L.-G.; Wu, D.; Li, A.-D. Self-Catalysis by Aminosilanes and Strong Surface Oxidation by O2 Plasma in Plasma-Enhanced Atomic Layer Deposition of High-Quality SiO2. Chem. Commun. 2015, 51, 1341–1344. [Google Scholar] [CrossRef]
- Jeong, U.; Kim, Y. Colorimetric Detection of Heavy Metal Ions Using Aminosilane. J. Ind. Eng. Chem. 2015, 31, 393–396. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Liu, Y.-M.; Bai, X.-L.; Yuan, H.; Hu, Y.-K.; Yu, X.-P.; Liao, X. Turn-on Fluorescent Probe for Dopamine Detection in Solutions and Live Cells Based on in Situ Formation of Aminosilane-Functionalized Carbon Dots. Anal. Chim. Acta 2021, 1157, 338394. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Fau, M.; Karbarz, M.; Donten, M.; Stojek, Z.; Nowicka, A.M. Hydrogel with Chains Functionalized with Carboxyl Groups as Universal 3D Platform in DNA Biosensors. Biosens. Bioelectron. 2014, 54, 222–228. [Google Scholar] [CrossRef]
- Feinle, A.; Leichtfried, F.; Straßer, S.; Hüsing, N. Carboxylic Acid-Functionalized Porous Silica Particles by a Co-Condensation Approach. J. Sol-Gel Sci. Technol. 2017, 81, 138–146. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic Aerogels from the Polycondensation of Resorcinol with Formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Salihovic, M.; Hüsing, N.; Bernardi, J.; Presser, V.; Elsaesser, M.S. Carbon aerogels with improved flexibility by sphere templating. RSC Adv. 2018, 8, 27326. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon Nanomaterials for Electronics, Optoelectronics, Photovoltaics, and Sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent Developments in Carbon Nanomaterial Sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Biju, V. Chemical Modifications and Bioconjugate Reactions of Nanomaterials for Sensing, Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Spehar-Délèze, A.-M.; Almadaghi, S.; O’Sullivan, C.K. Development of Solid-State Electrochemiluminescence (ECL) Sensor Based on Ru(Bpy)32+-Encapsulated Silica Nanoparticles for the Detection of Biogenic Polyamines. Chemosensors 2015, 3, 178–189. [Google Scholar] [CrossRef]
- Ahmad, V.; Ahmad, A.; Khan, S.A.; Ahmad, A.; Abuzinadah, M.F.; Karim, S.; Sajid Jamal, Q.M. Biomedical Applications of Aerogel; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Gao, H.; Ma, Y.; Song, P.; Leng, J.; Wang, Q. Gas Sensor Based on RGO/ZnO Aerogel for Efficient Detection of NO2 at Room Temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 10058–10069. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Santiago, K.S.; Jia, H.W.; Ahmed, M.M.M.; Lin, Y.F.; Yeh, J.M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, S.; Zhou, X.; Dong, X.; Jiang, Y.; Chen, Y.; Yuan, N.; Ding, J. A Highly Sensitive Piezoresistive Sensor Based on CNT-RGO Aerogel for Human Motion Detection. J. Compos. Mater. 2021, 55, 3661–3669. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhang, S.; Lai, X.; Zeng, X. Superhydrophobic MXene@carboxylated Carbon Nanotubes/Carboxymethyl Chitosan Aerogel for Piezoresistive Pressure Sensor. Chem. Eng. J. 2021, 425, 130462. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Reis, C.P.; Sabri, F.; Simón-Vázquez, R. Safety and Efficacy Assessment of Aerogels for Biomedical Applications. Biomed. Pharmacother. 2021, 144, 112356. [Google Scholar] [CrossRef] [PubMed]
- Karamikamkar, S.; Yalcintas, E.P.; Haghniaz, R.; de Barros, N.R.; Mecwan, M.; Nasiri, R.; Davoodi, E.; Nasrollahi, F.; Erdem, A.; Kang, H.; et al. Aerogel-Based Biomaterials for Biomedical Applications: From Fabrication Methods to Disease-Targeting Applications. Adv. Sci. 2023, 10, 2204681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Zhuo, H.; Liu, L.; Jing, S.; Zhong, L.; Peng, X.; Sun, R.C. Compressible, Elastic, and Pressure-Sensitive Carbon Aerogels Derived from 2D Titanium Carbide Nanosheets and Bacterial Cellulose for Wearable Sensors. Chem. Mater. 2019, 31, 3301–3312. [Google Scholar] [CrossRef]
- Yang, M.; Choy, K. leong. A Nature-Derived, Flexible and Three Dimensional (3D) Nano-Composite for Chronic Wounds PH Monitoring. Mater. Lett. 2021, 288, 129335. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Chen, W.; Li, X.; Weng, W.; Yin, C.; Dong, R.; Sun, W.; Li, G. Three-Dimensional Reduced Graphene Oxide Aerogel Modified Electrode for the Sensitive Quercetin Sensing and Its Application. Mater. Sci. Eng. C 2018, 89, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Keerthi, M.; Boopathy, G.; Chen, S.M.; Chen, T.W.; Lou, B.S. A Core-Shell Molybdenum Nanoparticles Entrapped f-MWCNTs Hybrid Nanostructured Material Based Non-Enzymatic Biosensor for Electrochemical Detection of Dopamine Neurotransmitter in Biological Samples. Sci. Rep. 2019, 9, 13075. [Google Scholar] [CrossRef]
- Liu, L.; Du, R.; Zhang, Y.; Yu, X. A Novel Sandwich-Type Immunosensor Based on Three-Dimensional Graphene-Au Aerogels and Quaternary Chalcogenide Nanocrystals for the Detection of Carcino Embryonic Antigen. New J. Chem. 2017, 41, 9008–9013. [Google Scholar] [CrossRef]
- Chen, Y.; Li, G.; Mu, W.; Wan, X.; Lu, D.; Gao, J.; Wen, D. Nonenzymatic Sweat Wearable Uric Acid Sensor Based on N-Doped Reduced Graphene Oxide/Au Dual Aerogels. Anal. Chem. 2023, 95, 3864–3872. [Google Scholar] [CrossRef]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D Porous Graphene Aerogel@GOx Based Microfluidic Biosensor for Electrochemical Glucose Detection. Analyst 2020, 145, 5141–5147. [Google Scholar] [CrossRef] [PubMed]
- Ruiyi, L.; Fangchao, C.; Haiyan, Z.; Xiulan, S.; Zaijun, L. Electrochemical Sensor for Detection of Cancer Cell Based on Folic Acid and Octadecylamine-Functionalized Graphene Aerogel Microspheres. Biosens. Bioelectron. 2018, 119, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Chen, Y.C.; Jiang, K.J.; Wu, J.C.; Chen-Yang, Y.W. Three-Dimensional Arrayed Amino Aerogel Biochips for Molecular Recognition of Antigens. Biomaterials 2011, 32, 7347–7354. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, D.K.; Chen, Y.C.; Su, H.J.; Wu, J.C.; Chen-Yang, Y.W. A Novel Three-Dimensional Aerogel Biochip for Molecular Recognition of Nucleotide Acids. Acta Biomater. 2010, 6, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Vincent Edwards, J.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, Characterization and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Kämpf, M.M.; Zimmermann, T.; Maniura-Weber, K.; Faccio, G. A Protein-Nanocellulose Paper for Sensing Copper Ions at the Nano- to Micromolar Level. Adv. Funct. Mater. 2017, 27, 1604291. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Ma, C.B.; Wang, Q.; Yang, M.; Du, Y. Point-of-Care Assay for Drunken Driving with Pd@Pt Core-Shell Nanoparticles-Decorated Ploy(Vinyl Alcohol) Aerogel Assisted by Portable Pressure. Meter. Theranostics 2020, 10, 5064–5073. [Google Scholar] [CrossRef]
- Guan, S.; Xu, B.; Yang, Y.; Zhu, X.; Chen, R.; Ye, D.; Liao, Q. Gold Nanowire Aerogel-Based Biosensor for Highly Sensitive Ethanol Detection in Simulated Sweat. ACS Appl. Nano Mater. 2022, 5, 11091–11099. [Google Scholar] [CrossRef]
- Wen, D.; Herrmann, A.K.; Borchardt, L.; Simon, F.; Liu, W.; Kaskel, S.; Eychmüller, A. Controlling the Growth of Palladium Aerogels with High-Performance toward Bioelectrocatalytic Oxidation of Glucose. J. Am. Chem. Soc. 2014, 136, 2727–2730. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-Capped Bimetallic AuPt Hydrogels Enable Robust Biosensor for Organophosphorus Pesticide Detection. Small 2019, 15, 1900632. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Sun, W.; Han, R.; Luo, C.; Wang, X.; Wei, Q. A Highly Selective and Sensitive Detection of Insulin with Chemiluminescence Biosensor Based on Aptamer and Oligonucleotide-AuNPs Functionalized Nanosilica @ Graphene Oxide Aerogel. Anal. Chim. Acta 2019, 1089, 152–164. [Google Scholar] [CrossRef]
- Tian, J.; Wang, D.; Zheng, Y.; Jing, T. A High Sensitive Electrochemical Avian Influenza Virus H7 Biosensor Based on CNTs/MoSx Aerogel. Int. J. Electrochem. Sci. 2017, 12, 2658–2668. [Google Scholar] [CrossRef]
- Trembecka-Wójciga, K.; Sobczak, J.J.; Sobczak, N. A Comprehensive Review of Graphene-Based Aerogels for Biomedical Applications. The Impact of Synthesis Parameters onto Material Microstructure and Porosity. In Archives of Civil and Mechanical Engineering; Springer: London, UK, 2023; Volume 23. [Google Scholar] [CrossRef]
- Jahed, F.S.; Hamidi, S.; Zamani-Kalajahi, M.; Siahi-Shadbad, M. Biomedical Applications of Silica-Based Aerogels: A Comprehensive Review. Macromol. Res. 2023, 31, 519–538. [Google Scholar] [CrossRef]
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as Biosensors: Viral Particle Detection by Bacteria Immobilized on Large Pore Aerogel. J. Non. Cryst. Solids 2001, 285, 303–308. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial Cellulose-based Biosensors. Med. Devices Sens. 2020, 3, e10102. [Google Scholar] [CrossRef]
- Gao, W.; Wen, D. Recent Advances of Noble Metal Aerogels in Biosensing. View 2021, 2, 20200124. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Sun, Q.; Liang, X.; Gu, P.; Huang, J.; Zu, G. Bioinspired Gradient Stretchable Aerogels for Ultrabroad-Range-Response Pressure-Sensitive Wearable Electronics and High-Efficient Separators. Angew. Chem.-Int. Ed. 2023, 62, e202213952. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Dna Electrochemical Biosensors for in Situ Probing of Pharmaceutical Drug Oxidative DNA Damage. Sensors 2021, 21, 1125. [Google Scholar] [CrossRef]
- Iyanagi, T. Molecular Mechanism of Phase I and Phase II Drug-Metabolizing Enzymes: Implications for Detoxification; International Review of Cytology. Int. Rev. Cytol. 2007, 260, 35–112. [Google Scholar] [CrossRef]
- Fan, Y.F.; Guo, Z.B.; Ge, G.B. Enzyme-Based Biosensors and Their Applications. Biosensors 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, D.; Yao, Q.; Ge, H.; Chung, J.; Fan, J.; Wang, J.; Peng, X.; Yoon, J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem.-Int. Ed. 2021, 60, 17268–17289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liew, S.S.; Wei, X.; Pu, K. Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem.-Int. Ed. 2021, 60, 26454–26475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Khoo, K.S.; Soto-Moscoso, M. Enzyme Immobilized Nanomaterials: An Electrochemical Bio-Sensing and Biocatalytic Degradation Properties toward Organic Pollutants. Top. Catal. 2023, 66, 691–706. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Qin, Y.; Liu, M.; Chen, G.; Hu, L.; Gu, W.; Zhu, C. AgCu@CuO Aerogels with Peroxidase-like Activities and Photoelectric Responses for Sensitive Biosensing. Chem. Commun. 2021, 57, 13788–13791. [Google Scholar] [CrossRef]
- Yin, D.; Bo, X.; Liu, J.; Guo, L. A Novel Enzyme-Free Glucose and H2O2 Sensor Based on 3D Graphene Aerogels Decorated with Ni3N Nanoparticles. Anal. Chim. Acta 2018, 1038, 11–20. [Google Scholar] [CrossRef]
- Li, X.; Tian, W.; Wan, C.; Liu, S.; Liu, X.; Su, J.; Chai, H.; Wu, Y. Hierarchically Porous Cellulose Nanofibril Aerogel Decorated with Polypyrrole and Nickel-Cobalt Layered Double Hydroxide for High-Performance Nonenzymatic Glucose Sensors. Front. Chem. Sci. Eng. 2023, 17, 1593–1607. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Hou, J.; Jia, Z.; Zhong, D.; Zhou, S.; Huo, D.; Yang, M.; Hou, C. Electrochemical Biointerface Based on Electrodeposition AuNPs on 3D Graphene Aerogel: Direct Electron Transfer of Cytochrome c and Hydrogen Peroxide Sensing. J. Electroanal. Chem. 2019, 842, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Tu, R.; Wang, Y.; Guo, X.; Hou, C.; Wang, Z. Introduction of Cascade Biocatalysis Systems into Metal-Organic Aerogel Nanostructures for Colorimetric Sensing of Glucose. ACS Appl. Nano Mater. 2022, 5, 8154–8160. [Google Scholar] [CrossRef]
- Ma, C.B.; Zhang, Y.; Liu, Q.; Du, Y.; Wang, E. Enhanced Stability of Enzyme Immobilized in Rationally Designed Amphiphilic Aerogel and Its Application for Sensitive Glucose Detection. Anal. Chem. 2020, 92, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Gu, W.; Li, H.; Du, D.; Lin, Y.; Zhu, C. A Dopamine-Induced Au Hydrogel Nanozyme for Enhanced Biomimetic Catalysis. Chem. Commun. 2019, 55, 9865–9868. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and Characterization of Immobilized Antibodies for Improved Immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Kumeria, T. Electrochemical Etching Methods for Producing Porous Silicon BT—Electrochemically Engineered Nanoporous Materials: Methods, Properties and Applications; Losic, D., Santos, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–36. [Google Scholar] [CrossRef]
- Crivianu-Gaita, V.; Thompson, M. Aptamers, Antibody ScFv, and Antibody Fab’ Fragments: An Overview and Comparison of Three of the Most Versatile Biosensor Biorecognition Elements. Biosens. Bioelectron. 2016, 85, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal Antibodies: Versatile Platforms for Cancer Immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody Production, Design and Use for Biosensor-Based Applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef]
- Sharma, S.; Byrne, H.; O’Kennedy, R.J. Antibodies and Antibody-Derived Analytical Biosensors. Essays Biochem. 2016, 60, 9–18. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody Orientation on Biosensor Surfaces: A Minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Heuer, C.; Jiang, X.; Segal, E. Aptasensors versus Immunosensors—Which Will Prevail? Eng. Life Sci. 2022, 22, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Avan, A.A.; Altaş Puntar, N.; Özyürek, M.; Güngör, Z.B.; Kucur, M.; Kamış, H.; Dicle, D.A. Ethylenediamine Grafted Carbon Nanotube Aerogels Modified Screen-Printed Electrode for Simultaneous Electrochemical Immunoassay of Multiple Tumor Markers. J. Electroanal. Chem. 2021, 900, 115700. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, H.; Kurth, F.; Burr, L.; Petropoulos, K.; Migliorelli, D.; Guenat, O.T.; Generelli, S. Nanocellulose Aerogel Inserts for Quantitative Lateral Flow Immunoassays. Biosens. Bioelectron. 2021, 192, 113491. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, J.; Lu, L.; Yu, Y.; Zuo, Y.; Tian, Q.; Li, P. Three-Dimensional Au Nanoparticles/Nano-Poly(3,4-Ethylene Dioxythiophene)- Graphene Aerogel Nanocomposite: A High-Performance Electrochemical Immunosensing Platform for Prostate Specific Antigen Detection. Sens. Actuators B Chem. 2018, 260, 990–997. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, L.; Jiao, L.; Wang, X.; Miao, L.; Li, H.; Zhou, F. Enzyme-Free Immunosorbent Assay of Prostate Specific Antigen Amplified by Releasing PH Indicator Molecules Entrapped in Mesoporous Silica Nanoparticles. Anal. Chem. 2018, 90, 8673–8679. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Tian, Q.; Xu, J.; Lu, L.; Ma, X.; Yu, Y. Aerogels Prepared from Polymeric β-Cyclodextrin and Graphene Aerogels as a Novel Host-Guest System for Immobilization of Antibodies: A Voltammetric Immunosensor for the Tumor Marker CA 15–3. Microchim. Acta 2018, 185, 517. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Z.; Sang, W.; Ding, B.; Chen, K.; Li, X.; Shen, Y.; Ni, Z. A Sensitive Electrochemical Platform Integrated with a 3D Graphene Aerogel for Point-of-Care Testing for Tumor Markers. J. Mater. Chem. B 2022, 10, 6928–6938. [Google Scholar] [CrossRef]
- Hu, T.; Bai, Z.; Wang, D.; Bai, Y.; Li, X.; Ni, Z. Electrochemical Aptasensor Based on 3D Graphene Aerogel for Prostate Specific Antigen Detection. Microchem. J. 2023, 195, 109436. [Google Scholar] [CrossRef]
- Zhou, W.; Jimmy Huang, P.-J.; Ding, J.; Liu, J. Aptamer-Based Biosensors for Biomedical Diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-Based Biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Sun, Y.; Han, R.; Dai, Y.; Zhu, X.; Liu, H.; Gao, D.; Luo, C.; Wang, X.; Wei, Q. Highly Selective and Sensitive Streptomycin Chemiluminescence Sensor Based on Aptamer and G-Quadruplex DNAzyme Modified Three-Dimensional Graphene Composite. Sens. Actuators B Chem. 2019, 301, 127122. [Google Scholar] [CrossRef]
- Hongxia, C.; Ji, H.; Zaijun, L.; Ruiyi, L.; Yongqiang, Y.; Xiulan, S. Electrochemical Aptasensor for Detection of Acetamiprid in Vegetables with Graphene Aerogel-Glutamic Acid Functionalized Graphene Quantum Dot/Gold Nanostars as Redox Probe with Catalyst. Sens. Actuators B Chem. 2019, 298, 126866. [Google Scholar] [CrossRef]
- Jin, W.; Ruiyi, L.; Nana, L.; Xiulan, S.; Haiyan, Z.; Guangli, W.; Zaijun, L. Electrochemical Detection of Carbendazim with Mulberry Fruit-like Gold Nanocrystal/Multiple Graphene Aerogel and DNA Cycle Amplification. Microchim. Acta 2021, 188, 284. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-X.; Huang, K.-J.; Liu, Y. Novel Electrochemical Dual-Aptamer-Based Sandwich Biosensor Using Molybdenum Disulfide/Carbon Aerogel Composites and Au Nanoparticles for Signal Amplification. Biosens. Bioelectron. 2015, 71, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, W. The Determination of Ochratoxin A Based on the Electrochemical Aptasensor by Carbon Aerogels and Methylene Blue Assisted Signal Amplification. Chem. Cent. J. 2018, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- de Dieu Habimana, J.J.; Sun, X. Minireview: Trends in Optical-Based Biosensors for Point-Of-Care Bacterial Pathogen Detection for Food Safety and Clinical Diagnostics. Anal. Lett. 2018, 51, 2933–2966. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Naseri, M.; Mohammadniaei, M.; Sun, Y.; Ashley, J. The Use of Aptamers and Molecularly Imprinted Polymers in Biosensors for Environmental Monitoring: A Tale of Two Receptors. Chemosensors 2020, 8, 32. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly Imprinted Polymers for Sample Preparation and Biosensing in Food Analysis: Progress and Perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef]
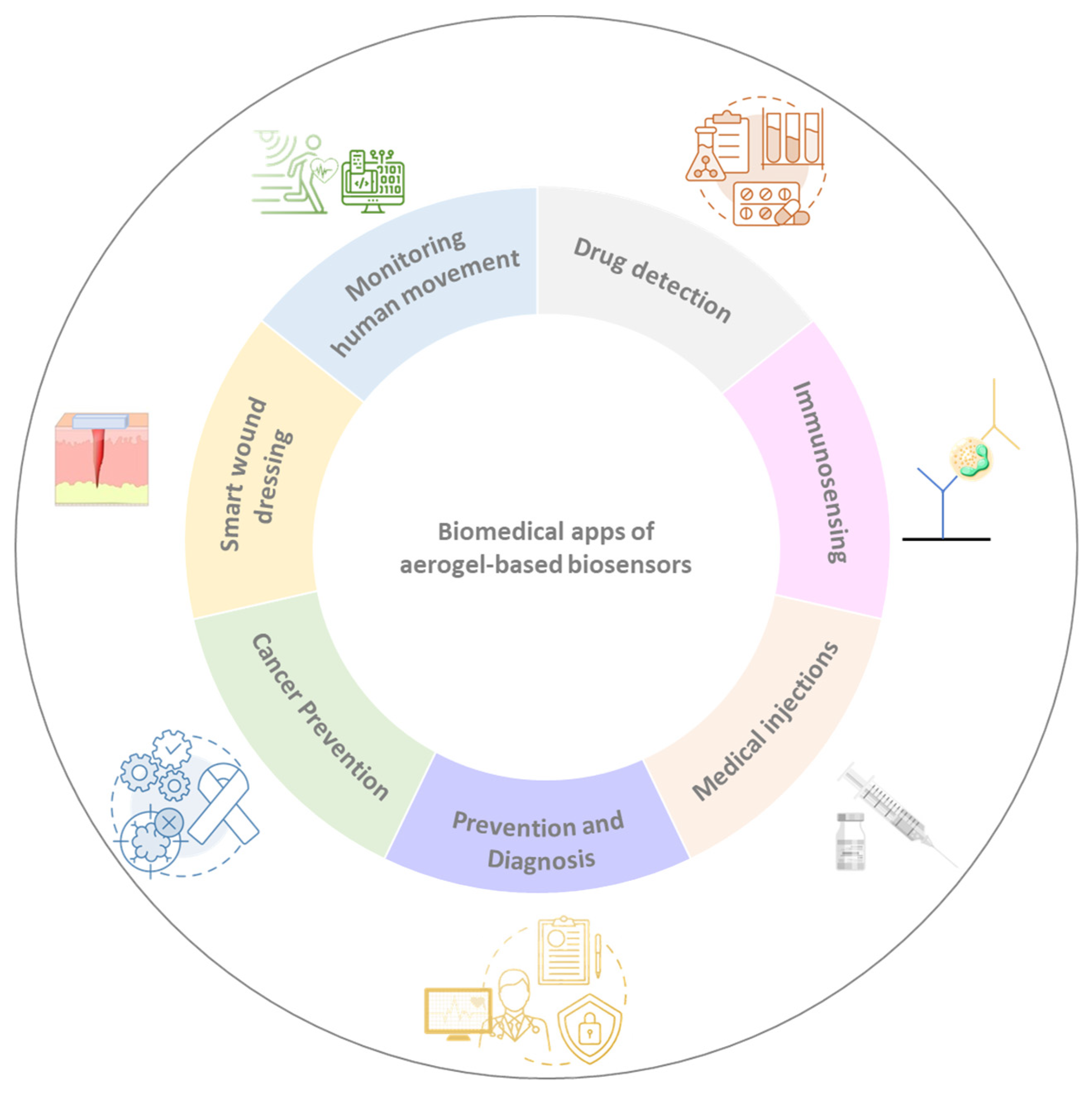
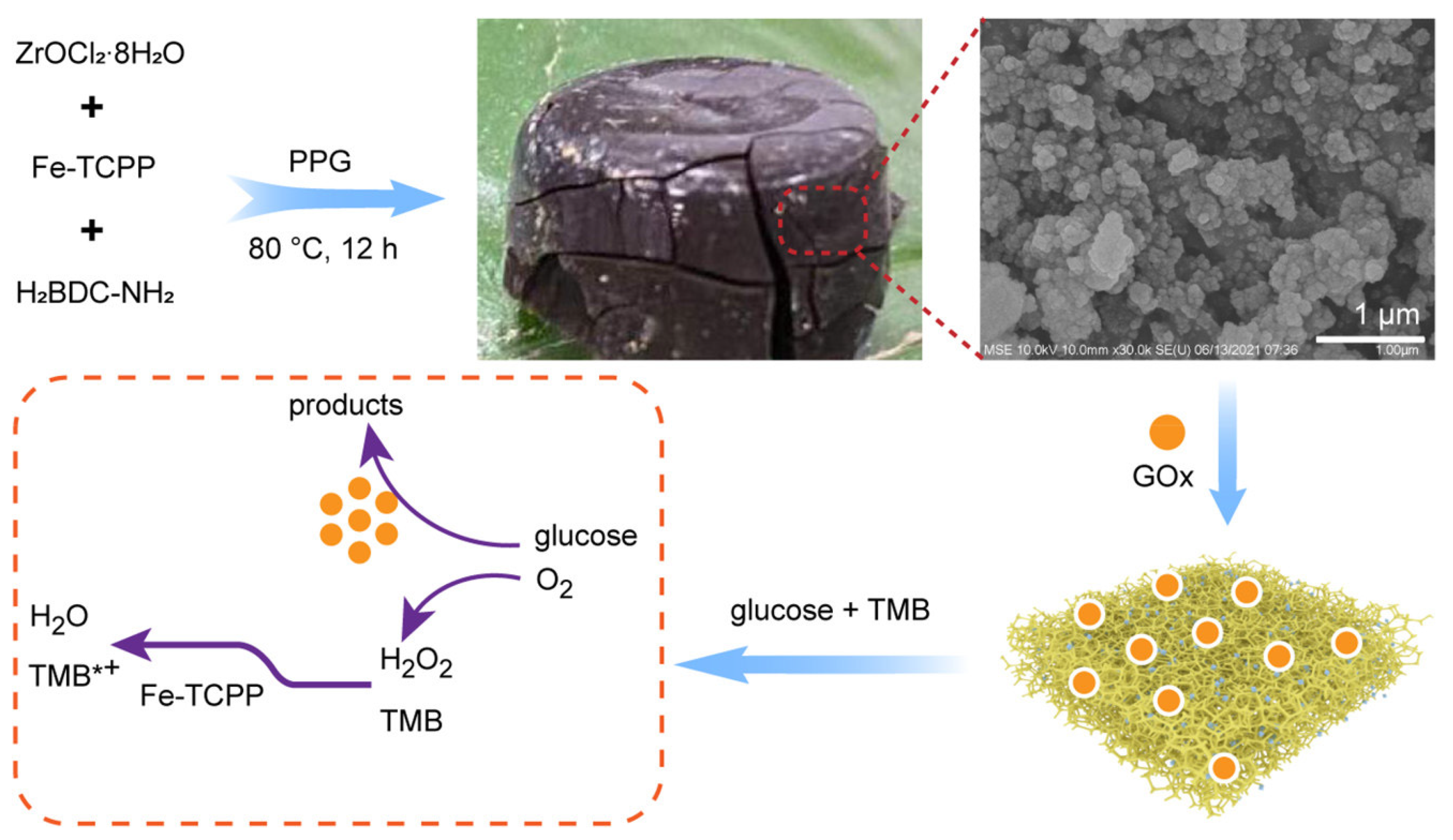
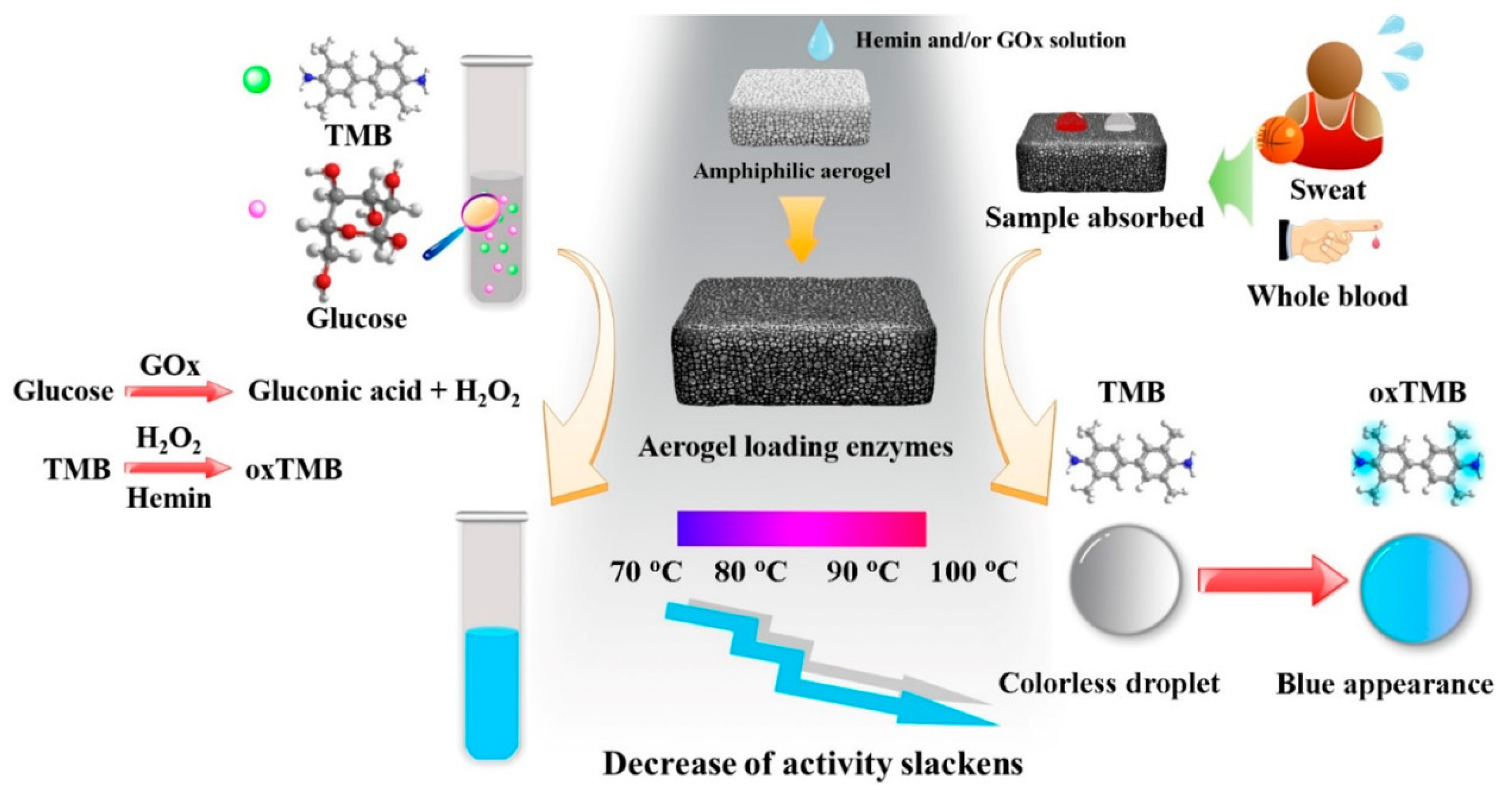

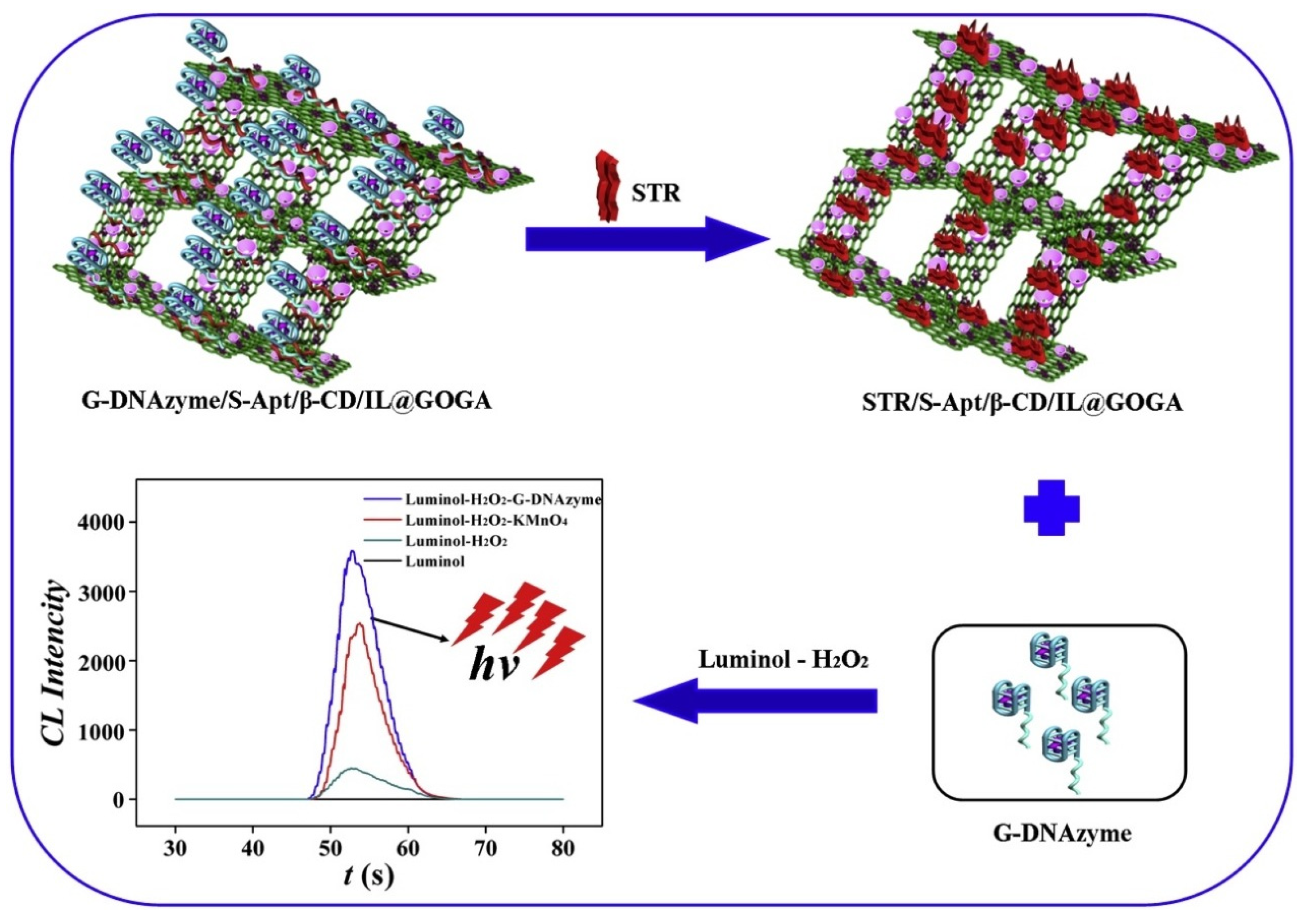
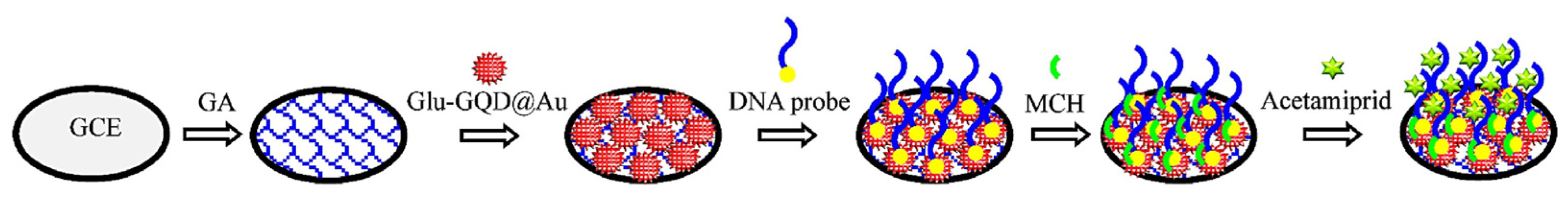
| Aerogel Matrix | Analyte/Stimuli | Detection Limit | Lineal Range | Biomedical Application | Reference |
|---|---|---|---|---|---|
| Carbon | Body pressure | 1.0 Pa | 0–10 kPa | Monitoring biosignals | [80] |
| Carbon nanofiber | pH in wound | −40.4 mV/pH | - | Chronic wound monitoring—Smart wound dressing | [81] |
| Graphene oxide | Quercetin | 0.065 μmol/L (3S0/S) | 0.1 μmol/L–100.0 μmol/L | Drug detection and quantification | [82] |
| MWCNTs/Mo nanoparticles | Dopamine | 1.26 nM | 0.01µM–1609 µM | Diagnosis and prevention | [83] |
| Graphene/Au | Carcino embryonic antigen | 0.15 pg/mL (S/N = 3) | 0.5 pg/mL–20 ng/mL | Immunosensing | [84] |
| Graphene oxide/Au | Uric acid | 3.7 μM (S/N = 3) | 5–600 μM | Metabolite monitoring | [85] |
| Graphene | Glucose | 0.87 mM (S/N = 3) | 1 mM–18 mM | Prevention and clinical diagnosis | [86] |
| Graphene microspheres | Cancer cells | 5 cell/mL (S/N = 3) | 5–105 cell/mL | Cancer detection, prevention and early treatment | [87] |
| Amino silica | Human interleukin-6 (IL6) | 0.00001 ng/ml | - | Antigen recognition | [88] |
| Silica | Nucleotide acids (human gene ATP5O) | - | 0.1–10 μM | Gene recognition | [89] |
| Peptide-Nanocellulose | Human neutrophil elastase | 0.13 units/milliliter | - | Biomarker for inflammatory diseases | [90] |
| Protein-Nanocellulose | Copper ions | 200 × 10−9 M | - | Point-of-care diagnostics | [91] |
| Poly(vinyl alcohol) | Alcohol | 0.50 mM (saliva) | 0–40 mM | Point-of-care diagnosis | [92] |
| Gold nanowire | Ethanol | 0.01 M | 0.01–0.5 M | Disease diagnosis | [93] |
| Palladium | Glucose | 2 mM | 2–20 mM | Prevention and clinical diagnosis | [94] |
| Au/Pt | Organophosphorus compounds | 0.185 ng/L | 0.5–1000 ng/L | Medical diagnosis | [95] |
| Nanosilica/Graphene oxide | Insulin | 1.6 × 10−12 moL/L | 7.5 × 10−12–5.0 × 10−9 moL/L | Medical treatments and injections | [96] |
| CNTs/MoSx | Avian Influenza Virus H7 | 0.43 ng/mL | 1–25 ng/mL | Immunosensing | [97] |
| Aerogel | System | Target | Detection Technique | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Carbon nanotube aerogel | Ethylenediamine grafted carbon nanotube aerogels modified screen-printed electrode | Alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) | Square Wave Voltammetry (SWV) | 5.0 × 10−12–1.0 ng/mL | 0.0010 ng mL−1 | [129] |
| Cellulose nanofiber aerogel | Cellulose nanofiber (CNF) aerogel material incorporated into LFIA strips | IgG | Lateral flow immunoassays | 0.72 ngmL−1–100 ngmL−1 | 4.6 ng mL−1–100 ng mL−1 (in human serum) | [130] |
| AuNPs/nano-PEDOT-graphene aerogel(GA) | Three-dimensional (3D) structural nano-PEDOT-graphene aerogel (nano-PEDOT-GA) composite | Metformin | Differential pulse voltammetry (DPV) | 0.0001–50 ng mL−1 | 0.03 pg mL−1 | [131] |
| Mesoporous silica | Releasing pH Indicator Molecules Entrapped in Mesoporous Silica Nanoparticles | Prostate specific antigen | Calorimetric | 0.5–8000 pg mL−1 | 0.36 pg mL−1 | [132] |
| Graphene aerogel | Graphene aerogel with β-cyclodextrin polymer (Pβ-CD) for immobilization of antibodies | Carbohydrate antigen | Differential pulse voltammetry (DPV) and Electrochemical Impedance Spectroscopy (EIS) | 0.1 mU mL−1–100 U mL−1 | 0.03 mU mL−1 | [133] |
| Graphene aerogel | Graphene aerogel via in situ chemical reduction of graphene oxide with L-ascorbic acid and then dehydration by freeze-drying | Alpha-fetoprotein | Electrochemical impedance spectroscopy (EIS) | 1.0 × 10−8–1.0 × 10−5 mg mL−1 | 7.9 pg mL−1 | [134] |
| Graphene aerogel microspheres | Folic acid (FA) and octadecylamine (OA)-functionalized graphene aerogel microspheres (FA-GAM-OA) | HepG2 | Cyclic voltammetry (CV) and differential pulse voltammograms (DPV) | 5–105 cell mL−1 | 5 cells mL−1 | [87] |
| Graphene aerogel | Immobilization of aptamers on the screen-printed electrode (SPE) surface modified by GA/AuNPs/Nafion. | Prostate specific antigen | Electrochemical impedance spectroscopy | 0.05–50 ng∙mL−1 | 0.0306 ng∙mL−1 | [135] |
| Aerogel | System | Target | Detection Technique | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Graphene oxide | Aptamer and oligonucleotide-AuNPs functionalized nanosilica @ graphene oxide aerogel | Insulin | Chemiluminescence | 7.5 × 10−12–5.0 × 10−9 | 1.6 × 10−12 | [96] |
| Graphene oxide | aptamer and G-quadruplex DNAzyme modified tgraphene composite | Streptomycin | Chemiluminescence | 1.4 × 10−12 to 2.8 × 10−9 | 9.2×10−14 | [138] |
| Graphene aerogel | Glutamic acid-functionalized graphene quantum dots/Au aerogel covalently connected with aptamer | Acetamiprid | Differential pulse voltammetry (DPV) | 1.0 fM–1 × 105 fM | 0.37 fM | [139] |
| Graphene aerogel | Gold nanocrystal/multiple graphene aerogel and DNA cycle amplification | Carbendazim | Differential pulse voltammetry (DPV) | 1.0 × 10−16–1.0 × 10−11 M | 4.4 × 10−17 M | [140] |
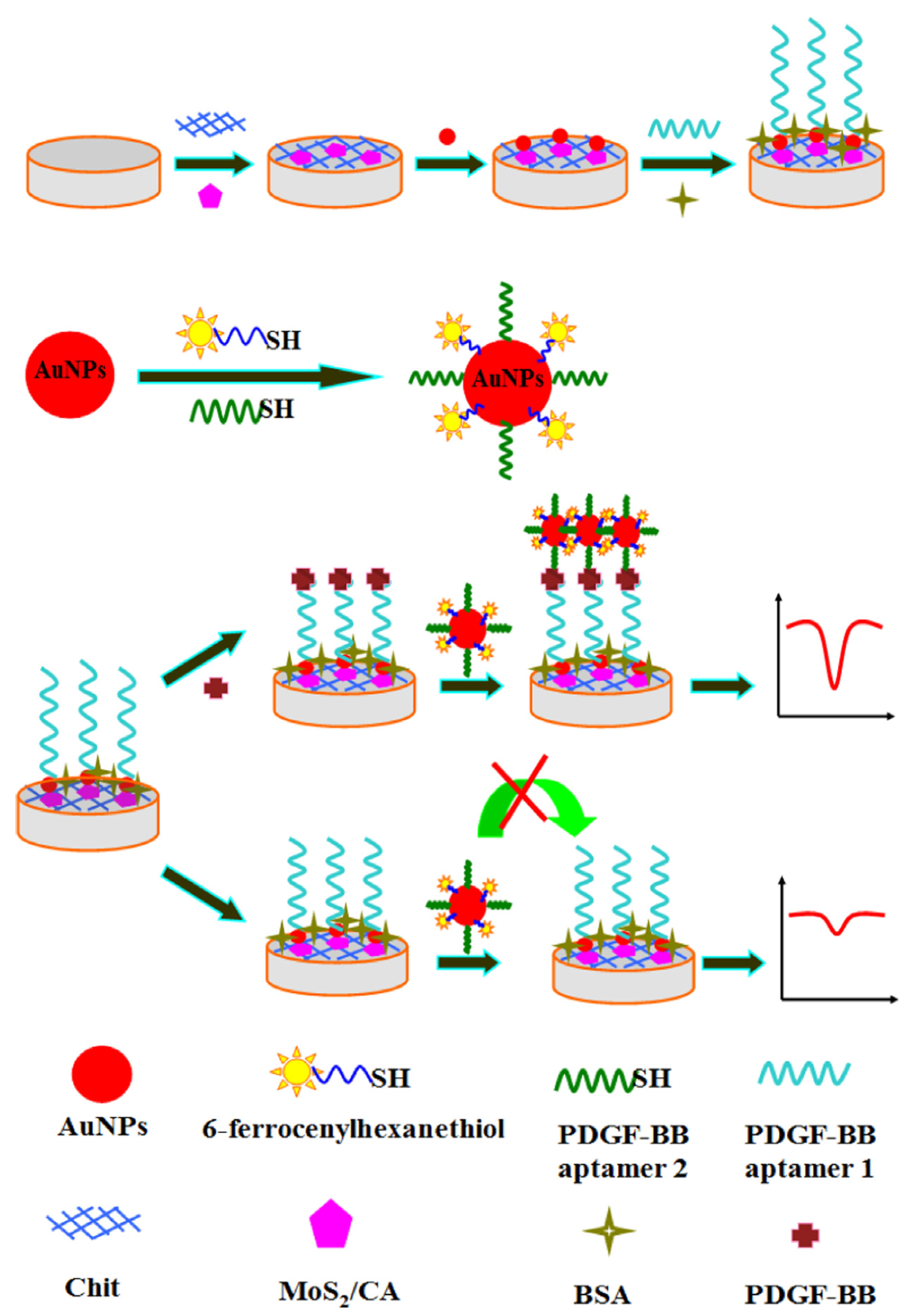
| Carbon aerogel | Electrochemical dual-aptamer-based sandwich biosensor using molybdenum disulfide/carbon aerogel and AuNPs | platelet-derived growth factor (PDGF-BB) | Differential pulse voltammetry (DPV) | 0.001–10 nM | 0.3 pM | [141] |
| Carbon aerogel | Carbon aerogel loaded with complementary DNA and aptamer immobilized on the Au electrode surface and methylene blue as signal amplification | Ochratoxin A | Differential pulse voltammetry (DPV) | 0.10–10 ng/mL | 1.0 × 10−4 ng/mL | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.M.R.; Merillas, B.; Pontinha, A.D.R. Trends on Aerogel-Based Biosensors for Medical Applications: An Overview. Int. J. Mol. Sci. 2024, 25, 1309. https://doi.org/10.3390/ijms25021309
Almeida CMR, Merillas B, Pontinha ADR. Trends on Aerogel-Based Biosensors for Medical Applications: An Overview. International Journal of Molecular Sciences. 2024; 25(2):1309. https://doi.org/10.3390/ijms25021309
Chicago/Turabian StyleAlmeida, Cláudio M. R., Beatriz Merillas, and Ana Dora Rodrigues Pontinha. 2024. "Trends on Aerogel-Based Biosensors for Medical Applications: An Overview" International Journal of Molecular Sciences 25, no. 2: 1309. https://doi.org/10.3390/ijms25021309
APA StyleAlmeida, C. M. R., Merillas, B., & Pontinha, A. D. R. (2024). Trends on Aerogel-Based Biosensors for Medical Applications: An Overview. International Journal of Molecular Sciences, 25(2), 1309. https://doi.org/10.3390/ijms25021309









