An Overview of Advances in Rare Cancer Diagnosis and Treatment
Abstract
1. Introduction
2. Rare Cancers
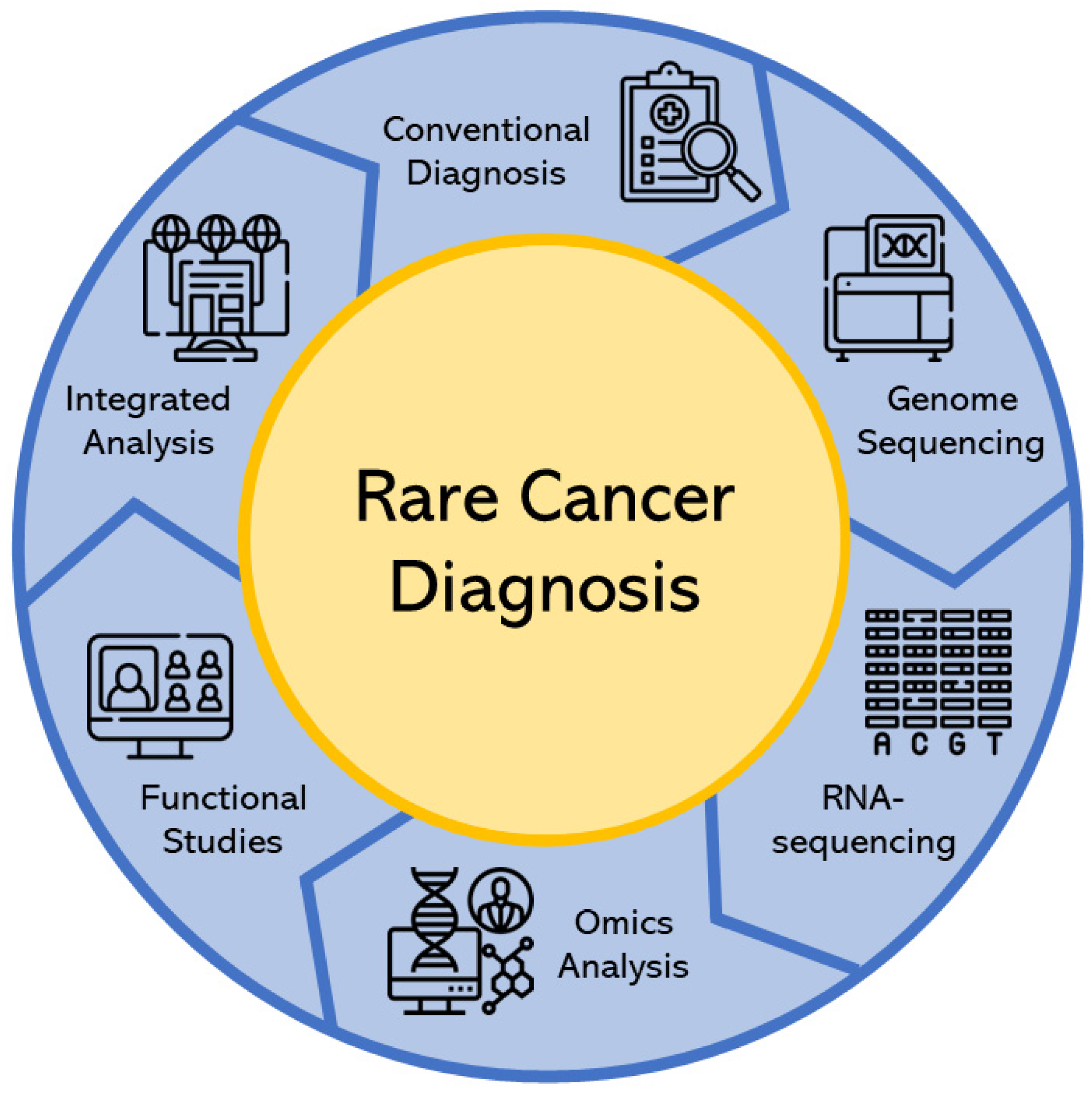
3. Rare Cancer Diagnosis
3.1. Conventional Diagnosis
3.2. Modern Diagnosis
3.2.1. Genome Sequencing
3.2.2. RNA-Sequencing Analysis
3.2.3. Omics Analysis
3.2.4. Functional Studies Analysis
3.2.5. Integrated Analysis
4. Rare Cancer Treatment
4.1. Conventional Therapy
4.2. Modern Therapy
4.2.1. Immunotherapy
Immune Checkpoint Inhibitors (ICIs)
Cytokines
Cancer Vaccines
Monoclonal Antibodies
Toxin-Based Immunotherapy
Adoptive Cell Therapy
Oncolytic Viruses
4.2.2. Targeted Therapy
Tyrosine Kinase Inhibitors (TKIs)
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors
Proteasome Inhibitors
Epigenetic Therapy
4.2.3. Transplantation
4.2.4. Drug Combination
4.3. Clinical Trials
4.4. Novel Advances in Other Types of Rare Cancer Treatment
5. Challenges and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1st GS | First-Generation Sequencing |
| ALL | Acute Lymphoblastic Leukemia |
| Allo-HSCT | Allogeneic Hematopoietic Stem Cell |
| BMT | Blood and Marrow Transplant |
| CAR | Chimeric Antigen Receptor |
| cART | Combined Anti-Retroviral Therapy |
| CGH | Comparative Genomic Hybridization |
| CML | Chronic Myeloid Leukemia |
| CNB | Core Needle Biopsy |
| CT | Computed Tomography |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| DSP | Digital Spatial Profiling |
| EFS | Event-Free Survival |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| FISH | Fluorescence in Situ Hybridization |
| FMRI | Functional Magnetic Resonance Imaging |
| FNAC | Fine Needle Aspiration Cytology |
| GvHD | Graft-versus-Host Disease |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| ICI | Immune Checkpoint Inhibitors |
| IFN-α | Interferon-α |
| IHC | Immunohistochemistry |
| IMC | Imaging Mass Cytometry |
| irAEs | Immune-related Adverse Events |
| ISH | In Situ Hybridization |
| MIBI | Multiplexed Ion Beam Imaging |
| mIHC | Multiplex IHC |
| NET | Neuroendocrine Tumor |
| NGS | Next/Second-Generation Sequencing |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| PARP | Poly (ADP-ribose) Polymerase |
| PD-1 | Programmed Cell Death Protein 1 |
| PDGFR | Platelet-derived Growth Factor Receptor |
| PD-L1 | Programmed Cell Death Protein 1 Ligand |
| PET | Positron Emission Tomography |
| PFS | Progression-free Survival |
| Poly-ICLC | Polyinosinic-Polycytidylic Acid with Poly-L-lysine and Carboxymethylcellulose |
| PRRT | Peptide Receptor Radionuclide Therapy |
| SBRT | Stereotactic Body Radiation Therapy |
| SIRT | Selective Internal Radiation Therapy |
| SNP | Single Nucleotide Polymorphisms |
| SSTR | Somatostatin Receptor |
| TKI | Tyrosine Kinase Inhibitor |
| TKIs | Tyrosine Kinase Inhibitors |
| USC | Uterine Serous Carcinomas |
| VATS | Video-Assisted Thoracoscopic Surgery |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| WGS | Whole Genome Sequencing |
References
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer 2015, 136, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, R.T.; Goodman, M.T.; Lynch, C.F.; Platz, C.E.; Havener, L.A.; Howe, H.L. The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep. 2010, 125, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; van der Zwan, J.M.; Casali, P.G.; Siesling, S.; Dei Tos, A.P.; Kunkler, I.; Otter, R.; Licitra, L.; Mallone, S.; Tavilla, A.; et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur. J. Cancer 2011, 47, 2493–2511. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Lozano, C.; Paramo-Rodriguez, L.; Cavero-Carbonell, C.; Corpas-Burgos, F.; Lopez-Maside, A.; Guardiola-Vilarroig, S.; Zurriaga, O. Rare Diseases: Needs and Impact for Patients and Families: A Cross-Sectional Study in the Valencian Region, Spain. Int. J. Environ. Res. Public. Health 2022, 19, 10366. [Google Scholar] [CrossRef]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Halicek, M.; Shahedi, M.; Little, J.V.; Chen, A.Y.; Myers, L.L.; Sumer, B.D.; Fei, B. Head and Neck Cancer Detection in Digitized Whole-Slide Histology Using Convolutional Neural Networks. Sci. Rep. 2019, 9, 14043. [Google Scholar] [CrossRef]
- Varadhachary, G.R.; Abbruzzese, J.L.; Lenzi, R. Diagnostic strategies for unknown primary cancer. Cancer 2004, 100, 1776–1785. [Google Scholar] [CrossRef]
- Singh, H.; Sethi, S.; Raber, M.; Petersen, L.A. Errors in cancer diagnosis: Current understanding and future directions. J. Clin. Oncol. 2007, 25, 5009–5018. [Google Scholar] [CrossRef]
- Bumgarner, R. Overview of DNA microarrays: Types, applications, and their future. Curr. Protoc. Mol. Biol. 2013, 22, Unit 22.1. [Google Scholar] [CrossRef]
- Chrzanowska, N.M.; Kowalewski, J.; Lewandowska, M.A. Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors. Molecules 2020, 25, 1864. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.L.; Reinin, G.; Chua, E. Full Spectrum Flow Cytometry as a Powerful Technology for Cancer Immunotherapy Research. Front. Mol. Biosci. 2021, 7, 612801. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Singh, S.; Singh, P.; Chauhan, P.; Zaidi, M.A. Tumor markers: A diagnostic tool. Natl. J. Maxillofac. Surg. 2016, 7, 17–20. [Google Scholar]
- Barba, D.; León-Sosa, A.; Lugo, P.; Suquillo, D.; Torres, F.; Surre, F.; Trojman, L.; Caicedo, A. Breast cancer, screening and diagnostic tools: All you need to know. Crit. Rev. Oncol./Hematol. 2021, 157, 103174. [Google Scholar] [CrossRef] [PubMed]
- Dabeer, S.; Khan, M.M.; Islam, S. Cancer diagnosis in histopathological image: CNN based approach. Inform. Med. Unlocked 2019, 16, 100231. [Google Scholar] [CrossRef]
- Sanger, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Hunkapiller, T.; Kaiser, R.J.; Koop, B.F.; Hood, L. Large-scale and automated DNA sequence determination. Science 1991, 254, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.B.; Barbara, M.; Iscove, N.N. Representative in vitro cDNA amplification from individual hemopoietic cells and colonies. Methods Mol. Cell. Biol. 1990, 2, 17–25. [Google Scholar]
- Temin, H.M.; Mizutani, S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 1970, 226, 1211–1213. [Google Scholar] [CrossRef]
- Lahens, N.F.R.; Ricciotti, E.; Smirnova, O.; Toorens, E.; Kim, E.J.; Baruzzo, G.; Hayer, K.E.; Ganguly, T.; Schug, J.; Grant, G.R. A comparison of Illumina and Ion Torrent sequencing platforms in the context of differential gene expression. BMC Genom. 2017, 18, 602. [Google Scholar] [CrossRef]
- Darwanto, A.; Hein, A.M.; Strauss, S.; Kong, Y.; Sheridan, A.; Richards, D.; Lader, E.; Ngowe, M.; Pelletier, T.; Adams, D.; et al. Use of the QIAGEN GeneReader NGS system for detection of KRAS mutations, validated by the QIAGEN Therascreen PCR kit and alternative NGS platform. BMC Cancer 2017, 17, 358. [Google Scholar] [CrossRef]
- Koitzsch, U.; Heydt, C.; Attig, H.; Immerschitt, I.; Merkelbach-Bruse, S.; Fammartino, A.; Buttner, R.H.; Kong, Y.; Odenthal, M. Use of the GeneReader NGS System in a clinical pathology laboratory: A comparative study. J. Clin. Pathol. 2017, 70, 725–728. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, N.; Chen, L.; Zhao, X.; Shan, Y.; Yang, F.; Wang, B.; Gao, H.; Xu, M.; Tang, P.; et al. Whole-genome sequencing and RNA sequencing analysis reveals novel risk genes and differential expression patterns in hepatoblastoma. Gene 2023, 897, 147991. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Camps, C.; Giacopuzzi, E.; Taylor, J.M.; Hashim, M.; Calpena, E.; Kaisaki, P.J.; Hashimoto, A.; Yu, J.; Sanders, E.; et al. Structural and non-coding variants increase the diagnostic yield of clinical whole genome sequencing for rare diseases. Genome Med. 2023, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, M.; Diamond, B.; Ziccheddu, B.; Rustad, E.; Maclachlan, K.; Papadimitriou, M.; Boyle, E.M.; Blaney, P.; Usmani, S.; Morgan, G.; et al. Impact of rare structural variant events in newly diagnosed multiple myeloma. Clin. Cancer Res. 2023, in press. [Google Scholar]
- Lee, C.H.; Gundem, G.; Lee, W.; Chen, Y.B.; Cross, J.R.; Dong, Y.; Redzematovic, A.; Mano, R.; Wei, E.Y.; Cheng, E.H.; et al. Persistent Severe Hyperlactatemia and Metabolic Derangement in Lethal SDHB-Mutated Metastatic Kidney Cancer: Clinical Challenges and Examples of Extreme Warburg Effect. JCO Precis. Oncol. 2017, 1, PO.16.00007. [Google Scholar] [CrossRef]
- Turro, E.; Astle, W.J.; Megy, K.; Graf, S.; Greene, D.; Shamardina, O.; Allen, H.L.; Sanchis-Juan, A.; Frontini, M.; Thys, C.; et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature 2020, 583, 96–102. [Google Scholar] [CrossRef]
- Sanders, L.M.; Rangaswami, A.; Bjork, I.; Lam, D.L.; Beale, H.C.; Kephart, E.T.; Durbin, A.; Learned, K.; Currie, R.; Lyle, A.G.; et al. Comparative RNA-seq analysis aids in diagnosis of a rare pediatric tumor. Cold Spring Harb. Mol. Case Stud. 2019, 5, a004317. [Google Scholar] [CrossRef]
- Pei, J.; Zhao, X.; Patchefsky, A.S.; Flieder, D.B.; Talarchek, J.N.; Testa, J.R.; Wei, S. Clinical application of RNA sequencing in sarcoma diagnosis: An institutional experience. Medicine 2019, 98, e16031. [Google Scholar] [CrossRef]
- Datta, S.; Malhotra, L.; Dickerson, R.; Chaffee, S.; Sen, C.K.; Roy, S. Laser capture microdissection: Big data from small samples. Histol. Histopathol. 2015, 30, 1255–1269. [Google Scholar]
- Jackson, K.; Milner, R.J.; Doty, A.; Hutchison, S.; Cortes-Hinojosa, G.; Riva, A.; Sahay, B.; Lejeune, A.; Bechtel, S. Analysis of canine myeloid-derived suppressor cells (MDSCs) utilizing fluorescence-activated cell sorting, RNA protection mediums to yield quality RNA for single-cell RNA sequencing. Vet. Immunol. Immunopathol. 2021, 231, 110144. [Google Scholar] [CrossRef] [PubMed]
- Welzel, G.; Seitz, D.; Schuster, S. Magnetic-activated cell sorting (MACS) can be used as a large-scale method for establishing zebrafish neuronal cell cultures. Sci. Rep. 2015, 5, 7959. [Google Scholar] [CrossRef]
- Lecault, V.; White, A.K.; Singhal, A.; Hansen, C.L. Microfluidic single cell analysis: From promise to practice. Curr. Opin. Chem. Biol. 2012, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Barrett, T.; Parikh, A.S.; Tirosh, I.; Puram, S.V. Single-cell sequencing and its applications in head and neck cancer. Oral. Oncol. 2019, 99, 104441. [Google Scholar] [CrossRef]
- Huang, D.; Ma, N.; Li, X.; Gou, Y.; Duan, Y.; Liu, B.; Xia, J.; Zhao, X.; Wang, X.; Li, Q.; et al. Advances in single-cell RNA sequencing and its applications in cancer research. J. Hematol. Oncol. 2023, 16, 98. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Elefant, N.; Paul, F.; Zaretsky, I.; Mildner, A.; Cohen, N.; Jung, S.; Tanay, A.; et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 2014, 343, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Selves, J.; Long-Mira, E.; Mathieu, M.C.; Rochaix, P.; Ilie, M. Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. Cancers 2018, 10, 108. [Google Scholar] [CrossRef]
- Ordonez, N.G. Value of PAX8, PAX2, napsin A, carbonic anhydrase IX, and claudin-4 immunostaining in distinguishing pleural epithelioid mesothelioma from metastatic renal cell carcinoma. Mod. Pathol. 2013, 26, 1132–1143. [Google Scholar] [CrossRef]
- Zhang, W.; Hubbard, A.; Jones, T.; Racolta, A.; Bhaumik, S.; Cummins, N.; Zhang, L.; Garsha, K.; Ventura, F.; Lefever, M.R.; et al. Fully automated 5-plex fluorescent immunohistochemistry with tyramide signal amplification and same species antibodies. Lab. Investig. 2017, 97, 873–885. [Google Scholar] [CrossRef]
- Ilie, M.; Beaulande, M.; Ben Hadj, S.; Chamorey, E.; Schiappa, R.; Long-Mira, E.; Lassalle, S.; Butori, C.; Cohen, C.; Leroy, S.; et al. Chromogenic Multiplex Immunohistochemistry Reveals Modulation of the Immune Microenvironment Associated with Survival in Elderly Patients with Lung Adenocarcinoma. Cancers 2018, 10, 326. [Google Scholar] [CrossRef]
- Giesen, C.; Wang, H.A.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schuffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Levenson, R.M.; Borowsky, A.D.; Angelo, M. Immunohistochemistry and mass spectrometry for highly multiplexed cellular molecular imaging. Lab. Investig. 2015, 95, 397–405. [Google Scholar] [CrossRef]
- Abel, E.J.; Bauman, T.M.; Weiker, M.; Shi, F.; Downs, T.M.; Jarrard, D.F.; Huang, W. Analysis and validation of tissue biomarkers for renal cell carcinoma using automated high-throughput evaluation of protein expression. Hum. Pathol. 2014, 45, 1092–1099. [Google Scholar] [CrossRef]
- Feng, Z.; Jensen, S.M.; Messenheimer, D.J.; Farhad, M.; Neuberger, M.; Bifulco, C.B.; Fox, B.A. Multispectral Imaging of T and B Cells in Murine Spleen and Tumor. J. Immunol. 2016, 196, 3943–3950. [Google Scholar] [CrossRef]
- Yeong, J.; Tan, T.; Chow, Z.L.; Cheng, Q.; Lee, B.; Seet, A.; Lim, J.X.; Lim, J.C.T.; Ong, C.C.H.; Thike, A.A.; et al. Multiplex immunohistochemistry/immunofluorescence (mIHC/IF) for PD-L1 testing in triple-negative breast cancer: A translational assay compared with conventional IHC. J. Clin. Pathol. 2020, 73, 557–562. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Decalf, J.; Albert, M.L.; Ziai, J. New tools for pathology: A user’s review of a highly multiplexed method for in situ analysis of protein and RNA expression in tissue. J. Pathol. 2019, 247, 650–661. [Google Scholar] [CrossRef]
- Toki, M.I.; Merritt, C.R.; Wong, P.F.; Smithy, J.W.; Kluger, H.M.; Syrigos, K.N.; Ong, G.T.; Warren, S.E.; Beechem, J.M.; Rimm, D.L. High-Plex Predictive Marker Discovery for Melanoma Immunotherapy-Treated Patients Using Digital Spatial Profiling. Clin. Cancer Res. 2019, 25, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.; Li, S.; Niedre, M. Performance of computer vision in vivo flow cytometry with low fluorescence contrast. J. Biomed. Opt. 2015, 20, 035005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, H.H.M.; Lee, R.Y.; Goh, S.; Tay, I.S.Y.; Lim, X.; Lee, B.; Chew, V.; Li, H.; Tan, B.; Lim, S.; et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000987. [Google Scholar] [CrossRef] [PubMed]
- Heskett, M.B.; Sanborn, J.Z.; Boniface, C.; Goode, B.; Chapman, J.; Garg, K.; Rabban, J.T.; Zaloudek, C.; Benz, S.C.; Spellman, P.T.; et al. Multiregion exome sequencing of ovarian immature teratomas reveals 2N near-diploid genomes, paucity of somatic mutations, and extensive allelic imbalances shared across mature, immature, and disseminated components. Mod. Pathol. 2020, 33, 1193–1206. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Kong, Q. Case report: Squamous cell carcinoma of the prostate-a clinicopathological and genomic sequencing-based investigation. Pathol. Oncol. Res. 2023, 29, 1611343. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Shin, N.; Chernyshov, K.; Calabro, F.; Cerbone, L.; Procopio, G.; Miheecheva, N.; Sagaradze, G.; Zaichikova, A.; Samarina, N.; et al. Case report: Metastatic urothelial cancer with an exceptional response to immunotherapy and comprehensive understanding of the tumor and the tumor microenvironment. Front. Oncol. 2022, 12, 1006017. [Google Scholar] [CrossRef]
- Song, W.; Wang, G.; Wang, C.; Liu, L.; Zhang, L.; Zhang, R.; Zhang, H.; Shi, K. Case Report: An unclassified T cell lymphoma subtype with co-expression of TCR alphabeta and gamma chains revealed by single cell sequencing. Front. Immunol. 2023, 14, 1184383. [Google Scholar] [CrossRef] [PubMed]
- Suter, P.; Dazert, E.; Kuipers, J.; Ng, C.K.Y.; Boldanova, T.; Hall, M.N.; Heim, M.H.; Beerenwinkel, N. Multi-omics subtyping of hepatocellular carcinoma patients using a Bayesian network mixture model. PLoS Comput. Biol. 2022, 18, e1009767. [Google Scholar] [CrossRef]
- He, D.N.; Wang, N.; Wen, X.L.; Li, X.H.; Guo, Y.; Fu, S.H.; Xiong, F.F.; Wu, Z.Y.; Zhu, X.; Gao, X.L.; et al. Multi-omics analysis reveals a molecular landscape of the early recurrence and early metastasis in pan-cancer. Front. Genet. 2023, 14, 1061364. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kosaka, T.; Nakamura, K.; Mikami, S.; Nishihara, H.; Oya, M. Squamous cell carcinoma of the prostate with SMARCA4 alteration in a Japanese patient. IJU Case Rep. 2022, 5, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Aryal, V.; Maharjan, R.; Singh, M.; Marhatta, A.; Bajracharya, A.; Dhakal, H.P. Malignant transformation of mature cystic teratoma into undifferentiated carcinoma: A case report. Clin. Case Rep. 2021, 9, e05240. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, T.; Baveja, P.; Sen, A. Myeloid sarcoma: An uncommon presentation of myeloid neoplasms; a case series of 4 rare cases reported in a tertiary care institute. Autops. Case Rep. 2021, 11, e2021339. [Google Scholar] [CrossRef]
- Burg, S.; Smeets, R.; Gosau, M.; Failing, K.; Grust, A.L.C. Case Report: Early detection of lung carcinoid in an asymptomatic individual by blood-test initiated PET-CT imaging. Front. Oncol. 2023, 13, 1177237. [Google Scholar] [CrossRef]
- Tuan Nguyen, T.; Thi Vinh Do, A.; Thi Nguyen, N.; Quoc Truong, T.; Ton, A.T. Flow-Cytometry in the Diagnosis of Diffuse Large B-Cell Lymphoma Based on Stomach Tissue Samples: A Case Report. Cureus 2022, 14, e21766. [Google Scholar] [CrossRef]
- Ichikawa, J.; Kawasaki, T.; Imada, H.; Wako, M.; Fujimaki, T.; Tatsuno, R.; Jubashi, T.; Haro, H. Case Report: Angiomatoid fibrous histiocytoma in the hand: A rare clinical presentation and diagnostic challenge. Front. Oncol. 2023, 13, 1280630. [Google Scholar] [CrossRef] [PubMed]
- Arneja, S.K.; Gujar, N. Renal cell carcinoma with t(6:11)(p21;q12). A case report highlighting distinctive immunohistologic features of this rare tumor. Int. J. Surg. Case Rep. 2015, 7C, 16–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, D.; Xiong, Y.; Wei, H.; Wang, S.; Ke, J.; Liang, P.; Zhang, H.; Yu, Y.; Zuo, Y.; Yang, L. Integrated analysis of ovarian cancer patients from prospective transcription factor activity reveals subtypes of prognostic significance. Heliyon 2023, 9, e16147. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.K.; Chen, Y.D.; Zheng, S. An integrated approach to the detection of colorectal cancer utilizing proteomics and bioinformatics. World J. Gastroenterol. 2004, 10, 3127–3131. [Google Scholar] [CrossRef]
- Menghi, F.; Orzan, F.N.; Eoli, M.; Farinotti, M.; Maderna, E.; Pisati, F.; Bianchessi, D.; Valletta, L.; Lodrini, S.; Galli, G.; et al. DNA microarray analysis identifies CKS2 and LEPR as potential markers of meningioma recurrence. Oncologist 2011, 16, 1440–1450. [Google Scholar] [CrossRef][Green Version]
- Allander, S.V.; Illei, P.B.; Chen, Y.; Antonescu, C.R.; Bittner, M.; Ladanyi, M.; Meltzer, P.S. Expression profiling of synovial sarcoma by cDNA microarrays: Association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am. J. Pathol. 2002, 161, 1587–1595. [Google Scholar] [CrossRef]
- Torres-Martin, M.; Lassaletta, L.; San-Roman-Montero, J.; De Campos, J.M.; Isla, A.; Gavilan, J.; Melendez, B.; Pinto, G.R.; Burbano, R.R.; Castresana, J.S.; et al. Microarray analysis of gene expression in vestibular schwannomas reveals SPP1/MET signaling pathway and androgen receptor deregulation. Int. J. Oncol. 2013, 42, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Tuan, J.; Pandha, H.; Corbishley, C.; Khoo, V. Basaloid carcinoma of the prostate: A literature review with case report. Indian J. Urol. 2012, 28, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wuthrick, E.; Blakaj, D.; Eroglu, Z.; Verschraegen, C.; Thapa, R.; Mills, M.; Dibs, K.; Liveringhouse, C.; Russell, J.; et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carci-noma: A randomised, open label, phase 2 trial. Lancet 2022, 400, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.G.; Blom, A.; Doumani, R.; Lewis, C.; Tarabadkar, E.S.; Anderson, A.; Ma, C.; Bestick, A.; Parvathaneni, U.; Bhatia, S.; et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016, 5, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.; Liu, S.; Johnson, B.; Prasad, S.; Mahvash, A.; Bhosale, P.; Rubin, M.L.; Rothschild, N.; Futreal, A.; Wistuba, I.I.; et al. 403MO Atezolizumab in combination with bevacizumab for patients with unresectable/metastatic anal cancer. Ann. Oncol. 2020, 31, S412. [Google Scholar] [CrossRef]
- Watanabe, K. Current chemotherapeutic approaches for hepatoblastoma. Int. J. Clin. Oncol. 2013, 18, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Casanova, M.; Chisholm, J.C.; Berlanga, P.; Chastagner, P.B.; Baruchel, S.; Amoroso, L.; Gallego Melcon, S.; Gerber, N.U.; Bisogno, G.; et al. Phase I results of a phase I/II study of weekly nab-paclitaxel in paediatric patients with recurrent/refractory solid tumours: A collaboration with innovative therapies for children with cancer. Eur. J. Cancer 2018, 100, 27–34. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Ntentas, G.; Darby, S.C.; Schaapveld, M.; Hauptmann, M.; Lugtenburg, P.J.; Janus, C.P.M.; Daniels, L.; van Leeuwen, F.E.; Cutter, D.J.; et al. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 2017, 129, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Triche, T., Jr.; Malvar, J.; Gaynon, P.; Sposto, R.; Yang, X.; Bittencourt, H.; Place, A.E.; Messinger, Y.; Fraser, C.; et al. A phase 1 study of azacitidine combined with chemotherapy in childhood leukemia: A report from the TACL consortium. Blood 2018, 131, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Slingluff, C.L.; Dasilva, D.; Schwarzenberger, P.; Ricciardi, T.; Macri, M.J.; Ryan, A.; Venhaus, R.R.; Bhardwaj, N. Phase 1/2 study of in situ vaccination with tremelimumab + intravenous (IV) durvalumab + poly-ICLC in patients with se-lect relapsed, advanced cancers with measurable, biopsy-accessible tumors. J. Clin. Oncol. 2018, 35, 15. [Google Scholar]
- Ao, Y.Q.; Gao, J.; Wang, S.; Jiang, J.H.; Deng, J.; Wang, H.K.; Xu, B.; Ding, J.Y. Immunotherapy of thymic epithelial tumors: Molecular understandings and clinical perspectives. Mol. Cancer 2023, 22, 70. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- McCrea, H.J.; Ivanidze, J.; O’Connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Gan, H.K.; Reardon, D.A.; Lassman, A.B.; Merrell, R.; van den Bent, M.; Butowski, N.; Lwin, Z.; Wheeler, H.; Fichtel, L.; Scott, A.M.; et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro-Oncology 2018, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Singh Achrol, A.; Aghi, M.K.; Bankiewicz, K.; Bexon, M.; Brem, S.; Brenner, A.; Chandhasin, C.; Chowdhary, S.; Coello, M.; et al. Targeting the IL4 receptor with MDNA55 in patients with recurrent glioblastoma: Results of a phase IIb trial. Neuro-Oncology 2023, 25, 1085–1097. [Google Scholar] [CrossRef]
- Ghisoli, M.; Barve, M.; Mennel, R.; Lenarsky, C.; Horvath, S.; Wallraven, G.; Pappen, B.O.; Whiting, S.; Rao, D.; Senzer, N.; et al. Three-year Follow up of GMCSF/bi-shRNA(furin) DNA-transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing’s Sarcoma. Mol. Ther. 2016, 24, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Biard, L.; Renaud, M.; Resche-Rigon, M.; Le Goff, J.; Dalle, S.; Heidelberger, V.; Da Meda, L.; Toullec, L.; Carcelain, G.; et al. PD-1 blockade with pembrolizumab in classic or endemic Kaposi’s sarcoma: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2022, 23, 491–500. [Google Scholar] [CrossRef]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; Maude, S.L.; Rives, S.; Hiramatsu, H.; Bittencourt, H.; Bader, P.; Baruchel, A.; Boyer, M.; De Moerloose, B.; Qayed, M.; et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J. Clin. Oncol. 2023, 41, 1664–1669. [Google Scholar] [CrossRef]
- Rao, S.; Anandappa, G.; Capdevila, J.; Dahan, L.; Evesque, L.; Kim, S.; Saunders, M.P.; Gilbert, D.C.; Jensen, L.H.; Samalin, E.; et al. A phase II study of retifanlimab (INCMGA00012) in patients with squamous carcinoma of the anal canal who have progressed following platinum-based chemotherapy (POD1UM-202). ESMO Open 2022, 7, 100529. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kizilbash, S.H.; Carlson, B.L.; Mladek, A.C.; Boakye-Agyeman, F.; Bakken, K.K.; Pokorny, J.L.; Schroeder, M.A.; Decker, P.A.; Cen, L.; et al. Delineation of MGMT Hypermethylation as a Biomarker for Veliparib-Mediated Temozolomide-Sensitizing Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108, djv369. [Google Scholar] [CrossRef]
- Koon, H.B.; Krown, S.E.; Lee, J.Y.; Honda, K.; Rapisuwon, S.; Wang, Z.; Aboulafia, D.; Reid, E.G.; Rudek, M.A.; Dezube, B.J.; et al. Phase II trial of imatinib in AIDS-associated Kaposi’s sarcoma: AIDS Malignancy Consortium Protocol 042. J. Clin. Oncol. 2014, 32, 402–408. [Google Scholar] [CrossRef]
- Rea, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.W.; Apperley, J.F.; Abdo, A.; et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood 2021, 138, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Devidas, M.; Wood, B.L.; Chen, Z.; Hayashi, R.J.; Hermiston, M.L.; Annett, R.D.; Archer, J.H.; Asselin, B.L.; August, K.J.; et al. Children’s Oncology Group Trial AALL1231: A Phase III Clinical Trial Testing Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia and Lymphoma. J. Clin. Oncol. 2022, 40, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Le Deley, M.C.; Dirksen, U.; Le Teuff, G.; Brennan, B.; Gaspar, N.; Hawkins, D.S.; Amler, S.; Bauer, S.; Bielack, S.; et al. High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J. Clin. Oncol. 2018, 36, JCO2018782516. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Yamanaka, T.; Taguchi, K.; Daga, H.; Ono, A.; Tanaka, K.; Kogure, Y.; Shimizu, J.; Kimura, T.; Fukuoka, J.; et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann. Oncol. 2015, 26, 363–368. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Clement, P.M.J.; Dirven, L.; Eoli, M.; Sepulveda-Sanchez, J.M.; Walenkamp, A.M.E.; Frenel, J.S.; Franceschi, E.; Weller, M.; Chinot, O.; De Vos, F.; et al. Impact of depatuxizumab mafodotin on health-related quality of life and neurological functioning in the phase II EORTC 1410/INTELLANCE 2 trial for EGFR-amplified recurrent glioblastoma. Eur. J. Cancer 2021, 147, 1–12. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro-Oncology 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Tobias, A.L.; Thaci, B.; Auffinger, B.; Rincon, E.; Balyasnikova, I.V.; Kim, C.K.; Han, Y.; Zhang, L.; Aboody, K.S.; Ahmed, A.U.; et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl. Med. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Katzenstein, H.M.; Malogolowkin, M.H.; Krailo, M.D.; Piao, J.; Towbin, A.J.; McCarville, M.B.; Tiao, G.M.; Dunn, S.P.; Langham, M.R.; McGahren, E.D.; et al. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: A Children’s Oncology Group study. Cancer 2022, 128, 1057–1065. [Google Scholar] [CrossRef]
- Guenther, L.M.; Dharia, N.V.; Ross, L.; Conway, A.; Robichaud, A.L.; Catlett, J.L., 2nd; Wechsler, C.S.; Frank, E.S.; Goodale, A.; Church, A.J.; et al. A Combination CDK4/6 and IGF1R Inhibitor Strategy for Ewing Sarcoma. Clin. Cancer Res. 2019, 25, 1343–1357. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 2145. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Sweet, K.; Atallah, E.L.; Radich, J.P.; Zhang, M.; Sahakian, E.; Mediavilla-Varela, M.; Vistocky, A.; Heinrich, M.C.; Thomp-son, J.E.; Mauro, M.J.; et al. Second Treatment Free Remission after Combination Therapy with Ruxolitinib Plus Tyrosine Kinase Inhibitors in Chronic Phase Chronic Myeloid Leukemia (CML). Blood 2021, 138, 2555. [Google Scholar] [CrossRef]
- Vicioso, Y.; Gram, H.; Beck, R.; Asthana, A.; Zhang, K.; Wong, D.P.; Letterio, J.; Parameswaran, R. Combination Therapy for Treating Advanced Drug-Resistant Acute Lymphoblastic Leukemia. Cancer Immunol. Res. 2019, 7, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Samaan, M.A.; Pavlidis, P.; Papa, S.; Powell, N.; Irving, P.M. Gastrointestinal toxicity of immune checkpoint inhibitors: From mechanisms to management. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.R.; Amato, C.M.; Hintzsche, J.; Robinson, W. Identification of somatic mutations and neoantigens to predict development of autoimmune adverse events to immune therapy in melanoma. J. Clin. Oncol. 2017, 35, 19. [Google Scholar] [CrossRef]
- Talpaz, M.; Hehlmann, R.; Quintas-Cardama, A.; Mercer, J.; Cortes, J. Re-emergence of interferon-alpha in the treatment of chronic myeloid leukemia. Leukemia 2013, 27, 803–812. [Google Scholar] [CrossRef]
- Jarmuzek, P.; Defort, P.; Kot, M.; Wawrzyniak-Gramacka, E.; Morawin, B.; Zembron-Lacny, A. Cytokine Profile in Development of Glioblastoma in Relation to Healthy Individuals. Int. J. Mol. Sci. 2023, 24, 16206. [Google Scholar] [CrossRef]
- Eng, C.; Fakih, M.; Amin, M.; Morris, V.; Hochster, H.S.; Boland, P.M.; Uronis, H. A phase II study of axalimogene filolisbac for patients with previously treated, unresectable, persistent/recurrent loco-regional or metastatic anal cancer. Oncotarget 2020, 11, 1334–1343. [Google Scholar] [CrossRef]
- Armeanu-Ebinger, S.; Hoh, A.; Wenz, J.; Fuchs, J. Targeting EpCAM (CD326) for immunotherapy in hepatoblastoma. Oncoimmunology 2013, 2, e22620. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Hijiya, N.; Maschan, A.; Rizzari, C.; Shimada, H.; Dufour, C.; Goto, H.; Kang, H.J.; Guinipero, T.; Karakas, Z.; Bautista, F.; et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia. Blood 2019, 134, 2036–2045. [Google Scholar] [CrossRef]
- Cortes, J.; Apperley, J.; Lomaia, E.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.A.; et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: A randomized, open-label phase 2 clinical trial. Blood 2021, 138, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hino, M.; Matsumura, I.; Fujisawa, S.; Ishizawa, K.; Sakaida, E.; Sekiguchi, N.; Ono, C.; Aizawa, M.; Tanetsugu, Y.; et al. Bosutinib in Japanese patients with newly diagnosed chronic-phase chronic myeloid leukemia: Final 3-year follow-up results of a phase 2 study. Int. J. Hematol. 2022, 116, 871–882. [Google Scholar] [CrossRef]
- Engert, F.; Schneider, C.; Weibeta, L.M.; Probst, M.; Fulda, S. PARP Inhibitors Sensitize Ewing Sarcoma Cells to Temozolomide-Induced Apoptosis via the Mitochondrial Pathway. Mol. Cancer Ther. 2015, 14, 2818–2830. [Google Scholar] [CrossRef] [PubMed]
- Muenchow, A.; Weller, S.; Hinterleitner, C.; Malenke, E.; Bugl, S.; Wirths, S.; Muller, M.R.; Schulze-Osthoff, K.; Aulitzky, W.E.; Kopp, H.G.; et al. The BCL-2 selective inhibitor ABT-199 sensitizes soft tissue sarcomas to proteasome inhibition by a concerted mechanism requiring BAX and NOXA. Cell Death Dis. 2020, 11, 701. [Google Scholar] [CrossRef]
- Chen, L.; Shi, H.; Zhang, W.; Zhu, Y.; Chen, H.; Wu, Z.; Qi, H.; Liu, J.; Zhong, M.; Shi, X.; et al. Carfilzomib suppressed LDHA-mediated metabolic reprogramming by targeting ATF3 in esophageal squamous cell carcinoma. Biochem. Pharmacol. 2024, 219, 115939. [Google Scholar] [CrossRef]
- Trerotola, M.; Relli, V.; Simeone, P.; Alberti, S. Epigenetic inheritance and the missing heritability. Hum. Genom. 2015, 9, 17. [Google Scholar] [CrossRef]
- Sahafnejad, Z.; Ramazi, S.; Allahverdi, A. An Update of Epigenetic Drugs for the Treatment of Cancers and Brain Diseases: A Comprehensive Review. Genes 2023, 14, 873. [Google Scholar] [CrossRef]
- Bellissimo, T.; Ganci, F.; Gallo, E.; Sacconi, A.; Tito, C.; De Angelis, L.; Pulito, C.; Masciarelli, S.; Diso, D.; Anile, M.; et al. Thymic Epithelial Tumors phenotype relies on miR-145-5p epigenetic regulation. Mol. Cancer 2017, 16, 88. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Tognon, M.; Martini, F. Epigenetic Dysregulations in Merkel Cell Polyomavirus-Driven Merkel Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 11464. [Google Scholar] [CrossRef] [PubMed]
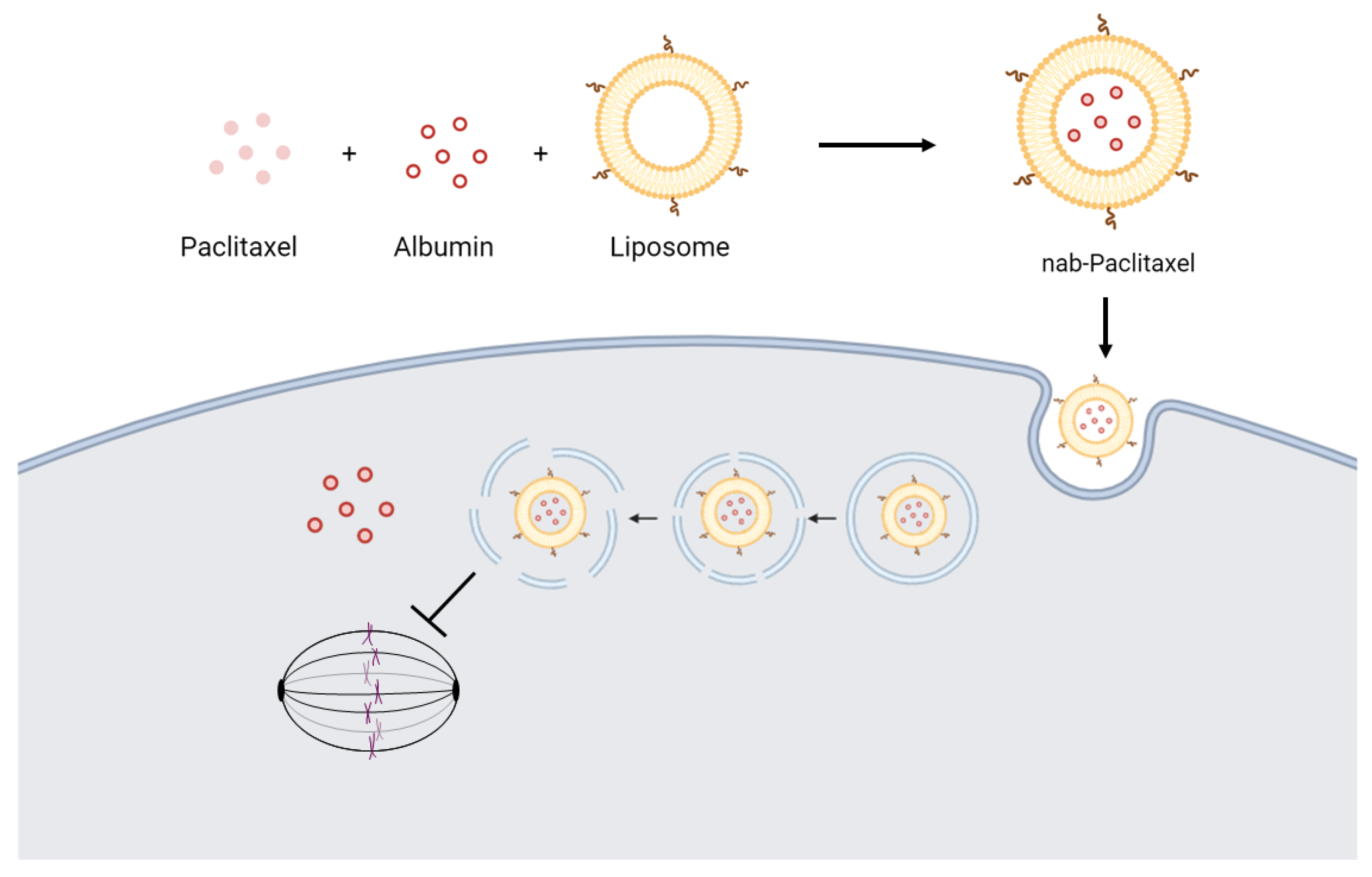
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf. B Biointerfaces 2023, 228, 113419. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Okamoto, Y.; Matsumoto, K.; Otagiri, M.; Chuang, V.T.G. When Albumin Meets Liposomes: A Feasible Drug Carrier for Biomedical Applications. Pharmaceuticals 2021, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Carvalho, M.A.; Castanheira, E.M.S. Functionalized Liposome and Albumin-Based Systems as Carriers for Poorly Water-Soluble Anticancer Drugs: An Updated Review. Biomedicines 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Taguchi, K.; Sakuragi, M.; Imoto, S.; Yamasaki, K.; Otagiri, M. Preparation, Characterization, and in Vitro/in Vivo Evaluation of Paclitaxel-Bound Albumin-Encapsulated Liposomes for the Treatment of Pancreatic Cancer. ACS Omega 2019, 4, 8693–8700. [Google Scholar] [CrossRef]
- Hamilton, B.K.; Liu, Y.; Hemmer, M.; Wang, T.; Chhabra, S.; Costa, L.J.; Kim, D.D.; Ringden, O.; Stuart, R.K.; Alousi, A.M.; et al. Cyclosporine in Combination with Mycophenolate Mofetil Leads to Increased Incidence of Graft-Versus-Host Disease and Inferior Outcomes after Myeloablative Allogeneic Hematopoietic Cell Transplantation: A CIBMTR Analysis. Biol. Blood Marrow Transplant. 2018, 24, S185. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; Porter, R.F.; Salter, J.T.; Yiannoutsos, C.; Burns, M.; Chiorean, E.G.; Loehrer, P.J., Sr. A Phase II Study of Pemetrexed in Patients with Recurrent Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2018, 13, 1940–1948. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Basu, S.; Parghane, R.V.; Kamaldeep; Chakrabarty, S. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front. Endocrinol. 2022, 13, 941832. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J. Clin. Oncol. 2018, 36, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Stary, J.; Zimmermann, M.; Campbell, M.; Castillo, L.; Dibar, E.; Donska, S.; Gonzalez, A.; Izraeli, S.; Janic, D.; Jazbec, J.; et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: Results of the randomized intercontinental trial ALL IC-BFM 2002. J. Clin. Oncol. 2014, 32, 174–184. [Google Scholar] [CrossRef]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
| Diagnostic Method | Description | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| DNA Microarray Analysis of Tumors |
|
|
| [10] |
| In Situ Hybridization |
|
|
| [11] |
| Flow Cytometry |
|
|
| [12] |
| Tumor Markers |
|
|
| [13] |
| Biopsy |
|
|
| [7] |
| Pressure Application |
|
|
| [14] |
| Fine Needle Aspiration Cytology (FNAC) and Core Needle Biopsy (CNB) |
|
|
| [14] |
| Histochemistry and Cytochemistry |
|
|
| [14] |
| Electron Microscopy |
|
|
| [14] |
| Immuno-histochemistry (IHC) |
|
|
| [14] |
| Histology |
|
|
| [15] |
| X-ray, Ultrasound, and Other Imaging Techniques |
|
|
| [15] |
| Diagnosis Technology | Tools | Examples |
|---|---|---|
| Genome sequencing | DNA-seq | |
| New generation sequencing | ||
| Third-generation sequencing | ||
| RNA-sequencing | Single-cell RNA-seq | |
| Massively parallel single-cell RNA-seq | ||
| Omics | Genomics | |
| Transcriptomics | ||
| Proteomics | ||
| Metabolomics | ||
| Integrated omics analysis | ||
| Liquid biopsies | ||
| Artificial intelligence and machine learning | ||
| Functional studies | Immunohistochemistry | |
| Flow cytometry | ||
| Fluorescence in situ hybridization | ||
| Functional magnetic resonance imaging (fMRI) | ||
| Positron emission tomography (PET) | ||
| Cell culture studies | ||
| Functional genomics | ||
| Integrated analysis | Genomic analysis
|
|
Transcriptomic analysis
| ||
Proteomic analysis
| ||
Epigenomic analysis
| ||
Imaging techniques
| ||
Clinical and pathological data
| ||
| Machine learning and bioinformatics |
| Challenges | Impact | Perspective |
|---|---|---|
| Low incidence | Small number of clinical samples | Intercontinental clinical trial collaboration for data collection |
| Termination of clinical trials due to a lack of participants | ||
| Lack of standardized protocol | Lack of homogeneity in the sample | Collaboration of healthcare professionals, researchers, and organizations in establishing international guidelines for rare cancer treatment |
| Delayed diagnosis due to rarity | Limited therapeutic options as the cancer advanced | The use of novel diagnosis tools and artificial intelligence integration in rare cancer diagnosis |
| Economic challenge in developing rare cancer drugs | Inadequate number of ongoing clinical trials | Orphan Drug Act pioneered by the U.S. Food and Drug Administration |
| Treatments | Rare Cancer Types | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Merkel Cell Carcinoma | Thymic Carcinoma | Glioblastoma Multiforme | Hepato-Blastoma | Ewing Sarcoma | Kaposi’s Sarcoma | Esophageal Cancer | Chronic Myeloid Leukemia | Acute Lymphoblastic Leukemia | Anal Cancer | |
| Surgery | Mohs micrographic surgery | VATS thymectomy and Robotic VATS | Fluorescence-Guided Surgery | Robot-assisted hepatectomy | Rotationplasty | Cryosurgery | Minimally invasive esophagectomy | Not Applicable | Not Applicable | Abdomino-perineal Resection (APR) |
| Radiation Therapy | Stereotactic body radiation therapy (SBRT) [70] | SBRT | Image-Guided Radiation Therapy | Not Applicable | Proton Therapy | Electron Beam Radiation Therapy | Chemo-radiation | Not Applicable | Not Applicable | Chemo-radiation Radio-frequency Ablation |
| Chemotherapy | Platinum + etoposide [71] | Alimta (Peme-trexed) [72] | Metronomic Temozolomide | Cisplatin, 5-fluorouracil, and Vincristine [73] | Nab-paclitaxel [74] | Liposomal Chemotherapy | Platin and Fluoro-pyrimidine | Daunorubicin [75] 5-Azacytidine [76] | Fludarabine, Cyclophos-phamide | |
| Immune Therapy | Poly-ICLC + Tremeli-mumab + Durvalumab [77] | PD-1/PD-L1 inhibitor [78] | Nivolumab [79] Cetuximab and Bevacizumab [80] ABT-414 [81] EGFRvIII CAR T Cells [82] MDNA55 [83] | EpCAM-specific monoclonal antibodies | Vigil [84] | Pembro-lizumab [85] | Pembro-lizumab [79] | IFN-α | Blinatumomab [86] CTL019 (Tisagenlecleucel) [87] | Retifanlimab (INCMGA00012) [88] Axalimogene Filolisbac (ADXS11-001) [50] |
| Targeted Therapy | Pazopanib + cabozantinib | Sorafenib | Veliparib [89] | Cabozantinib-S-Malate | Talazoparib, niraparib, olaparib, veliparib | Imatinib Mesylate [90] | EGFR VEGFR targeting agents | ABL001 [91] | Bortezomib [92] | Not Applicable |
| Transplant | Not Applicable | Not Applicable | Not Applicable | Liver transplantation | Autologous Stem Cell Transplantation [93] | Not Applicable | Not Applicable | Allogeneic Hematopoietic Stem Cell Transplantation | Allogeneic Hematopoietic Stem Cell Transplantation | Not Applicable |
| Combined Therapy | Nivolumab + Ipilimumab + SBRT [70] | Carboplatin + paclitaxel [94] Carboplatin + amrubicin | Oncolytic DNX-2401 virotherapy + pembrolizumab [95] ABT-414 + Temozolomide vs. Lomustine [96] Temozolomide + Radiation + Nivolumab [97] Oncolytic adenovirus + radiation + chemotherapy [98] | Cisplatin/5FU/ Vincristine [99] | CDK4/6 and IGF1R Inhibitor [100] | Valganciclovir and combined Antirretroviral Therapy (cART) [101] | Nivolumab + chemotherapy, nivolumab + ipilimumab [102] | Ruxolitinib + Tyrosine Kinase Inhibitors [103] | VAY736 antibody + EW-7197 [104] | 403MO Atezolizumab + bevacizumab [72] |
| Cancer Type | NCT Number | Phase | Treatment Arms | Ref. |
|---|---|---|---|---|
| Merkel cell carcinoma | NCT03071406 | Phase 2 | Nivolumab + Ipilimumab with or without SBRT | [105] |
| NCT02643303 | Phase 1 Phase 2 | Tremelimumab + IV Durvalumab + Poly-ICLC | [77] | |
| Thymic carcinoma | NCT03921671 | Phase 2 | Ramucirumab + Carbo-Paclitaxel | [94] |
| NCT00198133 | Phase 2 | Alimta (Pemetrexed) | [129] | |
| Glioblastoma multiforme | NCT02017717 | Phase 3 | Nivolumab | [106] |
| NCT01884740 | Phase 1 Phase 2 | Cetuximab + Bevacizumab | [80] | |
| NCT02573324 | Phase 3 | ABT-414 (Depatuxizumab mafodotin) | [81] | |
| NCT02858895 | Phase 2 | Convection-enhanced delivery of MDNA55 | [83] | |
| NCT02798406 | Phase 1 Phase 2 | Oncolytic DNX-2401 virotherapy plus pembrolizumab | [95] | |
| NCT02152982 | Phase 2 Phase 3 | Temozolomide with or without Veliparib | [89] | |
| NCT02343406 | Phase 2 | ABT-414 Alone or ABT-414 + Temozolomide vs. Lomustine or Temozolomide | [96] | |
| NCT02667587 | Phase 3 | Temozolomide + radiation therapy with Nivolumab | [97] | |
| NCT03072134 | Phase 1 | Neural stem cell-based virotherapy of newly diagnosed malignant glioma | [98] | |
| Hepatoblastoma | NCT02867592 | Phase 2 | Cabozantinib-S-Malate | [130] |
| NCT03698994 | Phase 2 | Ulixertinib | ||
| NCT03220035 | Phase 2 | Vemurafenib | ||
| NCT03213665 | Phase 2 | Tazemetostat | ||
| NCT00980460 | Phase 3 | Cisplatin/5FU/vincristine | [99] | |
| Ewing sarcoma | NCT01962103 | Phase 1 Phase 2 | Nab-paclitaxel | [74] |
| NCT02511132 | Phase 2 | Vigil | [84] | |
| Kaposi’s sarcoma | NCT00090987 | Phase 2 | Imatinib Mesylate | [90] |
| NCT03296553 | Phase 2 | Valganciclovir + cART | [101] | |
| Esophageal Cancer | NCT03189719 | Phase 3 | Chemo + Pembrolizumab | [79] |
| NCT03143153 | Phase 3 | Nivolumab + chemotherapy, nivolumab + the monoclonal antibody ipilimumab | [102] | |
| Chronic Myeloid Leukemia | NCT01844765 | Phase 2 | Nilotinib | [114] |
| NCT02467270 | Phase 2 | Ponatinib | [115] | |
| NCT03106779 | Phase 3 | ABL001 or Bosutinib | [91] | |
| NCT03128411 | Phase 2 | Bosutinib | [116] | |
| NCT03610971 | Phase 2 | Ruxolitinib + Tyrosine Kinase Inhibitors | [103] | |
| Childhood acute lymphoblastic leukemia | NCT04562792 | Phase 2 | Daunorubicin | [75] |
| NCT01861002 | Phase 1 | 5-Azacytidine | [76] | |
| NCT02101853 | Phase 3 | Blinatumomab | [86] | |
| NCT02435849 | Phase 2 | CTL019 (Tisagenlecleucel) | [87] | |
| NCT02112916 | Phase 3 | Combination chemotherapy with or without Bortezomib | [92] | |
| Anal cancer | NCT03597295 | Phase 2 | INCMGA00012 following platinum-based chemotherapy (POD1UM-202) | [88] |
| NCT02399813 | Phase 2 | Axalimogene Filolisbac (ADXS11-001) | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christyani, G.; Carswell, M.; Qin, S.; Kim, W. An Overview of Advances in Rare Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 1201. https://doi.org/10.3390/ijms25021201
Christyani G, Carswell M, Qin S, Kim W. An Overview of Advances in Rare Cancer Diagnosis and Treatment. International Journal of Molecular Sciences. 2024; 25(2):1201. https://doi.org/10.3390/ijms25021201
Chicago/Turabian StyleChristyani, Grania, Matthew Carswell, Sisi Qin, and Wootae Kim. 2024. "An Overview of Advances in Rare Cancer Diagnosis and Treatment" International Journal of Molecular Sciences 25, no. 2: 1201. https://doi.org/10.3390/ijms25021201
APA StyleChristyani, G., Carswell, M., Qin, S., & Kim, W. (2024). An Overview of Advances in Rare Cancer Diagnosis and Treatment. International Journal of Molecular Sciences, 25(2), 1201. https://doi.org/10.3390/ijms25021201






