Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review
Abstract
1. Introduction
2. Material and Methods
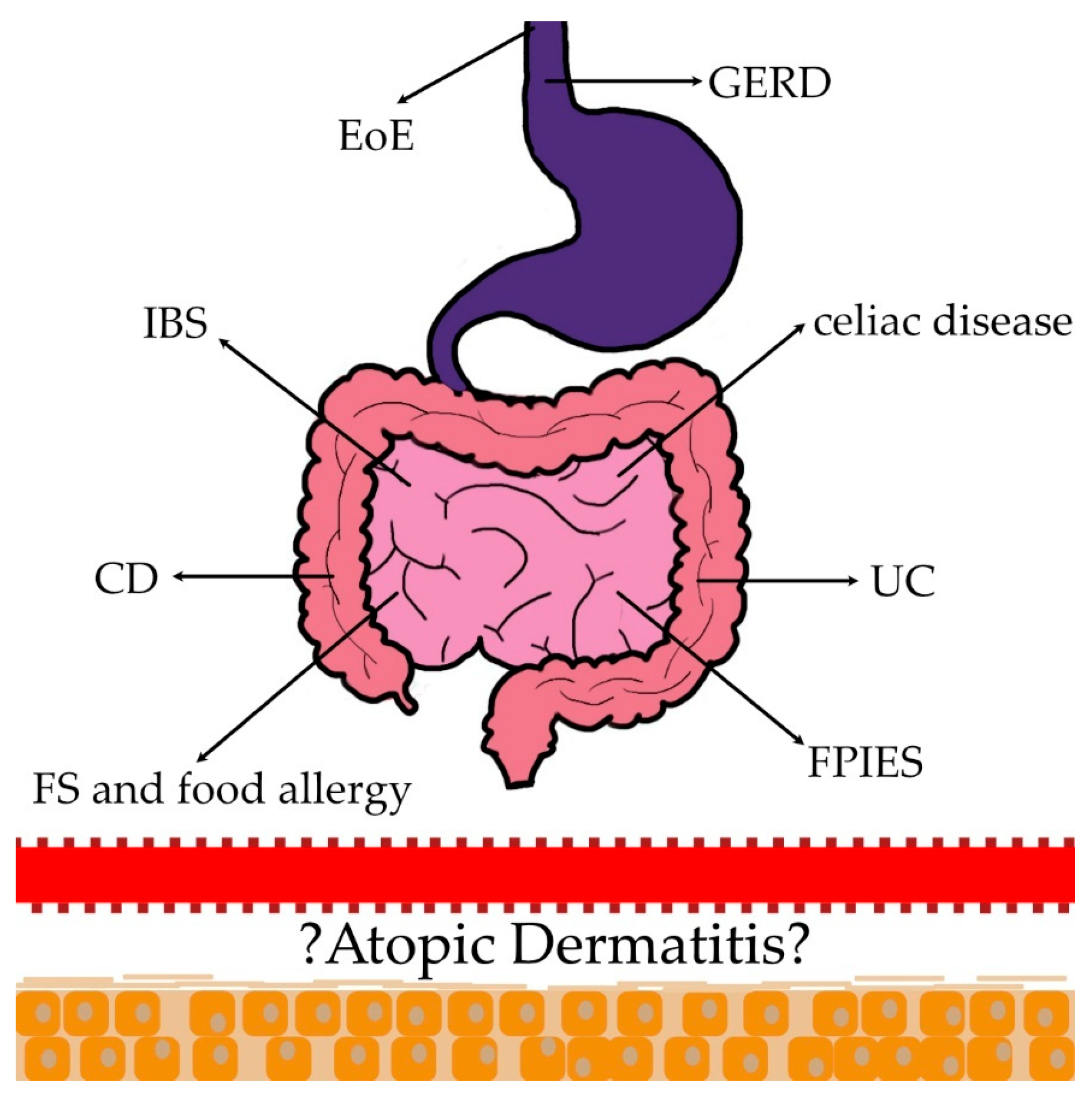
3. Discussion
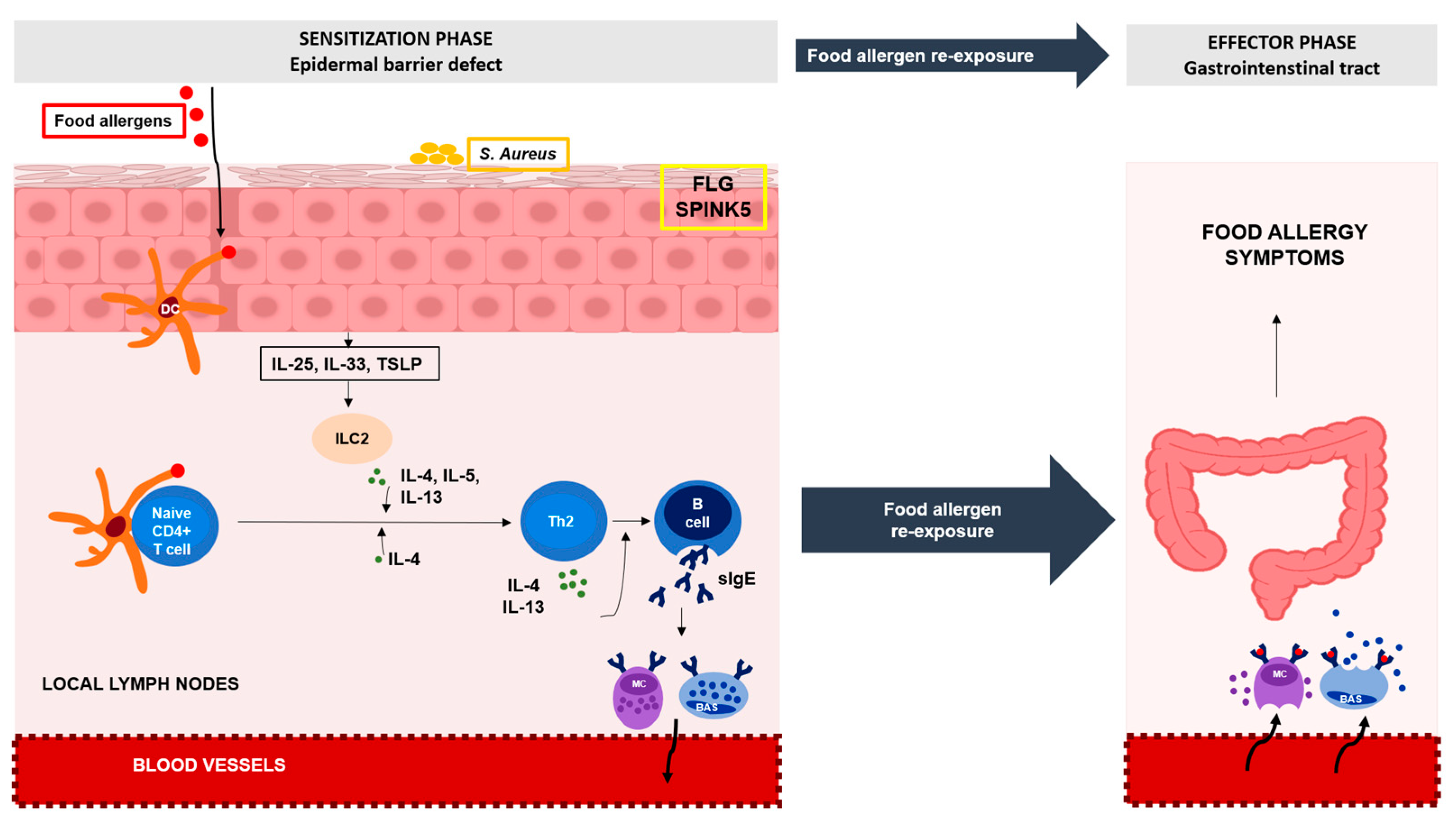
3.1. Food Sensitization (FS) and Food Allergy (FA)
3.2. Eosinophilic Esophagitis
3.3. Food Protein-Induced Enterocolitis Syndrome
3.4. Crohn’s Disease (CD) and Ulcerative Colitis (UC)
3.5. Celiac Disease
3.6. Irritable Bowel Syndrome (IBS)
3.7. Gastroesophageal Reflux Disease (GERD)
3.8. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, A.R.; Sandhu, V.K.; Drucker, A.M.; Fleming, P.; Lynde, C.W. Adult-Onset Atopic Dermatitis: Presentations and Progress. J. Cutan. Med. Surg. 2020, 24, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Patruno, C.; Potestio, L.; Napolitano, M. Clinical phenotypes of adult atopic dermatitis and related therapies. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef]
- Silverberg, J.I. Comorbidities and the impact of atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 144–151. [Google Scholar] [CrossRef]
- Mesjasz, A.; Zawadzka, M.; Chałubiński, M.; Trzeciak, M. Is Atopic Dermatitis Only a Skin Disease? Int. J. Mol. Sci. 2023, 24, 837. [Google Scholar] [CrossRef]
- Özer, M.; Aksoy, M.; Şimşek, M. Ocular complications of atopic dermatitis in children and adolescents. Alergol. Polska Pol. J. Allergol. 2022, 9, 133–138. [Google Scholar] [CrossRef]
- Wan, J.; Shin, D.B.; Syed, M.N.; Abuabara, K.; Lemeshow, A.R.; Fuxench, Z.C.C.; Gelfand, J.M. Malignancy risk in patients with atopic dermatitis: A population-based cohort study. Br. J. Dermatol. 2023, 189, 53–61. [Google Scholar] [CrossRef]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838. [Google Scholar] [CrossRef]
- Papapostolou, N.; Xepapadaki, P.; Gregoriou, S.; Makris, M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. J. Clin. Med. 2022, 11, 4232. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef]
- Eller, E.; Kjaer, H.F.; Høst, A.; Andersen, K.E.; Bindslev-Jensen, C. Food allergy and food sensitization in early childhood: Results from the DARC cohort. Allergy 2009, 64, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; the EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Perkin, M.; Logan, K.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.I.; Lack, G. Atopic Dermatitis and Disease Severity Are the Main Risk Factors for Food Sensitization in Exclusively Breastfed Infants. J. Investig. Dermatol. 2014, 134, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Eckert, J.K.; Koplin, J.J.; Lowe, A.J.; Gurrin, L.C.; Dharmage, S.C.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2015, 45, 255–264. [Google Scholar] [CrossRef]
- Yamamoto-Hanada, K.; Suzuki, Y.; Yang, L.; Saito-Abe, M.; Sato, M.; Mezawa, H.; Nishizato, M.; Kato, N.; Ito, Y.; Hashimoto, K.; et al. Persistent eczema leads to both impaired growth and food allergy: JECS birth cohort. PLoS ONE 2021, 16, e0260447. [Google Scholar] [CrossRef]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Factors Associated with the Development of Peanut Allergy in Childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Prim. 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Rajakulendran, M.; Lee, B.W.; Van Bever, H.P.S. Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know? Pediatr. Allergy Immunol. 2020, 31, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Curran-Everett, D.; Leung, D.Y. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1247–1248.e3. [Google Scholar] [CrossRef]
- O’Shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Spergel, J.M. The Immunologic Mechanisms of Eosinophilic Esophagitis. Curr. Allergy Asthma Rep. 2016, 16, 9. [Google Scholar] [CrossRef]
- González-Cervera, J.; Arias, Á.; Redondo-González, O.; Cano-Mollinedo, M.M.; Terreehorst, I.; Lucendo, A.J. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2017, 118, 582–590.e2. [Google Scholar] [CrossRef]
- Benninger, M.S.; Strohl, M.; Holy, C.E.; Hanick, A.L.; Bryson, P.C. Prevalence of atopic disease in patients with eosinophilic esophagitis. Int. Forum Allergy Rhinol. 2017, 7, 757–762. [Google Scholar] [CrossRef]
- Chehade, M.; Jones, S.M.; Pesek, R.D.; Burks, A.W.; Vickery, B.P.; Wood, R.A.; Leung, D.Y.; Furuta, G.T.; Fleischer, D.M.; Henning, A.K.; et al. Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population from the Consortium for Food Allergy Research. J. Allergy Clin. Immunol. Pract. 2018, 6, 1534–1544.e5. [Google Scholar] [CrossRef]
- Aw, M.; Penn, J.; Gauvreau, G.M.; Lima, H.; Sehmi, R. Atopic March: Collegium Internationale Allergologicum Update 2020. Int. Arch. Allergy Immunol. 2020, 181, 1–10. [Google Scholar] [CrossRef]
- Ochfeld, E.; Makhija, M.M. Eosinophilic Esophagitis: A review. LymphoSign J. 2017, 4, 119–135. [Google Scholar] [CrossRef]
- Hill, D.A.; Grundmeier, R.W.; Ramos, M.; Spergel, J.M. Eosinophilic Esophagitis is a Late Manifestation of the Atopic March. J. Allergy Clin. Immunol. 2018, 141, AB86. [Google Scholar] [CrossRef]
- Aceves, S.S. Food and aeroallergens in eosinophilic esophagitis. Curr. Opin. Gastroenterol. 2014, 30, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Dhami, S.; Sheikh, A. Estimating the prevalence of aero-allergy and/or food allergy in infants, children and young people with moderate-to-severe atopic eczema/dermatitis in primary care: Multi-centre, cross-sectional study. J. R. Soc. Med. 2015, 108, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; He, H.; Collins, M.H.; Abonia, J.; Myers, J.M.B.; Eby, M.; Johansson, H.; Kottyan, L.C.; Hershey, G.K.K.; Rothenberg, M.E. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J. Allergy Clin. Immunol. 2018, 141, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Page, B.; Vogel, M.; Bussmann, C.; Blanchard, C.; Straumann, A.; Simon, H. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid-treated eosinophilic esophagitis. Allergy 2018, 73, 239–247. [Google Scholar] [CrossRef]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Capucilli, P.; Hill, D.A. Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications. Clin. Rev. Allergy Immunol. 2019, 57, 111–127. [Google Scholar] [CrossRef]
- Agyemang, A.; Nowak-Wegrzyn, A. Food Protein-Induced Enterocolitis Syndrome: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 57, 261–271. [Google Scholar] [CrossRef]
- Leonard, S.A.; Pecora, V.; Fiocchi, A.G.; Nowak-Wegrzyn, A. Food protein-induced enterocolitis syndrome: A review of the new guidelines. World Allergy Organ. J. 2018, 11, 4. [Google Scholar] [CrossRef]
- Ruffner, M.A.; Wang, K.Y.; Dudley, J.W.; Cianferoni, A.; Grundmeier, R.W.; Spergel, J.M.; Brown-Whitehorn, T.F.; Hill, D.A. Elevated Atopic Comorbidity in Patients with Food Protein–Induced Enterocolitis. J. Allergy Clin. Immunol. Pract. 2019, 8, 1039–1046. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.H.; Brown-Whitehorn, T.F.; Cianferoni, A.; Schultz, F.; Warren, C.M.; Gupta, R.S. A population-based study of FPIES prevalence among US children. J. Allergy Clin. Immunol. 2019, 143, AB155. [Google Scholar] [CrossRef]
- Banerjee, A.; Wood, R.; Dantzer, J.; Dunlop, J.; Isola, J.; Keet, C. The Association of Food Protein-Induced Enterocolitis Syndrome (FPIES) with Personal and Familial Co-Morbidities. J. Allergy Clin. Immunol. 2022, 149, AB206. [Google Scholar] [CrossRef]
- Giusti, M.; Gasser, M.; Valentini, P.; Pescollderungg, L.; Eisendle, K. Food Protein-Induced Enterocolitis Syndrome in South Tyrol 2012–2016: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, E257–E259. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 918. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- de Lusignan, S.; Alexander, H.; Broderick, C.; Dennis, J.; McGovern, A.; Feeney, C.; Flohr, C. Atopic dermatitis and risk of autoimmune conditions: Population-based cohort study. J. Allergy Clin. Immunol. 2022, 150, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Schwarz, K.; Baurecht, H.; Hotze, M.; Fölster-Holst, R.; Rodríguez, E.; Lee, Y.A.; Franke, A.; Degenhardt, F.; Lieb, W.; et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J. Allergy Clin. Immunol. 2016, 137, 130–136. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, N.; Cheng, Y.; Chen, Y.; Li, Y.; Lu, Q.; Xia, Q.; Luo, D. Atopic dermatitis and risk of autoimmune diseases: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 96. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.H.; Koh, S.-J.; Park, H. Bidirectional relationship between atopic dermatitis and inflammatory bowel disease: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 83, 1385–1394. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Q.; Wang, F. The Bidirectional Association between Inflammatory Bowel Disease and Atopic Dermatitis: A Systematic Review and Meta-Analysis. Dermatology 2020, 236, 546–553. [Google Scholar] [CrossRef]
- Weng, Y.; Juan, C.; Ho, H.J.; Chang, Y.; Wu, C.; Chen, Y. Atopic dermatitis does not increase the risk of inflammatory bowel disease: A nationwide cohort study. J. Dermatol. 2021, 48, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, M.C.; Kirchgesner, J.; Wyss, R.; Jin, Y.; York, C.; Merola, J.F.; Mostaghimi, A.; Silverberg, J.I.; Schneeweiss, S.; Glynn, R.J. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: A cohort study. Br. J. Dermatol. 2022, 187, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Sumi, H.; Akamatsu, H. An association between ulcerative colitis and atopic dermatitis, diseases of impaired superficial barriers. J. Investig. Dermatol. 2004, 123, 999–1000. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Nasanbat, B.; Uchiyama, A.; Amalia, S.N.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; Motegi, S.-I. Kaempferol therapy improved MC903 induced-atopic dermatitis in a mouse by suppressing TSLP, oxidative stress, and type 2 inflammation. J. Dermatol. Sci. 2023, 111, 93–100. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.E.A.; Wijmenga, C. Coeliac disease and autoimmune disease—Genetic overlap and screening. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 507–515. [Google Scholar] [CrossRef]
- Shalom, G.; Kridin, K.; Raviv, K.-O.; Freud, T.; Comaneshter, D.; Friedland, R.; Cohen, A.D.; Ben-Amitai, D. Atopic Dermatitis and Celiac Disease: A Cross-Sectional Study of 116,816 Patients. Am. J. Clin. Dermatol. 2020, 21, 133–138. [Google Scholar] [CrossRef]
- Ivert, L.; Wahlgren, C.; Lindelöf, B.; Dal, H.; Bradley, M.; Johansson, E. Association between atopic dermatitis and autoimmune diseases: A population-based case–control study. Br. J. Dermatol. 2021, 185, 335–342. [Google Scholar] [CrossRef]
- Ress, K.; Annus, T.; Putnik, U.; Luts, K.; Uibo, R.; Uibo, O. Celiac Disease in Children with Atopic Dermatitis. Pediatr. Dermatol. 2014, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Pérez-Gilaberte, J.B.; Poblador-Plou, B.; Bliek-Bueno, K.; Gimeno-Miguel, A.; Prados-Torres, A. Prevalence and Comorbidity of Atopic Dermatitis in Children: A Large-Scale Population Study Based on Real-World Data. J. Clin. Med. 2020, 9, 1632. [Google Scholar] [CrossRef]
- Tsai, J.-D.; Wang, I.-C.; Shen, T.-C.; Lin, C.-L.; Wei, C.-C. A 8-year population-based cohort study of irritable bowel syndrome in childhood with history of atopic dermatitis. J. Investig. Med. 2018, 66, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kaya İslamoğlu, Z.G.; Unal, M.; Küçük, A. Atopic dermatitis in adults and irritable bowel syndrome: A cross-sectional study. Indian J. Dermatol. 2019, 64, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, Ö.; Balta, I.; Özuğuz, P.; Şen, B.; Rifaioğlu, E.; Ekiz, F.; Yüksel, I.; Çoban, Ş.; Başar, Ö. Irritable bowel syndrome in patients with chronic pruritus of undetermined origin. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Zhao, Y.; Liu, X.; Chang, C.; Zhang, J. Molecular Biology of Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2014, 47, 193–218. [Google Scholar] [CrossRef]
- Walker, M.M.; Warwick, A.; Ung, C.; Talley, N.J. The role of eosinophils and mast cells in intestinal functional disease. Curr. Gastroenterol. Rep. 2011, 13, 323–330. [Google Scholar] [CrossRef]
- Walker, M.M.; Talley, N.J.; Prabhakar, M.; Pennaneac’h, C.J.; Aro, P.; Ronkainen, J.; Storskrubb, T.; Harmsen, W.S.; Zinsmeister, A.R.; Agreus, L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment. Pharmacol. Ther. 2009, 29, 765–773. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14339. [Google Scholar] [CrossRef]
- Camilleri, M.; Carlson, P.; McKinzie, S.; Zucchelli, M.; D’amato, M.; Busciglio, I.; Burton, D.; Zinsmeister, A.R. Genetic susceptibility to inflammation and colonic transit in lower functional gastrointestinal disorders: Preliminary analysis. Neurogastroenterol. Motil. 2011, 23, 935-e398. [Google Scholar] [CrossRef]
- Bjorkman, D.J.; Popp, J.W. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am. J. Gastroenterol. 2006, 101, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Vicario, M.; Ramos, L.; Lobo, B.; Mosquera, J.L.; Alonso, C.; Sánchez, A.; Guilarte, M.; Antolín, M.; de Torres, I.; et al. The Jejunum of Diarrhea-Predominant Irritable Bowel Syndrome Shows Molecular Alterations in the Tight Junction Signaling Pathway That Are Associated with Mucosal Pathobiology and Clinical Manifestations. Am. J. Gastroenterol. 2012, 107, 736–746. [Google Scholar] [CrossRef]
- Piche, T. Tight junctions and IBS—the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol. Motil. 2014, 26, 296–302. [Google Scholar] [CrossRef]
- Trautmann, A.; Altznauer, F.; Akdis, M.; Simon, H.-U.; Blaser, K.; Akdis, C.A.; Disch, R.; Bröcker, E.-B. The Differential Fate of Cadherins during T-Cell-Induced Keratinocyte Apoptosis Leads to Spongiosis in Eczematous Dermatitis. J. Investig. Dermatol. 2001, 117, 927–934. [Google Scholar] [CrossRef]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal Reflux Disease. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.E. Gastroesophageal reflux disease and asthma: The two are directly related. Am. J. Med. 2000, 108, 153–158. [Google Scholar] [CrossRef]
- Feng, M.-C.; Tsai, Y.-G.; Chang, Y.-H.; Kuo, C.-H.; Lin, Y.-C.; Hung, C.-H. Allergic rhinitis as a key factor for the development of gastroesophageal reflux disease in children. J. Microbiol. Immunol. Infect. 2021, 54, 1167–1174. [Google Scholar] [CrossRef]
- Kung, Y.-M.; Tsai, P.-Y.; Chang, Y.-H.; Wang, Y.-K.; Hsieh, M.-S.; Hung, C.-H.; Kuo, C.-H. Allergic rhinitis is a risk factor of gastro-esophageal reflux disease regardless of the presence of asthma. Sci. Rep. 2019, 9, 15535. [Google Scholar] [CrossRef]
- Ahn, K.; Penn, R.B.; Rattan, S.; Panettieri, R.A.; Voight, B.F.; An, S.S. Mendelian Randomization Analysis Reveals a Complex Genetic Interplay among Atopic Dermatitis, Asthma, and Gastroesophageal Reflux Disease. Am. J. Respir. Crit. Care Med. 2023, 207, 130–137. [Google Scholar] [CrossRef]
- Brew, B.K.; Almqvist, C.; Lundholm, C.; Andreasson, A.; Lehto, K.; Talley, N.J.; Gong, T. Comorbidity of atopic diseases and gastro-oesophageal reflux: Evidence of a shared cause. Clin. Exp. Allergy 2022, 52, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Cavagni, G.; Deriu, F.M.; Zanotti, P.; Atherton, D.J. Gastrointestinal symptoms in atopic eczema. Arch. Dis. Child. 1998, 78, 230–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smirnova, J.; Montgomery, S.; Lindberg, M.; Svensson, Å.; von Kobyletzki, L. Associations of self-reported atopic dermatitis with comorbid conditions in adults: A population-based cross-sectional study. BMC Dermatol. 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Grieco, T.; Caviglia, M.; Cusano, G.; Sernicola, A.; Chello, C.; Del Duca, E.; Cantisani, C.; Taliano, A.; Sini, N.; Ianiro, G.; et al. Atopic Dermatitis and Ulcerative Colitis Successfully Treated with Upadacitinib. Medicina 2023, 59, 542. [Google Scholar] [CrossRef]
- Pessôa, R.; Clissa, P.B.; Sanabani, S.S. The Interaction between the Host Genome, Epigenome, and the Gut–Skin Axis Microbiome in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 14322. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, L.; Chen, Y.; Wang, H.; Xie, J. Gut microbiota and atopic dermatitis: A two-sample Mendelian randomization study. Front. Med. 2023, 10, 1174331. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Wang, Z.; Xu, X.; Wen, H.; Su, H.; Han, Y.; Luo, Y.; Zhang, Y.; Li, W.; et al. Differential changes in the gut microbiota between extrinsic and intrinsic atopic dermatitis. J. Autoimmun. 2023, 141, 103096. [Google Scholar] [CrossRef]
| Gastrointestinal Disease | OR | RR | 95% Cl | p-Value |
|---|---|---|---|---|
| The incidence of FS in AD infants [12] | 6.18 | 2.94–12.98 | p < 0.001 | |
| The incidence of FS to milk, raw egg, cod, sesame, and peanut in AD infants with a SCORAD > 20 [16] | 25.60 | 9.03–72.57 | p < 0.001 | |
| The incidence of FS to milk, raw egg, cod, sesame, and peanut in AD infant AD with a SCORAD < 20 [16] | 3.91 | 1.70–9.00 | p = 0.001 | |
| The incidence of AD in patients with EoE [26] | 2.85 | 1.87–4.34 | Not provided | |
| The incidence of AD in patients with IBDs [49] | 1.39 | 1.28–1.50 | p < 0.01 | |
| The incidence of IBDs in patients with AD [49] | 1.35 | 1.05–1.73 | p < 0.01 | |
| The incidence of CD in AD patients [48] | 1.66 | 1.50–1.84 | p = 0.374 | |
| The incidence of CD in AD patients [48] | 1.38 | 1.17–1.63 | p = 0.426 | |
| The incidence of CD in patients with AD [49] | 1.14 | 0.60–2.15 | ns | |
| The incidence of AD in patients with CD [50] | 2.06 | 1.61–2.64 | p < 0.01 | |
| The incidence of AD in patients with CD [49] | 1.69 | 1.51–1.89 | Not provided | |
| The incidence of AD in patients with UC [49] | 1.23 | 1.11–1.35 | Not provided | |
| The incidence of UC in patients with AD [49] | 1.53 | 1.07–2.18 | Not provided | |
| The incidence of UC in patients with AD [49] | 1.11 | 0.88–1.44 | ns | |
| The incidence of UC in patients with AD [48] | 1.95 | 1.57–2.44 | p = 0.009 | |
| The incidence of UC in patients with AD [48] | 1.49 | 1.05–2.11 | p = 0.196 | |
| The incidence of UC in patients with AD [50] | 1.48 | 1.06–2.04 | p < 0.05 | |
| The incidence of celiac disease in AD [48] | 1.98 | 1.51–2.60 | Not provided | |
| The incidence of celiac disease in AD [59] | 1.609 | 1.42–1.82 | p < 0.001 | |
| The incidence of celiac disease in AD children [61] | 4.18 | 1.12–15.64 | Not provided | |
| The incidence of IBS in AD children [63] | 1.90 | 1.56–2.31 | p < 0.001 | |
| The incidence of GERD in AD patients [82] | 1.23 | 1.10–1.38 | Not provided |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zysk, W.; Mesjasz, A.; Trzeciak, M.; Horvath, A.; Plata-Nazar, K. Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1194. https://doi.org/10.3390/ijms25021194
Zysk W, Mesjasz A, Trzeciak M, Horvath A, Plata-Nazar K. Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review. International Journal of Molecular Sciences. 2024; 25(2):1194. https://doi.org/10.3390/ijms25021194
Chicago/Turabian StyleZysk, Weronika, Alicja Mesjasz, Magdalena Trzeciak, Andrea Horvath, and Katarzyna Plata-Nazar. 2024. "Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review" International Journal of Molecular Sciences 25, no. 2: 1194. https://doi.org/10.3390/ijms25021194
APA StyleZysk, W., Mesjasz, A., Trzeciak, M., Horvath, A., & Plata-Nazar, K. (2024). Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review. International Journal of Molecular Sciences, 25(2), 1194. https://doi.org/10.3390/ijms25021194





