1. Background
A diploid human genome contains ~100 full-length copies of retrotransposition competent LINE-1 retroelements (
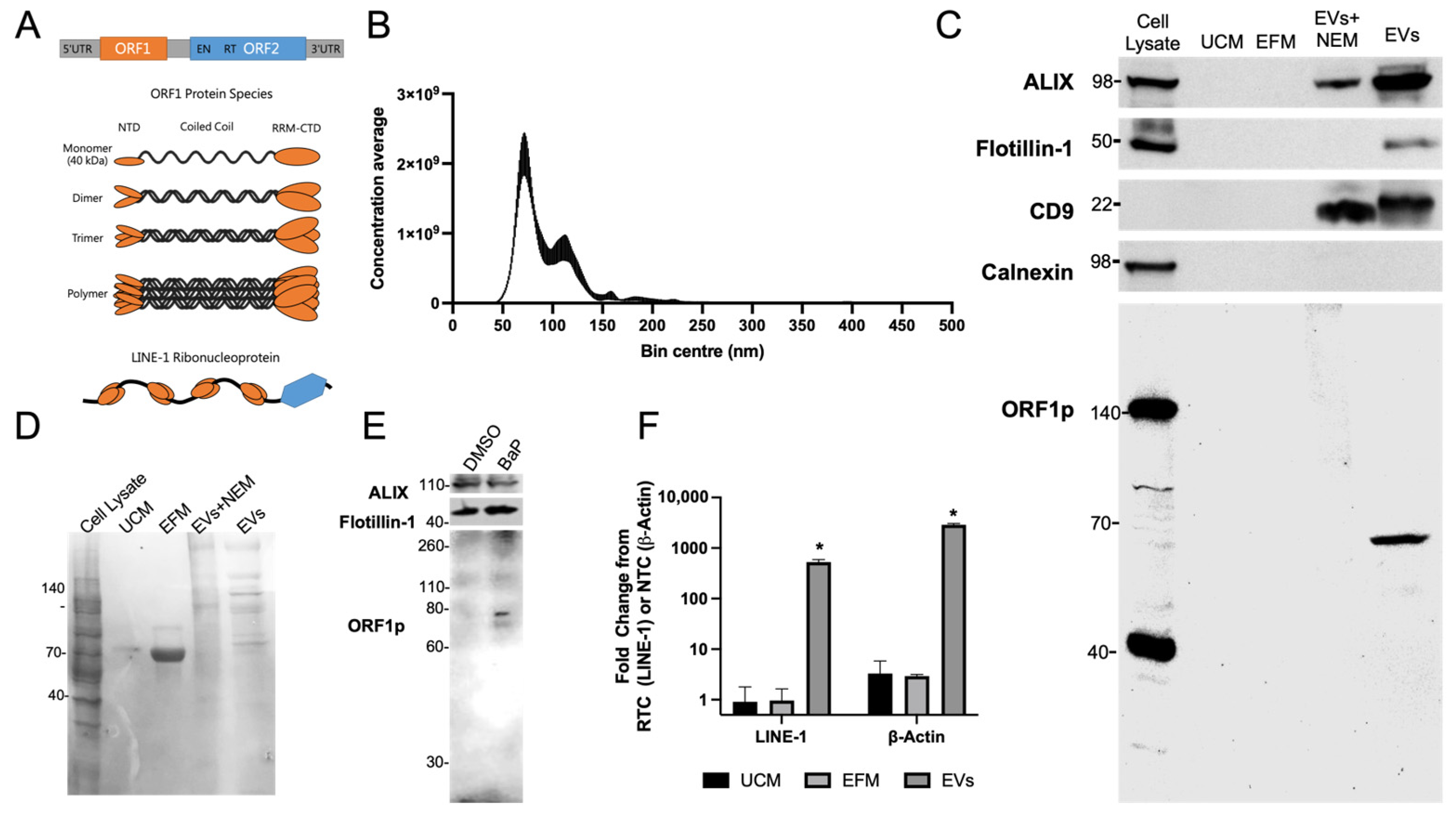
Figure 1A) [
1]. These retroelements are silenced epigenetically in nearly all healthy somatic cells by the interplay between DNA methylation and histone covalent modifications, a process orchestrated in part by retinoblastoma proteins and the NuRD corepressor complex [
2]. Disruption of epigenetic silencing can unleash LINE-1 retrotransposition, which entails propagation of LINE-1 DNA, or other DNAs, through a copy-and-paste mechanism using an RNA intermediate. LINE-encoded proteins (ORF1p and ORF2p) exhibit
cis- or
trans-preference and bind mRNAs to form ribonucleoprotein particles (RNPs). These particles mainly localize to the cytoplasm or are stored in stress granules [
3], but can also translocate to the nucleus where the endonuclease domain of ORF2p nicks a single strand of genomic DNA to expose a 3′-OH group that is used to prime and synthesize LINE-1 cDNA. This can lead to full-length or truncated insertions of LINE-1 or other sequences that modulate genome architecture and function.
The reactivation of LINE-1 retroelements can occur in several different contexts, particularly following DNA damage or disruption of epigenetic control by inflammation and oxidative injury [
4,
5]. Malignant transformation of somatic cells can also give rise to epigenetic disturbances that erode LINE-1 silencing and allow uncontrolled expression and accumulation of LINE-1 products [
6,
7]. Active LINEs are a source of endogenous mutagenesis, with reactivation in somatic cells causing a variety of genetic alterations, including aberrant splicing, exon skipping, gene fusions, and genome rearrangements that alter gene expression and cause genome instability [
8,
9,
10]. Furthermore, LINE-1 reactivation creates a positive feedback loop that perpetuates the aberrant behavior of cells carrying mutations in tumor suppressor genes and/or oncogenes [
11]. LINE-1 oncogenicity can also involve signaling pathways that are independent of retrotransposition [
12].
Given the multifaceted roles of LINE-1 in cancer, we and others have postulated that readouts of LINE-1 activity may serve as indicators of oncogenic transformation. This hypothesis is supported by the strong correlation between LINE-1 expression and malignancy [
6,
13,
14], tumor genomic instability [
7,
10], and cancer mortality [
7,
15]. LINE-1 DNA hypomethylation (i.e., activation) is also a common feature across many different cancer types [
16], and correlates with poor clinical outcomes and lung cancer mortality [
7,
15]. The clinical utility of LINE-1 methylation status is limited because it depends on the direct testing of tissue biopsies using low-throughput technologies.
Molecular biomarkers are sorely needed for lung cancer detection, especially because most cases are detected when curative interventions are no longer a viable option. Further, current screening with low-dose computerized tomography (LD-CT) is fraught with high false-positive rates leaving patients and providers with challenging follow-up decisions [
17]. Non-small cell lung cancers (NSCLCs) are strongly impacted by LINE-1 dysregulation. LINE-1 reactivation is prevalent during early stage NSCLCs, especially in smokers [
6,
7,
18], and the genome of NSCLC is strongly affected by LINE-1 insertions [
13,
19]. Thus, to harness the diagnostic potential of LINE-1 we have focused efforts on measurements of total plasma LINE-1 or LINE-1 analytes loaded onto extracellular vesicles (EVs) as lung cancer biomarkers. EVs are secreted membrane-bound vesicles containing curated DNA, RNA, and proteins from their cells of origin [
20]. EVs are released by nearly all cell types and can be collected from saliva, blood, urine, and cerebrospinal fluid [
21]. While the presence of LINE-1 products in EVs has been documented by us and others [
22,
23,
24], detailed analyses to determine if LINE-1 products in EVs can be used for cancer detection and future development of point-of-care diagnostics have not been systematically completed.
With these goals in mind, we used a panel of transformed and non-transformed lung epithelial cell lines and human plasma to define the relationship between cellular and EV LINE-1 contents and to evaluate LINE-1 profiles in healthy and diseased states. Here, we report that the LINE-1 cargo in EVs isolated from conditioned media paralleled LINE-1 levels in the cells of origin, under both constitutive and carcinogen-inducible conditions. Among ostensibly healthy subjects, plasma EV LINE-1 content was higher in females than males and in African Americans compared to Hispanic Americans. Among subjects with NSCLC, considerable heterogeneity in plasma EV LINE-1 levels was observed when stratified by cancer stage, race, sex, and tumor type. Further, ORF1p levels in whole plasma using an ELISA platform closely approximated ORF1p and LINE-1 mRNA levels in Evs, indicating that the majority of circulating LINE-1 is contained in Evs. We conclude that measurements of LINE-1 analytes in Evs and plasma may be of value as liquid biopsies to monitor tissue level expression and activity of LINE-1 retroelements.
2. Results
To characterize EVs released by H520 lung cancer cells, EVs were isolated by PEG precipitation of conditioned media for 48 h. H520 cells have high constitutive expression of LINE-1 [
24]. EV isolates ranged in diameter from 50 to 225 nm, with most particles concentrating at ~80 or 110 nm (
Figure 1B), a range consistent with EVs of endocytic origin, mainly exosomes. The preparations were free of contamination with particles of a high diameter. EV protein profiles were examined by Western blotting, using unconditioned medium (UCM) and EV-free medium (EFM) as negative controls and total cell lysate as a reference control (
Figure 1C). Traces of contaminating protein in EFM were removed by centrifugation coupled with several PEG resuspensions in fresh PBS as evidenced by the low abundance of ORF1p in EFM compared to the enriched EV sample. The exosome markers ALIX, Flotillin-1, and CD9 were enriched in EV fractions and absent in UCM and EFM. The absence of calnexin, a protein enriched in the endoplasmic reticulum, further validated the high quality of the EV preparations.
Next, we examined the LINE-1 ORF1p content in EVs. ORF1p is a 40 kDa protein that preferentially forms multimers resistant to denaturation under reducing SDS-PAGE conditions (
Figure 1A) [
25,
26]. The most abundant ORF1p species detected in H520 cell lysates were the monomeric and trimeric forms, while the dimeric form was predominant in EVs. These results are consistent with previous findings showing the ORF1p dimer in EV buoyant density gradients [
24]. N-ethyl maleimide treatment did not enhance ORF1p detection under reducing conditions. Protein loading was verified by Ponceau staining (
Figure 1D). Western blotting of EV isolates from H460 cells, another NSCLC cell line, challenged with the LINE-1 inducer benzo[a]pyrene (BaP), also yielded an ORF1p dimer in EVs (
Figure 1E), confirming ORF1p EV export in two different NSCLC cell lines.
To evaluate the presence of LINE-1 mRNA in EVs, intact H520 EVs were treated with RNAseA to remove contaminating RNA prior to lysis and isolation (
Figure 1F). β-Actin mRNA was used as a positive control. RNA was also collected from UCM and EFM to monitor background levels. LINE-1 contains no introns; thus, LINE-1 primers cannot distinguish genomic DNA from cDNA. Thus, each LINE-1 sample was normalized to a matched control lacking reverse transcriptase (RTC) to account for residual gDNA signal. As the exon-junction spanning β-Actin primers do not amplify gDNA, the signal was normalized to a non-template control (NTC). The presence of both β-Actin and LINE-1 mRNA was confirmed in EVs, with significant elevations of LINE-1 (526 ± 67.2 FC) detected over the background and β-Actin (2887.6 ± 182 FC) (
p < 0.05). LINE-1 and β-Actin mRNAs were not enriched in the EFM and UCM.
2.1. Profiles of LINE-1 Abundance in NSCLC Cells and Their Corresponding EVs under Constitutive Conditions
We next determined whether EV LINE-1 content serves as a proxy of cellular LINE-1 levels (
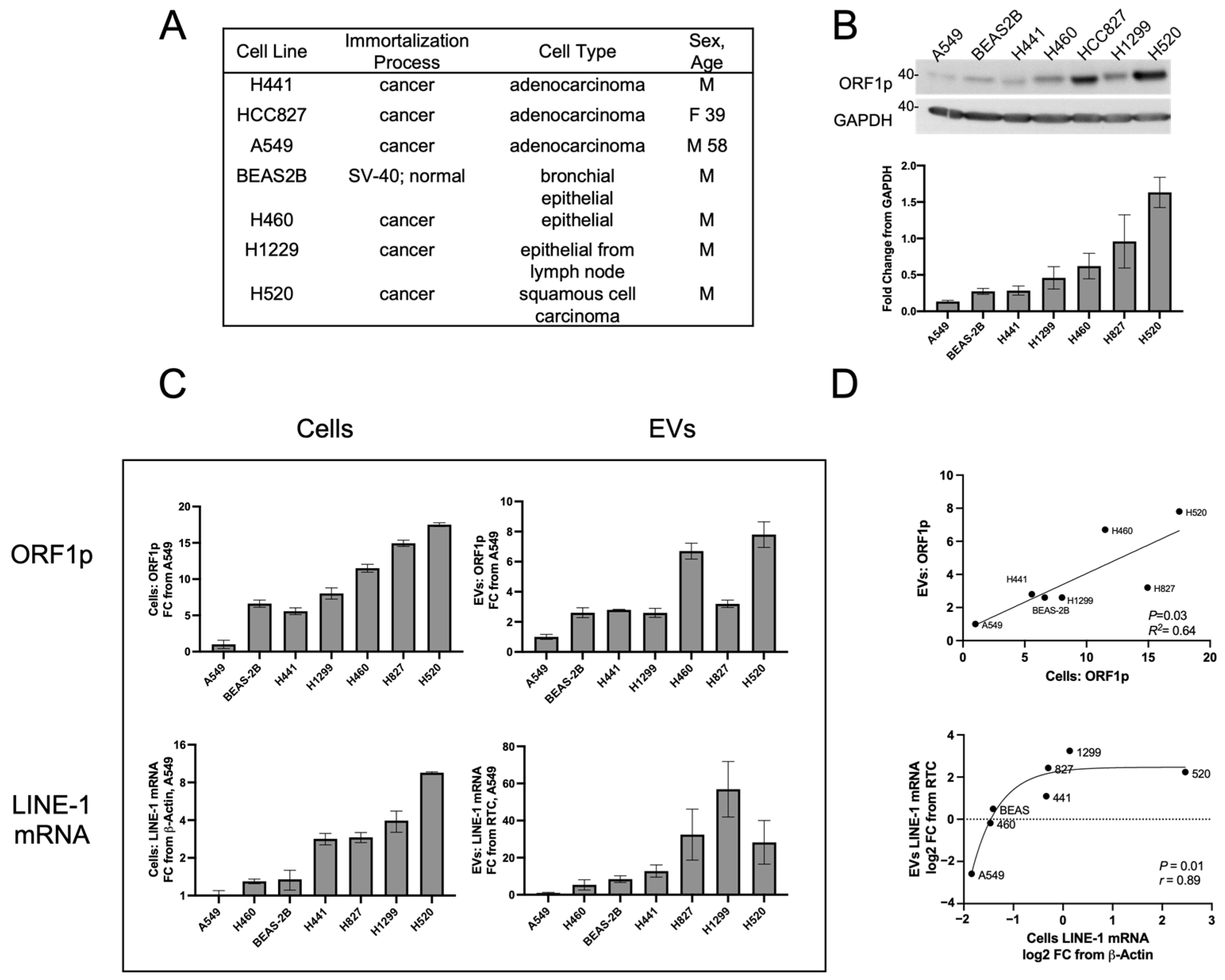
Figure 2). A panel of cells that included the non-transformed bronchial epithelial line, BEAS-2B, along with several NSCLC epithelial cell lines was examined (
Figure 2A). Cells were allowed to condition EV-depleted media for 48 h before the collection of cell and media fractions. Western blotting showed constitutive ORF1p expression across all lines (
Figure 2B). A549 cells exhibited the lowest levels of ORF1p, followed in ascending order by BEAS-2B, H441, H1299, H460, H827, and H520 cell lines. As A549 cells consistently exhibited the lowest LINE-1 levels, all subsequent LINE-1 measurements were expressed as a fold change relative to A549 cells or A549 EVs.
The next series of studies relied on PEG precipitation for EV isolation in order to facilitate comparisons between cells and plasma. PEG precipitation is incompatible with SDS-PAGE [
27] (
Figure S1), and therefore ELISA was used to measure ORF1p levels (
Figure 2C, top row). ORF1p measures in cell lysates using the ELISA platform displayed rank order profiles comparable to those seen by Western blotting (
Figure 2B). The BEAS-2B, H441, H1299, and H827 cell lines showed moderate ORF1p levels in EVs ranging from 2.6 to 3.2 FC relative to A549 EVs. The H460 and H520 cell lines had the highest ORF1p content with 6.7 and 7.8 FC, respectively. The linear regression showed a significant relationship between cellular and EV ORF1p content (
Figure 2D, top,
p = 0.03,
R2 = 0.64), indicating that ORF1p content between cells and their corresponding EVs is proportional.
Parallel analyses of LINE-1 mRNA expression in cells and Evs (
Figure 2C, bottom row) showed A549 cells to have the lowest expression levels, followed by H460 cells 1.30 FC, BEAS-2B (1.35), H441 (2.84), H827 (2.92), H1299 (3.97), and H520 (9.57). In Evs, H460 cells exhibited 5.29-fold enrichment relative to A549 followed by BEAS-2B (8.44), H441 (12.79), H520 (28.26), H827 (32.44), and H1299 (56.89). Log2 transformation followed by non-linear Spearman’s regression (
Figure 2D, bottom) showed a significant relationship (
r = 0.89,
p = 0.01) between these two variables. Curve fitting yielded a sigmoidal relationship (
r = 0.90) which may be artifactually driven by H520 cells or, alternatively, implicate loading constraints of LINE-1 mRNA in cells with high LINE-1 content. Together, these results demonstrate that constitutive expression of LINE-1 in cells can be approximated by measurements of EV LINE-1 cargo.
2.2. Cellular and EV LINE-1 Levels under Inducible Conditions
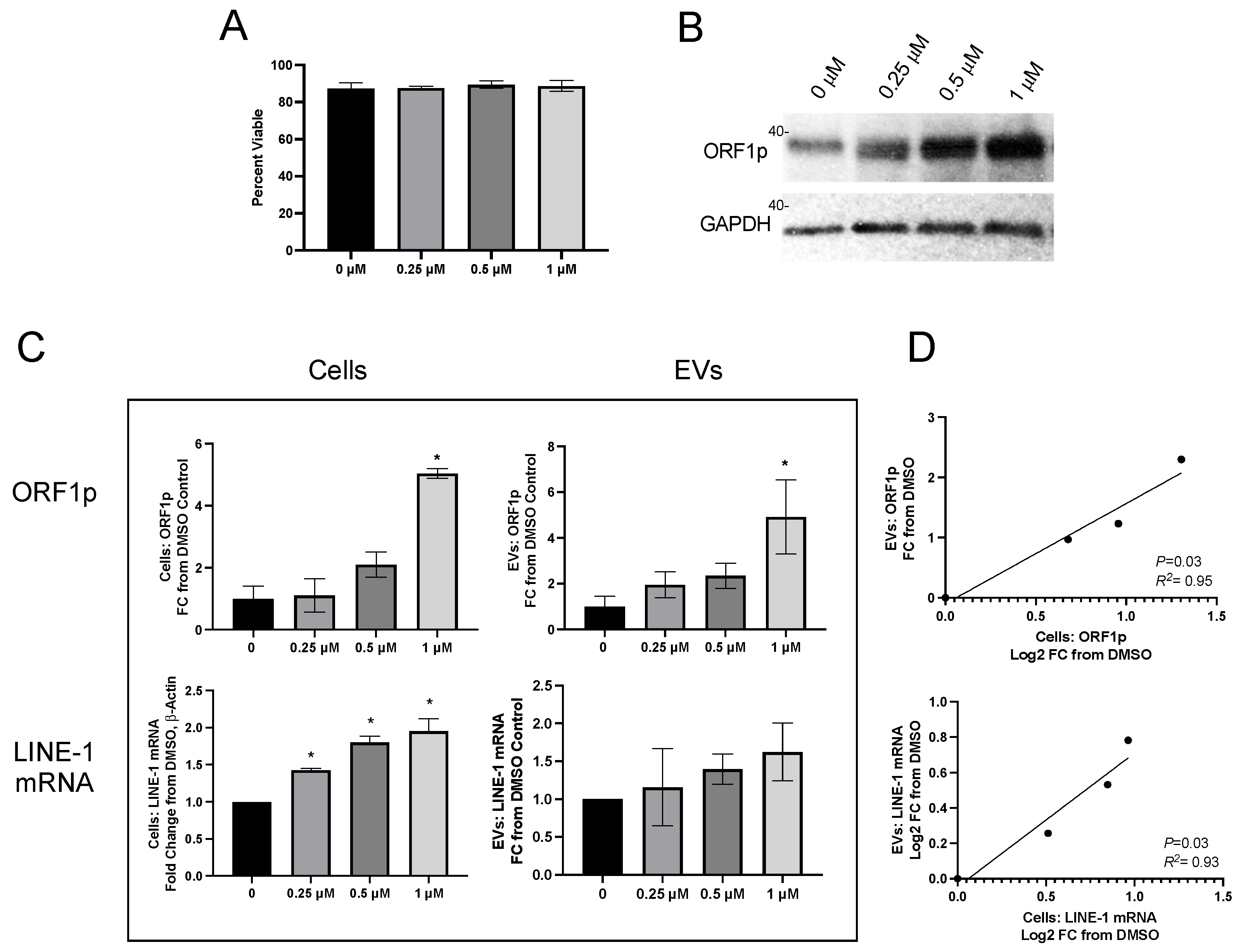
To determine if the remarkable cell-specific EV profiles seen under constitutive conditions were preserved upon the induction of LINE-1, cells were challenged with BaP, a known lung carcinogen and LINE-1 inducer. H460 cells were exposed to different BaP concentrations and allowed to condition their medium for 48 h (
Figure 3). We have previously shown that BaP treatment does not change the number of secreted EVs, though a minor shift in the size of exosomes was noted [
24]. There was not a statistically significant change in the number of BAPs secreted after BaP exposure, but it did appear that the size pattern of EVs was altered. See data below.
Carcinogen treatment did not compromise cell viability (
Figure 3A), but readily induced LINE-1 protein (
Figure 3B). ORF1p was quantified in cells and EVs using the ELISA platform (
Figure 3C, top row) and expressed as FC relative to DMSO. Cellular ORF1p exhibited concentration-dependent induction profiles (
p < 0.05), with a 5.04 ± 0.16 mean fold induction at 1 µM BaP and a 4.92 ± 1.62 mean fold induction in EVs at the same concentration. ORF1p increases in BaP-treated cells were proportional to their corresponding EVs, as established by linear regression (
Figure 3D, top;
p = 0.03,
R2= 0.95).
Cellular LINE-1 mRNA also increased as a function of increasing BaP concentrations (
Figure 3C, bottom row), with 1.43, 1.80, and 1.95-fold increases seen in cells treated with 0.25, 0.5, and 1 mM BaP, respectively (
p < 0.05). Mean LINE-1 mRNA in EVs exhibited a concentration-dependent trend, but the response was variable and not significantly different from DMSO. There was, however, a significant relationship between mean cellular and mean EV LINE-1 mRNA and ORF1p levels (
Figure 3D, bottom;
p = 0.048,
R2 = 0.91). Together, these results demonstrate that fluctuations in cellular LINE-1 expression following carcinogen induction are mirrored by their corresponding EVs.
2.3. LINE-1 in EV Isolates from Human Plasma of Ostensibly Healthy Individuals
We first examined the abundance of LINE-1 ORF1p in ostensibly healthy subjects to evaluate the degree of inter-individual variability (
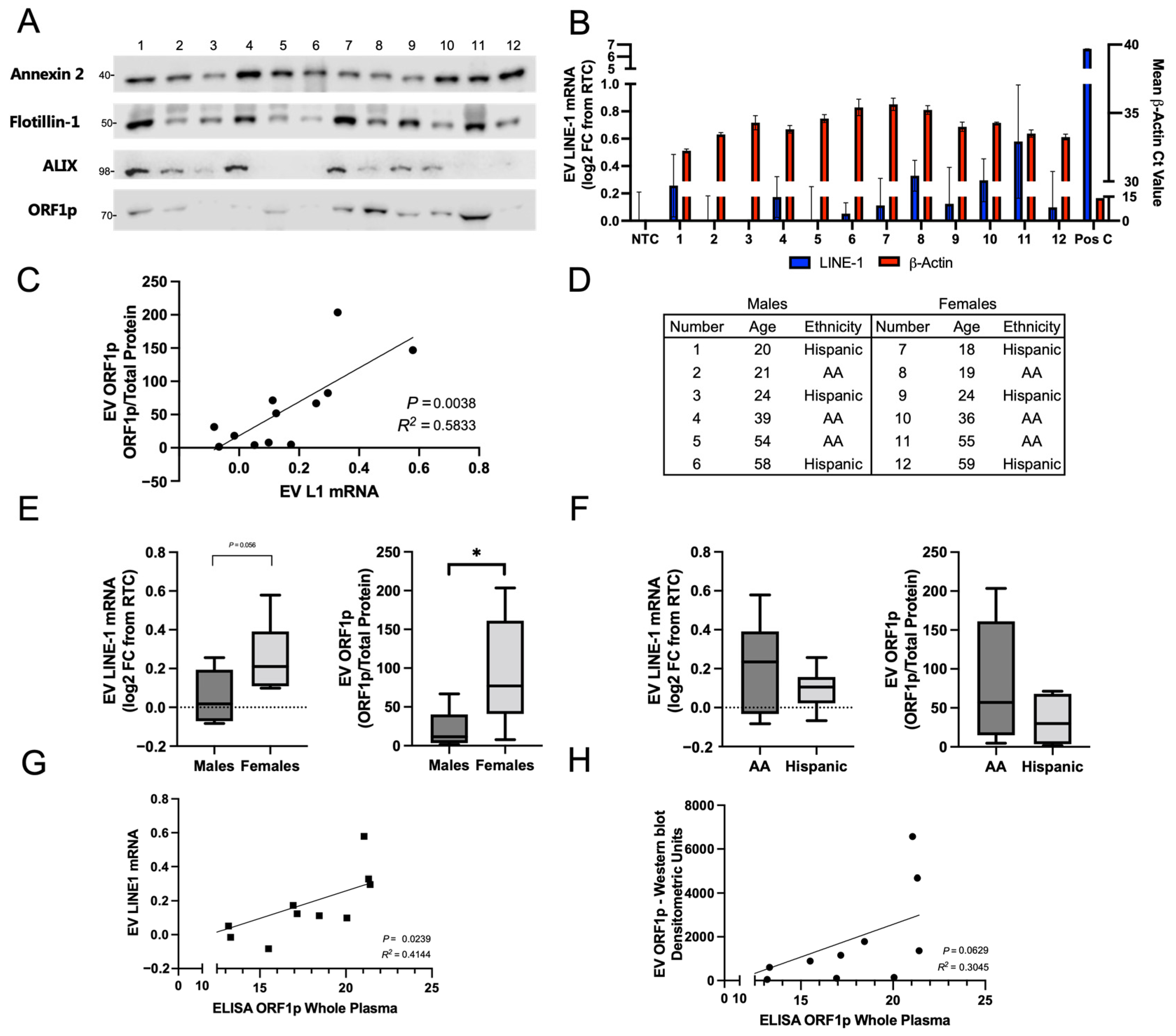
Figure 4). Twelve subjects (six African Americans and six Hispanics) were matched by age within 2 years, sex, and race (
Figure 4D). EVs were isolated by ultracentrifugation and normalized by total plasma volume (3 mL). For each subject, half of the preparation was used for measurements of LINE-1 mRNA and for quantification of ORF1p. EV proteins were visualized by Western blotting (
Figure 4A). The EV markers Annexin 2, Flotillin-1, and ALIX were used as positive controls. ORF1p dimer was present in all donors and exhibited significant variation across the cohort. Protein levels and a Ponceau stain are depicted in
Figure S2. β-Actin, a positive control, was detected in all samples, while LINE-1 mRNA could only be detected in trace amounts relative to the RTC. Despite low detection in human EVs, a positive correlation between EV LINE-1 mRNA and EV ORF1p levels was found (
Figure 4C;
p = 0.004,
R2 = 0.58). This relationship may reflect proportional EV cargo loading of ORF1p and LINE-1 mRNA, arguably in the form of high affinity LINE-1 ribonucleoprotein complexes [
28,
29,
30,
31].
In follow-up studies, we used densitometric measurements of ORF1p by Western blots normalized to total protein or mRNA levels to evaluate differences in LINE-1 EV cargo in our cohort by sex and race/ethnicity. ORF1p EV content was higher and exhibited a broader range in females than in males, with a mean ORF1p level of 94.01 arbitrary units versus 21.17 in males (
p = 0.04). Mean LINE-1 mRNA levels were generally higher in females, with EV LINE-1 mRNA content in females at 0.26 log2 FC relative to RTC, which was borderline significant (
p = 0.056) compared to males, with a mean of 0.053 log2 FC relative to RTC. While the profile of LINE-1 mRNA or ORF1p in African Americans compared to Hispanics was not significantly different, African Americans had slightly higher ORF1p content, and 2–3 times greater ranges of LINE-1 values compared to Hispanics. As we normalized EV inputs to total plasma volume, analyses were completed to rule out that the patterns observed were attributed to variations in EV abundance. Indeed, total protein levels exhibited no significant differences between groups (
Figure S2). Together, these results reveal that EV LINE-1 content varies considerably between individuals. Importantly, these findings can inform the creation of larger range finding studies to evaluate EV LINE-1 patterns in cancer patients.
2.4. Relationship between Whole Plasma and EV ORF1p Levels
Our previous studies noted a relative absence of free LINE-1 products in plasma compared to the highly enriched quantities found in plasma EVs [
24]. Thus, we hypothesized that the majority of circulating LINE-1 may be present in EVs, and that these levels may be approximated using whole-plasma ELISA measurements. To test this hypothesis, we measured bulk plasma ORF1p in ostensibly healthy subjects using ELISA and compared these values to measurements of LINE-1 mRNA (
Figure 4G) and EV ORF1p (
Figure 4H). Remarkably, a significant relationship between bulk plasma ELISA and EV LINE-1 mRNA levels was found (
R2 = 0.41,
p = 0.024), along with a near-significant relationship with EV ORF1p levels (
R2 = 0.30,
p = 0.063). These results support the hypothesis that circulating LINE-1 is predominantly contained within EVs and that performing a whole-plasma ELISA may approximate these values with a reasonable degree of fidelity.
2.5. EV LINE-1 mRNA in EVs Isolated from Lung Cancer Patients
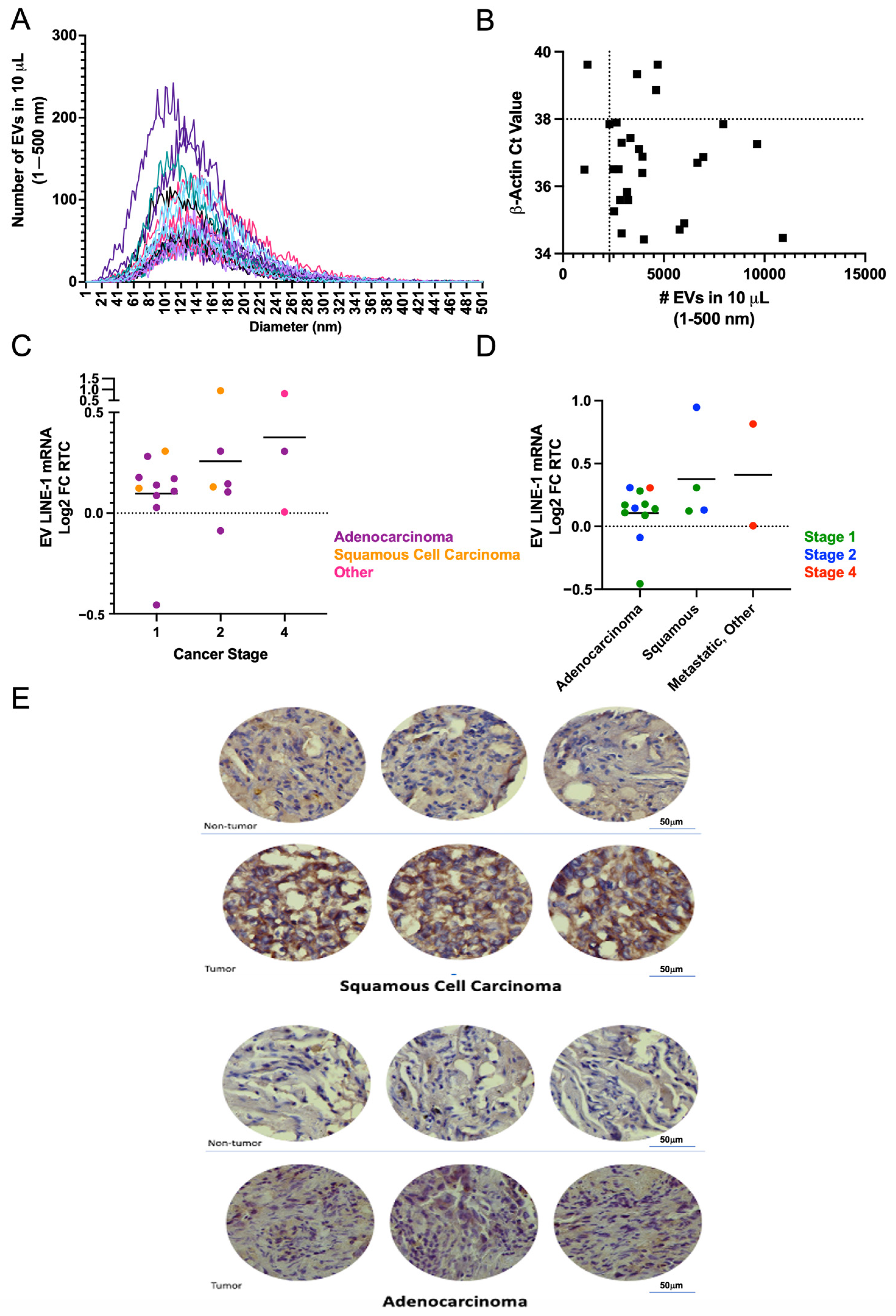
Using a single-blinded approach, LINE-1 mRNA was measured in plasma EVs from 28 patients who underwent lung resection surgery for a lung lesion. EVs were isolated from 250 μL of plasma and processed for measurements of LINE-1 mRNA. The presence of EVs in these preparations was verified by NTA (
Figure 5A). As a quality control, samples from six donors with less than 2300 particles and a β-Actin Ct greater than 38 were excluded from further analysis (
Figure 5B). Stratification of EV mRNA profiles based on cancer stage and histopathology showed that Stage 1 subjects had lower EV LINE-1 levels than those with Stages II and IV (
Figure 5C). Notably, not all Stage II and IV tumors exhibited high LINE-1 levels suggesting that the heterogeneity of response may be present. While mean LINE-1 levels did not significantly differ between squamous cell carcinomas and adenocarcinomas, a trend for higher levels in squamous cell carcinomas and metastatic tumors emerged (
Figure 5D). The profile of EV LINE-1 mRNA content in NSCLCs paralleled the patterns of ORF1p expression in NSCLC tissue sections compared to non-tumor tissue (
Figure 5E).
3. Discussion
Aberrant expression of the oncogenic retrotransposon LINE-1 has emerged as a hallmark of NSCLC. Most of the evidence to date has arisen from measurements of LINE-1 encoded ORF1p in cancer tissues at the time of resection, thus limiting the strength of inferences that can be made about the trajectory of illness, treatment response, or clinical outcome. To overcome these limitations, we have proposed measurements of LINE-1 cargo in human plasma-derived EVs as proxies of tissue LINE-1 expression. Here, we present proof of concept evidence that the LINE-1 cargo in EVs isolated from NSCLC cell lines mirrors their cellular content, and that measurements of LINE-1 analytes in plasma EVs can be used to stratify healthy subjects by gender and possibly race/ethnicity, and lung cancer patients by stage and histological type.
EV cargo need not be consistently proportional between cells and EVs as the loading of DNA, RNA, and protein may be subject to regulation via multiple biochemical pathways, and influenced by active and passive export mechanisms [
20,
32]. Thus, we initiated systematic studies to evaluate the levels of LINE-1 analytes in EVs and their cells of origin. Our findings under both constitutive and inducible conditions revealed that the proportionality between cellular and EV cargo is maintained for both LINE-1 mRNA and ORF1p, providing proof-of-principle that EV LINE-1 may serve as a “liquid biopsy” of LINE-1 tissue levels. While EV ORF1p can be readily detected in vitro and in vivo, EV LINE-1 mRNA was more difficult to detect, a limitation likely caused by the stoichiometry of LINE-1 ribonucleoprotein complex formation, in which one 6 kb LINE-1 mRNA molecule binds up to 240 ORF1p molecules and one molecule of ORF2p [
29]. In our studies, ORF1p was the target protein of interest given its high abundance compared to ORF2p, which exhibits low cellular abundance due to deficits during cellular processing [
28,
33,
34]. The finding that ORF1p is present in EVs isolated from cells and plasma, and detected as a dimer, was intriguing. We and others have previously reported the presence of an ORF1p dimer in cells, but the factors that dictate the appearance of the dimer relative to the monomer or the trimer have remained elusive [
24,
26,
28]. While ORF1p dimers may represent partially denatured trimers [
35], our data argue against this interpretation as dimers were selectively enriched in EVs and segregated from other LINE-1 products.
Given our interest in using LINE-1 readouts as biomarkers, we first explored EV LINE-1 cargo in the plasma of ostensibly healthy subjects to evaluate inter-individual variability. Our investigation revealed considerable heterogeneity in LINE-1 expression between individuals, with the source of variability likely reflecting genetic, environmental, and lifestyle interactions that influence circulating and tissue LINE-1 levels. For ostensibly healthy subjects, we identified sex-specific and possibly racial/ethnic differences. Females had higher EV LINE-1 levels than males and African Americans displayed wider ranges of EV LINE-1 values compared to Hispanics. These results are consistent with previous studies showing that males exhibit higher levels of LINE-1 hypermethylation than females [
36,
37] and that LINE-1 methylation can vary as a function of race and ethnicity [
38]. Moreover, we recently found greater numbers of ORF1p+ cells in African American lung cancer patients than Caucasians, with suggestive evidence that this difference may be related to lower survival rates in African Americans [
36]. Together, our findings confirm previous observations [
24] and lend support to the conclusion that LINE-1 expression in plasma and by extension in EVs can be influenced by phenotypic traits [
36,
37,
38].
As expected, levels of LINE-1 analytes in subjects with lung cancers were broadly distributed as a function of cancer stage and histological type. We found that mean EV LINE-1 mRNA increased with cancer stage and that higher LINE-1 levels were seen in squamous cell carcinomas. These results are consistent with other studies showing altered LINE-1 DNA methylation in NSCLC leading to variable retroelement expression [
7]. Saito et al. (2010) examined tumor LINE-1 methylation status in 379 cases of NSCLC and found that LINE-1 hypomethylation increases as a function of cancer stage and that squamous cell carcinoma, an NSCLC subtype linked to smoking [
39], exhibited lower median levels of LINE-1 methylation compared to adenocarcinomas [
7]. Consistent with these findings, the H520 squamous cell carcinoma line exhibited the highest levels of LINE-1 expression. In contrast, the lowest LINE-1 levels were seen in adenocarcinoma patients and the adenocarcinoma A549 cell line. Previous studies have shown that LINE-1 may be particularly useful in diagnostics for early stage cancers, as Stages IA and IB exhibit disparate levels of DNA methylation [
7] and LINE-1 hypomethylation can be used to identify early NSCLC among current smokers [
18].
A recent study by Taylor et al. (2023) examined circulating ORF1p levels in plasma using a novel single-molecule array (Simoa) assay and found that plasma ORF1p was elevated in individuals with various cancers, including NSCLC lung cancer [
40]. Among these individuals, a greater proportion of squamous cell carcinomas were LINE-1 positive compared to other histological types. This study also confirmed the presence of ORF1p within EVs; although, it also reported an abundance of free-floating ORF1p. While our methodology detected EV ORF1p in healthy subjects, using the Simoa assay, detection greatly depended on the protein sequence of the capture/detection nanobody. As such, differences may be explained by methodology-related differences in the detection of multimeric forms of ORF1p.
An additional consideration regarding the diagnostic use of LINE-1 EVs in complex matrices such as plasma is the relative enrichment of EVs from target tissues compared to other sources. We hypothesize that the bulk of LINE-1 readily detected in plasma originates from tissues that exhibit constitutive LINE-1 expression such as the brain [
41], esophagus, prostate, stomach, or heart muscle [
42].
Our findings raise significant questions regarding the functional consequences of circulating EV LINE-1. We and others have noted that EVs containing LINE-1 products possess reverse transcriptase activity [
22,
23,
24] and are capable of modifying the DNA of recipient cells [
23]. Enhanced EV LINE-1 export from tumors may therefore increase the potential for genomic aberrations and transformation of near or distant tissues that take up these EVs. Further mechanistic studies will be required to explore these dynamics. Future studies can examine whether the depletion of circulating NSCLC EVs reduces cancer progression.
In conclusion, our findings suggest that measurements of LINE-1 analytes in EVs may serve as a proxy for tracking changes in NSCLC. Efforts to understand the diagnostic potential of LINE-1 as a cancer biomarker will require the study of large cohorts along with the monitoring of environmental and lifestyle factors that may influence circulating LINE-1 levels.
4. Materials and Methods
4.1. Tissue Culture
Cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and confirmed to be free of mycoplasma contamination [
43]. All cell lines were cultured at 37 °C, 5% CO
2 in a humidified environment, and seeded to 70% confluence for 24 h. Cell lines were cultured as follows: BEAS-2B cells were cultured in LHC-9 medium, A549 cells in DMEM plus 10% fetal bovine serum (FBS), and the remaining lines in RPMI plus 10% FBS (Gibco/ThermoFisher Scientific, Waltham, MA, USA). To prepare for EV collection, cells were washed with DPBS and incubated in media containing EV-depleted FBS (Gibco/ThermoFisher Scientific) for 48 h. Conditioned media were collected, frozen, and processed for EV isolation upon thawing. To study inducible LINE-1 responses, 2 × 10
6 H460 cells were seeded in 150 mm dishes and allowed to attach overnight. The next day, cells were washed with DPBS and incubated with RPMI medium containing 10% EV-depleted FBS plus various concentrations of benzo(a)pyrene (BaP), a lung carcinogen and known inducer of LINE-1 in normal and transformed lung epithelial cell lines [
24,
44,
45], dissolved in 0.2% DMSO. Conditioned media were collected after 48 h and processed for EV isolation and measurement of cellular viability by trypan blue dye exclusion. Unconditioned media (UCM) was used as media control. Conditioned EV-free media (EFM) were used to measure the relative abundance of free versus EV-associated LINE-1 cargo.
4.2. EV Isolation and Characterization
For in vitro experiments, approximately 120 mL of conditioned media was centrifuged for 5 min at 500× g and concentrated to <12 mL using Centricon Plus-70 100 kDa MWCO centrifugal filter units (Millipore-Sigma, Darmstadt, Germany). The concentrate was centrifuged at 21,800× g for 30 min to pellet cellular debris, apoptotic bodies, and large vesicles. EVs were isolated using Polyethylene Glycol (PEG) or ultracentrifugation, as indicated. For PEG isolation, PEG-MW 6000 (Millipore-Sigma) was added to the supernatant to 10% w/v, incubated on ice for 15 min, and centrifuged at 21,800× g for 15 min. To remove protein contaminants, the EV pellet was resuspended in PBS, transferred to a clean tube, and reprecipitated in 10% PEG. PEG EV pellets were resuspended in PBS and confirmed to be free of debris using Nanosight Nanoparticle Tracking Analysis (NTA) (Malvern Panalytical, Malvern, UK). For ultracentrifugation, concentrated media were centrifuged at 100,000× g for 2 h at 4 °C. Pellets were washed in cold PBS and the centrifugation was repeated before resuspension of EV pellets in PBS. Protein was used to normalize EV input in in vitro experiments. Nanosight NTA of cell media preparations was performed by the Center for Nanotechnology in Drug Delivery at the University of North Carolina Chapel Hill. EVs in PBS were diluted and quantified with a NanoSight NS500 (Malvern) equipped with red laser illumination (532 nm). Each sample was read five times, using four samples per treatment.
4.3. Collection of Plasma EVs
Plasma was purchased from BioIVT (Westbury, NY, USA) from ostensibly healthy donors who were screened verbally to rule out serious health conditions such as prior metastatic disease, diabetes, or other inflammatory diseases. Plasma was collected using sodium citrate. Plasma was also obtained from patients undergoing surgical resection for a lung lesion at Houston Methodist Hospital. The collection of plasma and nodular tissue was approved by the Institutional Review Board at Houston Methodist Research Institute (Protocol # 00004763). Heparinized venous blood was collected prior to nodule resection and the final pathology report was obtained to identify tumor characteristics and staging for each patient. For plasma EV isolation, samples were thawed, and platelets were removed after two centrifugation cycles at 3600× g. Cleared plasma was diluted 4× with DPBS and centrifuged at 21,800× g for 30 min to pellet debris. The supernatant was ultracentrifuged as described above. EV inputs were normalized by input plasma volume.
4.4. Western Blotting
Cells and EVs were lysed in RIPA buffer and subjected to Western blotting as described [
46]. Immunoblots were imaged using a Konica Minolta SRX101A film developer (Konica Minolta Medical & Graphic, Inc., Tokyo, Japan). The ORF1p polyclonal antibody used was custom-made using the 14-amino acid N-terminus of the ORF1p protein (ORF1p1-14). The specificity of this antibody has been confirmed in previous studies [
47]. The monoclonal ORF1p antibody used was purchased from Millipore-Sigma (MABC1152). Antibodies to ALIX (CST, 92880), CD9 (CST, 13403), Flotillin-1 (CST, 18634), GAPDH (CST, 2118), and Calnexin (MA5-31501) were obtained commercially via Cell Signaling Technology (Danvers, MA, USA) and ThermoFisher.
4.5. ELISA
ORF1p was measured using a competitive indirect ELISA, where indicated, as this platform is not subject to PEG interference (
Figure S1) [
27]. Briefly, pre-blocked streptavidin-coated plates (Pierce/ThermoFisher) were incubated with biotinylated ORF1p1-14. After washing with PBS/0.01% Tween, diluted plasma, standards, and primary antibody were mixed, added to the plates, and incubated for 1 h. Standards were generated using known quantities of ORF1p1-14 and a matrix control of EV pellets derived from murine cells. After washing and incubation with HRP-linked secondary antibody (ThermoFisher), the ELISA was developed using TMB (Pierce/ThermoFisher) substrate and subsequently quenched with sulfuric acid. Absorbance was read at 450 nM.
4.6. LINE-1 mRNA Cargo and Quantification
EVs from H520 cells were used to evaluate LINE-1 mRNA content. Briefly, RNAseA was added to EVs in DPBS and incubated for 10 min at room temperature. RNA Secure (Invitrogen/ThermoFisher) was used for RNA extraction. EV protein was used to normalize EV input across cell lines and treatments. RNA was extracted from EV pellets using a Quick RNA kit (Zymo, Irvine, CA, USA) with DNAseI digestion (Turbo DNA Free, Invitrogen/ThermoFisher). RNA was eluted in equal volumes of nuclease-free water, and 3 μL was used for each RT-qPCR reaction. LINE-1 and β-Actin were quantified using Luna Universal One-Step RT-qPCR (NEB, Ipswich, MA, USA) according to the manufacturer’s protocol, with duplicate reactions for each sample. LINE-1 contains no introns; thus, LINE-1 primers cannot distinguish between residual genomic (g) DNA and cDNA. Thus, each LINE-1 sample was normalized to a matched control lacking reverse transcriptase (RTC) to control for residual gDNA. As exon-junction spanning β-Actin primers do not amplify gDNA, the β-Actin signal was normalized to a non-template control (NTC). All primers had amplification efficiencies of >90%. LINE-1 primer: Forward 5′ACACCTATTCCAAAATTGACCAC 3, Reverse 5′ TTCCCTCTACACACTGCTTTGA 3′, and probe 5′ TG GAAACTGAACAACCTGCTCCTGA 3′. β-Actin primers: Forward 5′ CTGGCACCCAGCACAATG 3′, Reverse 5′ GCCGATCCACACGGAGTACT 3′, Probe: 5′ ATCAAGATCATTGCTCCTCCTGAGCGC 3′.
4.7. Immunohistochemistry
Sections of human lung tumors and patient-matched non-tumor adjacent tissues were deparaffinized in xylene and rehydrated in a graded ethanol series. HistoZyme was performed for antibody retrieval. Endogenous peroxidase was inactivated by treating tissue sections with 3% H2O2/methanol for 15 min. The slides were washed five times for five min each and the sections blocked with 5% goat serum containing 0.1% Triton X-100 at room temperature for 1 h, followed by labeling with primary ORF1 antibody (Millipore) at 1:200 at 4 °C overnight. The primary antibody was labeled with HRP-conjugated secondary antibodies at 1:200 for 1 h at room temperature. The signal was visualized using a DAB substrate kit. Stained sections were dehydrated and mounted. Images were taken with Nikon eclipse Zyla SCMOS microscope at 40× magnification.
4.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.1.2. A p-value of less than 0.05 was considered significant. EV LINE-1 mRNA enrichment was compared to background levels using a one-tail t-test. The association between cellular and EV ORF1p with or without BaP treatment was established using simple linear regression. The relationship between cellular and EV mRNA was tested using Spearman’s non-linear regression. BaP treatments were compared to the DMSO control using a one-way ANOVA with Dunnett’s multiple comparisons or Kruskal–Wallace test with Dunn’s multiple comparisons, as indicated. The relationship between EV LINE-1 mRNA and ORF1p content in healthy plasma EVs was assessed by simple linear regression. The mean EV LINE-1 mRNA and ORF1p content were compared between males and females and African American and Hispanic American subjects using unpaired t-tests. Whole plasma ORF1p was compared to EV ORF1p levels using a simple linear regression.