Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation
Abstract
1. Introduction
2. Results
2.1. The Morphology of Astrocytes Cultured in Normal- or High-Glucose Medium
2.2. Cell Survival and NO Production of Rat Astrocytes Cultured in Normal- or High-Glucose Medium
2.3. Effect of LPS on NO Production by Astrocytes Exposed to Normal or High Glucose
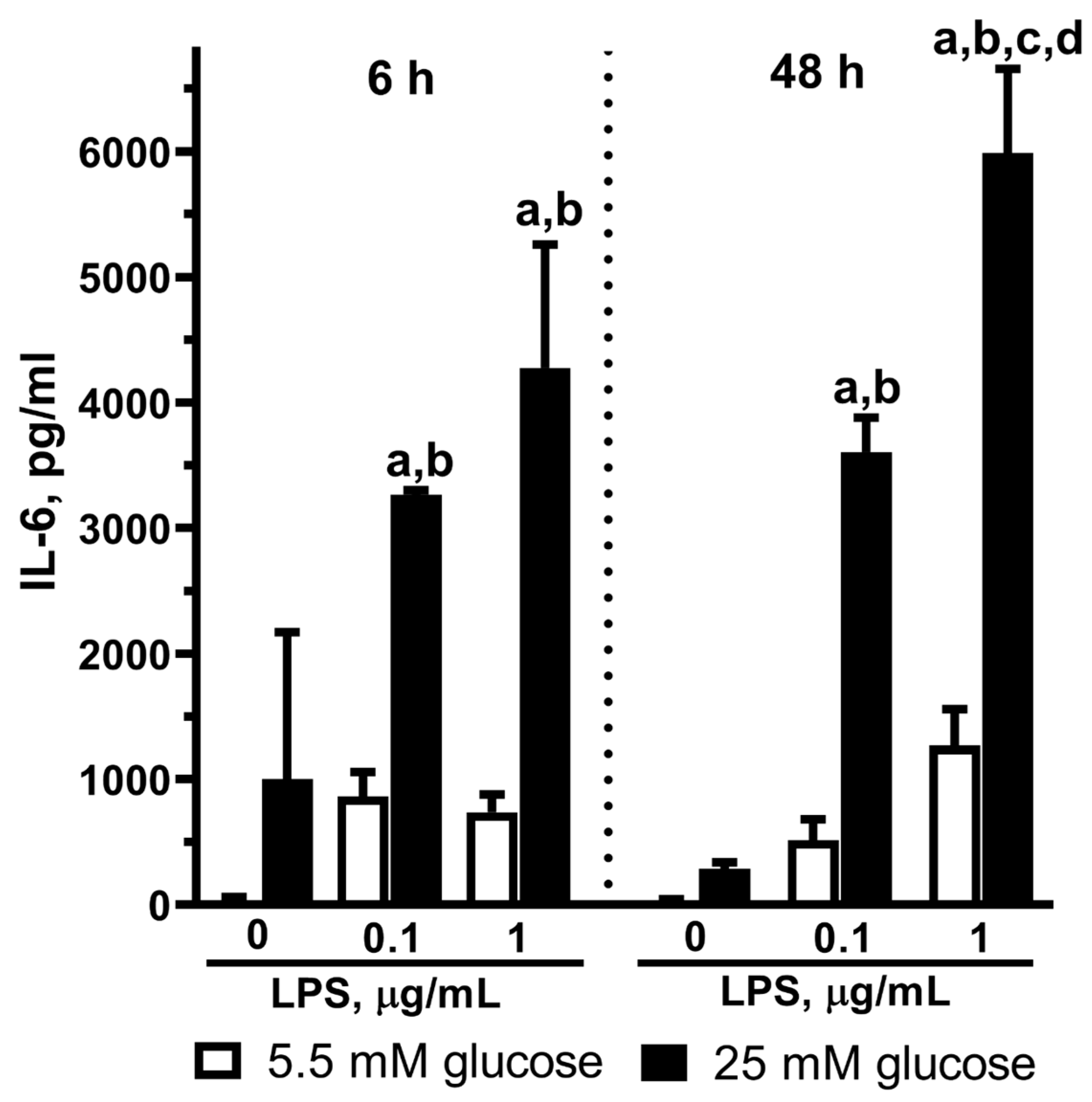
2.4. Effect of LPS on IL-6 Release in Astrocytes Exposed to Normal or High Glucose
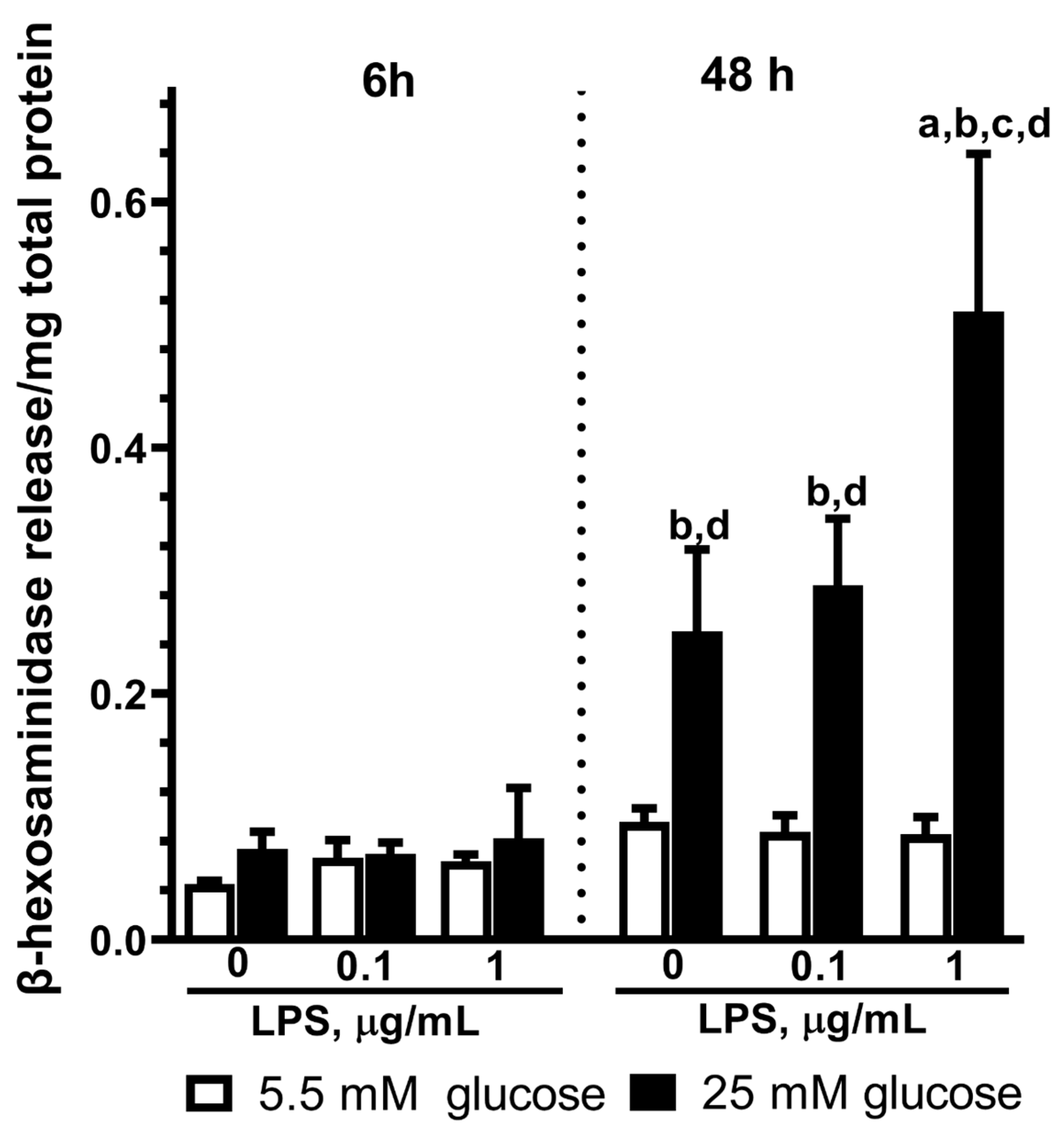
2.5. Effect of LPS on the β-Hexosaminidase Release by Astrocytes Exposed to Normal or High Glucose
3. Discussion
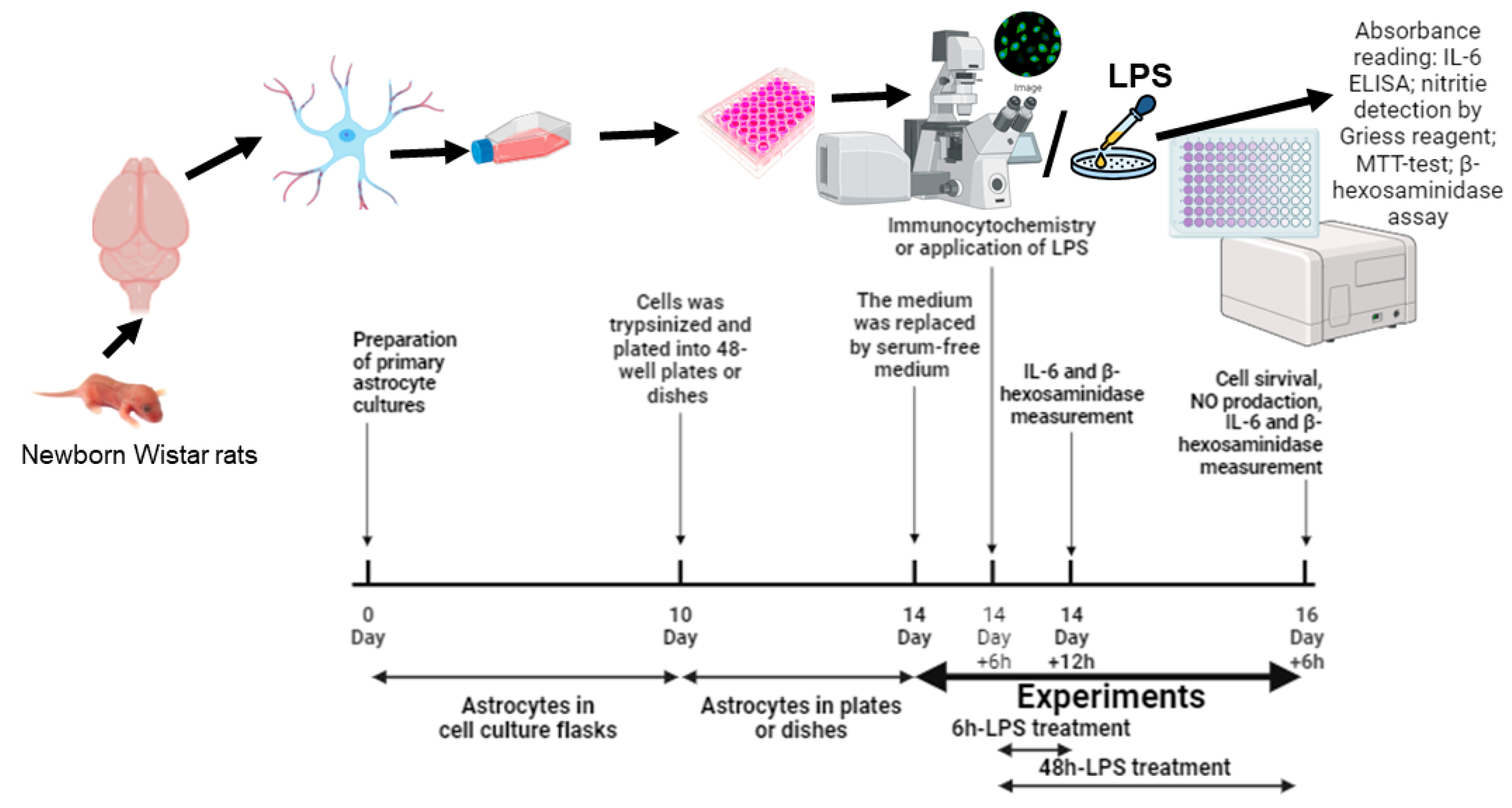
4. Materials and Methods
4.1. Reagents
4.2. Primary Astrocyte Cell Culture
4.3. Immunocytochemistry
4.4. MTT Assay
4.5. Measurement of Nitric Oxide Production
4.6. Measurement of IL-6 Production
4.7. β-Hexosaminidase Assay
4.8. Bradford Protein Assay
4.9. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef]
- Kwoun, M.O.; Ling, P.R.; Lydon, E.; Imrich, A.; Qu, Z.; Palombo, J.; Bistrian, B.R. Immunologic Effects of Acute Hyperglycemia in Nondiabetic Rats. J. Parenter. Enter. Nutr. 1997, 21, 91–95. [Google Scholar] [CrossRef]
- Sheetz, M.J.; King, G.L. Molecular Understanding of Hyperglycemia’s Adverse Effects for Diabetic Complications. JAMA 2002, 20, 2579–2588. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 6865, 813–820. [Google Scholar] [CrossRef]
- Yan, L. jun Redox Imbalance Stress in Diabetes Mellitus: Role of the Polyol Pathway. Anim. Model. Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Richa, R.; Yadawa, A.K.; Chaturvedi, C.M. Hyperglycemia and High Nitric Oxide Level Induced Oxidative Stress in the Brain and Molecular Alteration in the Neurons and Glial Cells of Laboratory Mouse, Mus Musculus. Neurochem. Int. 2017, 104, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Klein, J.P. Molecular Changes in the Diabetic Brain The Brain in Diabetes: Molecular Changes in Neurons and Their Implications for End-Organ Damage. Lancet Neurol. 2003, 9, 548–554. [Google Scholar] [CrossRef]
- Guyot, L.L.; Diaz, F.G.; O’regan, M.H.; Song, D.; Phillis, J.W. The Effect of Streptozotocin-Induced Diabetes on the Release of Excitotoxic and Other Amino Acids from the Ischemic Rat Cerebral Cortex. Neurosurgery 2001, 2, 385–390. [Google Scholar] [CrossRef]
- Atroba, M.W.; Grabowska, A.D.; Szukiewicz, D. Effects of Diabetes Mellitus-Related Dysglycemia on the Functions of Blood-Brain Barrier and the Risk of Dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.K.; Harik, L.; Gandhi, G.K.; Cruz, N.F.; Dienel, G.A. Reduced Gap Junctional Communication among Astrocytes in Experimental Diabetes: Contributions of Altered Connexin Protein Levels and Oxidative-Nitrosative Modifications. J. Neurosci. Res. 2011, 89, 2052–2067. [Google Scholar] [CrossRef]
- Li, X.; Cai, Y.; Zhang, Z.; Zhou, J. Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes. Diabetes Metab. J. 2022, 46, 222–238. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Wang, Z.; Zhang, X.; Yao, L.; Wang, F.; Liu, S.; Yin, J.; Ling, E.A.; Wang, L.; et al. High Glucose-Induced Expression of Inflammatory Cytokines and Reactive Oxygen Species in Cultured Astrocytes. Neuroscience 2012, 202, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Goriainov, S.V.; Astakhova, A.A.; Sergeeva, M.G. High Glucose Shifts the Oxylipin Profiles in the Astrocytes towards Pro-Inflammatory States. Metabolites 2021, 11, 311. [Google Scholar] [CrossRef]
- Cohen, S.; Liu, Q.; Wright, M.; Garvin, J.; Rarick, K.; Harder, D. High Glucose Conditioned Neonatal Astrocytes Results in Impaired Mitogenic Activity in Cerebral Microvessel Endothelial Cells in Co-Culture. Heliyon 2019, 5, e01795. [Google Scholar] [CrossRef]
- Li, W.; Choudhury, G.R.; Winters, A.; Prah, J.; Lin, W.; Liu, R.; Yang, S.H. Hyperglycemia Alters Astrocyte Metabolism and Inhibits Astrocyte Proliferation. Aging Dis. 2018, 9, 674–684. [Google Scholar] [CrossRef]
- Staricha, K.; Meyers, N.; Garvin, J.; Liu, Q.; Rarick, K.; Harder, D.; Cohen, S. Effect of High Glucose Condition on Glucose Metabolism in Primary Astrocytes. Brain Res. 2020, 1732, 146702. [Google Scholar] [CrossRef] [PubMed]
- Gorbatenko, V.O.; Goriainov, S.V.; Babenko, V.A.; Plotnikov, E.Y.; Sergeeva, M.G.; Chistyakov, D.V. Anti-Inflammatory Properties of Metformin During Cultivation of Primary Rat Astrocytes in a Medium with High Glucose Concentration. Biochemistry 2022, 87, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Tang, Y.; Yang, Q.W. Metabolic Changes Favor the Activity and Heterogeneity of Reactive Astrocytes. Trends Endocrinol. Metab. 2022, 33, 390–400. [Google Scholar] [CrossRef]
- Komorowska, J.; Wątroba, M.; Bednarzak, M.; Grabowska, A.D.; Szukiewicz, D. The Role of Glucose Concentration and Resveratrol in Modulating Neuroinflammatory Cytokines: Insights from an In Vitro Blood–Brain Barrier Model. Med. Sci. Monit. 2023, 29, e941044-1–e941044-19. [Google Scholar] [CrossRef]
- McConnell, H.L.; Li, Z.; Woltjer, R.L.; Mishra, A. Astrocyte Dysfunction and Neurovascular Impairment in Neurological Disorders: Correlation or Causation? Neurochem. Int. 2019, 128, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef] [PubMed]
- Nan, K.; Han, Y.; Fang, Q.; Huang, C.; Yu, L.; Ge, W.; Xiang, F.; Tao, Y.X.; Cao, H.; Li, J. HMGB1 Gene Silencing Inhibits Neuroinflammation via Down-Regulation of NF-ΚB Signaling in Primary Hippocampal Neurons Induced by Aβ 25–35. Int. Immunopharmacol. 2019, 67, 294–301. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Nogueira-Machado, J.A.; Volpe, C.M.D.O.; Veloso, C.A.; Chaves, M.M. HMGB1, TLR and RAGE: A Functional Tripod That Leads to Diabetic Inflammation. Expert. Opin. Ther. Targets 2011, 15, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Polozhintsev, A.I.; Sergeeva, M.G.; Reiser, G. Toll-like Receptors Control P38 and JNK MAPK Signaling Pathways in Rat Astrocytes Differently, When Cultured in Normal or High Glucose Concentrations. Neurochem. Int. 2019, 131, 104513. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Yang, E.; Pattani, S.; Zaheer, S.; Santillan, D.A.; Santillan, M.K.; Zaheer, A.; Holowka, D. Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins α-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS ONE 2015, 10, e0135776. [Google Scholar] [CrossRef]
- Li, Z.; Gu, Y.; Wen, R.; Shen, F.; Tian, H.L.; Yang, G.Y.; Zhang, Z. Lysosome Exocytosis Is Involved in Astrocyte ATP Release after Oxidative Stress Induced by H2O2. Neurosci. Lett. 2019, 705, 251–258. [Google Scholar] [CrossRef]
- Chen, Y.; Rennie, D.; Cormier, Y.; Dosman, J. Association between Obesity and Atopy in Adults. Int. Arch. Allergy Immunol. 2010, 153, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Nardin, P.; Tramontina, F.; Leite, M.C.; Tramontina, A.C.; Quincozes-Santos, A.; de Almeida, L.M.V.; Battastini, A.M.; Gottfried, C.; Gonçalves, C.A. S100B Content and Secretion Decrease in Astrocytes Cultured in High-Glucose Medium. Neurochem. Int. 2007, 50, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-M.; Ran, F.; Ni, H.-Z.; Sun, L.-L.; Xiao, L.; Li, X.-Q.; Li, W.-D. Metformin Inhibits High Glucose-Induced Smooth Muscle Cell Proliferation and Migration. Aging 2020, 12, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Fukushima, T.; Oike, H.; Kobori, M. High Glucose Increases the Expression of Proinflammatory Cytokines and Secretion of TNFα and β-Hexosaminidase in Human Mast Cells. Eur. J. Pharmacol. 2012, 687, 39–45. [Google Scholar] [CrossRef]
- Dragomir, E.; Manduteanu, I.; Voinea, M.; Costache, G.; Manea, A.; Simionescu, M. Aspirin Rectifies Calcium Homeostasis, Decreases Reactive Oxygen Species, and Increases NO Production in High Glucose-Exposed Human Endothelial Cells. J. Diabetes Complicat. 2004, 18, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Wu, H.J. Methylglyoxal and High Glucose Co-Treatment Induces Apoptosis or Necrosis in Human Umbilical Vein Endothelial Cells. J. Cell Biochem. 2008, 103, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Cantuária, A.P.C.; Figueiredo, T.M.; Freire, M.S.; Lima, S.M.F.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. The Effects of Glucose Concentrations Associated with Lipopolysaccharide and Interferon-Gamma Stimulus on Mediators’ Production of RAW 264.7 Cells. Cytokine 2018, 107, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, J.A.; Chan, P.H.; Chan, T.Y.; Gregory, G.A. Modification of Hypoxia-Induced Injury in Cultured Rat Astrocytes by High Levels of Glucose. Stroke 1993, 6, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, Y.H.; Liang, K.C.; Jheng, Y.S.; Jhao, J.J.; Su, M.T.; Lee-Chen, G.J.; Hsieh-Li, H.M. Exendin-4 Protected against Cognitive Dysfunction in Hyperglycemic Mice Receiving an Intrahippocampal Lipopolysaccharide Injection. PLoS ONE 2012, 7, e39656. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Schubert, P.; Morino, T.; Miyazaki, H.; Ogata, T.; Nakamura, Y.; Marchini, C.; Ferroni, S. Cascading Glia Reactions: A Common Pathomechanism and Its Differentiated Control by Cyclic Nucleotide Signaling. Ann. N. Y. Acad. Sci. 2000, 903, 24–33. [Google Scholar] [CrossRef]
- von Widdern, J.C.; Hohmann, T.; Dehghani, F. Abnormal Cannabidiol Affects Production of Pro-Inflammatory Mediators and Astrocyte Wound Closure in Primary Astrocytic-Microglial Cocultures. Molecules 2020, 25, 496. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. PHYSIOLOGY OF ASTROGLIA. Physiol. Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.; Montesinos, J.; Ureña-Peralta, J.R.; Guerri, C.; Pascual, M. TLR4 Participates in the Transmission of Ethanol-Induced Neuroinflammation via Astrocyte-Derived Extracellular Vesicles. J. Neuroinflammation 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Cerciat, M.; Unkila, M.; Garcia-Segura, L.M.; Arevalo, M.A. Selective Estrogen Receptor Modulators Decrease the Production of Interleukin-6 and Interferon-γ-Inducible Protein-10 by Astrocytes Exposed to Inflammatory Challenge in Vitro. Glia 2010, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Zhang, F.; Ying, C.J.; Chen, J.; Chen, L.; Dong, J.; Shi, Y.; Tang, M.; Hu, X.T.; Pan, Z.H.; et al. Inhibition of INOS Alleviates Cognitive Deficits and Depression in Diabetic Mice through Downregulating the NO/SGC/CGMP/PKG Signal Pathway. Behav. Brain Res. 2017, 322, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Shimizu, N.; Kunii, K.; Martyn, J.A.J.; Ueki, K.; Kaneki, M. A Role for INOS in Fasting Hyperglycemia and Impaired Insulin Signaling in the Liver of Obese Diabetic Mice. Diabetes 2005, 5, 1340–1348. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Dündar, M. Ameliorative Effects of Pentoxifylline on NOS Induced by Diabetes in Rat Kidney. Ren. Fail. 2016, 38, 605–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, N.R.; Zhang, P.; Lee, J.C.; Hogan, E.L. Extracellular Signal-Regulated Kinase and P38 Subgroups of Mitogen-Activated Protein Kinases Regulate Inducible Nitric Oxide Synthase and Tumor Necrosis Factor-Gene Expression in Endotoxin-Stimulated Primary Glial Cultures. J. Neurosci. 1998, 5, 1633–1641. [Google Scholar] [CrossRef]
- Ko, H.M.; Lee, S.H.; Bang, M.; Kim, K.C.; Jeon, S.J.; Park, Y.M.; Han, S.H.; Kim, H.Y.; Lee, J.; Shin, C.Y. Tyrosine Kinase Fyn Regulates INOS Expression in LPS-Stimulated Astrocytes via Modulation of ERK Phosphorylation. Biochem. Biophys. Res. Commun. 2018, 495, 1214–1220. [Google Scholar] [CrossRef]
- Park, G.H.; Jeon, S.J.; Ryu, J.R.; Choi, M.S.; Han, S.H.; Yang, S.I.; Ryu, J.H.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. Essential Role of Mitogen-Activated Protein Kinase Pathways in Protease Activated Receptor 2-Mediated Nitric-Oxide Production from Rat Primary Astrocytes. Nitric Oxide 2009, 21, 110–119. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Qin, A.P.; Zhang, H.L.; Qin, Z.H. Mechanisms of Lysosomal Proteases Participating in Cerebral Ischemia-Induced Neuronal Death. Neurosci. Bull. 2008, 24, 117–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuenzler, K.A.; Pearson, P.Y.; Schwartz, M.Z. IL-11 Pretreatment Reduces Cell Death after Intestinal Ischemia-Reperfusion. J. Surg. Res. 2002, 108, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, G.; Zhou, W.; Song, A.; Xu, T.; Luo, Q.; Wang, W.; Gu, X.S.; Duan, S. Regulated ATP Release from Astrocytes through Lysosome Exocytosis. Nat. Cell Biol. 2007, 9, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pappu, B.P.; Zeng, H.; Xue, L.; Morris, S.W.; Lin, X.; Wen, R.; Wang, D. B Cell Lymphoma 10 Is Essential for FcεR-Mediated Degranulation and IL-6 Production in Mast Cells. J. Immunol. 2007, 178, 49–57. [Google Scholar] [CrossRef]
- Chen, Q.; Mo, R.; Wu, N.; Zou, X.; Shi, C.; Gong, J.; Li, J.; Fang, K.; Wang, D.; Yang, D.; et al. Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front. Pharmacol. 2017, 8, 334. [Google Scholar] [CrossRef]
- Liu, B.; Cao, W.; Li, J.; Liu, J. Lysosomal Exocytosis of ATP Is Coupled to P2Y 2 Receptor in Marginal Cells in the Stria Vascular in Neonatal Rats. Cell Calcium 2018, 76, 62–71. [Google Scholar] [CrossRef]
- Dou, Y.; Wu, H.J.; Li, H.Q.; Qin, S.; Wang, Y.E.; Li, J.; Lou, H.F.; Chen, Z.; Li, X.M.; Luo, Q.M.; et al. Microglial Migration Mediated by ATP-Induced ATP Release from Lysosomes. Cell Res. 2012, 22, 1022–1033. [Google Scholar] [CrossRef]
- Ivanova, A.E.; Gorbacheva, L.R.; Strukova, S.M.; Pinelis, V.G.; Reiser, G. Activated Protein C and Thrombin Participate in the Regulation of Astrocyte Functions. Biochem. Suppl. Ser. A Membr. Cell Biol. 2014, 8, 50–59. [Google Scholar] [CrossRef]
- Gorbacheva, L.; Pinelis, V.; Ishiwata, S.; Strukova, S.; Reiser, G. Activated Protein C Prevents Glutamate- and Thrombin-Induced Activation of Nuclear Factor-ΚB in Cultured Hippocampal Neurons. Neuroscience 2010, 165, 1138–1146. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdyeva, A.; Kurtova, E.; Savinkova, I.; Galkov, M.; Gorbacheva, L. Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation. Int. J. Mol. Sci. 2024, 25, 1122. https://doi.org/10.3390/ijms25021122
Abdyeva A, Kurtova E, Savinkova I, Galkov M, Gorbacheva L. Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation. International Journal of Molecular Sciences. 2024; 25(2):1122. https://doi.org/10.3390/ijms25021122
Chicago/Turabian StyleAbdyeva, Ayna, Ekaterina Kurtova, Irina Savinkova, Maksim Galkov, and Liubov Gorbacheva. 2024. "Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation" International Journal of Molecular Sciences 25, no. 2: 1122. https://doi.org/10.3390/ijms25021122
APA StyleAbdyeva, A., Kurtova, E., Savinkova, I., Galkov, M., & Gorbacheva, L. (2024). Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation. International Journal of Molecular Sciences, 25(2), 1122. https://doi.org/10.3390/ijms25021122





