The Value of Urinary NGAL, KIM-1, and IL-18 Measurements in the Early Detection of Kidney Injury in Oncologic Patients Treated with Cisplatin-Based Chemotherapy
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Groups
2.2. Episodes of Acute Kidney Injury
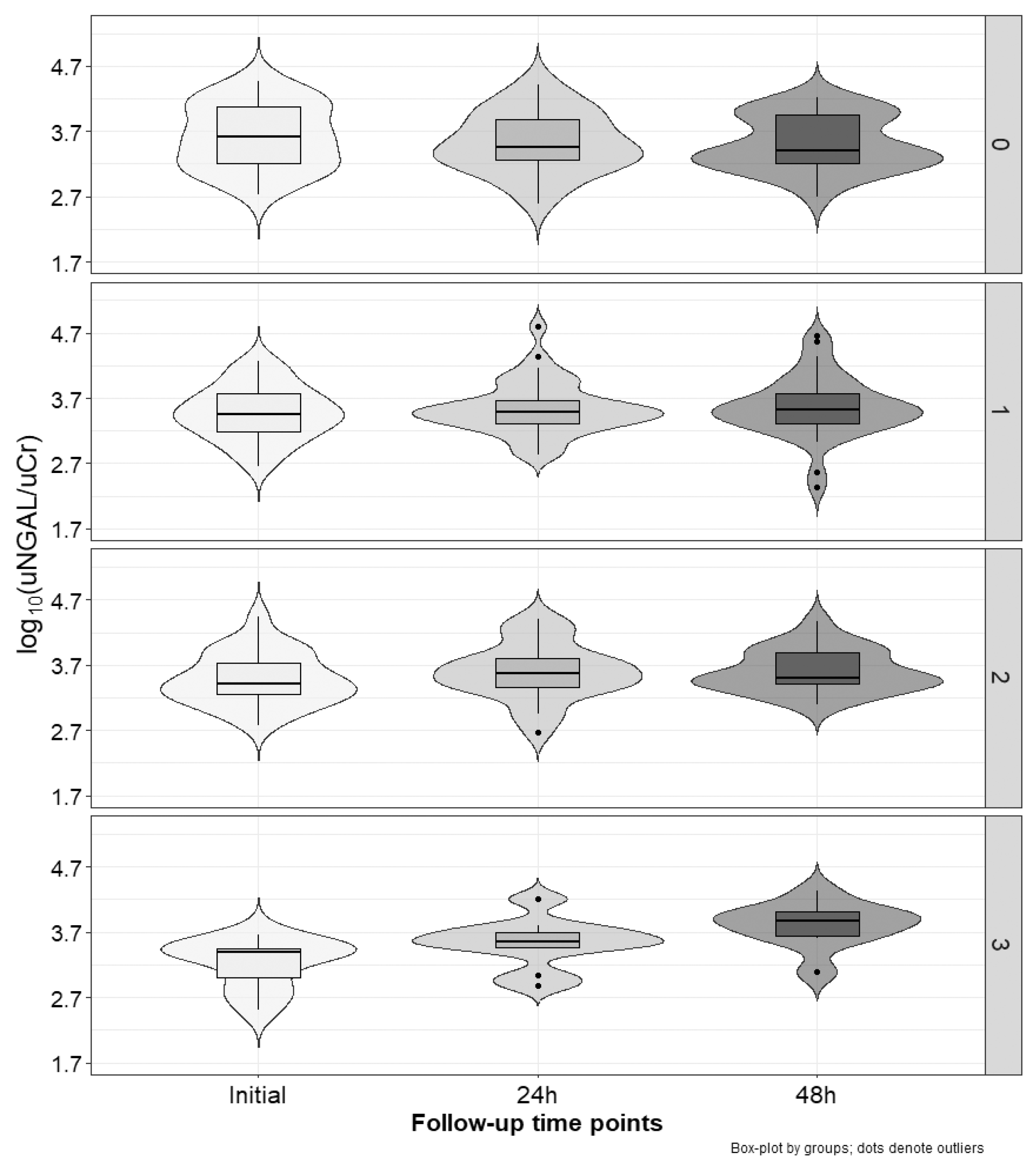
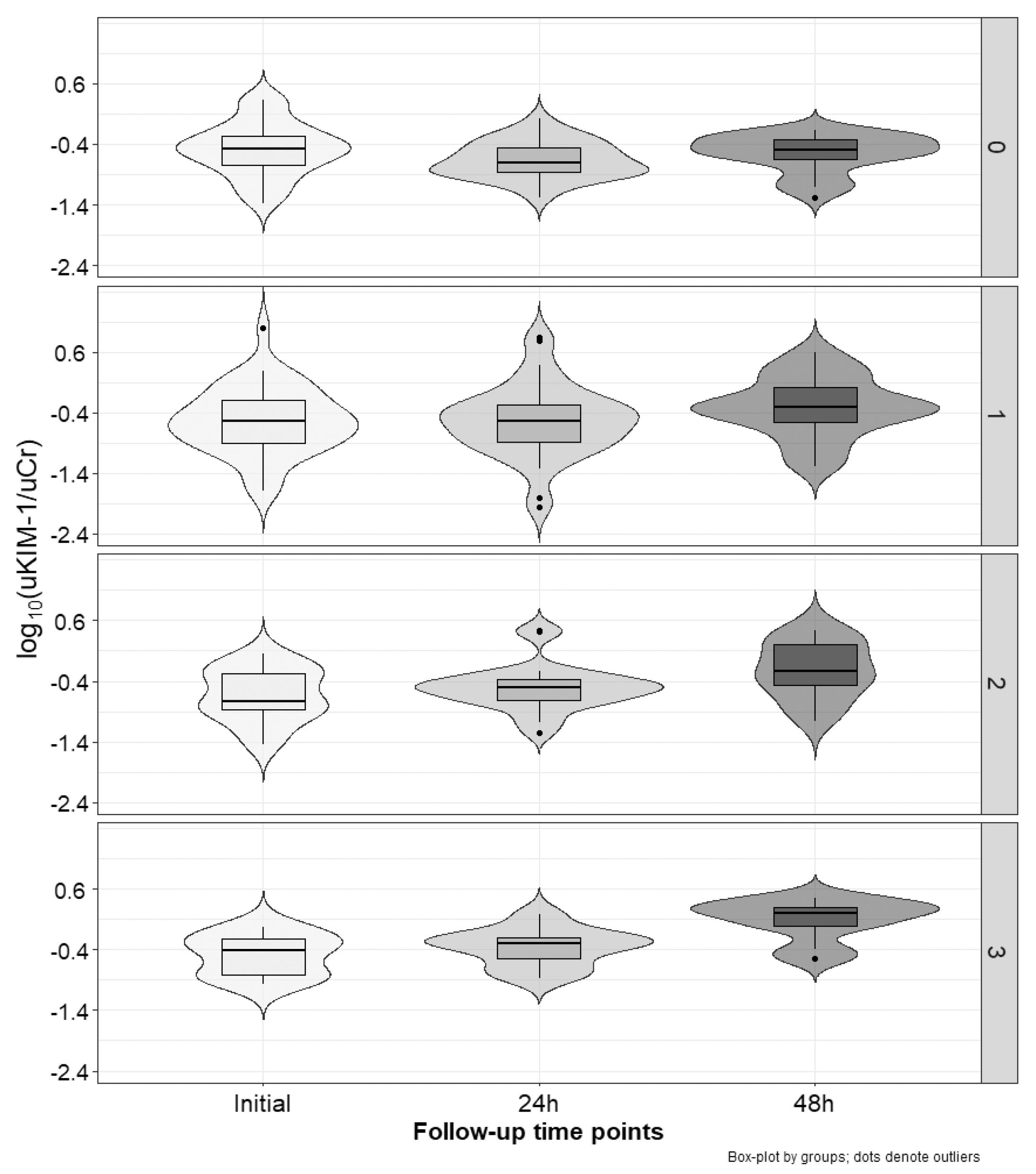
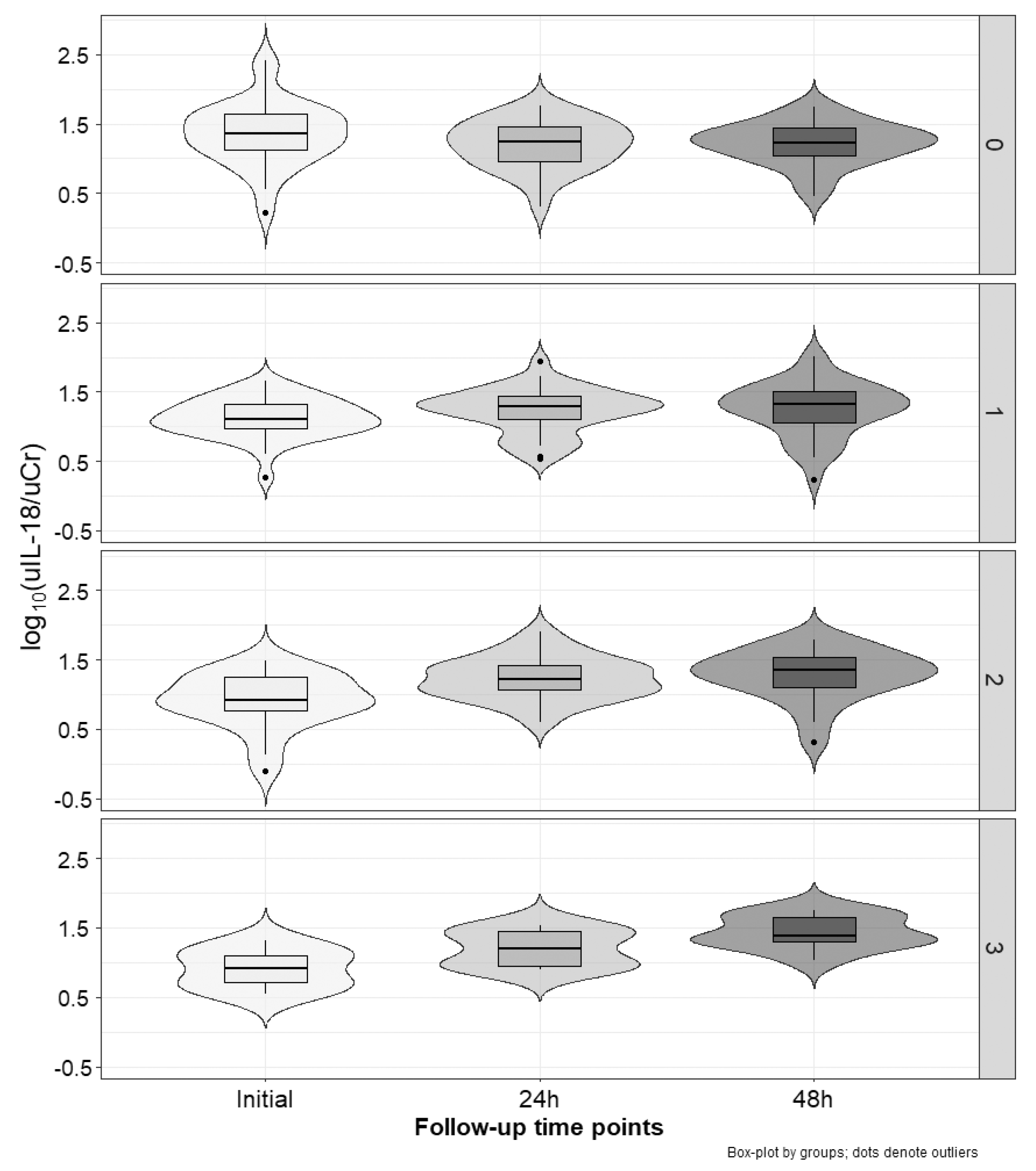
2.3. Marker Concentration Changes during the Cycles
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Protocol
4.3. Measurement of NGAL, KIM-1, and IL-18
4.4. Data Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular Mechanisms of Cisplatin-Induced Nephrotoxicity: A Balance on the Knife Edge between Renoprotection and Tumor Toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef]
- Oka, T.; Kimura, T.; Suzumura, T.; Yoshimoto, N.; Nakai, T.; Yamamoto, N.; Matsuura, K.; Mitsuoka, S.; Yoshimura, N.; Kudoh, S.; et al. Magnesium Supplementation and High Volume Hydration Reduce the Renal Toxicity Caused by Cisplatin-Based Chemotherapy in Patients with Lung Cancer: A Toxicity Study. BMC Pharmacol. Toxicol. 2014, 15, 70. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Watanabe, K.; Tsukiyama, I.; Matsushita, H.; Yabushita, H.; Matsuura, K.; Wakatsuki, A. Nephroprotective Effects of Hydration with Magnesium in Patients with Cervical Cancer Receiving Cisplatin. Anticancer Res. 2015, 35, 2199–2204. [Google Scholar]
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Friedl, A.; Stoesz, S.P.; Buckley, P.; Gould, M.N. Neutrophil Gelatinase-Associated Lipocalin in Normal and Neoplastic Human Tissues. Cell Type-Specific Pattern of Expression. Histochem. J. 1999, 31, 433–441. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. Molecular Characterization and Pattern of Tissue Expression of the Gene for Neutrophil Gelatinase-Associated Lipocalin from Humans. Genomics 1997, 45, 17–23. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengeløv, H.; Borregaard, N. Isolation and Primary Structure of NGAL, a Novel Protein Associated with Human Neutrophil Gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Kanda, J.; Mori, K.; Kawabata, H.; Kuwabara, T.; Mori, K.P.; Imamaki, H.; Kasahara, M.; Yokoi, H.; Mizumoto, C.; Thoennissen, N.H.; et al. An AKI Biomarker Lipocalin 2 in the Blood Derives from the Kidney in Renal Injury but from Neutrophils in Normal and Infected Conditions. Clin. Exp. Nephrol. 2015, 19, 99–106. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual Action of Neutrophil Gelatinase-Associated Lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Simsek, A.; Tugcu, V.; Tasci, A.I. New Biomarkers for the Quick Detection of Acute Kidney Injury. ISRN Nephrol. 2012, 2013, 394582. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil Gelatinase-Associated Lipocalin—An Emerging Troponin for Kidney Injury. Nephrol. Dial. Transplant. 2008, 23, 3737–3743. [Google Scholar] [CrossRef]
- Sancho-Martínez, S.M.; Blanco-Gozalo, V.; Quiros, Y.; Prieto-García, L.; Montero-Gómez, M.J.; Docherty, N.G.; Martínez-Salgado, C.; Morales, A.I.; López-Novoa, J.M.; López-Hernández, F.J. Impaired Tubular Reabsorption Is the Main Mechanism Explaining Increases in Urinary NGAL Excretion Following Acute Kidney Injury in Rats. Toxicol. Sci. 2020, 175, 75–86. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney Injury Molecule-1 (KIM-1), a Putative Epithelial Cell Adhesion Molecule Containing a Novel Immunoglobulin Domain, Is up-Regulated in Renal Cells after Injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney Injury Molecule-1: A Tissue and Urinary Biomarker for Nephrotoxicant-Induced Renal Injury. Am. J. Physiol. Ren. Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, C.R.; Xiao, S.; Sabbisetti, V.; Yeung, M.Y.; Hsiao, L.-L.; Ichimura, T.; Kuchroo, V.; Bonventre, J.V. KIM-1-Mediated Phagocytosis Reduces Acute Injury to the Kidney. J. Clin. Investig. 2015, 125, 1620–1636. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.P.V.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney Injury Molecule-1 Is a Phosphatidylserine Receptor That Confers a Phagocytic Phenotype on Epithelial Cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef]
- Lim, A.I.; Chan, L.Y.Y.; Lai, K.N.; Tang, S.C.W.; Chow, C.W.; Lam, M.F.; Leung, J.C.K. Distinct Role of Matrix Metalloproteinase-3 in Kidney Injury Molecule-1 Shedding by Kidney Proximal Tubular Epithelial Cells. Int. J. Biochem. Cell Biol. 2012, 44, 1040–1050. [Google Scholar] [CrossRef]
- Guo, L.; Takino, T.; Endo, Y.; Domoto, T.; Sato, H. Shedding of Kidney Injury Molecule-1 by Membrane-Type 1 Matrix Metalloproteinase. J. Biochem. 2012, 152, 425–432. [Google Scholar] [CrossRef]
- Gandhi, R.; Yi, J.; Ha, J.; Shi, H.; Ismail, O.; Nathoo, S.; Bonventre, J.V.; Zhang, X.; Gunaratnam, L. Accelerated Receptor Shedding Inhibits Kidney Injury Molecule-1 (KIM-1)-Mediated Efferocytosis. Am. J. Physiol. Ren. Physiol. 2014, 307, F205–F221. [Google Scholar] [CrossRef]
- Wawrocki, S.; Druszczynska, M.; Kowalewicz-Kulbat, M.; Rudnicka, W. Interleukin 18 (IL-18) as a Target for Immune Intervention. Acta Biochim. Pol. 2016, 63, 59–63. [Google Scholar] [CrossRef]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 Processes IFN-Gamma-Inducing Factor and Regulates LPS-Induced IFN-Gamma Production. Nature 1997, 386, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Ohtsuki, T.; Nukada, Y.; Tanimoto, T.; Namba, M.; Okura, T.; Takakura-Yamamoto, R.; Torigoe, K.; Gu, Y.; Su, M.S.; et al. Involvement of Caspase-1 and Caspase-3 in the Production and Processing of Mature Human Interleukin 18 in Monocytic THP.1 Cells. J. Biol. Chem. 1997, 272, 26595–26603. [Google Scholar] [CrossRef]
- Xin, C.; Yulong, X.; Yu, C.; Changchun, C.; Feng, Z.; Xinwei, M. Urine Neutrophil Gelatinase-Associated Lipocalin and Interleukin-18 Predict Acute Kidney Injury after Cardiac Surgery. Ren. Fail. 2008, 30, 904–913. [Google Scholar] [CrossRef]
- Buelow, M.W.; Dall, A.; Regner, K.; Weinberg, C.; Bartz, P.J.; Sowinski, J.; Rudd, N.; Katzmark, L.; Tweddell, J.S.; Earing, M.G. Urinary Interleukin-18 and Urinary Neutrophil Gelatinase-Associated Lipocalin Predict Acute Kidney Injury Following Pulmonary Valve Replacement Prior to Serum Creatinine. Congenit. Heart Dis. 2012, 7, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Striz, I.; Krasna, E.; Eliska, K.; Honsova, E.; Eva, H.; Lacha, J.; Jiri, L.; Petrickova, K.; Katerina, P.; Jaresova, M.; et al. Interleukin 18 (IL-18) Upregulation in Acute Rejection of Kidney Allograft. Immunol. Lett. 2005, 99, 30–35. [Google Scholar] [CrossRef]
- Dong, M.; Zhao, M.; Cui, M.; Sun, J.; Meng, X.; Sun, W.; Wang, L.; Du, P. Interleukin-18 Binding Protein Attenuates Renal Injury of Adriamycin-Induced Mouse Nephropathy. Int. J. Clin. Exp. Pathol. 2019, 12, 3005–3012. [Google Scholar]
- Gonul, Y.; Kazandı, S.; Kocak, A.; Ahsen, A.; Bal, A.; Karavelioglu, A.; Hazman, O.; Turamanlar, O.; Kokulu, S.; Yuksel, S. Interleukin-18 Binding Protein Pretreatment Attenuates Kidney Injury Induced by Hepatic Ischemia Reperfusion. Am. J. Med. Sci. 2016, 352, 200–207. [Google Scholar] [CrossRef]
- Wang, J.; Long, Q.; Zhang, W.; Chen, N. Protective Effects of Exogenous Interleukin 18-Binding Protein in a Rat Model of Acute Renal Ischemia-Reperfusion Injury. Shock 2012, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Fu, J.; Jiao, C.; Hao, X.; Feng, Z.; Zhu, L.; Zhang, Y.; Zhou, G.; Li, H.; Yang, W.; et al. IL-18 Deficiency Ameliorates the Progression from AKI to CKD. Cell Death Dis. 2022, 13, 957. [Google Scholar] [CrossRef]
- Hosohata, K.; Washino, S.; Kubo, T.; Natsui, S.; Fujisaki, A.; Kurokawa, S.; Ando, H.; Fujimura, A.; Morita, T. Early Prediction of Cisplatin-Induced Nephrotoxicity by Urinary Vanin-1 in Patients with Urothelial Carcinoma. Toxicology 2016, 359–360, 71–75. [Google Scholar] [CrossRef]
- Peres, L.A.B.; da Cunha, A.D.; Assumpção, R.A.B.; Schäfer, A.; da Silva, A.L.; Gaspar, A.D.; Scarpari, D.F.; Alves, J.B.F.; Girelli Neto, R.; de Oliveira, T.F.T. Evaluation of the Cisplatin Nephrotoxicity Using the Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Patients with Head and Neck Cancer. J. Bras. Nefrol. 2014, 36, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Shinke, H.; Masuda, S.; Togashi, Y.; Ikemi, Y.; Ozawa, A.; Sato, T.; Kim, Y.H.; Mishima, M.; Ichimura, T.; Bonventre, J.V.; et al. Urinary Kidney Injury Molecule-1 and Monocyte Chemotactic Protein-1 Are Noninvasive Biomarkers of Cisplatin-Induced Nephrotoxicity in Lung Cancer Patients. Cancer Chemother. Pharmacol. 2015, 76, 989–996. [Google Scholar] [CrossRef]
- Shahbazi, F.; Sadighi, S.; Dashti-Khavidaki, S.; Shahi, F.; Mirzania, M. Urine Ratio of Neutrophil Gelatinase-Associated Lipocalin to Creatinine as a Marker for Early Detection of Cisplatin-Associated Nephrotoxicity. Iran. J. Kidney Dis. 2015, 9, 306–310. [Google Scholar] [PubMed]
- Maeda, A.; Ando, H.; Ura, T.; Muro, K.; Aoki, M.; Saito, K.; Kondo, E.; Takahashi, S.; Ito, Y.; Mizuno, Y.; et al. Differences in Urinary Renal Failure Biomarkers in Cancer Patients Initially Treated with Cisplatin. Anticancer Res. 2017, 37, 5235–5239. [Google Scholar] [CrossRef]
- Gaspari, F.; Cravedi, P.; Mandalà, M.; Perico, N.; de Leon, F.R.; Stucchi, N.; Ferrari, S.; Labianca, R.; Remuzzi, G.; Ruggenenti, P. Predicting Cisplatin-Induced Acute Kidney Injury by Urinary Neutrophil Gelatinase-Associated Lipocalin Excretion: A Pilot Prospective Case-Control Study. Nephron Clin. Pract. 2010, 115, c154–c160. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.; Elmorsy, E.; Abdelwahab, H.; Algohary, O.; Naguib, M.; El Wahab, A.A.; Eldeeb, A.; Eltoraby, E.; Abdelsalam, A.; Sabry, A.; et al. Urinary Biomarkers for Early Detection of Platinum Based Drugs Induced Nephrotoxicity. BMC Nephrol. 2018, 19, 219. [Google Scholar] [CrossRef]
- Lin, H.Y.-H.; Lee, S.-C.; Lin, S.-F.; Hsiao, H.-H.; Liu, Y.-C.; Yang, W.-C.; Hwang, D.-Y.; Hung, C.-C.; Chen, H.-C.; Guh, J.-Y. Urinary Neutrophil Gelatinase-Associated Lipocalin Levels Predict Cisplatin-Induced Acute Kidney Injury Better than Albuminuria or Urinary Cystatin C Levels. Kaohsiung J. Med. Sci. 2013, 29, 304–311. [Google Scholar] [CrossRef]
- George, B.; Wen, X.; Mercke, N.; Gomez, M.; O’Bryant, C.; Bowles, D.W.; Hu, Y.; Hogan, S.L.; Joy, M.S.; Aleksunes, L.M. Profiling of Kidney Injury Biomarkers in Patients Receiving Cisplatin: Time-Dependent Changes in the Absence of Clinical Nephrotoxicity. Clin. Pharmacol. Ther. 2017, 101, 510–518. [Google Scholar] [CrossRef]
- Miyoshi, T.; Uoi, M.; Omura, F.; Tsumagari, K.; Maesaki, S.; Yokota, C. Risk Factors for Cisplatin-Induced Nephrotoxicity: A Multicenter Retrospective Study. Oncology 2021, 99, 105–113. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, M.J.D.L.; Neefjes, E.C.W.; Toffoli, E.C.; Oosterling-Jansen, J.E.W.; Vergeer, M.R.; Leemans, C.R.; Kooistra, M.P.; Voortman, J.; Verheul, H.M.W. Incidence and Risk Factors for Acute Kidney Injury in Head and Neck Cancer Patients Treated with Concurrent Chemoradiation with High-Dose Cisplatin. BMC Cancer 2019, 19, 1066. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Faig, J.; Haughton, M.; Taylor, R.C.; D’Agostino, R.B.; Whelen, M.J.; Porosnicu Rodriguez, K.A.; Bonomi, M.; Murea, M.; Porosnicu, M. Retrospective Analysis of Cisplatin Nephrotoxicity in Patients with Head and Neck Cancer Receiving Outpatient Treatment With Concurrent High-Dose Cisplatin and Radiotherapy. Am. J. Clin. Oncol. 2018, 41, 432–440. [Google Scholar] [CrossRef]
- Tuladhar, S.M.; Püntmann, V.O.; Soni, M.; Punjabi, P.P.; Bogle, R.G. Rapid Detection of Acute Kidney Injury by Plasma and Urinary Neutrophil Gelatinase-Associated Lipocalin after Cardiopulmonary Bypass. J. Cardiovasc. Pharmacol. 2009, 53, 261–266. [Google Scholar] [CrossRef]
- Elmedany, S.M.; Naga, S.S.; Elsharkawy, R.; Mahrous, R.S.; Elnaggar, A.I. Novel Urinary Biomarkers and the Early Detection of Acute Kidney Injury after Open Cardiac Surgeries. J. Crit. Care 2017, 40, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Liangos, O.; Tighiouart, H.; Perianayagam, M.C.; Kolyada, A.; Han, W.K.; Wald, R.; Bonventre, J.V.; Jaber, B.L. Comparative Analysis of Urinary Biomarkers for Early Detection of Acute Kidney Injury Following Cardiopulmonary Bypass. Biomarkers 2009, 14, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Domański, L.; Dziedziejko, V.; Kajdy, A.; Stefańska, K.; Kwiatkowski, S. The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage. Int. J. Mol. Sci. 2021, 22, 6109. [Google Scholar] [CrossRef]
- Małyszko, J.; Kozłowska, K.; Kozłowski, L.; Małyszko, J. Nephrotoxicity of Anticancer Treatment. Nephrol. Dial. Transplant. 2017, 32, 924–936. [Google Scholar] [CrossRef]
- Pianta, T.J.; Pickering, J.W.; Succar, L.; Chin, M.; Davidson, T.; Buckley, N.A.; Mohamed, F.; Endre, Z.H. Dexamethasone Modifies Cystatin C-Based Diagnosis of Acute Kidney Injury During Cisplatin-Based Chemotherapy. Kidney Blood Press. Res. 2017, 42, 62–75. [Google Scholar] [CrossRef]
- Tekce, B.K.; Uyeturk, U.; Tekce, H.; Uyeturk, U.; Aktas, G.; Akkaya, A. Does the Kidney Injury Molecule-1 Predict Cisplatin-Induced Kidney Injury in Early Stage? Ann. Clin. Biochem. 2015, 52, 88–94. [Google Scholar] [CrossRef]
- Bunel, V.; Tournay, Y.; Baudoux, T.; De Prez, E.; Marchand, M.; Mekinda, Z.; Maréchal, R.; Roumeguère, T.; Antoine, M.-H.; Nortier, J.L. Early Detection of Acute Cisplatin Nephrotoxicity: Interest of Urinary Monitoring of Proximal Tubular Biomarkers. Clin. Kidney J. 2017, 10, 639–647. [Google Scholar] [CrossRef]
- Ghadrdan, E.; Ebrahimpour, S.; Sadighi, S.; Chaibakhsh, S.; Jahangard-Rafsanjani, Z. Evaluation of Urinary Neutrophil Gelatinase-Associated Lipocalin and Urinary Kidney Injury Molecule-1 as Biomarkers of Renal Function in Cancer Patients Treated with Cisplatin. J. Oncol. Pharm. Pract. 2020, 26, 1643–1649. [Google Scholar] [CrossRef]
- McMahon, K.R.; Chui, H.; Rassekh, S.R.; Schultz, K.R.; Blydt-Hansen, T.D.; Mammen, C.; Pinsk, M.; Cuvelier, G.D.E.; Carleton, B.C.; Tsuyuki, R.T.; et al. Urine Neutrophil Gelatinase-Associated Lipocalin and Kidney Injury Molecule-1 to Detect Pediatric Cisplatin-Associated Acute Kidney Injury. Kidney360 2022, 3, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Hall-Craggs, M.; Little, J.R.; Sadler, J.H.; Trump, B.F. Structural Changes Following Hypothermic Preservation of Human Cadaveric Kidneys. Hum. Pathol. 1980, 11, 23–36. [Google Scholar] [CrossRef]
- Brezis, M.; Rosen, S.; Silva, P.; Epstein, F.H. Renal Ischemia: A New Perspective. Kidney Int. 1984, 26, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Brezis, M.; Heyman, S.N. Diversity of Injuries in Renal Ischemia. Int. J. Artif. Organs. 1986, 9, 7–8. [Google Scholar] [CrossRef]
- Dobyan, D.C.; Levi, J.; Jacobs, C.; Kosek, J.; Weiner, M.W. Mechanism of Cis-Platinum Nephrotoxicity: II. Morphologic Observations. J. Pharmacol. Exp. Ther. 1980, 213, 551–556. [Google Scholar]
- Safirstein, R.; Miller, P.; Dikman, S.; Lyman, N.; Shapiro, C. Cisplatin Nephrotoxicity in Rats: Defect in Papillary Hypertonicity. Am. J. Physiol. 1981, 241, F175–F185. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J. Understanding Renal Functional Reserve. Intensive Care Med. 2017, 43, 917–920. [Google Scholar] [CrossRef]
- Sharma, A.; Mucino, M.J.; Ronco, C. Renal Functional Reserve and Renal Recovery after Acute Kidney Injury. Nephron Clin. Pract. 2014, 127, 94–100. [Google Scholar] [CrossRef]
- Spasojević-Dimitrijeva, B.; Kotur-Stevuljević, J.; Đukić, M.; Paripović, D.; Miloševski-Lomić, G.; Spasojević-Kalimanovska, V.; Pavićević, P.; Mitrović, J.; Kostić, M. Serum Neutrophil Gelatinase-Associated Lipocalin and Urinary Kidney Injury Molecule-1 as Potential Biomarkers of Subclinical Nephrotoxicity after Gadolinium-Based and Iodinated-Based Contrast Media Exposure in Pediatric Patients with Normal Kidney Function. Med. Sci. Monit. 2017, 23, 4299–4305. [Google Scholar] [CrossRef]
- Breglia, A.; Godi, I.; Virzì, G.M.; Guglielmetti, G.; Iannucci, G.; De Cal, M.; Brocca, A.; Carta, M.; Giavarina, D.; Ankawi, G.; et al. Subclinical Contrast-Induced Acute Kidney Injury in Patients Undergoing Cerebral Computed Tomography. Cardiorenal. Med. 2020, 10, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A.; Somkereki, C.; Nicoară, T.R.; Oprica, M.; Demian, L. Neutrophil Gelatinase-Associated Lipocalin Monitoring Reveals Persistent Subclinical Kidney Injury Following Intraarterial Administration of Iodinated Contrast Agents. Sci. Rep. 2022, 12, 19464. [Google Scholar] [CrossRef] [PubMed]
- Boutin, L.; Latosinska, A.; Mischak, H.; Deniau, B.; Asakage, A.; Legrand, M.; Gayat, E.; Mebazaa, A.; Chadjichristos, C.E.; Depret, F. Subclinical and Clinical Acute Kidney Injury Share Similar Urinary Peptide Signatures and Prognosis. Intensive Care Med. 2023, 49, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Dépret, F.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Fournier, M.-C.; Jaber, S.; Damoisel, C.; Lu, Q.; Monnet, X.; et al. Incidence and Outcome of Subclinical Acute Kidney Injury Using penKid in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 822–829. [Google Scholar] [CrossRef]

| Cisplatin-Based CTH Group [N = 21] | Non-Cisplatin Based CTH Group [N = 11] | p | |
|---|---|---|---|
| Men [N; (%)] | 14 (66.7%) | 5 (45.4%) | 0.28 |
| Age [yrs] | 56 ± 8 | 58 ± 11 | 0.47 |
| Body mass index [kg/m2] | 25.3 ± 5.4 | 25.2 ± 4.7 | 0.96 |
| Co-morbidity | |||
| Hypertension [N; (%)] | 8 (38.1%) | 6 (54.6%) | 0.46 |
| Diabetes [N; (%)] | 4 (19.0%) | 4 (36.7%) | 0.40 |
| Coronary heart disease [N; (%)] | 3 (14.3%) | 3 (27.3%) | 0.39 |
| ACE-I [N; (%)] | 5 (23.8%) | 4 (36.4%) | 0.68 |
| Cisplatin dose per cycle [mg/m2] | 69 ± 28.8 | - | - |
| Cancer types [N; (%)] | |||
| Head and neck | 9 (42.9%) | - | - |
| Bile duct cancer | 3 (14.3%) | - | - |
| Lung cancer | 2 (9.5%) | 1 (9.1%) | - |
| Gastric cancer | 2 (9.5%) | - | - |
| Gallbladder cancer | 2 (9.5%) | - | - |
| Urinary bladder cancer | 1 (4.8%) | - | - |
| Ovary cancer | - | 5 (45.4%) | - |
| Colon cancer | - | 2 (18.2%) | - |
| Rectal cancer | - | 1 (9.1%) | - |
| Pancreas cancer | - | 1 (9.1%) | - |
| Cancer of unknown origin | 2 (9.5%) | 1 (9.1%) | - |
| Initial serum creatinine in C1 [mg/dL] | 0.65 (0.55–0.91) | 0.57 (0.42–0.91) | 0.40 |
| Initial uNGAL/uCr in C1 [ng/μmol] | 2.85 (1.80–4.79) | 3.42 (1.60–4.25) | 0.78 |
| Initial uKIM-1/uCr in C1 [ng/μmol] | 0.22 (0.11–0.49) | 0.20 (0.11–0.34) | 0.72 |
| Initial uIL-18/uCr in C1 [ng/μmol] | 15.5 (8.2–20.5) | 17.1 (13.0–29.4) | 0.45 |
| Cycle episodes [N] | 68 | 36 | - |
| Patient Number (Cycle) /Measured Marker | Day 1 | Day 2 | Day 3 | Day 4 | ||
|---|---|---|---|---|---|---|
| 015 (C3) | sCr | [mg/dL] | 0.75 | 0.52 | 0.72 | 1.14 |
| uNGAL/uCr | [ng/μmol] | 2.2 | 2.4 | 3.2 | 2.3 | |
| uKIM-1/uCr | [ng/μmol] | 0.35 | 0.25 | 0.58 | 0.74 | |
| uIL-18/uCr | [ng/μmol] | 9.5 | 38.3 | 51.2 | 41.3 | |
| 016 (C1) | sCr | [mg/dL] | 0.65 | 0.48 | 0.67 | 0.95 |
| uNGAL/uCr | [ng/μmol] | 8.1 | 5.9 | 12.5 | 18.4 | |
| uKIM-1/uCr | [ng/μmol] | 0.66 | 0.49 | 2.76 | 3.05 | |
| uIL-18/uCr | [ng/μmol] | 7.2 | 13.0 | 60.2 | 32.8 | |
| 017 (C3) | sCr | [mg/dL] | 0.44 | 0.44 | 0.69 | 0.65 |
| uNGAL/uCr | [ng/μmol] | 2.7 | 4.2 | 36.8 | 130.5 | |
| uKIM-1/uCr | [ng/μmol] | 1.28 | 1.21 | 1.62 | - | |
| uIL-18/uCr | [ng/μmol] | 16.7 | 13.6 | 17.2 | 23.9 | |
| 032 (C1) | sCr | [mg/dL] | 0.91 | 1.03 | 1.24 | 1.58 |
| uNGAL/uCr | [ng/μmol] | 2.8 | 2.7 | 10.4 | 8.1 | |
| uKIM-1/uCr | [ng/μmol] | 0.95 | 0.61 | 1.95 | 1.50 | |
| uIL-18/uCr | [ng/μmol] | 11.4 | 34.5 | 54.9 | 137.1 | |
| Relative Values | No Increased Marker 1 N [%] | One Increased Marker 1 N [%] | Two Increased Markers 1 N [%] | Three Increased Markers 1 N [%] | p | |
|---|---|---|---|---|---|---|
| uNGAL/uCr | Δ0–24 h [%] 2 | 5.2 (−50.3–44.1) | 28.6 (−16.9–63.3) | 42.4 (−23–112.4) | 69.8 (34.5–159) | <0.01 |
| Δ0–48 h [%] 3 | 1.6 (−47.2–29.3) | 9.1 (−28–99.1) | 46.9 (−8.8–137) | 185.5 (129.9–279.6) | <0.001 | |
| uKIM-1/uCr | Δ0–24 h [%] 2 | −27.2 (−49.8–27.5) | 10.1 (−36.2–41) | 19.5 (−26.7–122.4) | 25.5 (−2.1–94.7) | <0.01 |
| Δ0–48 h [%] 3 | −2.5 (−28.8–27.1) | 71.8 (26.6–149.5) | 152.3 (107.1–261.7) | 186.7 (160.8–355.9) | <0.001 | |
| uIL-18/uCr | Δ0–24 h [%] 2 | −14.1 (−43.8–20.8) | 33.4 (−13.6–77.5) | 80.8 (30.1–158.5) | 115.1 (30.8–199.2) | <0.001 |
| Δ0–48 h [%] 3 | −9.3 (−36.5–15.7) | 38.3 (−12.4–111.1) | 136.9 (38.6–243.2) | 261 (122.6–471.6) | <0.001 | |
| sCr [mg/dL] | Δ0–24 h [%] 4 | −0.5 (−8.8–4) | 1.6 (−10.9–6.1) | −2.6 (−7.1–5.2) | 1.2 (−11.4–16.7) | 0.89 |
| Δ0–48 h [%] 5 | −3 (−11.3–3.1) | 1.1 (−8.8–6.9) | −5.2 (−11.8–8.4) | −2.9 (−11.4–16.7) | 0.74 |
| KIM-1 N = 47 [64.2% +] | IL-18 N = 39 [54.1% +] | NGAL N = 30 [41.6% +] | N [%] |
|---|---|---|---|
| + | - | - | 17 [16.7] |
| + | + | - | 14 [13.7] |
| + | - | + | 6 [5.9] |
| + | + | + | 10 [9.8] |
| - | + | - | 11 [10.8] |
| - | - | + | 10 [9.8] |
| - | + | + | 4 [3.9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumilas, D.; Owczarek, A.J.; Brzozowska, A.; Niemir, Z.I.; Olszanecka-Glinianowicz, M.; Chudek, J. The Value of Urinary NGAL, KIM-1, and IL-18 Measurements in the Early Detection of Kidney Injury in Oncologic Patients Treated with Cisplatin-Based Chemotherapy. Int. J. Mol. Sci. 2024, 25, 1074. https://doi.org/10.3390/ijms25021074
Szumilas D, Owczarek AJ, Brzozowska A, Niemir ZI, Olszanecka-Glinianowicz M, Chudek J. The Value of Urinary NGAL, KIM-1, and IL-18 Measurements in the Early Detection of Kidney Injury in Oncologic Patients Treated with Cisplatin-Based Chemotherapy. International Journal of Molecular Sciences. 2024; 25(2):1074. https://doi.org/10.3390/ijms25021074
Chicago/Turabian StyleSzumilas, Dawid, Aleksander Jerzy Owczarek, Aniceta Brzozowska, Zofia Irena Niemir, Magdalena Olszanecka-Glinianowicz, and Jerzy Chudek. 2024. "The Value of Urinary NGAL, KIM-1, and IL-18 Measurements in the Early Detection of Kidney Injury in Oncologic Patients Treated with Cisplatin-Based Chemotherapy" International Journal of Molecular Sciences 25, no. 2: 1074. https://doi.org/10.3390/ijms25021074
APA StyleSzumilas, D., Owczarek, A. J., Brzozowska, A., Niemir, Z. I., Olszanecka-Glinianowicz, M., & Chudek, J. (2024). The Value of Urinary NGAL, KIM-1, and IL-18 Measurements in the Early Detection of Kidney Injury in Oncologic Patients Treated with Cisplatin-Based Chemotherapy. International Journal of Molecular Sciences, 25(2), 1074. https://doi.org/10.3390/ijms25021074








