Cellulase Promotes Mycobacterial Biofilm Dispersal in Response to a Decrease in the Bacterial Metabolite Gamma-Aminobutyric Acid
Abstract
1. Introduction
2. Results
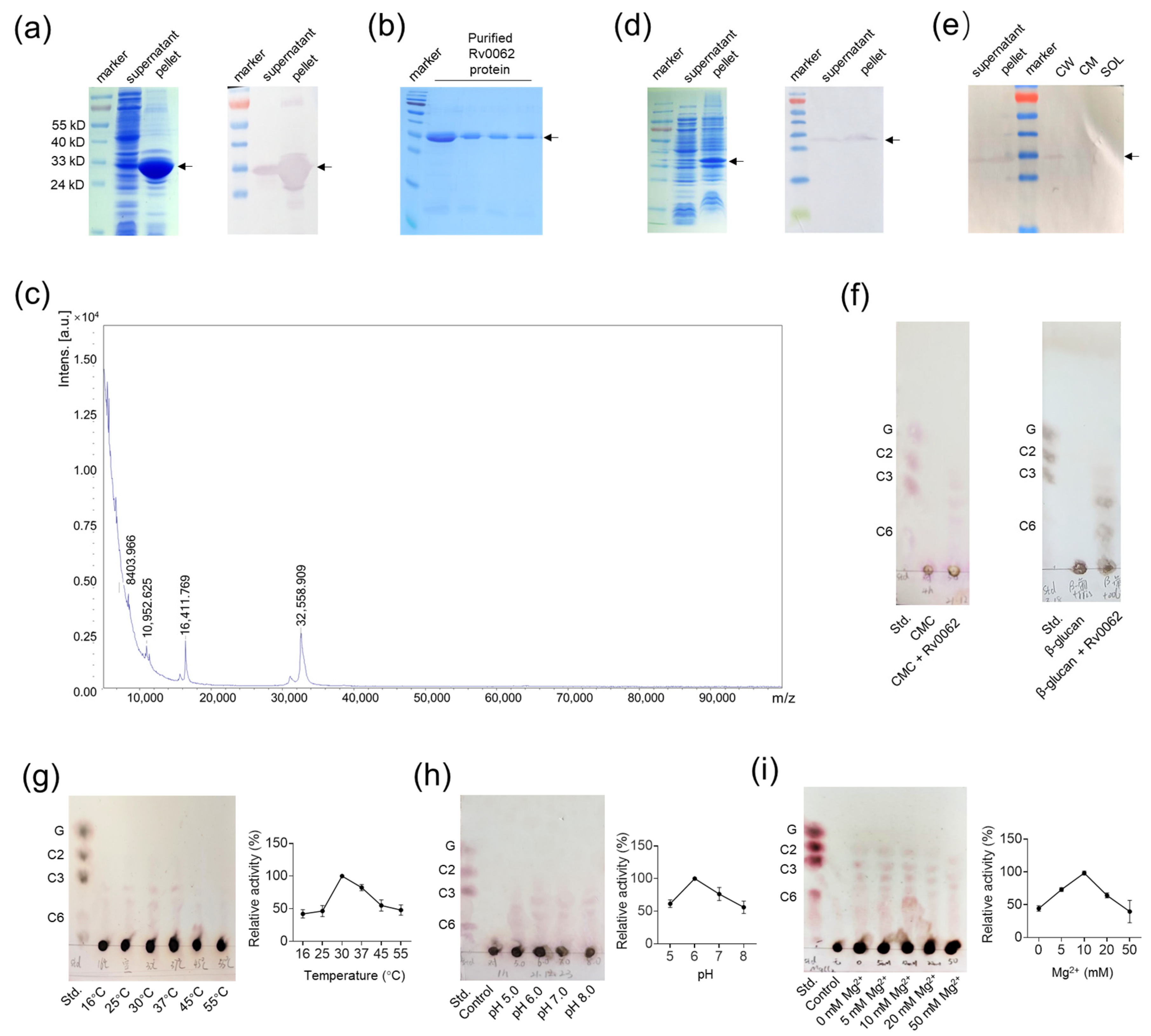
2.1. Overexpression, Purification, and Cellulase Properties of Rv0062 Protein
2.2. Cellulase Hydrolyzes the Cellulose in Mycobacterial Biofilm
2.3. Cellulase Promotes the Dispersal of Mycobacterial Biofilm
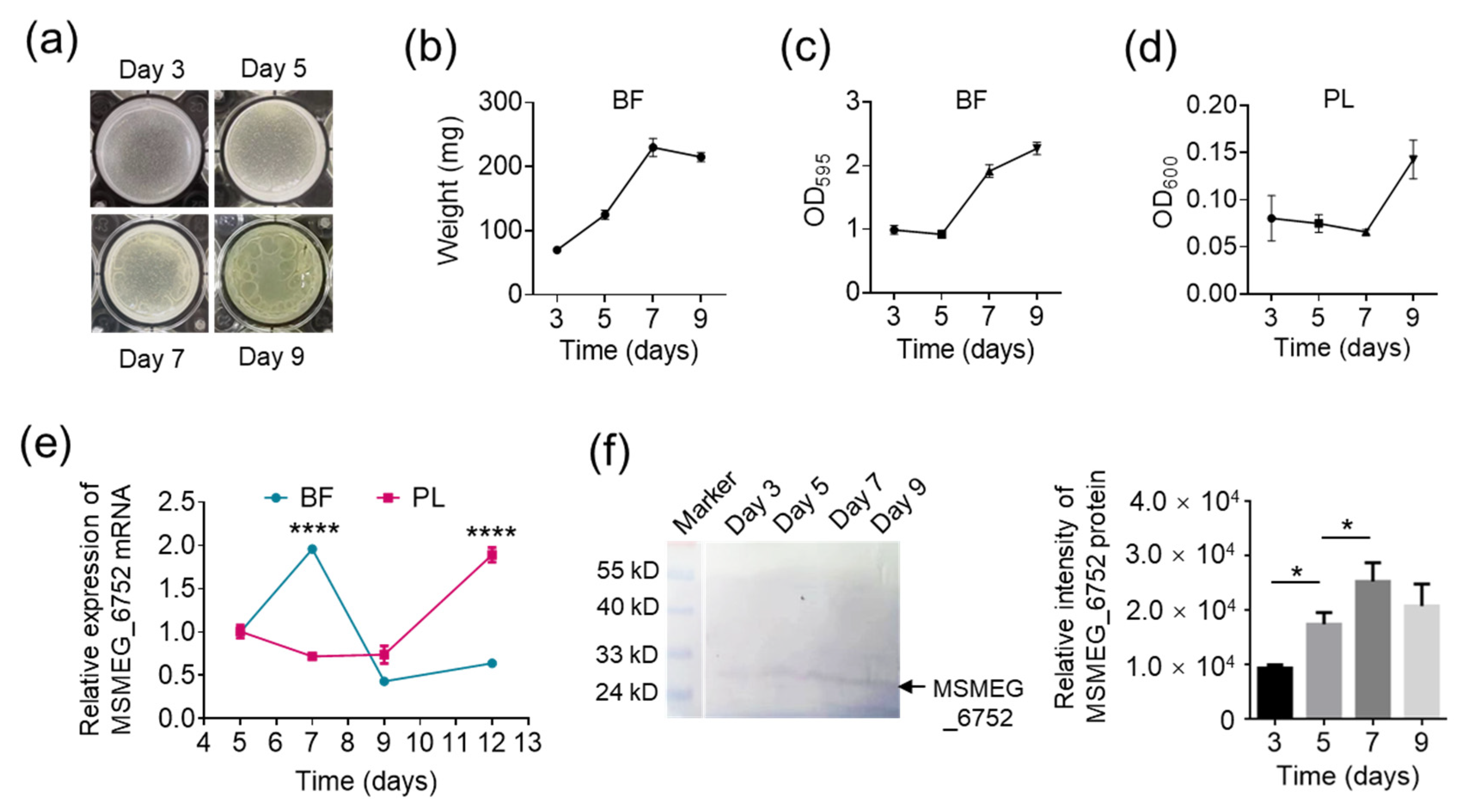
2.4. The Expression of Cellulase Is Upregulated during Mycobacterial Biofilm Dispersal
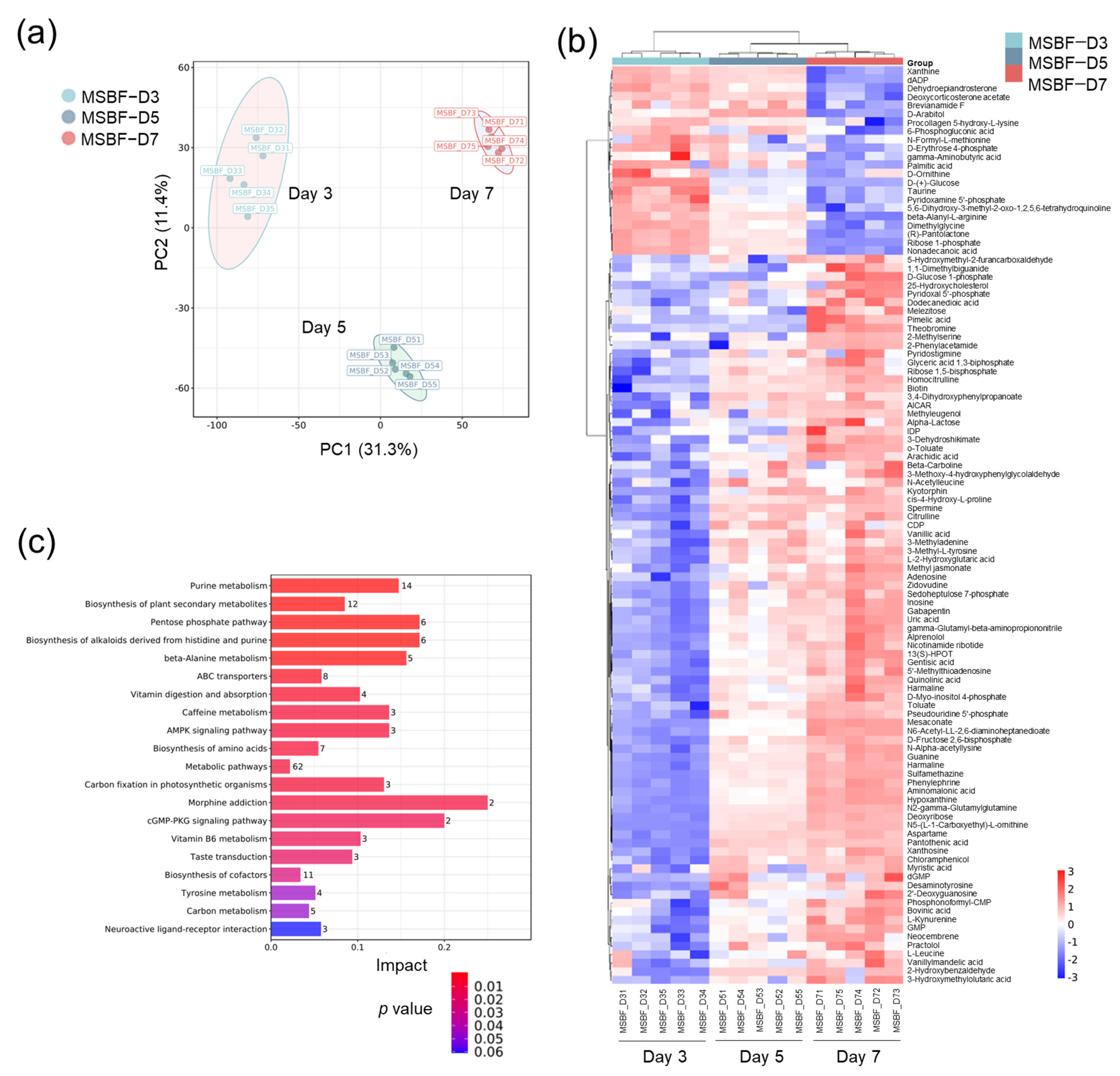
2.5. Metabolome of Mycobacterial Biofilm
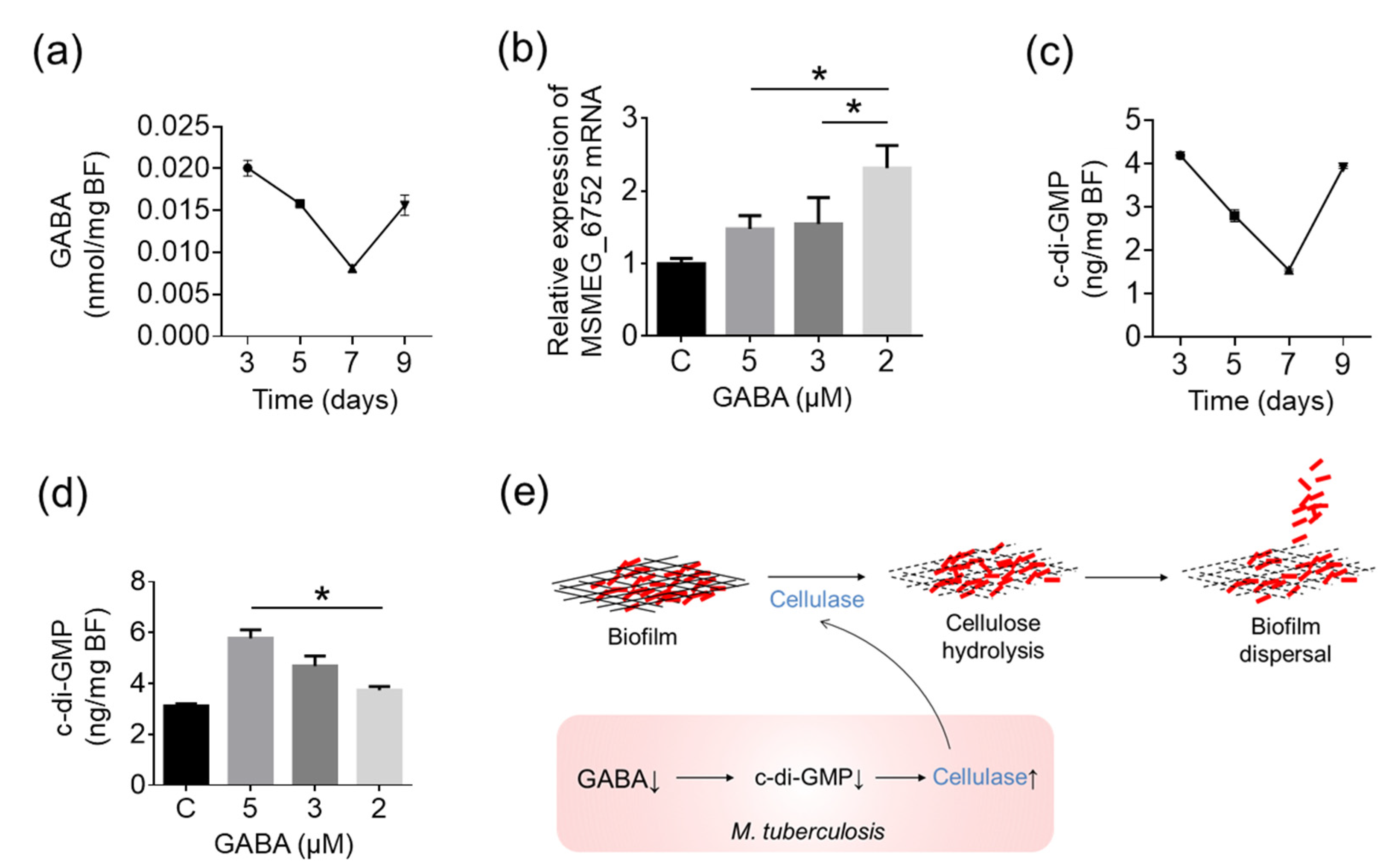
2.6. A Decrease in GABA Induces the Cellulase Expression through the Down-Regulation of the c-di-GMP Level
3. Discussion
4. Materials and Methods
4.1. Overexpression of Rv0062 in E. coli and M. smegmatis Strains
4.2. Purification of Rv0062 Protein
4.3. Cellulase Activity
4.4. TLC Analysis
4.5. Biofilm Growth
4.6. Hydrolysis of Biofilm by Rv0062
4.7. Congo Red Staining
4.8. Crystal Violet Staining
4.9. Isolation of Bacterial RNA and RT-qPCR
4.10. Western Blot
4.11. Infection of Mice
4.12. Lung Histopathology
4.13. Calcofluor White Staining
4.14. Analysis of Bacterial Metabolites in Biofilm LC/MS-MS
4.15. GABA Detection
4.16. Growth of Biofilm In Vitro with a Decrease in the GABA Level
4.17. c-di-GMP Detection
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Kjelleberg, S.; Rice, S.A. Dispersal from Microbial Biofilms. Microbiol. Spectr. 2015, 3, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report. 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports (accessed on 27 October 2022).
- Cantillon, D.; Wroblewska, J.; Cooper, I.; Newport, M.J.; Waddell, S.J. Three-dimensional low shear culture of Mycobacterium bovis BCG induces biofilm formation and antimicrobial drug tolerance. NPJ Biofilms Microbiomes 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Anand, M.; Bhatt, A.; Kremer, L.; Jacobs, W.R., Jr.; Hatfull, G.F. GroEL1: A dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 2005, 123, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.K.; Baughn, A.D.; Sambandan, D.; Hsu, T.; Trivelli, X.; Guerardel, Y.; Alahari, A.; Kremer, L.; Jacobs, W.R., Jr.; Hatfull, G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008, 69, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.K.; Trivelli, X.; Guerardel, Y.; Kremer, L.; Hatfull, G.F. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J. Biol. Chem. 2010, 285, 17380–17389. [Google Scholar] [CrossRef] [PubMed]
- Kulka, K.; Hatfull, G.; Ojha, A.K. Growth of Mycobacterium tuberculosis biofilms. J. Vis. Exp. 2012, 60, 3820. [Google Scholar]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Ojha, A.K. Mycobacterial Biofilms: Revisiting Tuberculosis Bacilli in Extracellular Necrotizing Lesions. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Nyka, W. Studies on Mycobacterium tuberculosis in lesions of the human lung. A new method of staining tubercle bacilli in tissue sections. Am. Rev. Respir. Dis. 1963, 88, 670–679. [Google Scholar]
- Wong, K.W.; Jacobs, W.R., Jr. Postprimary Tuberculosis and Macrophage Necrosis: Is There a Big ConNECtion? mBio 2016, 7, e01589-15. [Google Scholar] [CrossRef] [PubMed]
- Orme, I.M. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis 2014, 94, 8–14. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bajeli, S.; Kaushal, D.; Radotra, B.D.; Kumar, A. Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat. Commun. 2021, 12, 1606. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm dispersal: Multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005, 13, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Trivedi, A.; Mavi, P.S.; Bhatt, D.; Kumar, A. Thiol reductive stress induces cellulose-anchored biofilm formation in Mycobacterium tuberculosis. Nat. Commun. 2016, 7, 11392. [Google Scholar] [CrossRef]
- Mba Medie, F.; Ben Salah, I.; Drancourt, M.; Henrissat, B. Paradoxical conservation of a set of three cellulose-targeting genes in Mycobacterium tuberculosis complex organisms. Microbiology 2010, 156, 1468–1475. [Google Scholar] [CrossRef]
- Varrot, A.; Leydier, S.; Pell, G.; Macdonald, J.M.; Stick, R.V.; Henrissat, B.; Gilbert, H.J.; Davies, G.J. Mycobacterium tuberculosis strains possess functional cellulases. J. Biol. Chem. 2005, 280, 20181–20184. [Google Scholar] [CrossRef]
- Van Wyk, N.; Navarro, D.; Blaise, M.; Berrin, J.G.; Henrissat, B.; Drancourt, M.; Kremer, L. Characterization of a mycobacterial cellulase and its impact on biofilm- and drug-induced cellulose production. Glycobiology 2017, 27, 392–399. [Google Scholar] [CrossRef]
- Sha, S.; Shi, X.; Deng, G.; Chen, L.; Xin, Y.; Ma, Y. Mycobacterium tuberculosis Rv1987 induces Th2 immune responses and enhances Mycobacterium smegmatis survival in mice. Microbiol Res. 2017, 197, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.B.; Dhakshnamoorthy, V.; Lee, C.R. Molecular characterization of SCO0765 as a cellotriose releasing endo-β-1,4-cellulase from Streptomyces coelicolor A(3). J. Microbiol. 2016, 54, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Zhang, Z.; Zhu, Z.; Sathitsuksanoh, N.; Yang, Y.; Zhang, Y.H. The noncellulosomal family 48 cellobiohydrolase from Clostridium phytofermentans ISDg: Heterologous expression, characterization, and processivity. Appl. Microbiol. Biotechnol. 2010, 86, 525–533. [Google Scholar] [CrossRef]
- Dagorn, A.; Chapalain, A.; Mijouin, L.; Hillion, M.; Duclairoir-Poc, C.; Chevalier, S.; Taupin, L.; Orange, N.; Feuilloley, M.G. Effect of GABA, a bacterial metabolite, on Pseudomonas fluorescens surface properties and cytotoxicity. Int. J. Mol. Sci. 2013, 14, 12186–12204. [Google Scholar] [CrossRef]
- Dagorn, A.; Hillion, M.; Chapalain, A.; Lesouhaitier, O.; Duclairoir Poc, C.; Vieillard, J.; Chevalier, S.; Taupin, L.; Le Derf, F.; Feuilloley, M.G.J. Gamma-aminobutyric acid acts as a specific virulence regulator in Pseudomonas aeruginosa. Microbiology 2013, 159, 339–351. [Google Scholar] [CrossRef]
- Gallegos-Monterrosa, R.; Kankel, S.; Götze, S.; Barnett, R.; Stallforth, P.; Kovács, Á.T. Lysinibacillus fusiformis M5 Induces Increased Complexity in Bacillus subtilis 168 Colony Biofilms via Hypoxanthine. J. Bacteriol. 2017, 199, e00204-17. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Mallard, C.; Barke, T.; Hancock, L.E.; Self, W.T. A selenium-dependent xanthine dehydrogenase triggers biofilm proliferation in Enterococcus faecalis through oxidant production. J. Bacteriol. 2011, 193, 1643–1652. [Google Scholar] [CrossRef]
- Li, B.; Maezato, Y.; Kim, S.H.; Kurihara, S.; Liang, J.; Michael, A.J. Polyamine-independent growth and biofilm formation, and functional spermidine/spermine N-acetyltransferases in Staphylococcus aureus and Enterococcus faecalis. Mol. Microbiol. 2019, 111, 159–175. [Google Scholar] [CrossRef]
- Simm, R.; Morr, M.; Kader, A.; Nimtz, M.; Römling, U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004, 53, 1123–1134. [Google Scholar] [CrossRef]
- Boehm, A.; Steiner, S.; Zaehringer, F.; Casanova, A.; Hamburger, F.; Ritz, D.; Keck, W.; Ackermann, M.; Schirmer, T.; Jenal, U. Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 2009, 72, 1500–1516. [Google Scholar] [CrossRef]
- Gjermansen, M.; Nilsson, M.; Yang, L.; Tolker-Nielsen, T. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: Genetic elements and molecular mechanisms. Mol. Microbiol. 2010, 75, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Newell, P.D.; Monds, R.D.; O’Toole, G.A. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. USA 2009, 106, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Chakrabarty, A.M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1994, 60, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Meyenhofer, M.F.; Fine, D.H. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J. Bacteriol. 2003, 185, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Ragunath, C.; Ramasubbu, N.; Fine, D.H. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J. Bacteriol. 2003, 185, 4693–4698. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Cullen, M.C.; Rickard, A.H.; Zeef, L.A.; Davies, D.G.; Gilbert, P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 2004, 186, 7312–7326. [Google Scholar] [CrossRef] [PubMed]
- Schleheck, D.; Barraud, N.; Klebensberger, J.; Webb, J.S.; McDougald, D.; Rice, S.A.; Kjelleberg, S. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS ONE 2009, 4, e5513. [Google Scholar] [CrossRef]
- Thormann, K.M.; Saville, R.M.; Shukla, S.; Spormann, A.M. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 2005, 187, 1014–1021. [Google Scholar] [CrossRef]
- Hay, A.J.; Zhu, J. Host intestinal signal-promoted biofilm dispersal induces Vibrio cholerae colonization. Infect. Immun. 2015, 83, 317–323. [Google Scholar] [CrossRef]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Maddula, V.S.; Pierson, E.A.; Pierson, L.S., 3rd. Altering the ratio of phenazines in Pseudomonas chlororaphis (aureofaciens) strain 30-84: Effects on biofilm formation and pathogen inhibition. J. Bacteriol. 2008, 190, 2759–2766. [Google Scholar] [CrossRef]
- Freeman, R.; Geier, H.; Weigel, K.M.; Do, J.; Ford, T.E.; Cangelosi, G.A. Roles for cell wall glycopeptidolipid in surface adherence and planktonic dispersal of Mycobacterium avium. Appl. Environ. Microbiol. 2006, 72, 7554–7558. [Google Scholar] [CrossRef]
- Noe, F.F.; Nickerson, W.J. Metabolism of 2-pyrrolidone and gamma-aminobutyric acid by Pseudomonas aeruginosa. J. Bacteriol. 1958, 75, 674–681. [Google Scholar] [CrossRef]
- Tunnicliff, G. Inhibition of 4-aminobutyrate aminotransferase from Pseudomonas fluorescens by ATP. Biochem. Mol. Biol. Int. 1993, 31, 41–47. [Google Scholar]
- Richard, H.T.; Foster, J.W. Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 2003, 52, 167–186. [Google Scholar]
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; de Vroom, E.; van der Marel, G.A.; van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Sharma, I.M.; Prakash, S.; Dhanaraman, T.; Chatterji, D. Characterization of a dual active enzyme, DcpA, involved in cyclic-di-guanosine monophosphate turnover in Mycobacterium smegmatis. Microbiology 2014, 160, 2304–2314. [Google Scholar] [CrossRef][Green Version]
- Gupta, K.; Kumar, P.; Chatterji, D. Identification, activity and disulfide connectivity of c-di-GMP regulating proteins in Mycobacterium tuberculosis. PLoS ONE 2010, 5, e15072. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Q.; Deng, W.; Chen, T.; Liu, M.; Xie, J. Mycobacterium tuberculosis Rv3402c enhances mycobacterial survival within macrophages and modulates the host pro-inflammatory cytokines production via NF-kappa B/ERK/p38 signaling. PLoS ONE 2014, 9, e94418. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Izzo, A.A.; Brandt, L.; Orme, I.M. Decreased survival of guinea pigs infected with Mycobacterium tuberculosis after multiple BCG vaccinations. Vaccine 2006, 24, 280–286. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Hu, J.; Leng, G.; Liu, X.; Cui, Z.; Wang, W.; Ma, Y.; Sha, S. Cellulase Promotes Mycobacterial Biofilm Dispersal in Response to a Decrease in the Bacterial Metabolite Gamma-Aminobutyric Acid. Int. J. Mol. Sci. 2024, 25, 1051. https://doi.org/10.3390/ijms25021051
Zhang J, Liu Y, Hu J, Leng G, Liu X, Cui Z, Wang W, Ma Y, Sha S. Cellulase Promotes Mycobacterial Biofilm Dispersal in Response to a Decrease in the Bacterial Metabolite Gamma-Aminobutyric Acid. International Journal of Molecular Sciences. 2024; 25(2):1051. https://doi.org/10.3390/ijms25021051
Chicago/Turabian StyleZhang, Jiaqi, Yingying Liu, Junxing Hu, Guangxian Leng, Xining Liu, Zailin Cui, Wenzhen Wang, Yufang Ma, and Shanshan Sha. 2024. "Cellulase Promotes Mycobacterial Biofilm Dispersal in Response to a Decrease in the Bacterial Metabolite Gamma-Aminobutyric Acid" International Journal of Molecular Sciences 25, no. 2: 1051. https://doi.org/10.3390/ijms25021051
APA StyleZhang, J., Liu, Y., Hu, J., Leng, G., Liu, X., Cui, Z., Wang, W., Ma, Y., & Sha, S. (2024). Cellulase Promotes Mycobacterial Biofilm Dispersal in Response to a Decrease in the Bacterial Metabolite Gamma-Aminobutyric Acid. International Journal of Molecular Sciences, 25(2), 1051. https://doi.org/10.3390/ijms25021051





