Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation
Abstract
1. Introduction
2. Results
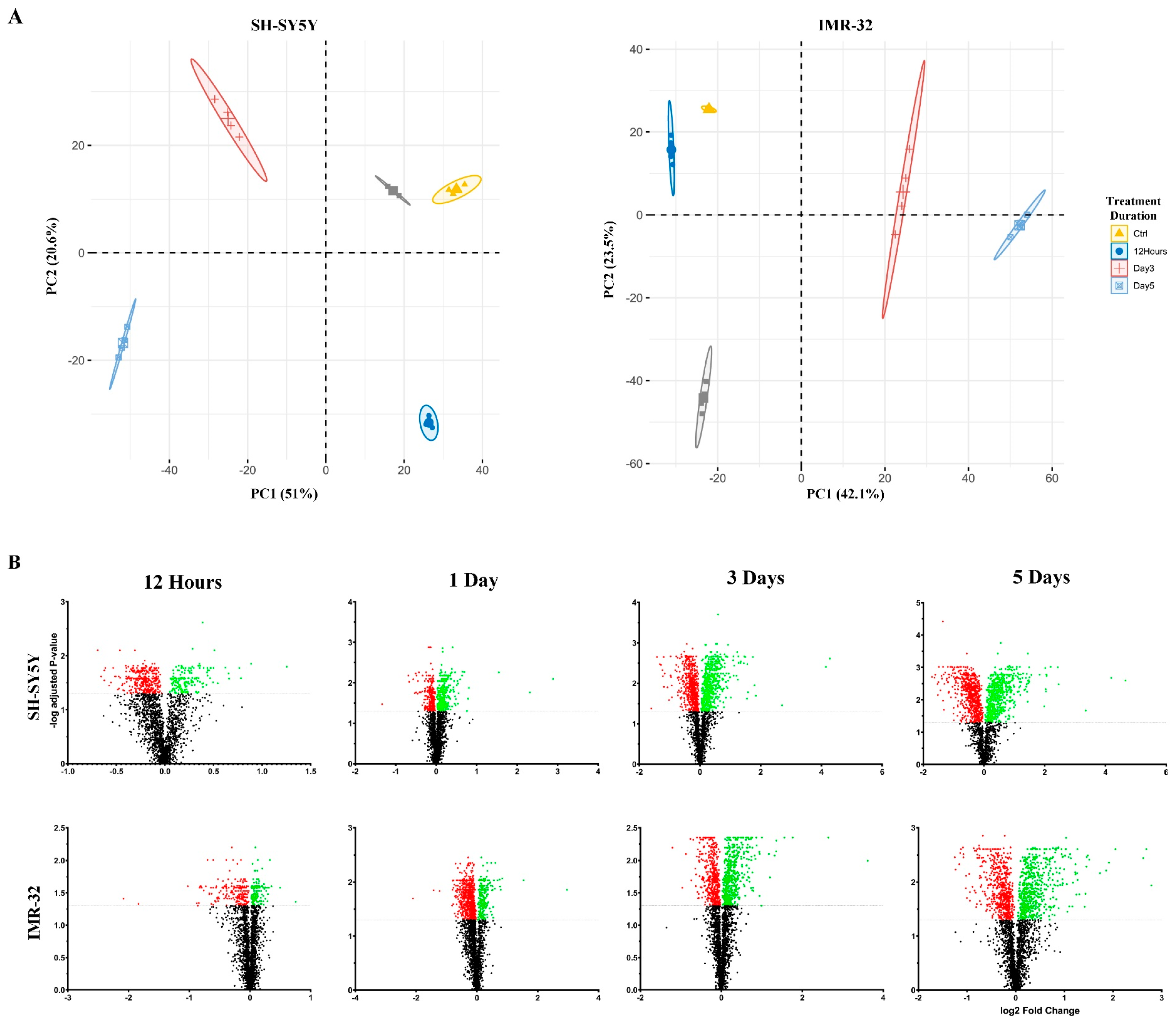
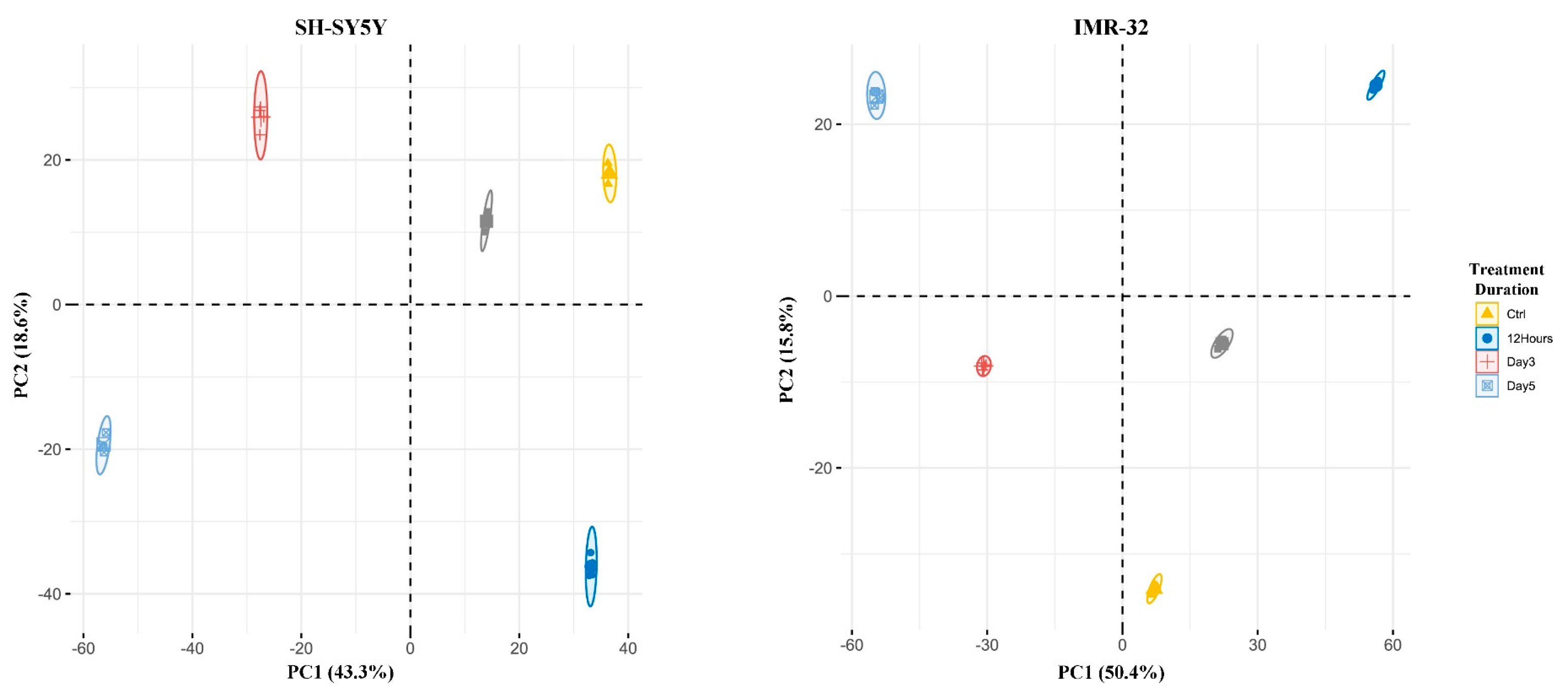
2.1. Quantitative Proteomic Profiling Reveals Temporal Remodeling of the Proteome during Neuronal Differentiation
2.2. Functional Enrichment Analysis Highlights Biological Processes Altered during Differentiation
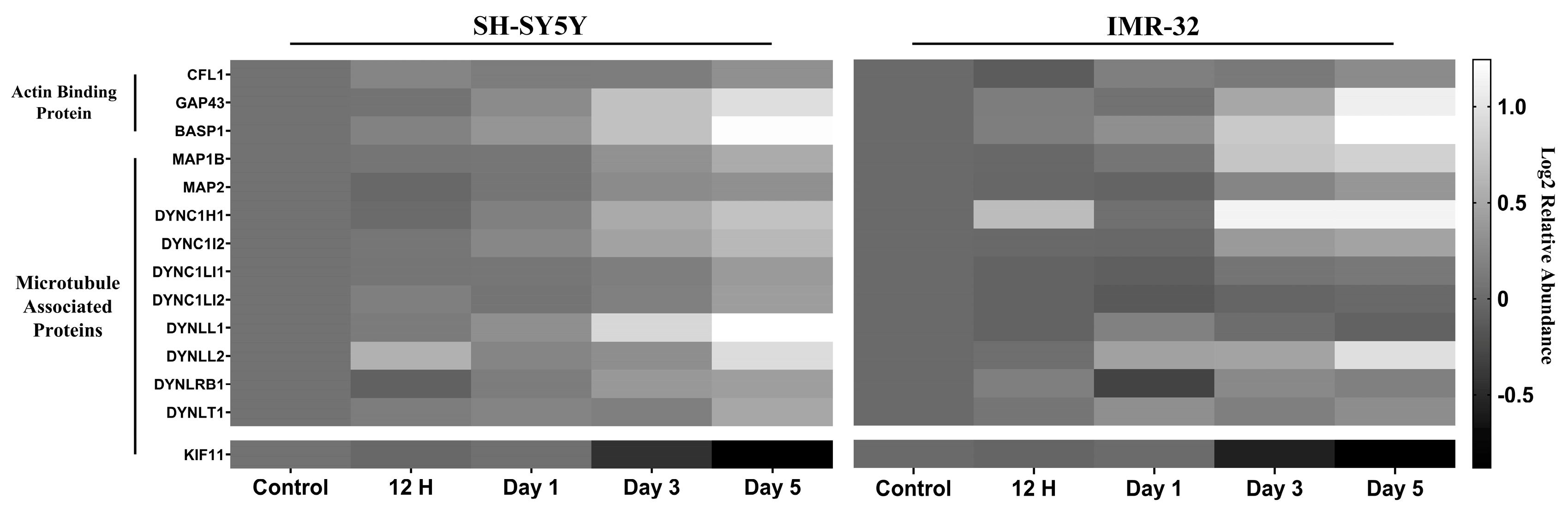
2.2.1. Differentiation Alters Expression Cytoskeletal Proteins
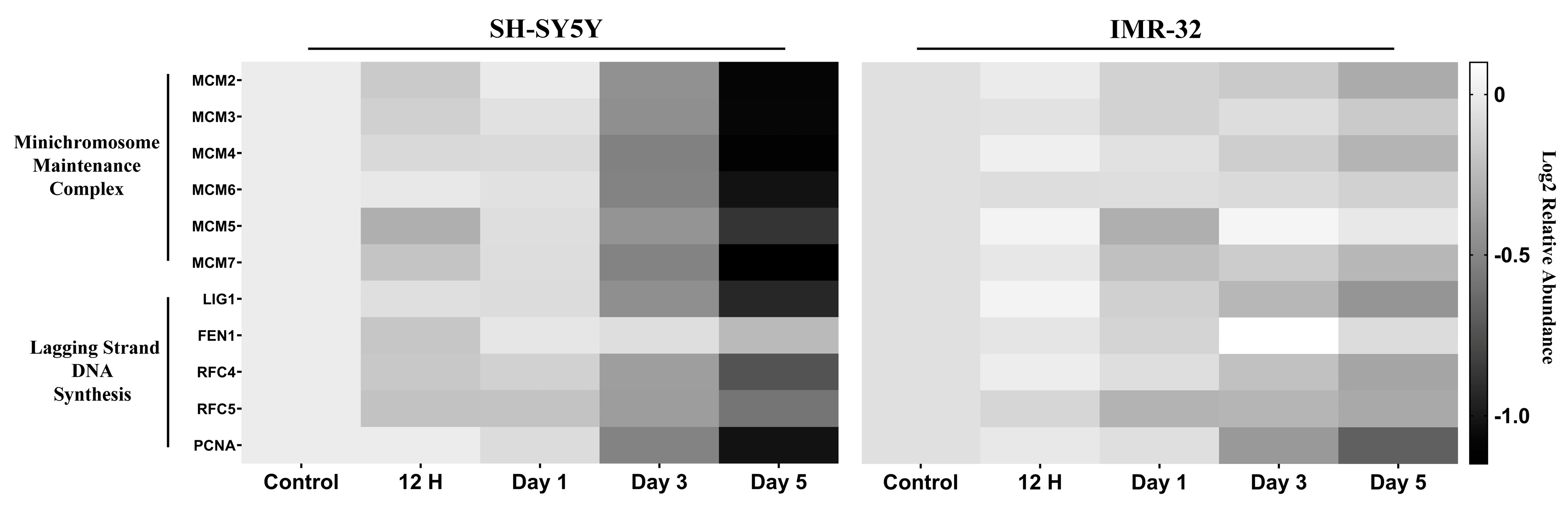
2.2.2. DNA Replication and Cell Cycle Proteins Are Downregulated upon Differentiation
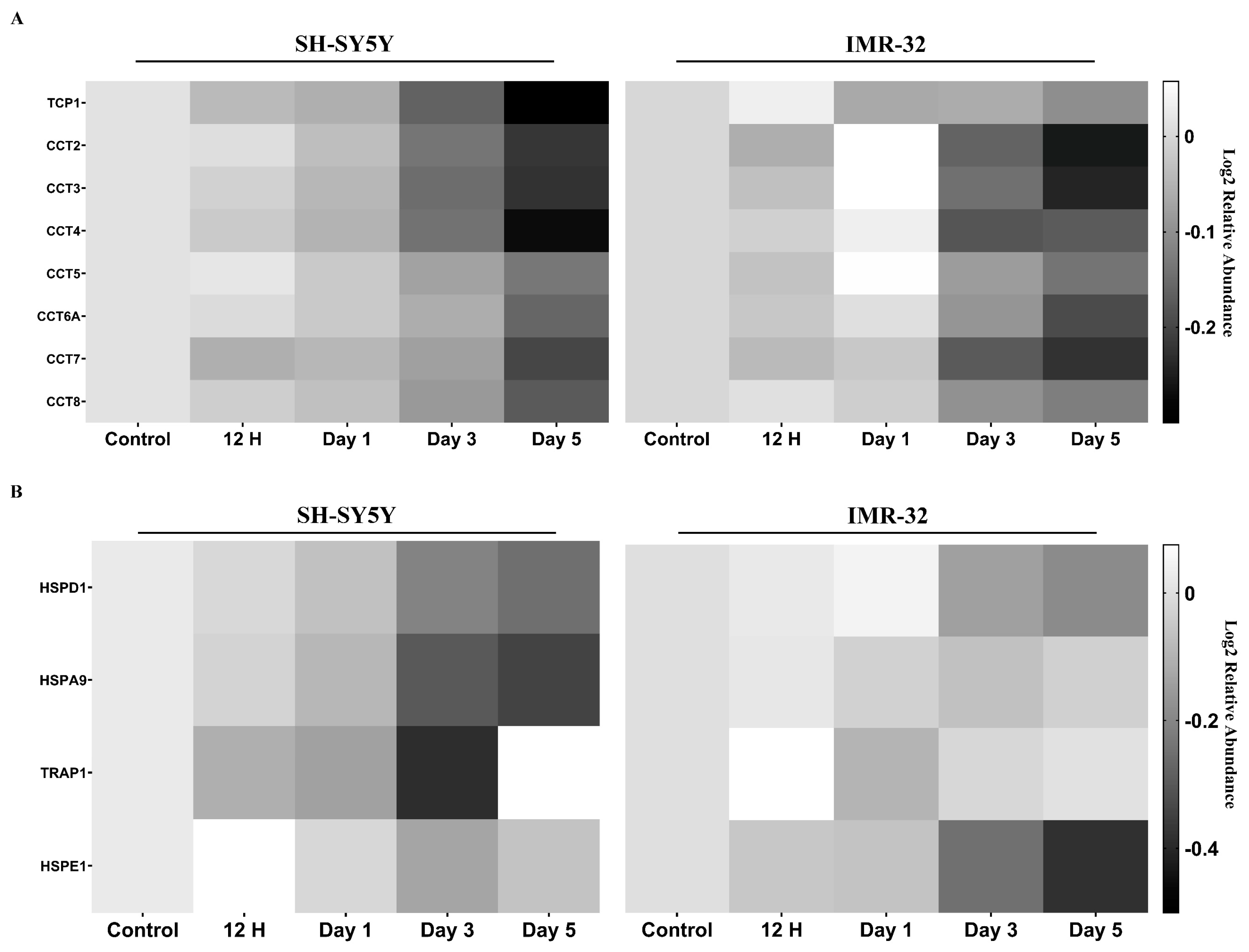
2.2.3. Molecular Chaperones Are Downregulated in Differentiated Cells
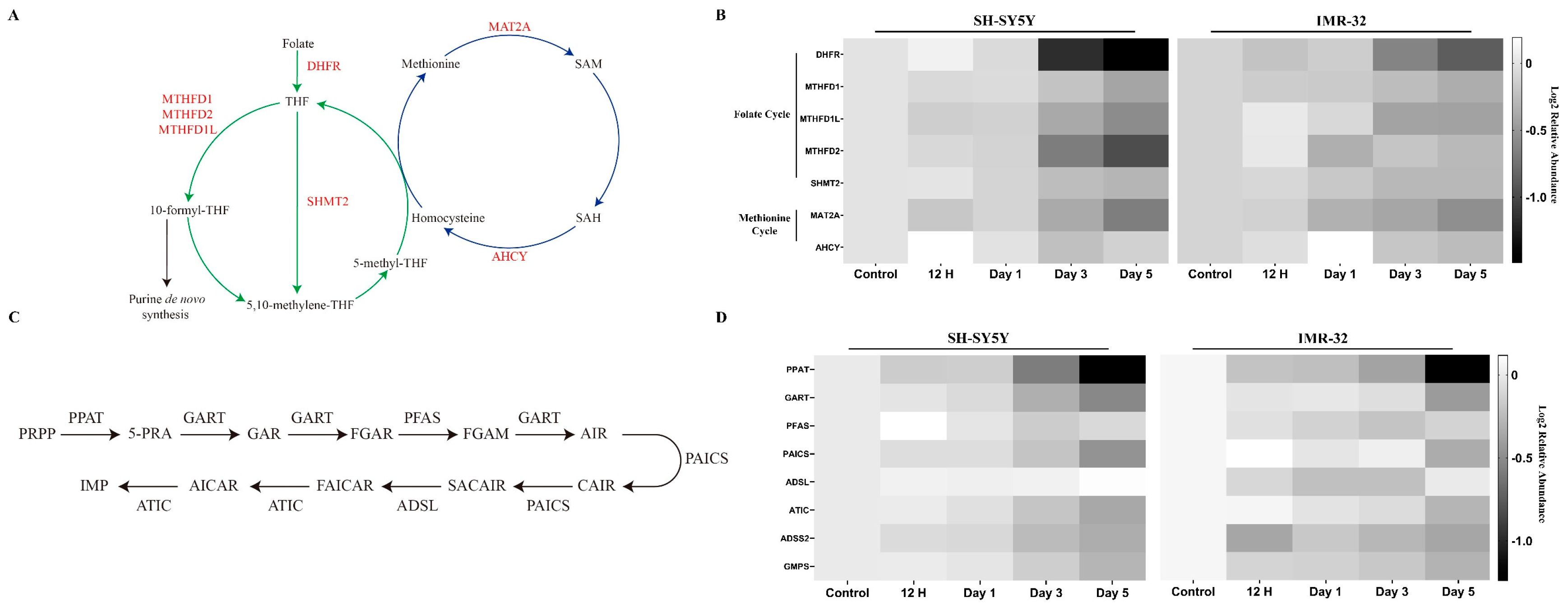
2.2.4. Key Enzymes in One-Carbon Metabolism and Purine Synthesis Are Reduced during Differentiation
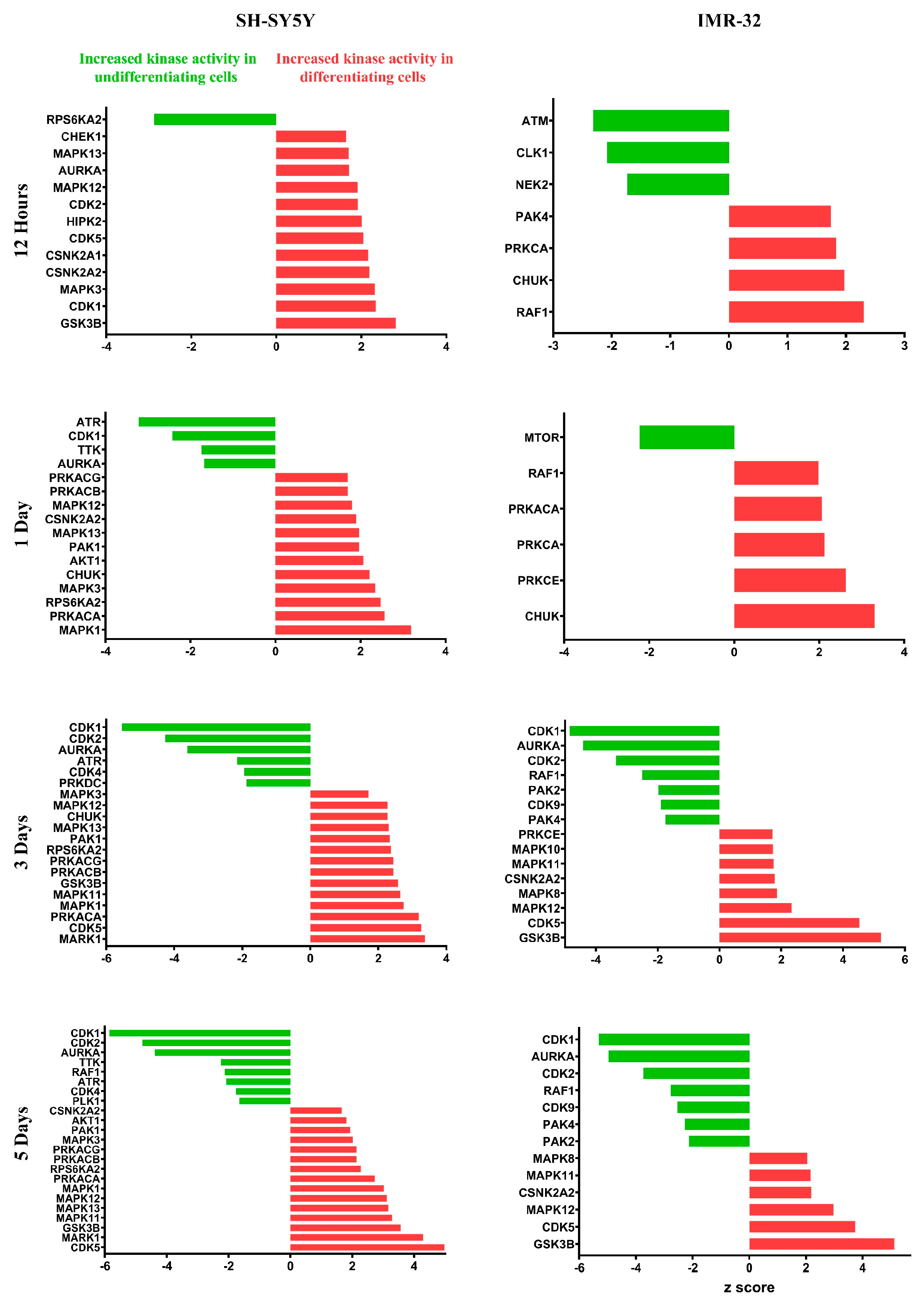
2.3. Quantitative Phosphoproteomic Profiling Reveals Alterations in Cytoskeletal Regulation and Cell Cycle Processes Following ATRA Treatment
3. Discussion
3.1. Cell Cycle Dynamics and Regulation of DNA Replication
3.2. Cytoskeletal Remodeling
3.3. Alterations in Molecular Chaperones
3.4. Metabolic Reprogramming of Folate, Methionine, and Purine Pathways
4. Materials and Methods
4.1. Cell Cultures and Treatment
4.2. Sample Preparation
4.3. Phosphopeptide Enrichment
4.4. LC-MS/MS Analysis
4.5. Proteomic Data Analysis
4.6. Statistical Analysis and Data Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stepchenko, A.G.; Portseva, T.N.; Ilyin, Y.V.; Georgieva, S.G.; Pankratova, E.V. Differentiation of IMR32 Neuroblastoma Is Accompanied by a Global Change in the Transcriptome. Dokl. Biochem. Biophys. 2021, 497, 90–94. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. In Neuronal Cell Culture; Amini, S., White, M.K., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1078, pp. 9–21. ISBN 978-1-62703-639-9. [Google Scholar]
- Neill, D.; Hughes, D.; Edwardson, J.A.; Rima, B.K.; Allsop, D. Human IMR-32 Neuroblastoma Cells as a Model Cell Line in Alzheimer’s Disease Research. J. Neurosci. Res. 1994, 39, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.W.K.; Cheung, W.M.W.; Kwong, Y.L. Differential Effects of 9-Cis, 13-Cis and All-Trans Retinoic Acids on the Neuronal Differentiation of Human Neuroblastoma Cells. NeuroReport 2003, 14, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gygi, S.P.; Paulo, J.A. Temporal Proteomic Profiling of SH-SY5Y Differentiation with Retinoic Acid Using FAIMS and Real-Time Searching. J. Proteome Res. 2021, 20, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Toto Nienguesso, A.; Navarrete Santos, A.; Schmidt, C. Quantitative Proteomics and In-Cell Cross-Linking Reveal Cellular Reorganisation during Early Neuronal Differentiation of SH-SY5Y Cells. Commun. Biol. 2022, 5, 551. [Google Scholar] [CrossRef] [PubMed]
- Murillo, J.R.; Goto-Silva, L.; Sánchez, A.; Nogueira, F.C.S.; Domont, G.B.; Junqueira, M. Quantitative Proteomic Analysis Identifies Proteins and Pathways Related to Neuronal Development in Differentiated SH-SY5Y Neuroblastoma Cells. EuPA Open Proteom. 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Puty, B.; Bittencourt, L.O.; Nogueira, I.C.; Buzalaf, M.A.R.; Oliveira, E.H.; Lima, R.R. Human Cultured IMR-32 Neuronal-like and U87 Glial-like Cells Have Different Patterns of Toxicity under Fluoride Exposure. PLoS ONE 2021, 16, e0251200. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.R.; Tam, N.W.; Teixeira, A.G.; Frampton, J.P. SH-SY5Y and LUHMES Cells Display Differential Sensitivity to MPP+, Tunicamycin, and Epoxomicin in 2D and 3D Cell Culture. Biotechnol. Prog. 2020, 36, e2942. [Google Scholar] [CrossRef]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of All-Trans-Retinoic Acid on Human SH-SY5Y Neuroblastoma as in Vitro Model in Neurotoxicity Research. NeuroToxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashler, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-Anchored Aspartyl Protease with Alzheimer’s Disease β-Secretase Activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef]
- Bell, M.; Zempel, H. SH-SY5Y-Derived Neurons: A Human Neuronal Model System for Investigating TAU Sorting and Neuronal Subtype-Specific TAU Vulnerability. Rev. Neurosci. 2022, 33, 1–15. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; Júnior, M.L.C.D.F.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between Proliferative and Neuron-like SH-SY5Y Cells as an in Vitro Model for Parkinson Disease Studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; McNeill, E.M.; Muley, P.D. Role of All- Trans Retinoic Acid in Neurite Outgrowth and Axonal Elongation. J. Neurobiol. 2006, 66, 739–756. [Google Scholar] [CrossRef]
- Bochman, M.L.; Schwacha, A. The Mcm Complex: Unwinding the Mechanism of a Replicative Helicase. Microbiol. Mol. Biol. Rev. 2009, 73, 652–683. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell. Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Bahr, T.; Katuri, J.; Liang, T.; Bai, Y. Mitochondrial Chaperones in Human Health and Disease. Free Radic. Biol. Med. 2022, 179, 363–374. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the Brotherhood of the Mitochondrial Chaperones Mortalin and Heat Shock Protein 60. Cell Stress Chaperones 2006, 11, 116. [Google Scholar] [CrossRef]
- Cicero, S.; Herrup, K. Cyclin-Dependent Kinase 5 Is Essential for Neuronal Cell Cycle Arrest and Differentiation. J. Neurosci. 2005, 25, 9658–9668. [Google Scholar] [CrossRef]
- Tsai, L.-H.; Takahashi, T.; Caviness, V.S.; Harlow, E. Activity and Expression Pattern of Cyclin-Dependent Kinase 5 in the Embryonic Mouse Nervous System. Development 1993, 119, 1029–1040. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Loss of Cdk2 and Cdk4 Induces a Switch from Proliferation to Differentiation in Neural Stem Cells. Stem Cells 2012, 30, 1509–1520. [Google Scholar] [CrossRef]
- Nikolic, M.; Dudek, H.; Kwon, Y.T.; Ramos, Y.F.; Tsai, L.H. The Cdk5/P35 Kinase Is Essential for Neurite Outgrowth during Neuronal Differentiation. Genes Dev. 1996, 10, 816–825. [Google Scholar] [CrossRef]
- Milletti, G.; Colicchia, V.; Cecconi, F. Cyclers’ Kinases in Cell Division: From Molecules to Cancer Therapy. Cell Death Differ. 2023, 30, 2035–2052. [Google Scholar] [CrossRef]
- Busino, L.; Chiesa, M.; Draetta, G.F.; Donzelli, M. Cdc25A Phosphatase: Combinatorial Phosphorylation, Ubiquitylation and Proteolysis. Oncogene 2004, 23, 2050–2056. [Google Scholar] [CrossRef]
- Braithwaite, A.W.; Del Sal, G.; Lu, X. Some P53-Binding Proteins That Can Function as Arbiters of Life and Death. Cell Death Differ. 2006, 13, 984–993. [Google Scholar] [CrossRef]
- Maiorano, D.; Lutzmann, M.; Méchali, M. MCM Proteins and DNA Replication. Curr. Opin. Cell Biol. 2006, 18, 130–136. [Google Scholar] [CrossRef]
- Ibarra, A.; Schwob, E.; Méndez, J. Excess MCM Proteins Protect Human Cells from Replicative Stress by Licensing Backup Origins of Replication. Proc. Natl. Acad. Sci. USA 2008, 105, 8956–8961. [Google Scholar] [CrossRef]
- Blair, K.; Tehseen, M.; Raducanu, V.-S.; Shahid, T.; Lancey, C.; Rashid, F.; Crehuet, R.; Hamdan, S.M.; De Biasio, A. Mechanism of Human Lig1 Regulation by PCNA in Okazaki Fragment Sealing. Nat. Commun. 2022, 13, 7833. [Google Scholar] [CrossRef]
- Stodola, J.L.; Burgers, P.M. Mechanism of Lagging-Strand DNA Replication in Eukaryotes. In DNA Replication; Masai, H., Foiani, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1042, pp. 117–133. ISBN 978-981-10-6954-3. [Google Scholar]
- Salomoni, P.; Calegari, F. Cell Cycle Control of Mammalian Neural Stem Cells: Putting a Speed Limit on G1. Trends Cell Biol. 2010, 20, 233–243. [Google Scholar] [CrossRef]
- Lange, C.; Calegari, F. Cdks and Cyclins Link G1 Length and Differentiation of Embryonic, Neural and Hematopoietic Stem Cells. Cell Cycle 2010, 9, 1893–1900. [Google Scholar] [CrossRef]
- Ambriz, X.; De Lanerolle, P.; Ambrosio, J.R. The Mechanobiology of the Actin Cytoskeleton in Stem Cells during Differentiation and Interaction with Biomaterials. Stem Cells Int. 2018, 2018, 2891957. [Google Scholar] [CrossRef]
- Del Castillo, U.; Norkett, R.; Gelfand, V.I. Unconventional Roles of Cytoskeletal Mitotic Machinery in Neurodevelopment. Trends Cell Biol. 2019, 29, 901–911. [Google Scholar] [CrossRef]
- Preis, P.N.; Saya, H.; Nádasdi, L.; Hochhaus, G.; Levin, V.; Sadée, W. Neuronal Cell Differentiation of Human Neuroblastoma Cells by Retinoic Acid plus Herbimycin A. Cancer Res. 1988, 48, 6530–6534. [Google Scholar]
- Moriyama, K.; Iida, K.; Yahara, I. Phosphorylation of Ser-3 of Cofilin Regulates Its Essential Function on Actin. Genes Cells 1996, 1, 73–86. [Google Scholar] [CrossRef]
- Marsick, B.M.; Flynn, K.C.; Santiago-Medina, M.; Bamburg, J.R.; Letourneau, P.C. Activation of ADF/Cofilin Mediates Attractive Growth Cone Turning toward Nerve Growth Factor and Netrin-1. Dev. Neurobiol. 2010, 70, 565–588. [Google Scholar] [CrossRef]
- Miller, K.E.; Suter, D.M. An Integrated Cytoskeletal Model of Neurite Outgrowth. Front. Cell. Neurosci. 2018, 12, 447. [Google Scholar] [CrossRef]
- Mosevitsky, M.I. Nerve Ending “Signal” Proteins GAP-43, MARCKS, and BASP1. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 245, pp. 245–325. ISBN 978-0-12-364649-1. [Google Scholar]
- Laux, T.; Fukami, K.; Thelen, M.; Golub, T.; Frey, D.; Caroni, P. Gap43, Marcks, and Cap23 Modulate Pi(4,5)P2 at Plasmalemmal Rafts, and Regulate Cell Cortex Actin Dynamics through a Common Mechanism. J. Cell Biol. 2000, 149, 1455–1472. [Google Scholar] [CrossRef]
- Galjart, N.; Perez, F. A Plus-End Raft to Control Microtubule Dynamics and Function. Curr. Opin. Cell Biol. 2003, 15, 48–53. [Google Scholar] [CrossRef]
- Muroyama, A.; Lechler, T. Microtubule Organization, Dynamics and Functions in Differentiated Cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, J.; Zhao, Q.; Chen, J.-R.; Liu, H.; Peng, G.; Wu, Y.; Chen, C.; He, Q.; Shi, H.; et al. Mutations of MAP1B Encoding a Microtubule-Associated Phosphoprotein Cause Sensorineural Hearing Loss. JCI Insight 2020, 5, e136046. [Google Scholar] [CrossRef]
- Del Río, J.A.; González-Billault, C.; Ureña, J.M.; Jiménez, E.M.; Barallobre, M.J.; Pascual, M.; Pujadas, L.; Simó, S.; La Torre, A.; Wandosell, F.; et al. MAP1B Is Required for Netrin 1 Signaling in Neuronal Migration and Axonal Guidance. Curr. Biol. 2004, 14, 840–850. [Google Scholar] [CrossRef]
- Gobrecht, P.; Leibinger, M.; Andreadaki, A.; Fischer, D. Sustained GSK3 Activity Markedly Facilitates Nerve Regeneration. Nat. Commun. 2014, 5, 4561. [Google Scholar] [CrossRef]
- Scales, T.M.E.; Lin, S.; Kraus, M.; Goold, R.G.; Gordon-Weeks, P.R. Nonprimed and DYRK1A-Primed GSK3β-Phosphorylation Sites on MAP1B Regulate Microtubule Dynamics in Growing Axons. J. Cell Sci. 2009, 122, 2424–2435. [Google Scholar] [CrossRef]
- Trivedi, N.; Marsh, P.; Goold, R.G.; Wood-Kaczmar, A.; Gordon-Weeks, P.R. Glycogen Synthase Kinase-3β Phosphorylation of MAP1B at Ser1260 and Thr1265 Is Spatially Restricted to Growing Axons. J. Cell Sci. 2005, 118, 993–1005. [Google Scholar] [CrossRef]
- Villarroel-Campos, D.; Gonzalez-Billault, C. The MAP1B Case: An Old MAP That Is New Again: Novel Roles for MAP1B. Dev. Neurobiol. 2014, 74, 953–971. [Google Scholar] [CrossRef]
- Mendieta, I.; Rodríguez-Gómez, G.; Rueda-Zarazúa, B.; Rodríguez-Castelán, J.; Álvarez-León, W.; Delgado-González, E.; Anguiano, B.; Vázquez-Martínez, O.; Díaz-Muñoz, M.; Aceves, C. Molecular Iodine Synergized and Sensitized Neuroblastoma Cells to the Antineoplastic Effect of ATRA. Endocr. Relat. Cancer 2020, 27, 699–710. [Google Scholar] [CrossRef]
- Schneider, L.; Giordano, S.; Zelickson, B.R.; Johnson, M.S.; Benavides, G.A.; Ouyang, X.; Fineberg, N.; Darley-Usmar, V.M.; Zhang, J. Differentiation of SH-SY5Y Cells to a Neuronal Phenotype Changes Cellular Bioenergetics and the Response to Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 2007–2017. [Google Scholar] [CrossRef]
- Itoh, T.J.; Hotani, H. Microtubule-Stabilizing Activity of Microtubule-Associated Proteins (MAPs) Is Due to Increase in Frequency of Rescue in Dynamic Instability: Shortening Length Decreases with Binding of MAPs onto Microtubules. Cell Struct. Funct. 1994, 19, 279–290. [Google Scholar] [CrossRef]
- Ozer, R.S.; Halpain, S. Phosphorylation-Dependent Localization of Microtubule-Associated Protein MAP2c to the Actin Cytoskeleton. Mol. Biol. Cell 2000, 11, 3573–3587. [Google Scholar] [CrossRef]
- Dehmelt, L.; Smart, F.M.; Ozer, R.S.; Halpain, S. The Role of Microtubule-Associated Protein 2c in the Reorganization of Microtubules and Lamellipodia during Neurite Initiation. J. Neurosci. 2003, 23, 9479–9490. [Google Scholar] [CrossRef]
- Inoue, D.; Obino, D.; Pineau, J.; Farina, F.; Gaillard, J.; Guerin, C.; Blanchoin, L.; Lennon-Duménil, A.; Théry, M. Actin Filaments Regulate Microtubule Growth at the Centrosome. EMBO J. 2019, 38, e99630. [Google Scholar] [CrossRef]
- He, Y.; Francis, F.; Myers, K.A.; Yu, W.; Black, M.M.; Baas, P.W. Role of Cytoplasmic Dynein in the Axonal Transport of Microtubules and Neurofilaments. J. Cell Biol. 2005, 168, 697–703. [Google Scholar] [CrossRef]
- Roossien, D.H.; Lamoureux, P.; Miller, K.E. Cytoplasmic Dynein Pushes the Cytoskeletal Meshwork Forward during Axonal Elongation. J. Cell Sci. 2014, 127, 3593–3602. [Google Scholar] [CrossRef]
- Kahn, O.I.; Ha, N.; Baird, M.A.; Davidson, M.W.; Baas, P.W. TPX2 Regulates Neuronal Morphology through Kinesin-5 Interaction: TPX2 and Kinesin-5 in Neuronal Morphology. Cytoskeleton 2015, 72, 340–348. [Google Scholar] [CrossRef]
- Wilson, A.E.; Kosater, W.M.; Liberles, D.A. Evolutionary Processes and Biophysical Mechanisms: Revisiting Why Evolved Proteins Are Marginally Stable. J. Mol. Evol. 2020, 88, 415–417. [Google Scholar] [CrossRef]
- Nisaa, K.; Ben-Zvi, A. Chaperone Networks Are Shaped by Cellular Differentiation and Identity. Trends Cell Biol. 2022, 32, 470–474. [Google Scholar] [CrossRef]
- Vonk, W.I.M.; Rainbolt, T.K.; Dolan, P.T.; Webb, A.E.; Brunet, A.; Frydman, J. Differentiation Drives Widespread Rewiring of the Neural Stem Cell Chaperone Network. Mol. Cell 2020, 78, 329–345.e9. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, X.; Guo, W. The Impact of Amino Acid Metabolism on Adult Neurogenesis. Biochem. Soc. Trans. 2023, 51, 233–244. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine Metabolism in Health and Cancer: A Nexus of Diet and Precision Medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef]
- Villa, E.; Ali, E.S.; Sahu, U.; Ben-Sahra, I. Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef]
- Sardana, S.; Nederstigt, A.E.; Baggelaar, M.P. S -Palmitoylation during Retinoic Acid-Induced Neuronal Differentiation of SH-SY5Y Neuroblastoma Cells. J. Proteome Res. 2023, 22, 2421–2435. [Google Scholar] [CrossRef]
- Jiang, X.; Bomgarden, R.; Brown, J.; Drew, D.L.; Robitaille, A.M.; Viner, R.; Huhmer, A.R. Sensitive and Accurate Quantitation of Phosphopeptides Using TMT Isobaric Labeling Technique. J. Proteome Res. 2017, 16, 4244–4252. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Leeming, M.G.; O’Callaghan, S.; Licata, L.; Iannuccelli, M.; Lo Surdo, P.; Micarelli, E.; Ang, C.-S.; Nie, S.; Varshney, S.; Ameen, S.; et al. Phosphomatics: Interactive Interrogation of Substrate–Kinase Networks in Global Phosphoproteomics Datasets. Bioinformatics 2021, 37, 1635–1636. [Google Scholar] [CrossRef]
- Wiredja, D.D.; Koyutürk, M.; Chance, M.R. The KSEA App: A Web-Based Tool for Kinase Activity Inference from Quantitative Phosphoproteomics. Bioinformatics 2017, 33, 3489–3491. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, T.C.N.; Lu, S.N.; Chu, C.N.; Lee, J.; Liu, X.; Ngai, S.M. Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation. Int. J. Mol. Sci. 2024, 25, 1047. https://doi.org/10.3390/ijms25021047
Leung TCN, Lu SN, Chu CN, Lee J, Liu X, Ngai SM. Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation. International Journal of Molecular Sciences. 2024; 25(2):1047. https://doi.org/10.3390/ijms25021047
Chicago/Turabian StyleLeung, Thomas C. N., Scott Ninghai Lu, Cheuk Ning Chu, Joy Lee, Xingyu Liu, and Sai Ming Ngai. 2024. "Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation" International Journal of Molecular Sciences 25, no. 2: 1047. https://doi.org/10.3390/ijms25021047
APA StyleLeung, T. C. N., Lu, S. N., Chu, C. N., Lee, J., Liu, X., & Ngai, S. M. (2024). Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation. International Journal of Molecular Sciences, 25(2), 1047. https://doi.org/10.3390/ijms25021047




