Radiosynthesis and Bioevaluation of 99mTc-Labeled Isocyanide Ubiquicidin 29-41 Derivatives as Potential Agents for Bacterial Infection Imaging
Abstract
1. Introduction
2. Results
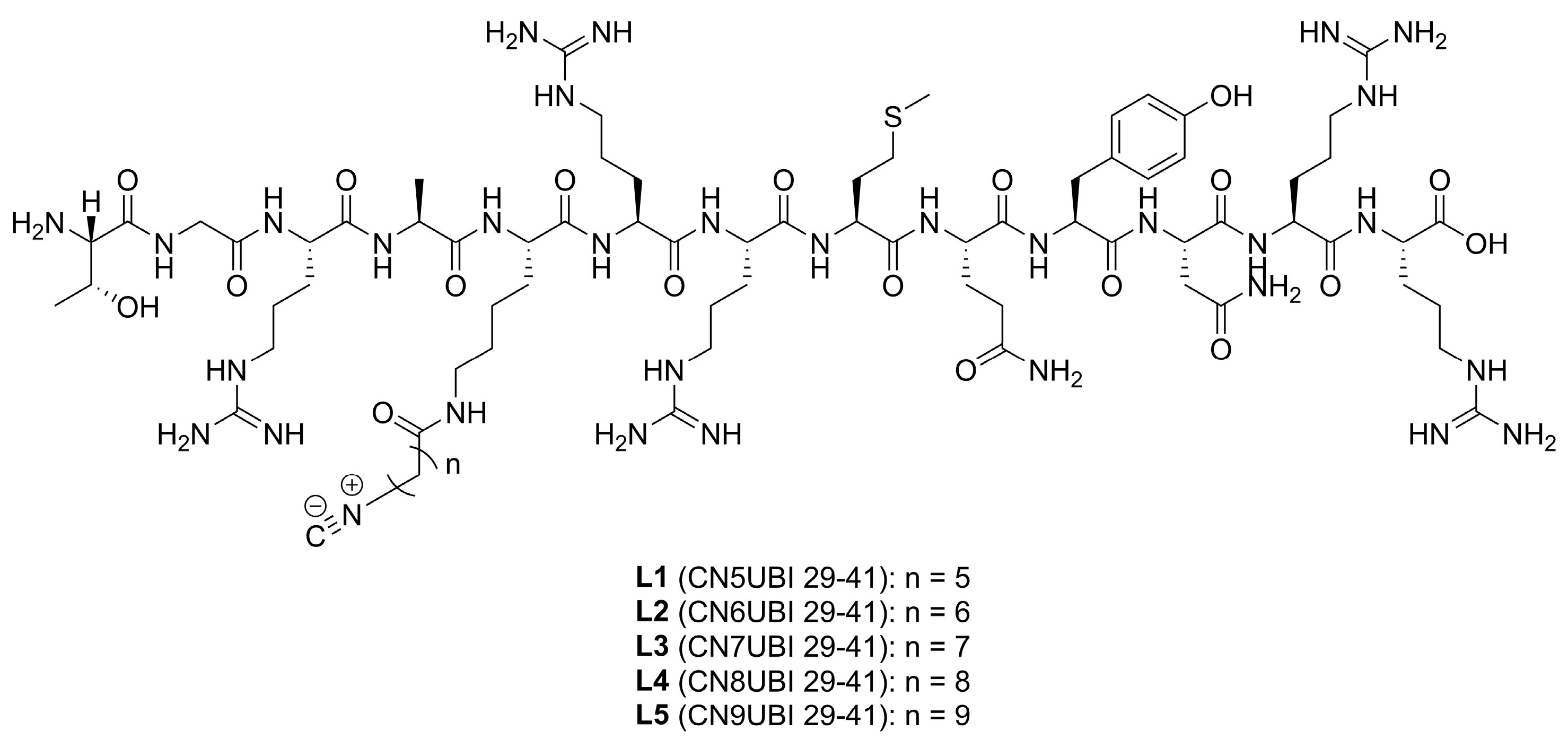
2.1. Ligand Characterization
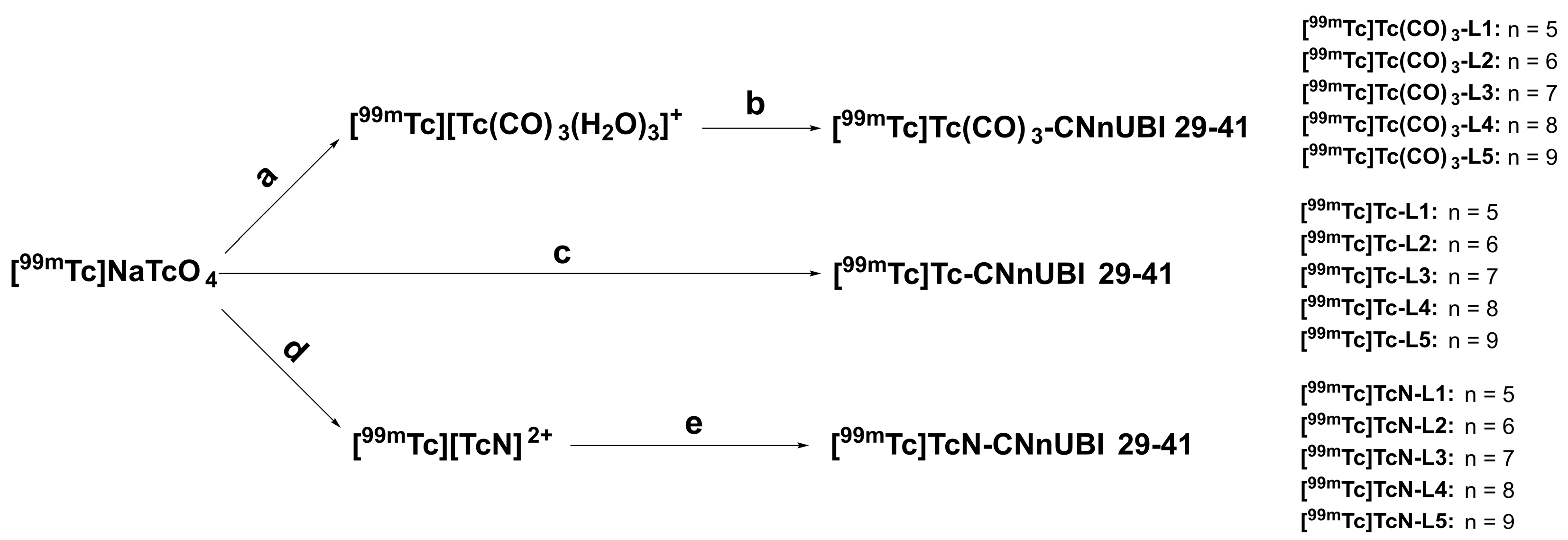
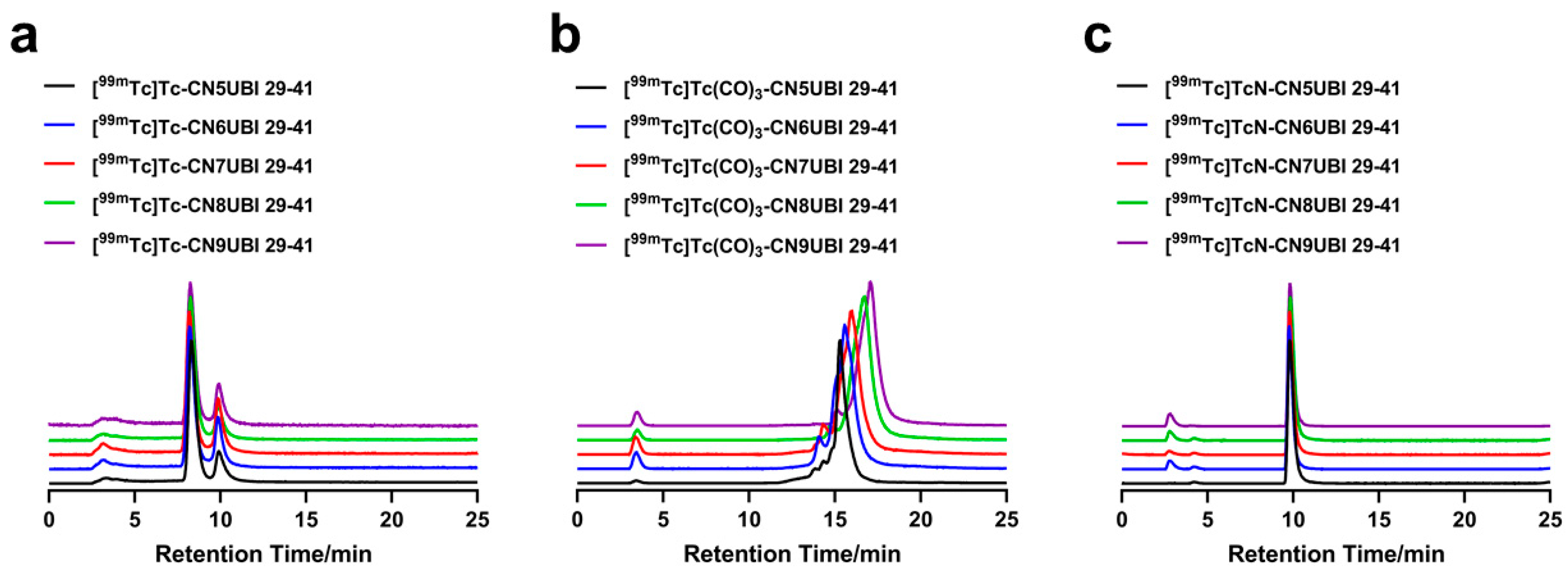
2.2. Radiochemistry and Quality Control
2.3. In Vitro Physicochemical Characterization
2.3.1. In Vitro Stability Tests
2.3.2. Determination of the Distribution Coefficients of the Complexes
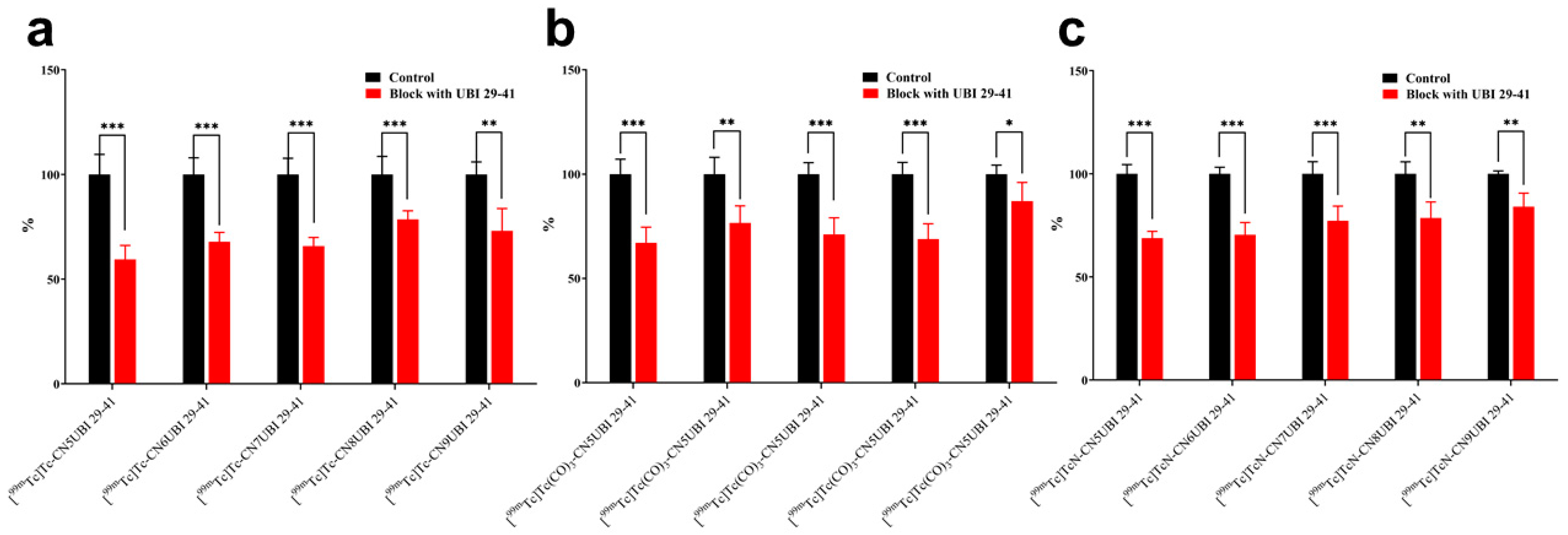
2.4. In Vitro Binding of Complexes to Bacteria
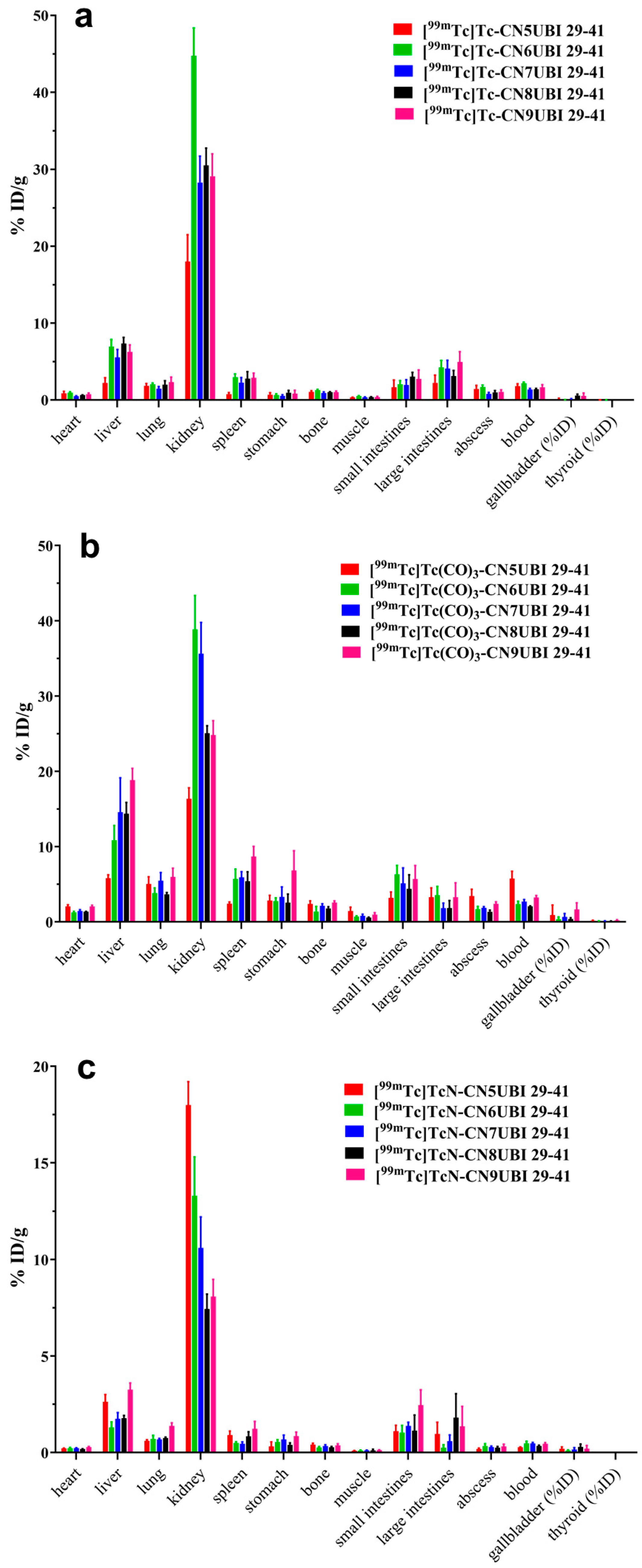
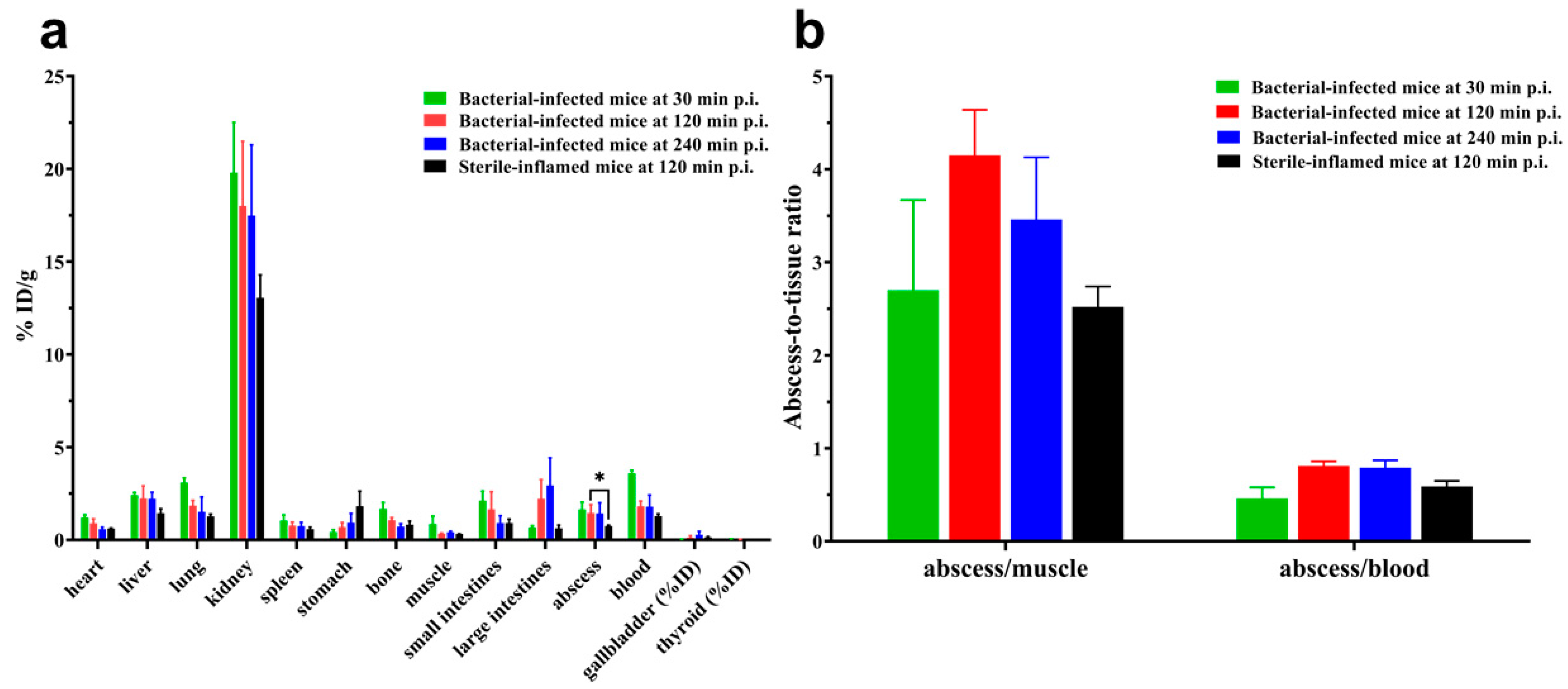
2.5. Biodistribution
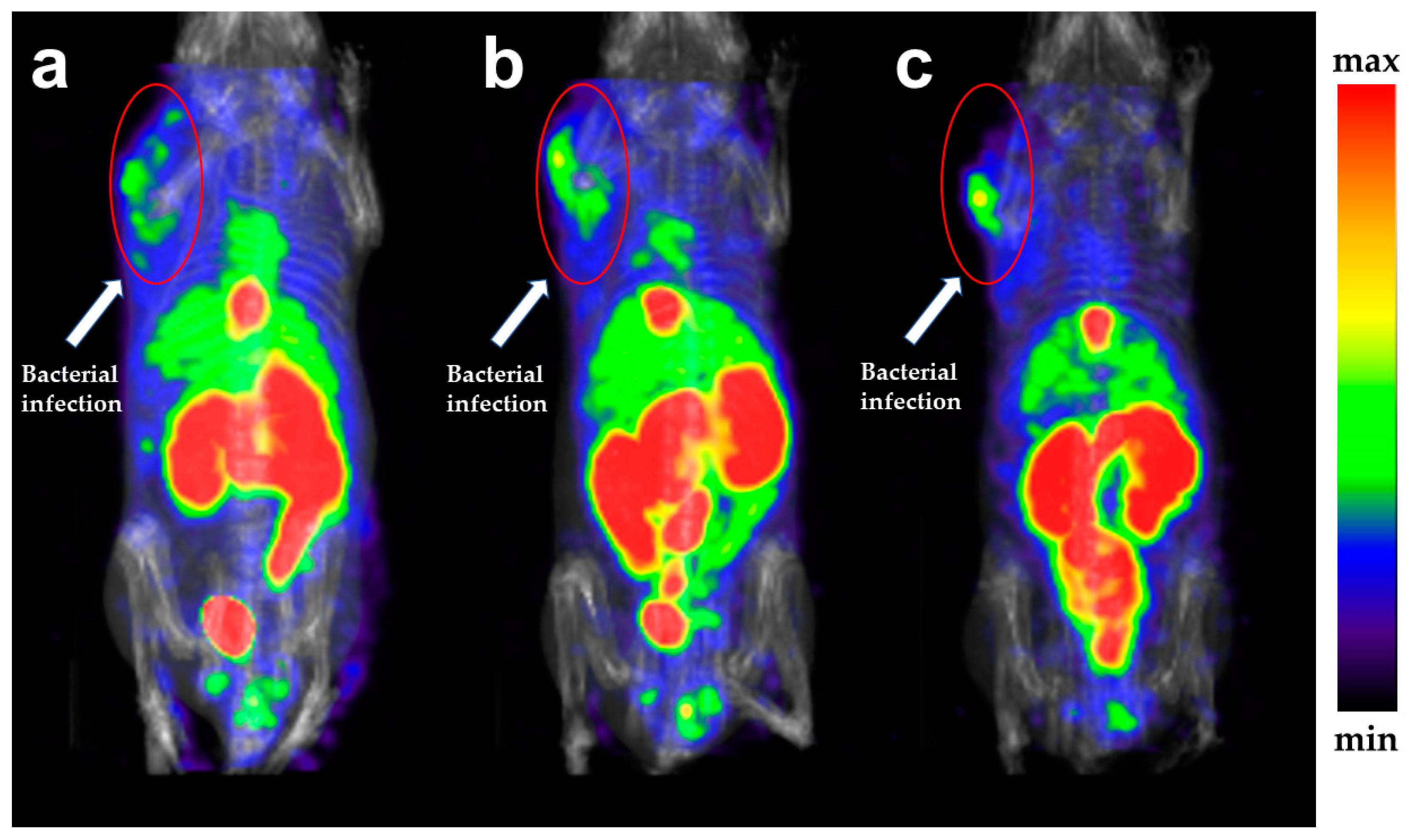
2.6. SPECT Imaging
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ligand Characterization
4.3. Radiochemistry and Quality Control
4.4. In Vitro Physicochemical Characterization
4.4.1. In Vitro Stability Study
4.4.2. Distribution Coefficient (Log D) Measurements
4.5. In Vitro Bacterial Binding Experiments
4.6. Biodistribution Studies in Mice with Bacterial Infection
4.7. Biodistribution Studies in Mice with Turpentine-Induced Abscesses
4.8. SPECT Imaging Study
4.9. Statistical and Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polvoy, I.; Flavell, R.; Ohliger, M.; Rosenberg, O.; Wilson, D. Nuclear imaging of bacterial infection- state of the art and future directions. J. Nucl. Med. 2020, 61, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Pijl, J.; Kwee, T.; Slart, R.; Glaudemans, A. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. [Google Scholar] [CrossRef]
- Palestro, C.J. A Brief History of Nuclear Medicine Imaging of Infection. J. Infect. Dis. 2023, 228, S237–S240. [Google Scholar] [CrossRef]
- Jain, S.K. New Approaches for Imaging Bacteria. Semin. Nucl. Med. 2023, 53, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, D.; Lane, H.; Jain, S. Molecular Imaging of Infections: Advancing the Search for the Hidden Enemy. J. Infect. Dis. 2023, 228, S233–S236. [Google Scholar] [CrossRef]
- Ordonez, A.; Sellmyer, M.; Gowrishankar, G.; Ruiz-Bedoya, C.; Tucker, E.; Palestro, C.; Hammoud, D.; Jain, S. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Transl. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fang, J.; Hong, S.; Liu, H.; Zhu, H.; Feng, L.; Zhuang, R.; Zhao, X.; Guo, Z.; Zhang, X. MicroPET imaging of bacterial infection with nitroreductase-specific responsive 18F-labelled nitrogen mustard analogues. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2645–2654. [Google Scholar] [CrossRef]
- Frickenstein, A.N.; Jones, M.A.; Behkam, B.; McNally, L.R. Imaging Inflammation and Infection in the Gastrointestinal Tract. Int. J. Mol. Sci. 2020, 21, 243. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Welling, M.M.; Paulusma-Annema, A.; Brouwer, C.P.J.M.; Lupetti, A.; Pauwels, E.K.J. 99mTc-Labeled UBI 29-41 Peptide for Monitoring the Efficacy of Antibacterial Agents in Mice Infected with Staphylococcus aureus. J. Nucl. Med. 2004, 45, 321–326. [Google Scholar]
- Rowe, S.P.; Auwaerter, P.G.; Sheikhbahaei, S.; Solnes, L.B.; Wright, W.F. Molecular Imaging of Infections: Emerging Techniques for Pathogen-Specific Diagnosis and Guided Therapy. J. Infect. Dis. 2023, 228, S241–S248. [Google Scholar] [CrossRef]
- Foss, C.; Renslo, A. Imaging-Selected Host Responses in the Context of Infections. J. Infect. Dis. 2023, 228, S302–S310. [Google Scholar] [CrossRef] [PubMed]
- Northrup, J.D.; Mach, R.H.; Sellmyer, M.A. Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets. Int. J. Mol. Sci. 2019, 20, 5808. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of peptides for antibacterial applications. Colloids Surf. B Biointerfaces 2021, 202, 111682. [Google Scholar] [CrossRef]
- Lupetti, A.; Welling, M.M.; Pauwels, E.K.; Nibbering, P.H. Radiolabelled antimicrobial peptides for infection detection. Lancet Infect. Dis. 2003, 3, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Claude, G.; Zeh, L.; Roca Jungfer, M.; Hagenbach, A.; Figueroa, J.S.; Abram, U. The Chemistry of Phenylimidotechnetium(V) Complexes with Isocyanides: Steric and Electronic Factors. Molecules 2022, 27, 8546. [Google Scholar] [CrossRef]
- Tim, V.D.W.; Filipe, E.; Stijn, D.S.; John, A.K.; Ora, I. SPECT/CT: Standing on the Shoulders of Giants, It Is Time to Reach for the Sky! J. Nucl. Med. 2020, 61, 1284–1291. [Google Scholar] [CrossRef]
- Ahmed, N.; Zia, M. Diagnostic modalities and radiopharmaceuticals with particular importance of technetium-99m (99mTc). Chin. J. Acad. Radiol. 2023, 6, 143–159. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Welling, M.M.; Paulusma-Annema, A.; Balter, H.S.; Pauwels, E.K.J.; Nibbering, P.H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 2000, 27, 292–301. [Google Scholar] [CrossRef]
- Bhatt Mitra, J.; Sharma, V.K.; Mukherjee, A.; Garcia Sakai, V.; Dash, A.; Kumar, M. Ubiquicidin-Derived Peptides Selectively Interact with the Anionic Phospholipid Membrane. Langmuir 2020, 36, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Arteaga De Murphy, C.; Pedraza-López, M.; Meléndez-Alafort, L.; Zhang, Y.; Rusckowski, M.; Hnatowich, D.J. In vitro and in vivo assessment of 99mTc-UBI specificity for bacteria. Nucl. Med. Biol. 2003, 30, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Alafort, L.; Ramírez, F.D.M.; Ferro-Flores, G.; de Murphy, C.A.; Pedraza-López, M.; Hnatowich, D.J. Lys and Arg in UBI: A specific site for a stable Tc-99m complex? Nucl. Med. Biol. 2003, 30, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Visentin, R.; Feitsma, H.I.J.; Lupetti, A.; Pauwels, E.K.J.; Nibbering, P.H. Infection detection in mice using 99mTc-labeled HYNIC and N2S2 chelate conjugated to the antimicrobial peptide UBI 29-41. Nucl. Med. Biol. 2004, 31, 503–509. [Google Scholar] [CrossRef]
- Welling, M.M.; Korsak, A.; Gorska, B.; Oliver, P.; Mikolajczak, R.; Balter, H.S.; Feitsma, H.I.J.; Pauwels, E.K.J. Kit with technetium-99m labelled antimicrobial peptide UBI 29-41 for specific infection detection. J. Labelled Compd. Radiopharm. 2005, 48, 683–691. [Google Scholar] [CrossRef]
- Gandomkar, M.; Najafi, R.; Mazidi, M.; Goudarzi, M.; Mirfallah, S.H. New peptide based freeze-dried kit [99mTc-HYNIC]-UBI 29-41 as a human specific infection imaging agent. Iran. J. Nucl. Med. 2008, 16, 25–30. [Google Scholar]
- Meléndez-Alafort, L.; Nadali, A.; Pasut, G.; Zangoni, E.; De Caro, R.; Cariolato, L.; Giron, M.C.; Castagliuolo, I.; Veronese, F.M.; Mazzi, U. Detection of sites of infection in mice using 99mTc-labeled PN2S-PEG conjugated to UBI and 99mTc-UBI: A comparative biodistribution study. Nucl. Med. Biol. 2009, 36, 57–64. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J. Current Status of and Perspectives on Radiolabelled Ubiquicidin 29-41 Derivatives for Bacterial Infection Imaging. Mini-Rev. Med. Chem. 2023, 23, 1500–1506. [Google Scholar] [CrossRef]
- Claude, G.; Genz, J.; Weh, D.; Roca Jungfer, M.; Hagenbach, A.; Gembicky, M.; Figueroa, J.S.; Abram, U. Mixed-Isocyanide Complexes of Technetium under Steric and Electronic Control. Inorg. Chem. 2022, 61, 16163–16176. [Google Scholar] [CrossRef]
- Claude, G.; Puccio, D.; Roca Jungfer, M.; Hagenbach, A.; Spreckelmeyer, S.; Abram, U. Technetium Complexes with an Isocyano-alkyne Ligand and Its Reaction Products. Inorg. Chem. 2023, 62, 12445–12452. [Google Scholar] [CrossRef] [PubMed]
- Claude, G.; Salsi, F.; Hagenbach, A.; Gembicky, M.; Neville, M.; Chan, C.; Figueroa, J.S.; Abram, U. Structural and Redox Variations in Technetium Complexes Supported by m-Terphenyl Isocyanides. Organometallics 2020, 39, 2287–2294. [Google Scholar] [CrossRef]
- Nazari, B.; Azizmohammadi, Z.; Rajaei, M.; Karami, M.; Javadi, H.; Assadi, M.; Asli, I.N. Role of 99mTc-ubiquicidin 29–41 scintigraphy to monitor antibiotic therapy in patients with orthopedic infection: A preliminary study. Nucl. Med. Commun. 2011, 32, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Zafar, J.; Khan, B.; Akhtar, A.; Qurieshi, S.; Fatima, S.; Ahmad, N.; Irfanullah, J. Utility of 99mTc-labelled antimicrobial peptide ubiquicidin (29-41) in the diagnosis of diabetic foot infection. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 737–743. [Google Scholar] [CrossRef]
- Sarda-Mantel, L.; Saleh-Mghir, A.; Welling, M.M.; Meulemans, A.; Vrigneaud, J.; Raguin, O.; Hervatin, F.; Martet, G.; Chau, F.; Lebtahi, R.; et al. Evaluation of 99mTc-UBI 29-41 scintigraphy for specific detection of experimental Staphylococcus aureus prosthetic joint infections. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Paez, D.; Sathekge, M.M.; Douis, H.; Giammarile, F.; Fatima, S.; Dhal, A.; Puri, S.K.; Erba, P.A.; Lazzeri, E.; Ferrando, R.; et al. Comparison of MRI, [18F]FDG PET/CT, and 99mTc-UBI 29-41 scintigraphy for postoperative spondylodiscitis—A prospective multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1864–1875. [Google Scholar] [CrossRef]
- Vallejo, E.; Martinez, I.; Tejero, A.; Hernandez, S.; Jimenez, L.; Bialostozky, D.; Sanchez, G.; Ilarraza, H.; Ferro-Flores, G. Clinical Utility of 99mTc-Labeled Ubiquicidin 29–41 Antimicrobial Peptide for the Scintigraphic Detection of Mediastinitis after Cardiac Surgery. Arch. Med. Res. 2008, 39, 768–774. [Google Scholar] [CrossRef]
- Welling, M.M.; Hensbergen, A.W.; Bunschoten, A.; Velders, A.H.; Scheper, H.; Smits, W.K.; Roestenberg, M.; van Leeuwen, F.W.B. Fluorescent imaging of bacterial infections and recent advances made with multimodal radiopharmaceuticals. Clin. Transl. Imaging 2019, 7, 125–138. [Google Scholar] [CrossRef]
- Bhatt Mitra, J.; Chatterjee, S.; Kumar, A.; Bandyopadhyay, A.; Mukherjee, A. Integrating a covalent probe with ubiquicidin fragment enables effective bacterial infection imaging. RSC Med. Chem. 2022, 13, 1239–1245. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Chen, D.; Madrid, K.; Peng, S.; Dong, X.; Zhang, M.; Gu, Y. Bacteria-Targeting Conjugates Based on Antimicrobial Peptide for Bacteria Diagnosis and Therapy. Mol. Pharmaceut. 2015, 12, 2505–2516. [Google Scholar] [CrossRef]
- Kunstler, J.; Veerendra, B.; Figueroa, S.D.; Sieckman, G.L.; Rold, T.L.; Hoffman, T.J.; Smith, C.J.; Pietzsch, H. Organometallic 99mTc(III) ‘4 + 1’ Bombesin(7−14) Conjugates: Synthesis, Radiolabeling, and In Vitro/In Vivo Studies. Bioconjugate Chem. 2007, 18, 1651–1661. [Google Scholar] [CrossRef]
- Aalbersberg, E.A.; van Andel, L.; Geluk-Jonker, M.M.; Beijnen, J.H.; Stokkel, M.P.M.; Hendrikx, J.J.M.A. Automated synthesis and quality control of [99mTc]Tc-PSMA for radioguided surgery (in a [68Ga]Ga-PSMA workflow). EJNMMI Radiopharm. Chem. 2020, 5, 10. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Jia, F.; Tang, Z.; Zhang, J.; Liu, B.; Wang, X.Y. Preparation, characterization and biodistribution of a new technetium-99m nitrido complex with 2-methoxyisobutylisomtrile and comparison with 99mTc-MIBI. J. Labelled Compd. Radiopharm. 2002, 45, 1029–1043. [Google Scholar] [CrossRef]
- Hirano, T.; Otake, H.; Kazama, K.; Wakabayashi, K.; Zama, A.; Shibasaki, T.; Tamura, M.; Endo, K. Technetium-99m(V)-DMSA and thallium-201 in brain tumor imaging: Correlation with histology and malignant grade. J. Nucl. Med. 1997, 38, 1741–1749. [Google Scholar]
- Demir, F.; Demir, M. Comparison of 99mTc-DMSA, 99mTc-DTPA and 99mTc-MAG3 Renal Scintigraphy in the Calculation of Relative Renal Function. J. Urol. Surg. 2020, 7, 130–133. [Google Scholar] [CrossRef]
- Fahmy, H.; Yassin, H.; Muhamed, I.; Mohamed, S.; Hassan, S. Evaluation of the Efficiency of 99mTc-DMSA as a Radiopharmaceutical in Dynamic Renal Scans. Erciyes. Med. J. 2018, 40, 140–147. [Google Scholar] [CrossRef]
- Othman, S.; Al-Hawas, A.; Al-Maqtari, R. Renal Cortical Imaging in Children: 99mTc MAG3 Versus: 99mTc DMSA. Clin. Nucl. Med. 2012, 37, 351–355. [Google Scholar] [CrossRef]
- Valind, K.; Minarik, D.; Garpered, S.; Persson, E.; Jögi, J.; Trägårdh, E. [18F]PSMA-1007 PET is comparable to [99mTc]Tc-DMSA SPECT for renal cortical imaging. Eur. J. Hybrid Imaging 2023, 7, 25. [Google Scholar] [CrossRef]
- Welling, M.; Lupetti, A.; Balter, H.; Lanzzeri, S.; Souto, B.; Rey, A.; Savio, E.; Paulusma-Annema, A.; Pauwels, E.; Nibbering, P. 99mTc-labeled antimicrobial peptides for detection of bacterial and Candida albicans infections. J. Nucl. Med. 2001, 42, 788–794. [Google Scholar]
- Welling, M.M.; Mongera, S.; Lupetti, A.; Balter, H.S.; Bonetto, V.; Mazzi, U.; Pauwels, E.K.J.; Nibbering, P.H. Radiochemical and biological characteristics of 99mTc-UBI 29–41 for imaging of bacterial infections. Nucl. Med. Biol. 2002, 29, 413–422. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Iqbal, J.; Khan, M.A.; Irfanullah, J.; Jehangir, M.; Khan, B.; Ul-Haq, I.; Muhammad, G.; Nadeem, M.A.; Afzal, M.S.; et al. 99mTc-Labeled Antimicrobial Peptide Ubiquicidin (29-41) Accumulates Less in Escherichia coli Infection than in Staphlococcus aureus Infection. J. Nucl. Med. 2004, 45, 849. [Google Scholar]
- Kiritsis, C.; Shegani, A.; Makrypidi, K.; Roupa, I.; Lazopoulos, A.; Panagiotopoulou, A.; Triantopoulou, S.; Paravatou-Petsotas, M.; Pietzsch, H.; Pelecanou, M.; et al. Synthesis and preclinical evaluation of rhenium and technetium-99m “4 + 1” mixed-ligand complexes bearing quinazoline derivatives as potential EGFR imaging agents. Bioorgan. Med. Chem. 2022, 73, 117012. [Google Scholar] [CrossRef] [PubMed]
- Sagnou, M.; Tsoukalas, C.; Triantis, C.; Raptopoulou, C.P.; Terzis, A.; Pirmettis, I.; Pelecanou, M.; Papadopoulos, M. A new tricarbonyl fac-[M(acac)(isc)(CO)3] complex (M=Re, 99mTc) with acetylacetonate (acac) and isocyanide (isc) in a 2+1 combination. Inorg. Chim. Acta 2010, 363, 1649–1653. [Google Scholar] [CrossRef]
- Hayes, T.; Powell, A.; Barnes, C.; Benny, P. Synthesis and stability of 2+1 complexes of N,N-diethylbenzoylthiourea with [MI(CO)3]+ (M = Re, 99mTc). J. Coord. Chem. 2015, 68, 3432–3448. [Google Scholar] [CrossRef]
- Shegani, A.; Ischyropoulou, M.; Roupa, I.; Kiritsis, C.; Makrypidi, K.; Papasavva, A.; Raptopoulou, C.; Psycharis, V.; Hennkens, H.M.; Pelecanou, M.; et al. Synthesis and evaluation of new mixed “2 + 1” Re, 99mTc and 186Re tricarbonyl dithiocarbamate complexes with different monodentate ligands. Bioorgan. Med. Chem. 2021, 47, 116373. [Google Scholar] [CrossRef]
- Oshikiri, S.; Uehara, T.; Suzuki, H.; Koike-Satake, M.; Hino, A.; Arano, Y. Zn Complex of Diaminedithiol Tetradentate Ligand as a Stable Precursor for 99mTc-Labeled Compounds. Molecules 2020, 25, 254. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Y.; Chu, T. 99mTc/Re complexes bearing bisnitroimidazole or mononitroimidazole as potential bioreductive markers for tumor: Synthesis, physicochemical characterization and biological evaluation. Eur. J. Med. Chem. 2012, 58, 50–63. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D. 99mTc-Labeled Small Peptides as Diagnostic Radiopharmaceuticals. Chem. Rev. 1999, 99, 2235–2268. [Google Scholar] [CrossRef]


| Ligand | [99mTc][Tc(Ⅰ)]+ Core | [99mTc][Tc(CO)3(H2O)3]+ Core | [99mTc][Tc(Ⅴ)N]2+ Core |
|---|---|---|---|
| L1: CN5UBI 29-41 | −2.66 ± 0.02 | −2.29 ± 0.04 | −2.71 ± 0.03 |
| L2: CN6UBI 29-41 | −2.62 ± 0.03 | −2.20 ± 0.03 | −2.65 ± 0.03 |
| L3: CN7UBI 29-41 | −2.57 ± 0.03 | −2.09 ± 0.04 | −2.58 ± 0.04 |
| L4: CN8UBI 29-41 | −2.54 ± 0.01 | −1.97 ± 0.02 | −2.55 ± 0.03 |
| L5: CN9UBI 29-41 | −2.52 ± 0.01 | −1.91 ± 0.03 | −2.41 ± 0.03 |
| Compound | Chemical Formula | HPLC Purity | MS Analysis (m/z) | |
|---|---|---|---|---|
| Calculated [M + 3H]3+ | Found [M + 3H]3+ | |||
| L1: CN5UBI 29-41 | C75H130N32O19S | 99.91% | 606.4 | 606.5 |
| L2: CN6UBI 29-41 | C76H132N32O19S | 95.13% | 611.0 | 610.9 |
| L3: CN7UBI 29-41 | C77H134N32O19S | 96.41% | 615.7 | 615.6 |
| L4: CN8UBI 29-41 | C78H136N32O19S | 97.02% | 620.4 | 620.3 |
| L5: CN9UBI 29-41 | C79H138N32O19S | 94.29% | 625.1 | 624.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Han, P.; Yin, G.; Wang, Q.; Feng, J.; Ruan, Q.; Xiao, D.; Zhang, J. Radiosynthesis and Bioevaluation of 99mTc-Labeled Isocyanide Ubiquicidin 29-41 Derivatives as Potential Agents for Bacterial Infection Imaging. Int. J. Mol. Sci. 2024, 25, 1045. https://doi.org/10.3390/ijms25021045
Jiang Y, Han P, Yin G, Wang Q, Feng J, Ruan Q, Xiao D, Zhang J. Radiosynthesis and Bioevaluation of 99mTc-Labeled Isocyanide Ubiquicidin 29-41 Derivatives as Potential Agents for Bacterial Infection Imaging. International Journal of Molecular Sciences. 2024; 25(2):1045. https://doi.org/10.3390/ijms25021045
Chicago/Turabian StyleJiang, Yuhao, Peiwen Han, Guangxing Yin, Qianna Wang, Junhong Feng, Qing Ruan, Di Xiao, and Junbo Zhang. 2024. "Radiosynthesis and Bioevaluation of 99mTc-Labeled Isocyanide Ubiquicidin 29-41 Derivatives as Potential Agents for Bacterial Infection Imaging" International Journal of Molecular Sciences 25, no. 2: 1045. https://doi.org/10.3390/ijms25021045
APA StyleJiang, Y., Han, P., Yin, G., Wang, Q., Feng, J., Ruan, Q., Xiao, D., & Zhang, J. (2024). Radiosynthesis and Bioevaluation of 99mTc-Labeled Isocyanide Ubiquicidin 29-41 Derivatives as Potential Agents for Bacterial Infection Imaging. International Journal of Molecular Sciences, 25(2), 1045. https://doi.org/10.3390/ijms25021045







