Transpulmonary Plasma Endothelin-1 Arterial:Venous Ratio Differentiates Survivors from Non-Survivors in Critically Ill Patients with COVID-19-Induced Acute Respiratory Distress Syndrome
Abstract
1. Introduction
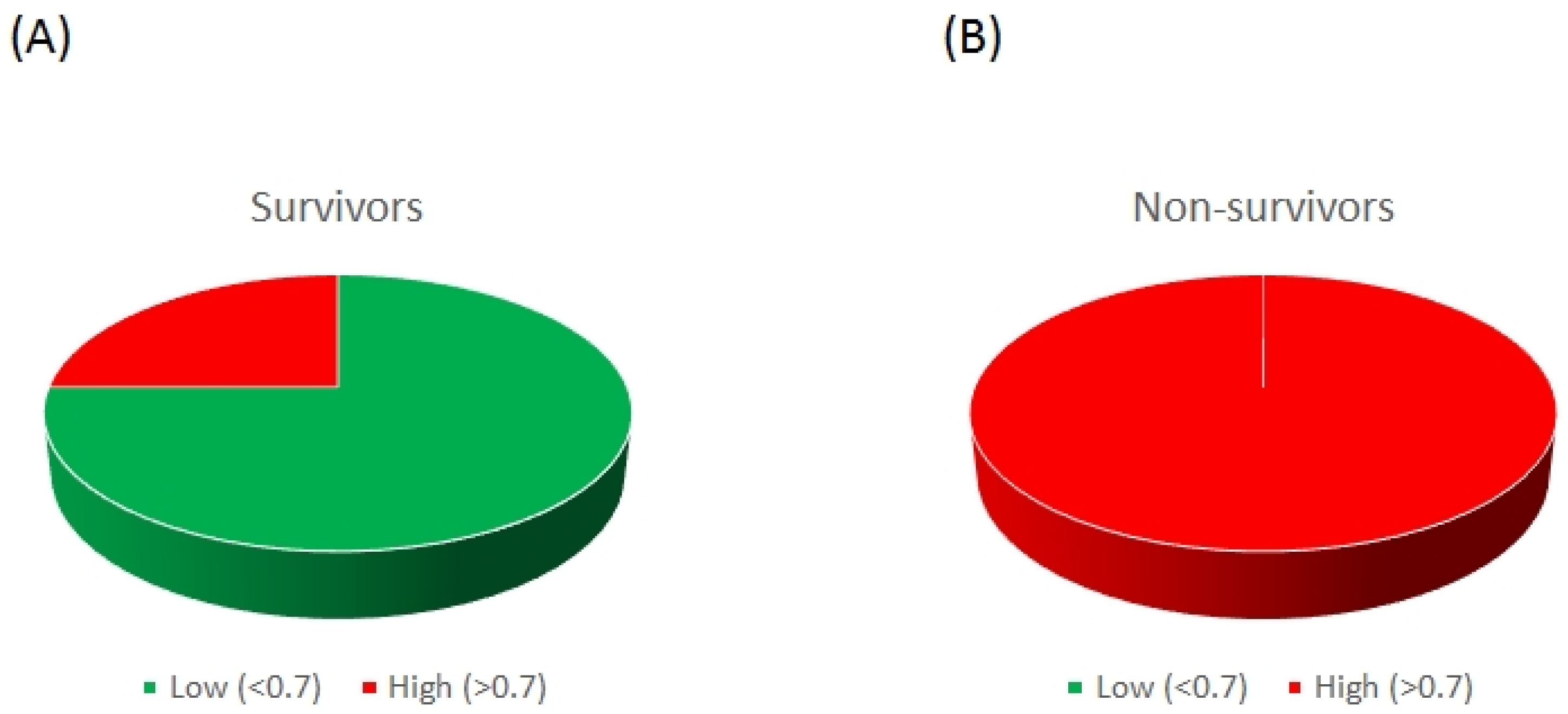
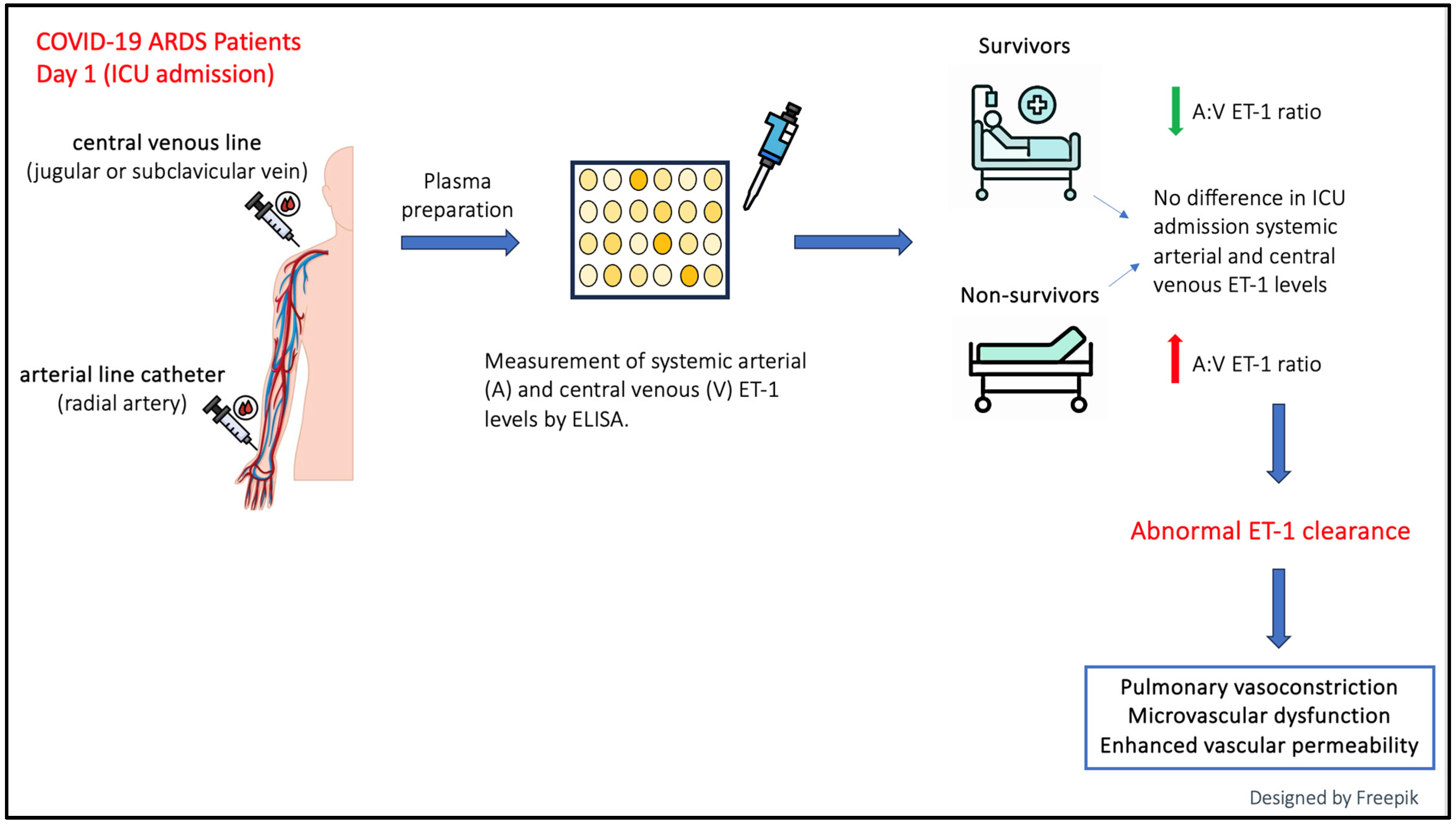
2. Results
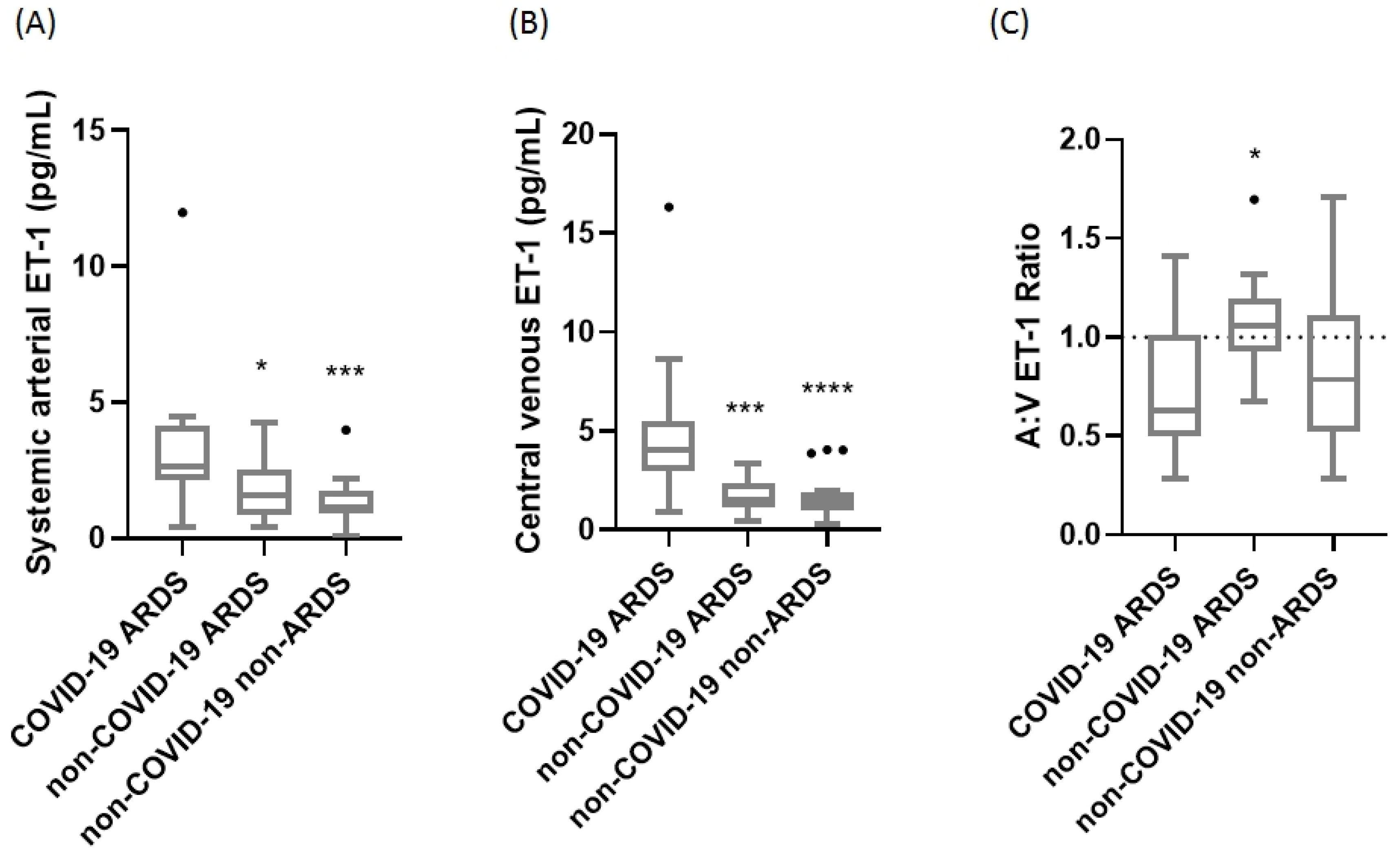
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, D.K.; Titus, A.; Caulfield, T.R.; David Freeman, W. Endotheliitis, endothelin, and endothelin receptor blockers in COVID-19. Med. Hypotheses 2021, 150, 110564. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Liu, R.; Lan, Y.; Lei, Y.; Zeng, F.; Tang, X.; He, H. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences. Front. Med. 2022, 9, 829771. [Google Scholar] [CrossRef]
- Beloncle, F.M. Is COVID-19 different from other causes of acute respiratory distress syndrome? J. Intensive Med. 2023, 3, 212–219. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals 2021, 14, 695. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef]
- Ragnoli, B.; Da Re, B.; Galantino, A.; Kette, S.; Salotti, A.; Malerba, M. Interrelationship between COVID-19 and Coagulopathy: Pathophysiological and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 8945. [Google Scholar] [CrossRef]
- Harrison, D.G. Cellular and molecular mechanisms of endothelial cell dysfunction. J. Clin. Investig. 1997, 100, 2153–2157. [Google Scholar] [CrossRef]
- Mather, K.J.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes 2004, 53, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Banecki, K.; Dora, K.A. Endothelin-1 in Health and Disease. Int. J. Mol. Sci. 2023, 24, 11295. [Google Scholar] [CrossRef] [PubMed]
- D’Orléans-Juste, P.; Labonté, J.; Bkaily, G.; Choufani, S.; Plante, M.; Honoré, J.C. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol. Ther. 2002, 95, 221–238. [Google Scholar] [CrossRef]
- Dupuis, J.; Stewart, D.J.; Cernacek, P.; Gosselin, G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation 1996, 94, 1578–1584. [Google Scholar] [CrossRef]
- Dupuis, J.; Cernacek, P.; Tardif, J.C.; Stewart, D.J.; Gosselin, G.; Dyrda, I.; Bonan, R.; Crépeau, J. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am. Heart J. 1998, 135, 614–620. [Google Scholar] [CrossRef]
- Stewart, D.J.; Levy, R.D.; Cernacek, P.; Langleben, D. Increased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease? Ann. Intern. Med. 1991, 114, 464–469. [Google Scholar] [CrossRef]
- Langleben, D.; DeMarchie, M.; Laporta, D.; Spanier, A.H.; Schlesinger, R.D.; Stewart, D.J. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993, 148, 1646–1650. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.M.; Rudehill, A. Elevated plasma levels of endothelin in patients with sepsis syndrome. Circ. Shock 1991, 33, 222–227. [Google Scholar]
- Stewart, D.J.; Kubac, G.; Costello, K.B.; Cernacek, P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J. Am. Coll. Cardiol. 1991, 18, 38–43. [Google Scholar] [CrossRef]
- Yamane, K. Endothelin and Collagen Vascular Disease: A Review with Special Reference to Raynaud’s Phenomenon and Systemic Sclerosis. Intern. Med. 1994, 33, 579–582. [Google Scholar] [CrossRef][Green Version]
- Uguccioni, M.; Pulsatelli, L.; Grigolo, B.; Facchini, A.; Fasano, L.; Cinti, C.; Fabbri, M.; Gasbarrini, G.; Meliconi, R. Endothelin-1 in idiopathic pulmonary fibrosis. J. Clin. Pathol. 1995, 48, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.A.; McMurtry, I.F.; Rodman, D.M. Role of endothelin-1 in lung disease. Respir. Res. 2001, 2, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.; Jais, X.; Yaici, A.; Sitbon, O.; Humbert, M.; Simonneau, G. Clinical Challenges in Pulmonary Hypertension. Chest 2005, 128, 622S–628S. [Google Scholar] [CrossRef]
- Clozel, M.; Salloukh, H. Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann. Med. 2005, 37, 2–12. [Google Scholar] [CrossRef]
- Fonseca, C.; Abraham, D.; Renzoni, E.A. Endothelin in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Kolettis, T.M.; Barton, M.; Langleben, D.; Matsumura, Y. Endothelin in Coronary Artery Disease and Myocardial Infarction. Cardiol. Rev. 2013, 21, 249–256. [Google Scholar] [CrossRef]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Ambrosino, P.; Bachetti, T.; D’anna, S.E.; Galloway, B.; Bianco, A.; D’agnano, V.; Papa, A.; Motta, A.; Perrotta, F.; Maniscalco, M. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 136. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 is increased in the plasma of patients hospitalised with COVID-19. J. Mol. Cell. Cardiol. 2022, 167, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Turgunova, L.; Mekhantseva, I.; Laryushina, Y.; Alina, A.; Bacheva, I.; Zhumadilova, Z.; Turmukhambetova, A. The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19. J. Pers. Med. 2023, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Miedema, J.; Schreurs, M.; van der Sar-van der Brugge, S.; Paats, M.; Baart, S.; Bakker, M.; Hoek, R.; Dik, W.A.; Endeman, H.; Van Der Velden, V.; et al. Antibodies Against Angiotensin II Receptor Type 1 and Endothelin A Receptor Are Associated With an Unfavorable COVID19 Disease Course. Front. Immunol. 2021, 12, 684142. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.H.; Groh, L.A.; Leentjens, J.; Janssen, N.A.F.; Netea, M.G.; Thijssen, D.H.J.; Hannink, G.; et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb. Res. 2022, 209, 106–114. [Google Scholar] [CrossRef]
- Shahbazi, S.; Vahdat Shariatpanahi, Z.; Shahbazi, E. Bosentan for high-risk outpatients with COVID-19 infection: A randomized, double blind, placebo-controlled trial. EClinicalMedicine 2023, 62, 102117. [Google Scholar] [CrossRef]
- Simonson, M.S. Endothelins: Multifunctional renal peptides. Physiol. Rev. 1993, 73, 375–411. [Google Scholar] [CrossRef]
- Galié, N.; Manes, A.; Branzi, A. The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 2004, 61, 227–237. [Google Scholar] [CrossRef]
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pr. 2014, 2014, 62–78. [Google Scholar] [CrossRef]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef]
- Langleben, D.; Barst, R.J.; Badesch, D.; Groves, B.M.; Tapson, V.F.; Murali, S.; Bourge, R.C.; Ettinger, N.; Shalit, E.; Clayton, L.M.; et al. Continuous infusion of epoprostenol improves the net balance between pulmonary endothelin-1 clearance and release in primary pulmonary hypertension. Circulation 1999, 99, 3266–3271. [Google Scholar] [CrossRef]
- Michel, R.P.; Langleben, D.; Dupuis, J. The endothelin system in pulmonary hypertension. Can. J. Physiol. Pharmacol. 2003, 81, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D.; Dupuis, J.; Langleben, I.; Hirsch, A.M.; Baron, M.; Senécal, J.L.; Giovinazzo, M. Etiology-specific endothelin-1 clearance in human precapillary pulmonary hypertension. Chest 2006, 129, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D. Endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Clin. Chest Med. 2007, 28, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Amoura, Z.; Langleben, D. Treatment of pulmonary arterial hypertension by endothelin receptor antagonists in 2008. Rev. Med. Interne 2008, 29, 283–289. [Google Scholar] [CrossRef]
- Star, G.P.; Giovinazzo, M.; Langleben, D. Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: A potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc. Res. 2010, 80, 349–354. [Google Scholar] [CrossRef]
- Horn, E.M.; Chakinala, M.; Oudiz, R.; Joseloff, E.; Rosenzweig, E.B. Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm. Circ. 2020, 10, 2045894020922799. [Google Scholar] [CrossRef]
- Badagliacca, R.; Sciomer, S.; Petrosillo, N. Endothelin receptor antagonists for pulmonary arterial hypertension and COVID-19: Friend or foe? J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2020, 39, 729–730. [Google Scholar] [CrossRef]
- Nabeh, O.A.; Matter, L.M.; Khattab, M.A.; Esraa, M. The possible implication of endothelin in the pathology of COVID-19-induced pulmonary hypertension. Pulm. Pharmacol. Ther. 2021, 71, 102082. [Google Scholar] [CrossRef]
- Shilin, D.S.; Shapovalov, K.G. Changes in Some Vascular Biomarkers in Patients with Severe COVID-19 with Various Degrees of Pulmonary Hypertension. Bull. Exp. Biol. Med. 2022, 173, 433–436. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, X.; Xu, S. Aprocitentan, a dual endothelin-1 (ET-1) antagonist for treating resistant hypertension: Mechanism of action and therapeutic potential. Drug Discov. Today 2023, 28, 103788. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, T.; Wang, R.; Gong, D.; Wang, S.; Du, G.; Fang, L. Insights into Endothelin Receptors in Pulmonary Hypertension. Int. J. Mol. Sci. 2023, 24, 10206. [Google Scholar] [CrossRef]
- Nahar, S.; Kanda, S.; Chatha, U.; Odoma, V.A.; Pitliya, A.; AlEdani, E.M.; Bhangu, J.K.; Javed, K.; Manshahia, P.K.; Yu, A.K. Current Status of Endothelin Receptor Antagonists in Pulmonary Arterial Hypertension: A Combined Study Results and Pharmacology-Based Review. Cureus 2023, 15, e42748. [Google Scholar] [CrossRef]
- Druml, W.; Steltzer, H.; Waldhäusl, W.; Lenz, K.; Hammerle, A.; Vierhapper, H.; Gasic, S.; Wagner, O.F. Endothelin-1 in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993, 148, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Sanai, L.; Haynes, W.G.; MacKenzie, A.; Grant, I.S.; Webb, D.J. Endothelin production in sepsis and the adult respiratory distress syndrome. Intensive Care Med. 1996, 22, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, C.; Hirata, Y.; Nagura, T.; Tsunoda, Y.; Amaha, K. Circulating endothelin-1 concentrations in acute respiratory failure. Chest 1993, 104, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Albertine, K.H.; Wang, Z.M.; Michael, J.R. Expression of endothelial nitric oxide synthase, inducible nitric oxide synthase, and endothelin-1 in lungs of subjects who died with ARDS. Chest 1999, 116, 101S–102S. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Tasaka, S.; Saito, F.; Yamada, W.; Shiraishi, Y.; Ogawa, Y.; Koh, H.; Hasegawa, N.; Fujishima, S.; Hashimoto, S.; et al. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology 2007, 12, 740–743. [Google Scholar] [CrossRef]
- Bhavsar, T.M.; Cerreta, J.M.; Liu, M.; Reznik, S.E.; Cantor, J.O. Phosphoramidon, an endothelin-converting enzyme inhibitor, attenuates lipopolysaccharide-induced acute lung injury. Exp. Lung Res. 2008, 34, 141–154. [Google Scholar] [CrossRef]
- Fujii, Y.; Magder, S.; Cernacek, P.; Goldberg, P.; Guo, Y.; Hussain, S.N. Endothelin receptor blockade attenuates lipopolysaccharide-induced pulmonary nitric oxide production. Am. J. Respir. Crit. Care Med. 2000, 161, 982–989. [Google Scholar] [CrossRef]
- Kuklin, V.; Kirov, M.; Sovershaev, M.; Andreasen, T.; Ingebretsen, O.C.; Ytrehus, K.; Bjertnaes, L. Tezosentan-induced attenuation of lung injury in endotoxemic sheep is associated with reduced activation of protein kinase C. Crit. Care 2005, 9, R211–R217. [Google Scholar] [CrossRef]
- Manitsopoulos, N.; Nikitopoulou, I.; Maniatis, N.A.; Magkou, C.; Kotanidou, A.; Orfanos, S.E. Highly Selective Endothelin-1 Receptor A Inhibition Prevents Bleomycin-Induced Pulmonary Inflammation and Fibrosis in Mice. Respiration 2018, 95, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.P.; Bardou, M.; Goirand, F.; Dumas, M. Hypoxic pulmonary vasoconstriction. Gen. Pharmacol. 1999, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Morio, Y.; Morris, K.G.; Rodman, D.M.; McMurtry, I.F. Mechanism of hypoxic pulmonary vasoconstriction involves ET(A) receptor-mediated inhibition of K(ATP) channel. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L434–L442. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y.; et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020, 5, e140289. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Hutchings, S.D.; Watchorn, J.; Trovato, F.; Napoli, S.; Mujib, S.F.; Hopkins, P.; McPhail, M. Microcirculatory, Endothelial, and Inflammatory Responses in Critically Ill Patients With COVID-19 Are Distinct from Those Seen in Septic Shock: A Case Control Study. Shock 2021, 55, 752–758. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Dimopoulou, I.; Jahaj, E.; Keskinidou, C.; Mastora, Z.; Orfanos, S.E.; Kotanidou, A. Selection of the Appropriate Control Group Is Essential in Evaluating the Cytokine Storm in COVID-19. In Vivo 2021, 35, 1295–1298. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]


| Characteristics | COVID-19 ARDS | Non-COVID-19 ARDS | Non-COVID-19 Non-ARDS |
|---|---|---|---|
| Number of patients, N | 18 | 14 | 20 |
| Age (years) | 73 (66–78) | 64 (48–76) | 62 (45–73) |
| Sex, N (%) | |||
| Male | 13 (72.2) | 7 (50.0) | 11 (55.0) |
| Female | 5 (27.8) | 7 (50.0) | 9 (45.0) |
| Comorbidities, N (%) | |||
| Hypertension | 11 (61.1) | 7 (50.0) | 6 (30.0) |
| Dyslipidemia | 4 (22.2) | 4 (28.6) | 5 (25.0) |
| Diabetes | 4 (22.2) | 6 (42.9) | 3 (15.0) |
| Coronary disease | 4 (22.2) | 2 (14.3) | 2 (10.0) |
| Cancer | 3 (16.7) | 1 (7.1) | 4 (20.0) |
| COPD | 2 (11.1) | 0 (0.0) | 1 (5.0) |
| Chronic renal failure | 0 (0.0) | 2 (14.3) | 0 (0.0) |
| Liver failure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| APACHE II | 16 (10–21) | 16 (13–23) | 15 (11–20) |
| SOFA | 8 (6–9) | 10 (9–10) | 7 (6–10) |
| Admission diagnosis | |||
| Medical | 18 (100.0) | 11 (78.6) | 7 (35.0) |
| Surgical | 0 (0.0) | 3 (21.4) | 6 (30.0) |
| Trauma | 0 (0.0) | 0 (0.0) | 7 (35.0) |
| Mean arterial pressure (mmHg) | 92 (85–102) | 80 (76–88) ## | 85 (79–90) # |
| White blood cell count (×103 per μL) | 9.9 (8.2–13.2) | 12.6 (7.9–16.6) | 14.7 (8.7–18.5) |
| Neutrophils (%) | 88.2 (81.2–92.3) | 86.3 (76.3–90.9) | 89.6 (76.0–94.5) |
| Platelets (×103 per μL) | 266 (118–371) | 220 (159–322) | 233 (169–282) |
| PO2 (mmHg) | 98.3 (76.6–117.8) | 108.6 (85.6–145) | 124.5 (92.2–167.0) |
| HCO3 (mEq/L) | 22.9 (20.0–25.8) | 22.1 (19.7–24.6) | 21.4 (19.4–23.2) |
| PaO2/FiO2 (mmHg) | 131 (95–177) | 175 (100–209) | 310 (202–381) # |
| PCO2 (mmHg) | 44.3 (40.8–50.8) | 51.6 (38.7–56.0) | 37.3 (34.5–42.3) # § |
| Urea (mg/dL) | 55 (47–67) | 56 (33–87) | 32 (26–43) # § |
| Creatinine (mg/dL) | 0.95 (0.68–1.33) | 1.10 (0.68–2.88) | 0.80 (0.60–0.98) |
| Total bilirubin (mg/dL) | 0.69 (0.39–1.37) | 0.55 (0.30–1.10) | 0.63 (0.52–0.80) |
| LDH (U/L) | 431 (449–615) | 228 (215–489) ## | 328 (255–425) # |
| CRP (mg/dL) | 9.8 (6.2–13.4) | 17.3 (6.3–28.0) | 6.5 (2.0–13.8) § |
| Fibrinogen (mg/dL) | 623 (527–718) | 575 (424–763) | 418 (255–535) ### § |
| pH | 7.33 (7.29–7.36) | 7.29 (7.24–7.38) | 7.37 (7.31–7.42) |
| Mean vasopressor dose (mL/h) | 5 (4–7) | 12 (4–25) # | 12 (4–20) |
| Tidal volume (VT) (mL) | 420 (408–463) | 450 (380–500) | 440 (405–480) |
| Respiratory rate (RR) (mechanical breaths/min) | 27 (22–29) | 24 (20–30) | 22 (18–26) # |
| Positive end-expiratory pressure (PEEP) (cmH2O) | 12 (10–13) | 11 (9–15) | 8 (6–10) #### § |
| Volume control (VC), N (%) | 18 (100.0) | 14 (100.0) | 19 (95.0) |
| sICAM-1 (ng/mL) | 630 (482–1014) | 595 (490–1464) | 447 (293–650) # § |
| sVCAM-1 (ng/mL) | 1488 (797–2166) | 1395 (933–2414) | 1147 (484–1703) |
| sE-selectin (ng/mL) | 78.9 (60.4–108.8) | 142.9 (107.3–226.5) ## | 67.6 (57.1–117.9) §§ |
| vWf (pg/mL) | 12313 (4581–15254) | 7558 (4637–18020) | 10632 (3723–17280) |
| ICU outcomes | |||
| Length of ICU stay, days | 28 (6–40) | 25 (8–51) | 14 (9–25) |
| Mortality, N (%) | 5 (27.8) | 4 (28.6) | 2 (10.0) |
| Characteristics | Survivors | Non-Survivors | p-Value |
|---|---|---|---|
| Number of patients, N | 12 | 5 | |
| Age (years) | 73 (63 79) | 74 (65 78) | >0.99 |
| Sex, N (%) | >0.99 | ||
| Male | 8 (66.7) | 4 (80.0) | |
| Female | 4 (33.3) | 1 (20.0) | |
| Comorbidities, N (%) | >0.99 | ||
| Hypertension | 8 (66.7) | 3 (60.0) | |
| Dyslipidemia | 3 (25.0) | 1 (20.0) | |
| Diabetes | 3 (25.0) | 0 (0.0) | |
| Coronary disease | 3 (25.0) | 1 (20.0) | |
| Cancer | 2 (16.7) | 1 (20.0) | |
| COPD | 1 (8.3) | 1 (20.0) | |
| APACHE II | 15 (10–19) | 20 (9–23) | 0.51 |
| SOFA | 7 (4–9) | 7 (6–12) | 0.44 |
| Mean arterial pressure (mmHg) | 92 (88–101) | 100 (81–118) | 0.65 |
| White blood cell count (×103 per μL) | 9.9 (9.0–12.5) | 12.9 (4.9–24.9) | 0.72 |
| Neutrophils (%) | 87.2 (81.5–91.3) | 92.9 (73.9–93.5) | 0.16 |
| Platelets (×103 per μL) | 266 (136–386) | 317 (54–605) | 0.89 |
| PCO2 (mmHg) | 44.3 (41.4–48.3) | 41.0 (36.3–65.0) | 0.65 |
| HCO3 (mEq/L) | 21.9 (19.7–26.6) | 25.2 (21.0–25.9) | 0.57 |
| PaO2/FiO2 (mmHg) | 125 (83–193) | 132 (107–195) | 0.88 |
| Glucose (mg/dL) | 179 (139–220) | 137 (120–188) | 0.16 |
| Urea (mg/dL) | 52 (40–57) | 65 (57–80) | 0.06 |
| Creatinine (mg/dL) | 0.8 (0.6–1.3) | 1.2 (1.0–1.3) | 0.13 |
| Total bilirubin (mg/dL) | 0.7 (0.4–0.9) | 1.5 (0.4–1.9) | 0.51 |
| LDH (U/L) | 431 (344–589) | 566 (372–4170) | 0.51 |
| CRP (mg/dL) | 10.9 (6.5–14.1) | 8.2 (5.4–24.8) | 0.57 |
| pH | 7.32 (7.29–7.36) | 7.35 (7.24–7.40) | 0.51 |
| Mean vasopressor dose (mL/h) | 5 (3–5) | 4 (3–31) | 0.79 |
| Tidal volume (VT) (mL) | 430 (420–465) | 410 (375–465) | 0.51 |
| Respiratory rate (RR) (mechanical breaths/min) | 24 (21–28) | 28 (28–30) | 0.04 * |
| Positive end-expiratory pressure (PEEP) (cmH2O) | 12.0 (10.3–14.8) | 10.0 (8.0–12.5) | 0.23 |
| Dexamethasone administration, N (%) | 10 (83.3) | 4 (80.0) | >0.99 |
| Day of death | N/A | 6 (4–23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassiliou, A.G.; Roumpaki, A.; Keskinidou, C.; Athanasiou, N.; Tsipilis, S.; Jahaj, E.; Vrettou, C.S.; Giannopoulou, V.; Halioti, A.; Ferentinos, G.; et al. Transpulmonary Plasma Endothelin-1 Arterial:Venous Ratio Differentiates Survivors from Non-Survivors in Critically Ill Patients with COVID-19-Induced Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2024, 25, 10640. https://doi.org/10.3390/ijms251910640
Vassiliou AG, Roumpaki A, Keskinidou C, Athanasiou N, Tsipilis S, Jahaj E, Vrettou CS, Giannopoulou V, Halioti A, Ferentinos G, et al. Transpulmonary Plasma Endothelin-1 Arterial:Venous Ratio Differentiates Survivors from Non-Survivors in Critically Ill Patients with COVID-19-Induced Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences. 2024; 25(19):10640. https://doi.org/10.3390/ijms251910640
Chicago/Turabian StyleVassiliou, Alice G., Anastasia Roumpaki, Chrysi Keskinidou, Nikolaos Athanasiou, Stamatios Tsipilis, Edison Jahaj, Charikleia S. Vrettou, Vassiliki Giannopoulou, Asimenia Halioti, Georgios Ferentinos, and et al. 2024. "Transpulmonary Plasma Endothelin-1 Arterial:Venous Ratio Differentiates Survivors from Non-Survivors in Critically Ill Patients with COVID-19-Induced Acute Respiratory Distress Syndrome" International Journal of Molecular Sciences 25, no. 19: 10640. https://doi.org/10.3390/ijms251910640
APA StyleVassiliou, A. G., Roumpaki, A., Keskinidou, C., Athanasiou, N., Tsipilis, S., Jahaj, E., Vrettou, C. S., Giannopoulou, V., Halioti, A., Ferentinos, G., Dimopoulou, I., Kotanidou, A., Langleben, D., & Orfanos, S. E. (2024). Transpulmonary Plasma Endothelin-1 Arterial:Venous Ratio Differentiates Survivors from Non-Survivors in Critically Ill Patients with COVID-19-Induced Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences, 25(19), 10640. https://doi.org/10.3390/ijms251910640






