Readdressing the Localization of Apolipoprotein E (APOE) in Mitochondria-Associated Endoplasmic Reticulum (ER) Membranes (MAMs): An Investigation of the Hepatic Protein–Protein Interactions of APOE with the Mitochondrial Proteins Lon Protease (LONP1), Mitochondrial Import Receptor Subunit TOM40 (TOMM40) and Voltage-Dependent Anion-Selective Channel 1 (VDAC1)
Abstract
1. Introduction
2. Results
2.1. APOE Accumulates in the MAMs of Human Cultured Hepatocytes, Equally Evident for APOE3 and APOE4
2.2. The Co-Localization of MERC-forming Proteins and Expression of MAM Markers Is Similar in the Presence of APOE3 and APOE4 in Cultured Hepatocytes and the Livers of Mice
2.3. Identification of TOMM40 and Other Mitochondrial and Non-Mitochondrial Proteins as APOE Interaction Partners in Human Cultured Hepatocytes
2.4. LONP1, TOMM40 and VDAC1 Share Cellular Localizations with APOE, Particularly in MAMs, Which Was Equally Evident in APOE3- and APOE4-Transfected Cells
2.5. The UPR and ER Stress Provokes the Accumulation of APOE and LONP1 in MAMs, Strengthening Their Interaction
3. Discussion
4. Materials and Methods
4.1. Cultivation of Human Hepatoma Cells
4.2. APOE3 and APOE4 Plasmid DNA Construction and Transformation and Transient Transfection of Human Hepatoma Cells
4.3. Isolation of Subcellular Fractions
4.4. Co-Immunoprecipitation (co-IP)
4.5. Proteome Analysis
4.6. APOE-Targeted Replacement Mice
4.7. Western Blotting
4.8. Proximity Ligation Assay (PLA)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dose, J.; Huebbe, P.; Nebel, A.; Rimbach, G. APOE genotype and stress response—A mini review. Lipids Health Dis. 2016, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Rueter, J.; Rimbach, G.; Huebbe, P. Functional diversity of apolipoprotein E: From subcellular localization to mitochondrial function. Cell. Mol. Life Sci. 2022, 79, 499. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Nandi, A.; Counts, N.; Jiao, L.; Prettner, K.; Kuhn, M.; Seligman, B.; Tortorice, D.; Vigo, D.; et al. The global macroeconomic burden of Alzheimer’s disease and other dementias: Estimates and projections for 152 countries or territories. Lancet Glob. Health 2024, 12, e1534–e1543. [Google Scholar] [CrossRef]
- Liu, M.; Kuhel, D.G.; Shen, L.; Hui, D.Y.; Woods, S.C. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R903–R908. [Google Scholar] [CrossRef]
- Davignon, J.; Bouthillier, D.; Nestruck, A.C.; Sing, C.F. Apolipoprotein E polymorphism and atherosclerosis: Insight from a study in octogenarians. Trans. Am. Clin. Climatol. Assoc. 1988, 99, 100–110. [Google Scholar]
- Nebel, A.; Croucher, P.J.P.; Stiegeler, R.; Nikolaus, S.; Krawczak, M.; Schreiber, S. No association between microsomal triglyceride transfer protein (MTP) haplotype and longevity in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 7906–7909. [Google Scholar] [CrossRef]
- De Feo, E.; Cefalo, C.; Arzani, D.; Amore, R.; Landolfi, R.; Grieco, A.; Ricciardi, W.; Miele, L.; Boccia, S. A case–control study on the effects of the apolipoprotein E genotypes in nonalcoholic fatty liver disease. Mol. Biol. Rep. 2012, 39, 7381–7388. [Google Scholar] [CrossRef]
- Huebbe, P.; Bilke, S.; Rueter, J.; Schloesser, A.; Campbel, G.; Glüer, C.-C.; Lucius, R.; Röcken, C.; Tholey, A.; Rimbach, G. Human APOE4 Protects High-Fat and High-Sucrose Diet Fed Targeted Replacement Mice against Fatty Liver Disease Compared to APOE3. Aging Dis. 2024, 15, 259–281. [Google Scholar] [CrossRef]
- Oriá, R.B.; Patrick, P.D.; Zhang, H.; Lorntz, B.; Costa, C.M.D.C.; Brito, G.A.C.; Barrett, L.J.; Lima, A.A.M.; Guerrant, R.L. APOE4 protects the cognitive development in children with heavy diarrhea burdens in northeast brazil. Pediatr. Res. 2005, 57, 310–316. [Google Scholar] [CrossRef]
- Huebbe, P.; Rimbach, G. Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors. Ageing Res. Rev. 2017, 37, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Garai, K.; Mustafi, S.M.; Baban, B.; Frieden, C. Structural differences between apolipoprotein E3 and E4 as measured by 19F NMR. Protein Sci. 2010, 19, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Knouff, C.; Hinsdale, M.E.; Mezdour, H.; Altenburg, M.K.; Watanabe, M.; Quarfordt, S.H.; Sullivan, P.M.; Maeda, N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Investig. 1999, 103, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Maugeais, C.; Glick, J.M.; Rader, D.J. Markedly increased secretion of VLDL triglycerides induced by gene transfer of apolipoprotein E isoforms in apoE-deficient mice. J. Lipid Res. 2000, 41, 253–259. [Google Scholar] [CrossRef]
- Bergeron, N.; Havel, R.J. Prolonged postprandial responses of lipids and apolipoproteins in triglyceride-rich lipoproteins of individuals expressing an apolipoprotein epsilon 4 allele. J. Clin. Investig. 1996, 97, 65–72. [Google Scholar] [CrossRef][Green Version]
- Theendakara, V.; Peters-Libeu, C.A.; Spilman, P.; Poksay, K.S.; Bredesen, D.E.; Rao, R.V. Direct Transcriptional Effects of Apolipoprotein E. J. Neurosci. 2016, 36, 685–700. [Google Scholar] [CrossRef]
- Panin, L.E.; Russkikh, G.S.; Polyakov, L.M. Detection of apolipoprotein A-I, B, and E immunoreactivity in the nuclei of various rat tissue cells. Biochemistry 2000, 65, 1419–1423. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Wong, J.S.; Guo, L.S.; Krisans, S.; Havel, R.J. Apolipoprotein E localization in rat hepatocytes by immunogold labeling of cryothin sections. J. Lipid Res. 1990, 31, 1589–1603. [Google Scholar]
- James, R.; Searcy, J.L.; Le Bihan, T.; Martin, S.F.; Gliddon, C.M.; Povey, J.; Deighton, R.F.; Kerr, L.E.; McCulloch, J.; Horsburgh, K. Proteomic Analysis of Mitochondria in APOE Transgenic Mice and in Response to an Ischemic Challenge. J. Cereb. Blood Flow Metab. 2011, 32, 164–176. [Google Scholar] [CrossRef]
- Vance, J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990, 265, 7248–7256. [Google Scholar]
- De Stefani, D.; Bononi, A.; Romagnoli, A.; Messina, A.; De Pinto, V.; Pinton, P.; Rizzuto, R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012, 19, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, N.; Sánchez-Álvarez, M.; Bachmann, M.; Costiniti, V.; Del Pozo, M.A.; Giacomello, M. Protein Localization at Mitochondria-ER Contact Sites in Basal and Stress Conditions. Front. Cell Dev. Biol. 2017, 5, 107. [Google Scholar] [CrossRef]
- Poston, C.N.; Krishnan, S.C.; Bazemore-Walker, C.R. In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J. Proteom. 2013, 79, 219–230. [Google Scholar] [CrossRef]
- Horner, S.M.; Wilkins, C.; Badil, S.; Iskarpatyoti, J.; Gale, M. Proteomic analysis of mitochondrial-associated ER membranes (MAM) during RNA virus infection reveals dynamic changes in protein and organelle trafficking. PLoS ONE 2015, 10, e0117963. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Navarro-Lérida, I.; Sánchez-Alvarez, M.; Bosch, M.; Calvo, C.; López, J.A.; Calvo, E.; Ferguson, C.; Giacomello, M.; Serafini, A.; et al. Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci. Rep. 2016, 6, 27351. [Google Scholar] [CrossRef]
- Anastasia, I.; Ilacqua, N.; Raimondi, A.; Lemieux, P.; Ghandehari-Alavijeh, R.; Faure, G.; Mekhedov, S.L.; Williams, K.J.; Caicci, F.; Valle, G.; et al. Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Rep. 2021, 34, 108873. [Google Scholar] [CrossRef]
- Tambini, M.D.; Pera, M.; Kanter, E.; Yang, H.; Guardia-Laguarta, C.; Holtzman, D.; Sulzer, D.; Area-Gomez, E.; Schon, E.A. ApoE4 upregulates the activity of mitochondria-associated ER membranes. EMBO Rep. 2016, 17, 27–36. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, Y.-F.; Chan, H.-C.; Chung, C.-H.; Peng, H.-Y.; Ho, Y.-C.; Chen, C.-H.; Chang, K.-C.; Tang, C.-H.; Lee, A.-S. Role of apolipoprotein E in electronegative low -density lipoprotein-induced mitochondrial dysfunction in cardiomyocytes. Metabolism 2020, 107, 154227. [Google Scholar] [CrossRef]
- Rueter, J.; Rimbach, G.; Treitz, C.; Schloesser, A.; Lüersen, K.; Tholey, A.; Huebbe, P. The mitochondrial BCKD complex interacts with hepatic apolipoprotein E in cultured cells in vitro and mouse livers in vivo. Cell. Mol. Life Sci. 2023, 80, D535–D539. [Google Scholar] [CrossRef]
- Stark, C.; Breitkreutz, B.J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A general repository for interaction datasets. Nucleic Acids Res. 2006, 34, D535–D539. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Kovács, I.A.; Luck, K.; Spirohn, K.; Wang, Y.; Pollis, C.; Schlabach, S.; Bian, W.; Kim, D.-K.; Kishore, N.; Hao, T.; et al. Network-based prediction of protein interactions. Nat. Commun. 2019, 10, 3279–3294. [Google Scholar] [CrossRef]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.-A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Lam, V.Y.M.; Ernst, I.M.A.; Huebbe, P.; Rimbach, G.; Halliwell, B. Variability in APOE genotype status in human-derived cell lines: A cause for concern in cell culture studies? Genes Nutr. 2014, 9, 364. [Google Scholar] [CrossRef]
- Moutaoufik, M.T.; Malty, R.; Amin, S.; Zhang, Q.; Phanse, S.; Gagarinova, A.; Zilocchi, M.; Hoell, L.; Minic, Z.; Gagarinova, M.; et al. Rewiring of the Human Mitochondrial Interactome during Neuronal Reprogramming Reveals Regulators of the Respirasome and Neurogenesis. iScience 2019, 19, 1114–1132. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- Harada, T.; Sada, R.; Osugi, Y.; Matsumoto, S.; Matsuda, T.; Hayashi-Nishino, M.; Nagai, T.; Harada, A.; Kikuchi, A. Palmitoylated CKAP4 regulates mitochondrial functions through an interaction with VDAC2 at ER–mitochondria contact sites. J. Cell Sci. 2020, 133, jcs249045. [Google Scholar] [CrossRef]
- Namba, T. BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites. Sci. Adv. 2019, 5, eaaw1386. [Google Scholar] [CrossRef]
- Shin, C.-S.; Meng, S.; Garbis, S.D.; Moradian, A.; Taylor, R.W.; Sweredoski, M.J.; Lomenick, B.; Chan, D.C. LONP1 and mtHSP70 cooperate to promote mitochondrial protein folding. Nat. Commun. 2021, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Polo, M.; Alegre, F.; Moragrega, A.B.; Gibellini, L.; Marti-Rodrigo, A.; Blas-Garcia, A.; Esplugues, J.V.; Apostolova, N. Lon protease: A novel mitochondrial matrix protein in the interconnection between drug-induced mitochondrial dysfunction and endoplasmic reticulum stress. Br. J. Pharmacol. 2017, 174, 4409–4429. [Google Scholar] [CrossRef] [PubMed]
- Hori, O.; Ichinoda, F.; Tamatani, T.; Yamaguchi, A.; Sato, N.; Ozawa, K.; Kitao, Y.; Miyazaki, M.; Harding, H.P.; Ron, D.; et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: Enhanced expression of Lon protease. J. Cell Biol. 2002, 157, 1151–1160. [Google Scholar] [CrossRef]
- van Vliet, A.R.; Agostinis, P. Mitochondria-Associated Membranes and er Stress. In Coordinating Organismal Physiology through the Unfolded Protein Response; Wiseman, R.L., Haynes, C.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 414, pp. 73–102. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Jia, L.; Shangguan, F.; Gong, S.; Lan, L.; Song, Z.; Xu, J.; Yan, C.; Chen, T.; et al. LonP1 Links Mitochondria–ER Interaction to Regulate Heart Function. Research 2023, 6, 0175. [Google Scholar] [CrossRef]
- Orr, A.L.; Kim, C.; Jimenez-Morales, D.; Newton, B.W.; Johnson, J.R.; Krogan, N.J.; Swaney, D.L.; Mahley, R.W. Neuronal Apolipoprotein E4 Expression Results in Proteome-Wide Alterations and Compromises Bioenergetic Capacity by Disrupting Mitochondrial Function. J. Alzheimer’s Dis. 2019, 68, 991–1011. [Google Scholar] [CrossRef]
- Hu, Y.; Meuret, C.; Go, S.; Yassine, H.N.; Nedelkov, D. Simple and Fast Assay for Apolipoprotein E Phenotyping and Glycotyping: Discovering Isoform-Specific Glycosylation in Plasma and Cerebrospinal Fluid. J. Alzheimer’s Dis. 2020, 76, 883–893. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2013, 25, 8–14. [Google Scholar] [CrossRef]
- Buttini, M.; Masliah, E.; Yu, G.-Q.; Palop, J.J.; Chang, S.; Bernardo, A.; Lin, C.; Wyss-Coray, T.; Huang, Y.; Mucke, L. Cellular Source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am. J. Pathol. 2010, 177, 563–569. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Y.; Xu, X.; Wang, G.; Liu, Y.; Wu, H.; Li, W.; Wang, Y.; Chen, Z.; Zhang, W.; et al. A mitochondrial FUNDC1/HSC70 interaction organizes the proteostatic stress response at the risk of cell morbidity. EMBO J. 2019, 38, e98786. [Google Scholar] [CrossRef]
- Baden, P.; Perez, M.J.; Raji, H.; Bertoli, F.; Kalb, S.; Illescas, M.; Spanos, F.; Giuliano, C.; Calogero, A.M.; Oldrati, M.; et al. Glucocerebrosidase is imported into mitochondria and preserves complex I integrity and energy metabolism. Nat. Commun. 2023, 14, 1930. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.; Qi, H.; Yap, M.C.; Tahbaz, N.; Milburn, L.A.; Lucchinetti, E.; Lou, P.-H.; Zaugg, M.; LaPointe, P.G.; Mercier, P.; et al. The ER chaperone calnexin controls mitochondrial positioning and respiration. Sci. Signal. 2020, 13, eaax6660. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Prabhu, B.M.; Galati, D.F.; Avadhani, N.G.; Anandatheerthavarada, H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006, 26, 9057–9068. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar]
- Reed, A.L.; Mitchell, W.; Alexandrescu, A.T.; Alder, N.N. Interactions of amyloidogenic proteins with mitochondrial protein import machinery in aging-related neurodegenerative diseases. Front. Physiol. 2023, 14, 1263420. [Google Scholar] [CrossRef]
- Wisniewski, T.; Frangione, B. Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 1992, 135, 235–238. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. 125I-labeled ApoE binds competitively to beta(1-40) fibrils with pathological chaperone proteins. Amyloid 2000, 7, 83–89. [Google Scholar] [CrossRef]
- Ngo, J.K.; Pomatto, L.C.D.; Davies, K.J.A. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013, 1, 258–264. [Google Scholar] [CrossRef]
- Babuharisankar, A.P.; Kuo, C.-L.; Chou, H.-Y.; Tangeda, V.; Fan, C.-C.; Chen, C.-H.; Kao, Y.-H.; Lee, A.Y.-L. Mitochondrial Lon-induced mitophagy benefits hypoxic resistance via Ca2+-dependent FUNDC1 phosphorylation at the ER-mitochondria interface. Cell Death Dis. 2023, 14, 199. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.M.; de Waal, R.M.W.; Verbeek, M.M. Heat shock proteins and amateur chaperones in amyloid-beta accumulation and clearance in alzheimer’s disease. Mol. Neurobiol. 2007, 35, 203–216. [Google Scholar] [CrossRef]
- Matsushima, Y.; Takahashi, K.; Yue, S.; Fujiyoshi, Y.; Yoshioka, H.; Aihara, M.; Setoyama, D.; Uchiumi, T.; Fukuchi, S.; Kang, D. Mitochondrial Lon protease is a gatekeeper for proteins newly imported into the matrix. Commun. Biol. 2021, 4, 974. [Google Scholar] [CrossRef]
- Hosp, F.; Vossfeldt, H.; Heinig, M.; Vasiljevic, D.; Arumughan, A.; Wyler, E.; Landthaler, M.; Hubner, N.; Wanker, E.E.; Lannfelt, L.; et al. Quantitative interaction proteomics of neurodegenerative disease proteins. Cell Rep. 2015, 11, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Hagl, S.; Hoehn, A.; Huebbe, P.; Pallauf, K.; Grune, T.; Frank, J.; Eckert, G.P.; Rimbach, G. Adenosine triphosphate concentrations are higher in the brain of APOE3- compared to APOE4-targeted replacement mice and can be modulated by curcumin. Genes Nutr. 2014, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Schmukler, E.; Solomon, S.; Simonovitch, S.; Goldshmit, Y.; Wolfson, E.; Michaelson, D.M.; Pinkas-Kramarski, R. Altered mitochondrial dynamics and function in APOE4-expressing astrocytes. Cell Death Dis. 2020, 11, 578. [Google Scholar] [CrossRef]
- Yin, J.; Reiman, E.M.; Beach, T.G.; Serrano, G.E.; Sabbagh, M.N.; Nielsen, M.; Caselli, R.J.; Shi, J. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology 2020, 94, e2404–e2411. [Google Scholar] [CrossRef]
- Dose, J.; Nebel, A.; Piegholdt, S.; Rimbach, G.; Huebbe, P. Influence of the APOE genotype on hepatic stress response: Studies in APOE targeted replacement mice and human liver cells. Free. Radic. Biol. Med. 2016, 96, 264–272. [Google Scholar] [CrossRef]
- Yu, H.; Sun, C.; Gong, Q.; Feng, D. Mitochondria-Associated Endoplasmic Reticulum Membranes in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 629669. [Google Scholar] [CrossRef]
- Pinti, M.; Gibellini, L.; Nasi, M.; De Biasi, S.; Bortolotti, C.A.; Iannone, A.; Cossarizza, A. Emerging role of Lon protease as a master regulator of mitochondrial functions. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 1300–1306. [Google Scholar] [CrossRef]
- Williamson, C.D.; Wong, D.S.; Bozidis, P.; Zhang, A.; Colberg-Poley, A.M. Isolation of Endoplasmic Reticulum, Mitochondria, and Mitochondria-Associated Membrane and Detergent Resistant Membrane Fractions from Transfected Cells and from Human Cytomegalovirus-Infected Primary Fibroblasts. Curr. Protoc. Cell Biol. 2015, 68, 3.27.1–3.27.33. [Google Scholar] [CrossRef]
- Wang, W.-X.; Prajapati, P.; Nelson, P.T.; Springer, J.E. The Mitochondria-Associated ER Membranes Are Novel Subcellular Locations Enriched for Inflammatory-Responsive MicroRNAs. Mol. Neurobiol. 2020, 57, 2996–3013. [Google Scholar] [CrossRef]
- Tubbs, E.; Rieusset, J. Study of Endoplasmic Reticulum and Mitochondria Interactions by In Situ Proximity Ligation Assay in Fixed Cells. J. Vis. Exp. 2016, 118, e54899. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Zabielski, P.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.J.; Bergen, H.R., 3rd; Dasari, S.; Walrand, S.; Short, K.R.; Johnson, M.L.; et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012, 16, 777–788. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
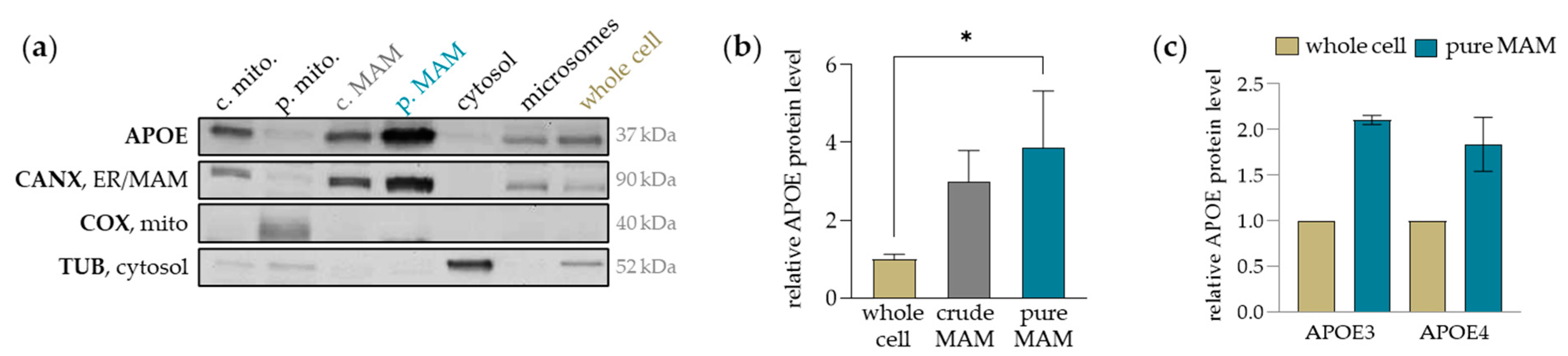
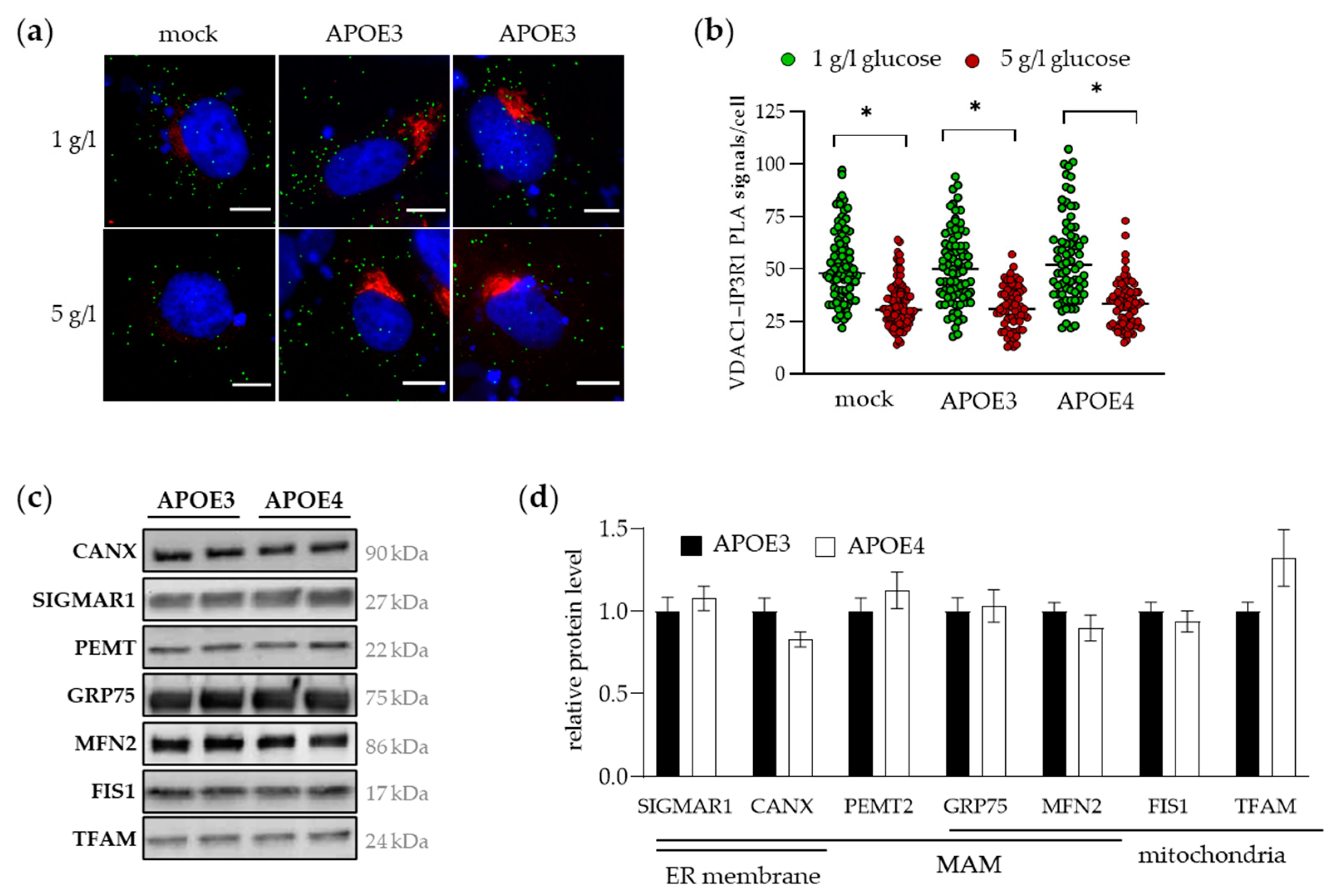

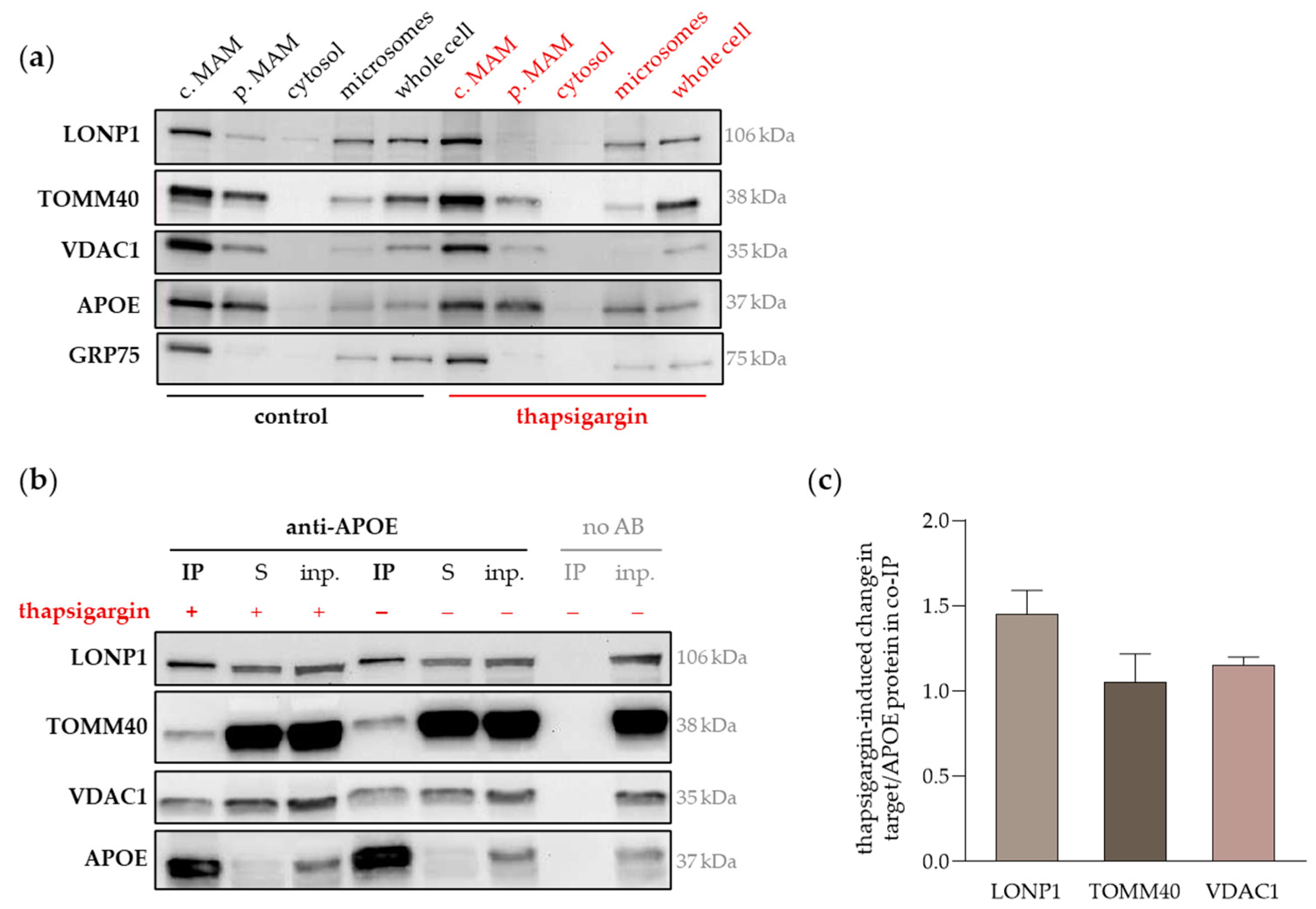
| Protein | Antibody | Company | |
|---|---|---|---|
| APOE | Apolipoprotein E | sc-13521 | Santa Cruz, Dallas, TX, USA |
| CANX | Calnexin | ab13504 | Abcam, Cambridge, UK |
| COX | Cytochrome C oxidase | ab110413 | Abcam, Cambridge, UK |
| GRP75 | Glucose-regulated protein 75 | ORB214063 | Biorbyt, Cambridge, UK |
| FIS1 | Mitochondrial fission 1 protein | 10956-1-AP | Proteintech, Planegg-Martinsried, Germany |
| LONP1 | Lon protease | 15440-1-AP | Proteintech, Planegg-Martinsried, Germany |
| MFN2 | Mitofusin 2 | 12186-1-AP | Proteintech, Planegg-Martinsried, Germany |
| PEMT | Phosphatidylethanolamine N-methyltransferase | ORB46023 | Biorbyt, Cambridge, UK |
| SIGMAR1 | Sigma non-opioid intracellular receptor 1 | 15168-1-AP | Proteintech, Planegg-Martinsried, Germany |
| TFAM | Mitochondrial transcription factor A | sc-166965 | Santa Cruz, Dallas, TX, USA |
| TOMM40 | Translocase of outer mitochondria membrane 40 | 18409-1-AP | Proteintech, Planegg-Martinsried, Germany |
| TUB | Tubulin | ab7291 | Abcam, Cambridge, UK |
| VDAC1 | Voltage-dependent anion-selective channel 1 | sc-8828 | Santa Cruz, Dallas, TX, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueter, J.; Rimbach, G.; Bilke, S.; Tholey, A.; Huebbe, P. Readdressing the Localization of Apolipoprotein E (APOE) in Mitochondria-Associated Endoplasmic Reticulum (ER) Membranes (MAMs): An Investigation of the Hepatic Protein–Protein Interactions of APOE with the Mitochondrial Proteins Lon Protease (LONP1), Mitochondrial Import Receptor Subunit TOM40 (TOMM40) and Voltage-Dependent Anion-Selective Channel 1 (VDAC1). Int. J. Mol. Sci. 2024, 25, 10597. https://doi.org/10.3390/ijms251910597
Rueter J, Rimbach G, Bilke S, Tholey A, Huebbe P. Readdressing the Localization of Apolipoprotein E (APOE) in Mitochondria-Associated Endoplasmic Reticulum (ER) Membranes (MAMs): An Investigation of the Hepatic Protein–Protein Interactions of APOE with the Mitochondrial Proteins Lon Protease (LONP1), Mitochondrial Import Receptor Subunit TOM40 (TOMM40) and Voltage-Dependent Anion-Selective Channel 1 (VDAC1). International Journal of Molecular Sciences. 2024; 25(19):10597. https://doi.org/10.3390/ijms251910597
Chicago/Turabian StyleRueter, Johanna, Gerald Rimbach, Stephanie Bilke, Andreas Tholey, and Patricia Huebbe. 2024. "Readdressing the Localization of Apolipoprotein E (APOE) in Mitochondria-Associated Endoplasmic Reticulum (ER) Membranes (MAMs): An Investigation of the Hepatic Protein–Protein Interactions of APOE with the Mitochondrial Proteins Lon Protease (LONP1), Mitochondrial Import Receptor Subunit TOM40 (TOMM40) and Voltage-Dependent Anion-Selective Channel 1 (VDAC1)" International Journal of Molecular Sciences 25, no. 19: 10597. https://doi.org/10.3390/ijms251910597
APA StyleRueter, J., Rimbach, G., Bilke, S., Tholey, A., & Huebbe, P. (2024). Readdressing the Localization of Apolipoprotein E (APOE) in Mitochondria-Associated Endoplasmic Reticulum (ER) Membranes (MAMs): An Investigation of the Hepatic Protein–Protein Interactions of APOE with the Mitochondrial Proteins Lon Protease (LONP1), Mitochondrial Import Receptor Subunit TOM40 (TOMM40) and Voltage-Dependent Anion-Selective Channel 1 (VDAC1). International Journal of Molecular Sciences, 25(19), 10597. https://doi.org/10.3390/ijms251910597






