Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis
Abstract
1. Introduction
2. Results
2.1. Determination of Antioxidant and Immune Indicators
2.2. Histological Analysis of the Liver
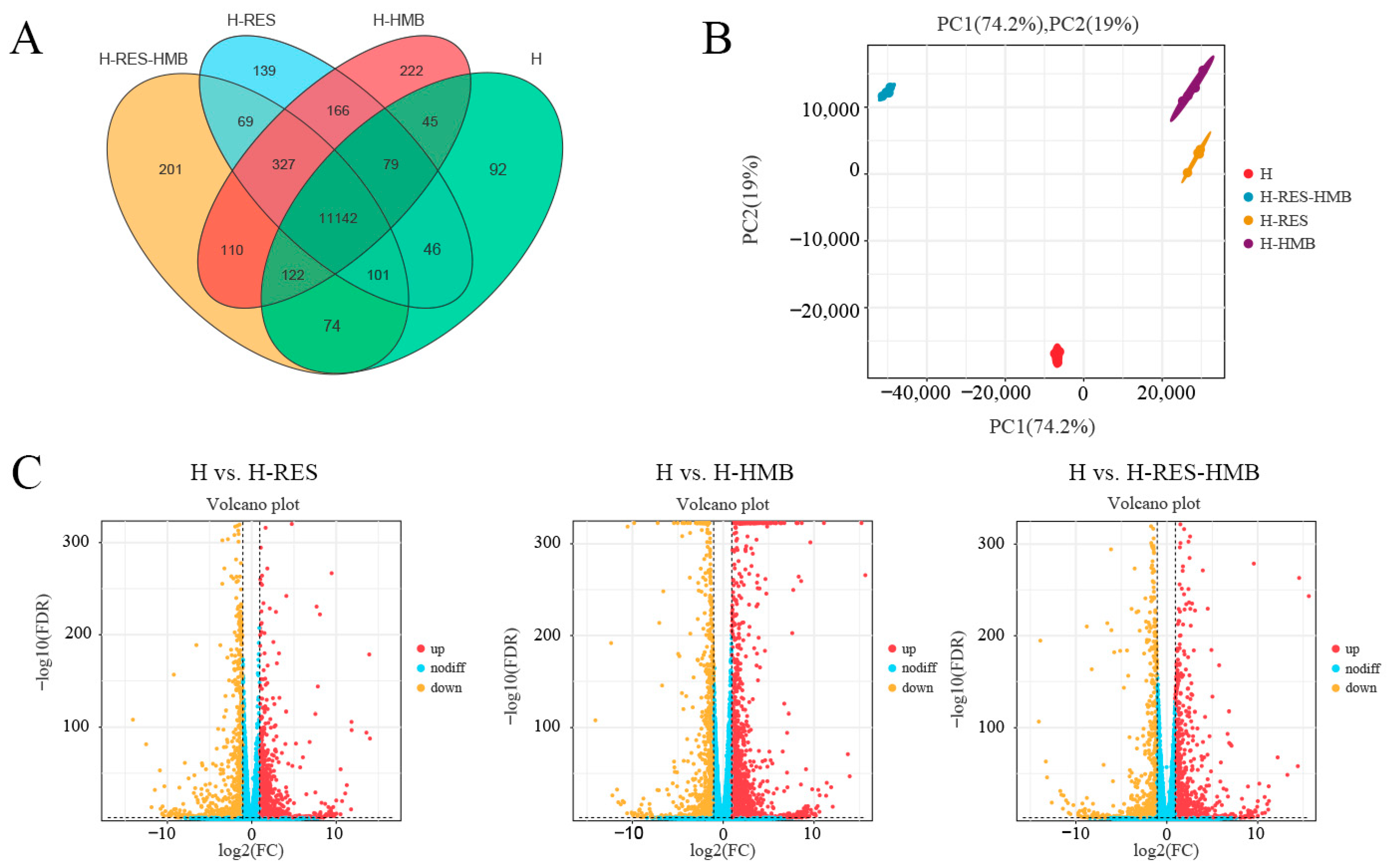
2.3. Identification of Differentially Expressed Genes and Functional Enrichment Analysis
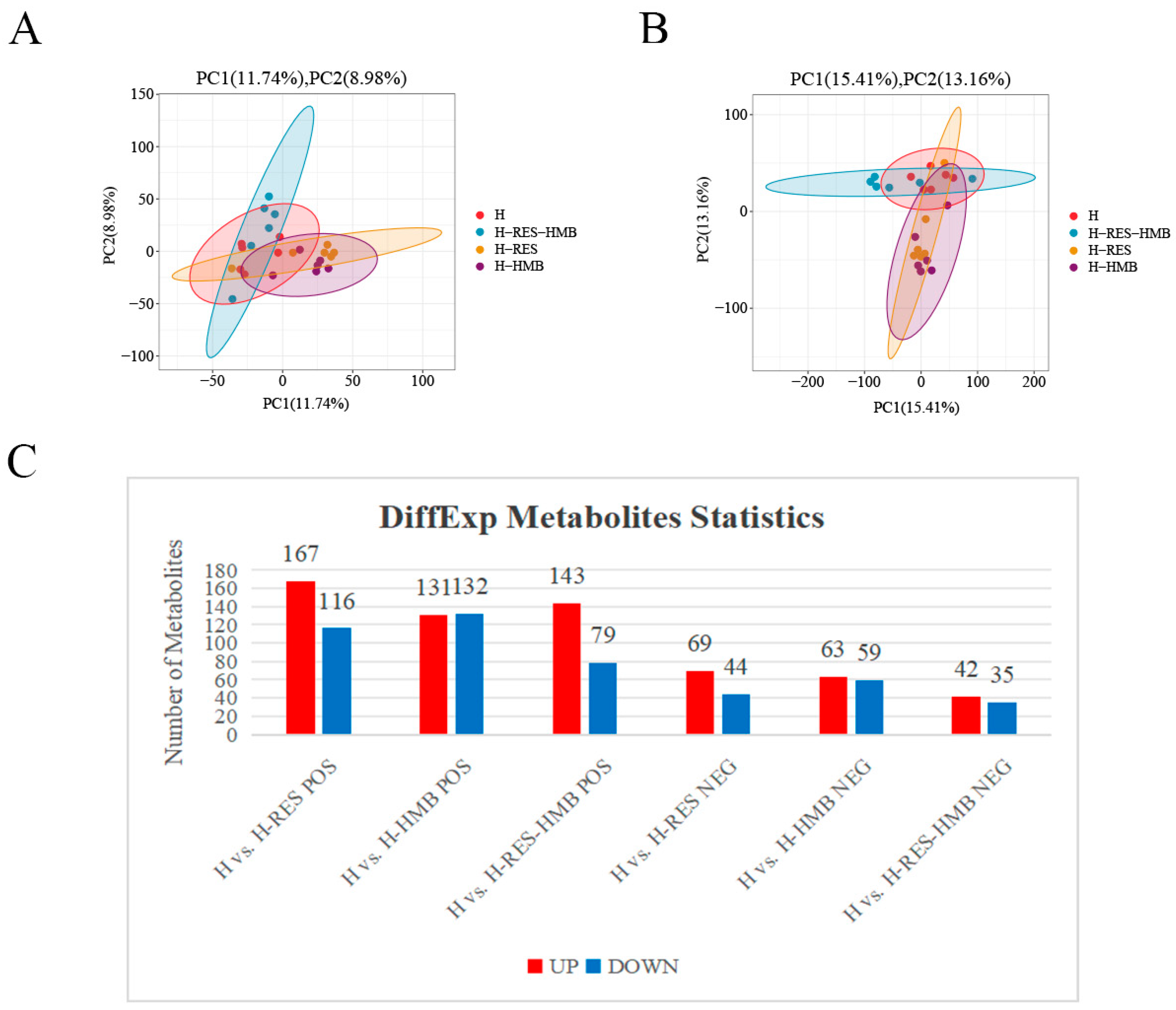
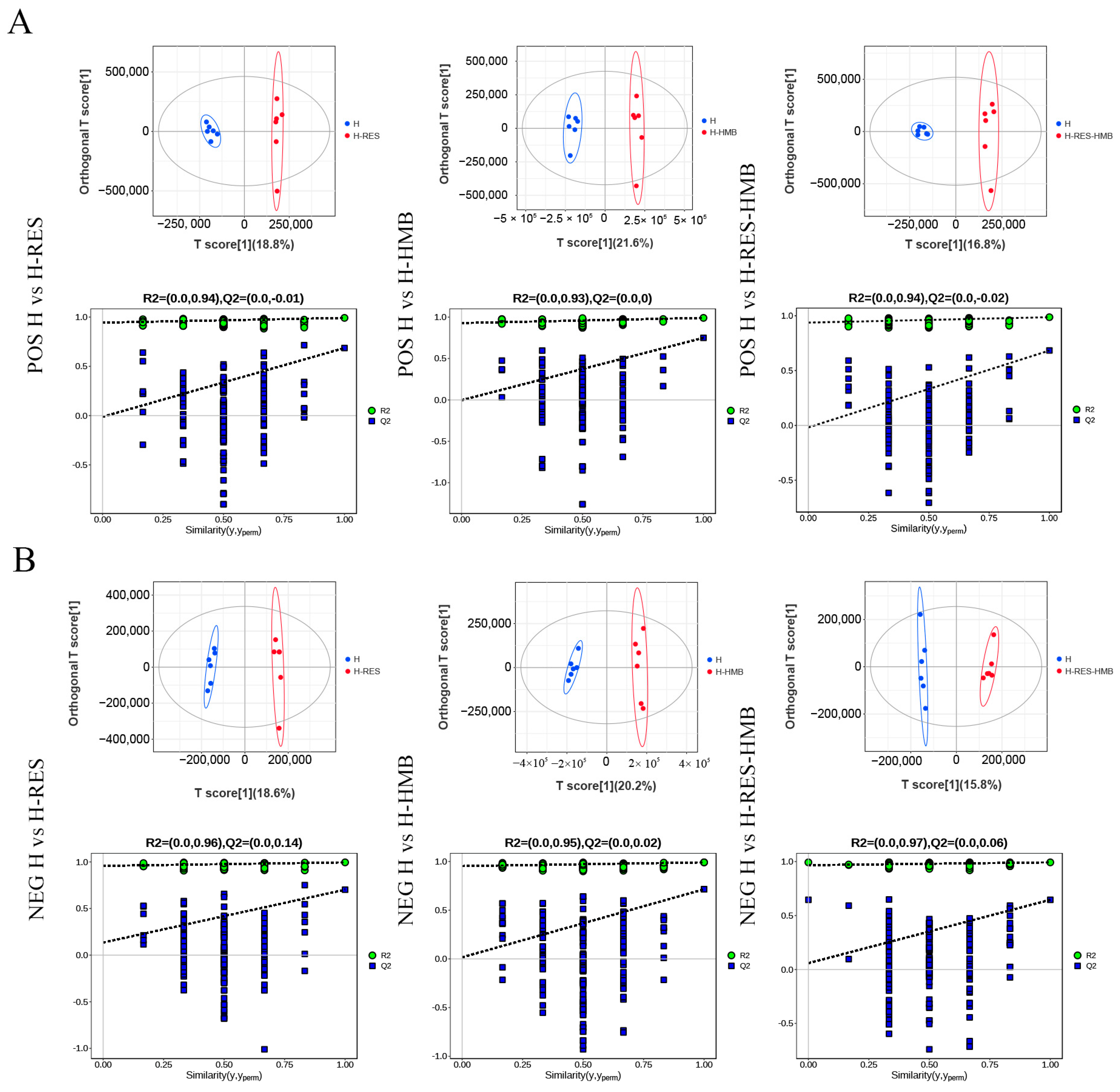
2.4. Metabolomic Profiling Based on LC-MS/MS
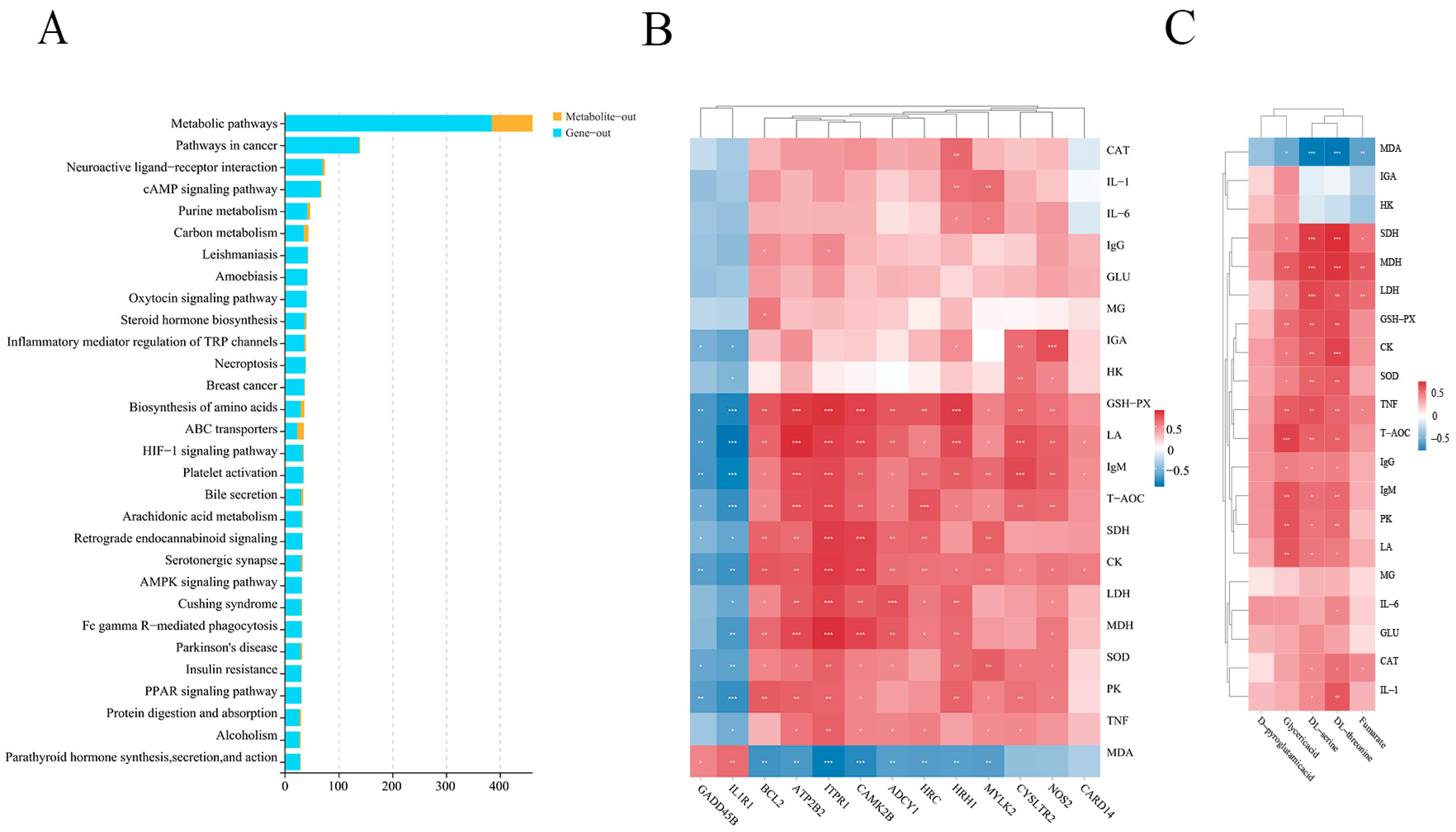
2.5. Integrative Analysis of the Transcriptome and Metabolome
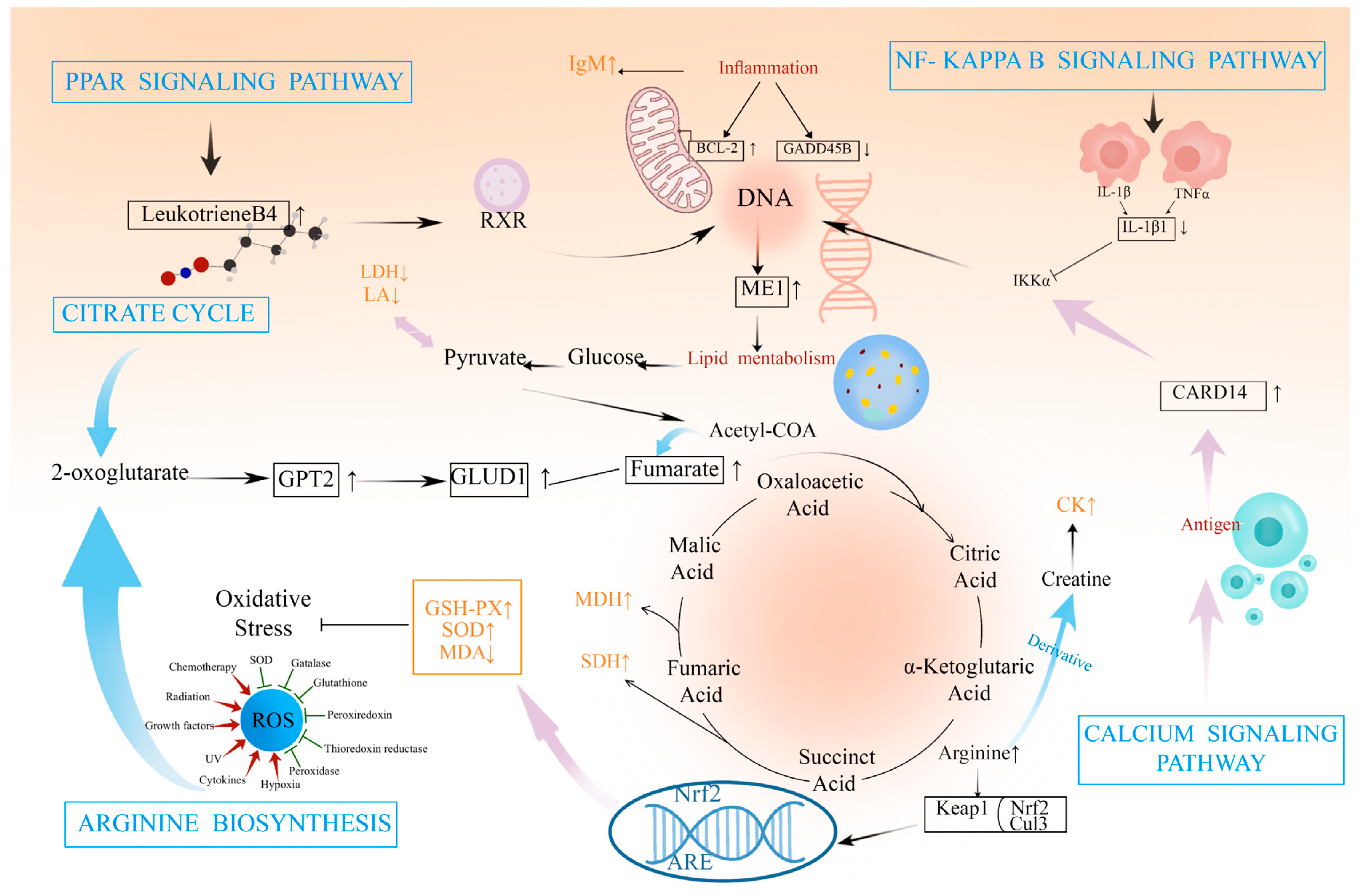
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Feed and Sample Collection
4.3. Determination of Antioxidant, Immune, and Glycolytic Indices in the Liver Tissues
4.4. Liver Histological Analysis
4.5. RNA Sequencing (RNA-Seq) and Data Analysis
4.6. Metabolite Extraction for LC-MS/MS Analysis
4.7. Integrative Analysis of the Metabolome and Transcriptome
4.8. Statistical Analysis
4.9. Verification of RNA-Seq Results with Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J.; et al. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Raza, S.H.A.; Han, L.J.; Ma, B.Y.; Althobaiti, F.; Kesba, H.; Shukry, M.; Ghamry, H.I.; Gao, Z.H.; Hou, S.Z.; et al. Effects of dietary concentrate: Forage ratio on development of gastrointestinal tract in black Tibetan sheep. J. Appl. Anim. Res. 2022, 50, 192–197. [Google Scholar] [CrossRef]
- Gui, L.S.; Raza, S.H.A.; Allam, F.; Zhou, L.; Hou, S.Z.; Khan, I.; Kakar, I.U.; Abd El-Aziz, A.H.; Jia, J.L.; Sun, Y.G.; et al. Altered milk yield and rumen microbial abundance in response to concentrate supplementation during the cold season in Tibetan sheep. Electron. J. Biotechnol. 2021, 53, 80–86. [Google Scholar] [CrossRef]
- Jin, S.J.; Pang, Q.; Yang, H.; Diao, X.P.; Shan, A.S.; Feng, X.J. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos). Food Chem. 2021, 363, 130263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, M.N.; Deng, J.; Zhang, C.H.; Liu, M.W. Resveratrol exhibits neuroprotection against paraquat-induced PC12 cells via heme oxygenase 1 upregulation by decreasing MiR-136-5p expression. Bioengineered 2022, 13, 7065–7081. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef]
- Meng, Q.; Li, J.; Wang, C.; Shan, A. Biological function of resveratrol and its application in animal production: A review. J. Anim. Sci. Biotechnol. 2023, 14, 25. [Google Scholar] [CrossRef]
- Zheng, C.B.; Song, B.; Duan, Y.H.; Zhong, Y.Z.; Yan, Z.M.; Zhang, S.Y.; Li, F.N. Dietary β-hydroxy-β-methylbutyrate improves intestinal function in weaned piglets after lipopolysaccharide challenge. Nutrition 2020, 78, 110839. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, J.; Ji, K.; Liu, H.; Degen, A.; Zhai, M.; Jiao, D.; Guo, J.; Zhao, Z.; Yang, G. Protective Effect of Resveratrol Improves Systemic Inflammation Responses in LPS-Injected Lambs. Animal 2019, 9, 872. [Google Scholar] [CrossRef]
- Ryu, C.H.; Kim, B.H.; Lee, S.; Bang, H.T.; Baek, Y.C. Effects of Supplemented Resveratrol on In Vitro Ruminal Fermentation and Growth Performance of Hanwoo Calves. Animal 2022, 12, 3420. [Google Scholar] [CrossRef] [PubMed]
- Cebulska, K.; Sobiech, P.; Milewski, S.; Ząbek, K. Efficacy of β-hydroxy-β-methylbutyric acid (HMB) for growing rate and its influence for health indicators in blood test of young early-weaning goats. Pol. J. Vet. Sci. 2019, 22, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Yang, M.; Yang, J.; Lin, S.; Wei, X.; Xu, X. Cellular crosstalk during liver regeneration: Unity in diversity. Cell Commun. Signal. CCS 2022, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, T.; Jin, Z.; Guo, H.; Wei, X.; Liu, Y.; Chen, Q.; He, J. Blood glucose concentration and risk of liver cancer: Systematic review and meta-analysis of prospective studies. Oncotarget 2017, 8, 50164–50173. [Google Scholar] [CrossRef]
- Casey, L.M.; Hughes, K.R.; Saunders, M.N.; Miller, S.D.; Pearson, R.M.; Shea, L.D. Mechanistic contributions of Kupffer cells and liver sinusoidal endothelial cells in nanoparticle-induced antigen-specific immune tolerance. Biomaterials 2022, 283, 121457. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Hu, S.D.; Li, X.Y.; He, W.L.; Wu, G.Y. Amino Acid Metabolism in the Liver: Nutritional and Physiological Significance. In Amino Acids in Nutrition and Health: Amino Acids in Systems Function and Health; Wu, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1265, pp. 21–37. [Google Scholar]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, Z. Liver-Mediated Adaptive Immune Tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Liu, J.; Fan, X.; You, G.; Zhao, L.; Zhou, H.; Li, J.; Lei, H. Investigation of the differences between the Tibetan and Han populations in the hemoglobin-oxygen affinity of red blood cells and in the adaptation to high-altitude environments. Hematology 2018, 23, 309–313. [Google Scholar] [CrossRef]
- Konishi, T.; Yoshidome, H.; Shida, T.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Takano, S.; Miyazaki, M.; Ohtsuka, M. Phosphorylated mTOR expression as a predictor of survival after liver resection for colorectal liver metastases. J. Surg. Oncol. 2021, 124, 598–606. [Google Scholar] [CrossRef]
- Li, S.; Han, B.; Li, J.; Lv, Z.; Jiang, H.; Liu, Y.; Yang, X.; Lu, J.; Zhang, Z. Resveratrol Alleviates Liver Fibrosis Induced by Long-Term Inorganic Mercury Exposure through Activating the Sirt1/PGC-1α Signaling Pathway. J. Agric. Food Chem. 2024, 72, 15985–15997. [Google Scholar] [CrossRef]
- Gezer, A.; Ustundag, H.; Mendil, A.S.; Bedir, G.; Duysak, L. Hepatoprotective effects of resveratrol on α-amanitin-induced liver toxicity in rats. Toxicon 2024, 247, 107855. [Google Scholar] [CrossRef] [PubMed]
- Farías, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Pei, X.M.; Teng, B.T.; Tam, E.W.; Yung, B.Y.; Siu, P.M. Oxidative stress and DNA damage signalling in skeletal muscle in pressure-induced deep tissue injury. Pflügers Arch. Eur. J. Physiol. 2013, 465, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Kulczynski, B.; Sidor, A.; Gramza-Michalowska, A. Characteristics of Selected Antioxidative and Bioactive Compounds in Meat and Animal Origin Products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Gong, M.Y.; Zhang, W.N.; Mo, J.T.; Zhang, S.M.; Zhu, Z.; Wang, X.N.; Zhang, B.; Qian, W.K.; Wu, Z.; et al. Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling. Cell Death Dis. 2022, 13, 630. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Fu, S.; Lv, R.; Wang, L.; Hou, H.; Liu, H.; Shao, S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018, 25, 259–266. [Google Scholar] [CrossRef]
- Sun, Y.B.; Geng, S.X.; Yuan, T.Y.; Liu, Y.; Zhang, Y.X.; Di, Y.T.; Li, J.T.; Zhang, L.Y. Effects of Manganese Hydroxychloride on Growth Performance, Antioxidant Capacity, Tibia Parameters and Manganese Deposition of Broilers. Animals 2021, 11, 3470. [Google Scholar] [CrossRef]
- Saber, Y.H.A.; Ibrahim, S.; Mahmoud, K.G.M.; Ahmed, W.M.; Ragab, R.S.A.; Seida, A.A.M. Expression profile of viability and stress response genes as a result of resveratrol supplementation in vitrified and in vitro produced cattle embryos. Mol. Biol. Rep. 2024, 51, 692. [Google Scholar] [CrossRef]
- Sen, U. Maturation of bovine oocytes under low culture temperature decreased glutathione peroxidase activity of both oocytes and blastocysts. Pol. J. Vet. Sci. 2021, 24, 93–99. [Google Scholar] [CrossRef]
- Kang, Y.J.; Song, W.; Lee, S.J.; Choi, S.A.; Chae, S.; Yoon, B.R.; Kim, H.Y.; Lee, J.H.; Kim, C.; Cho, J.Y.; et al. Inhibition of BCAT1-mediated cytosolic leucine metabolism regulates Th17 responses via the mTORC1-HIF1α pathway. Exp. Mol. Med. 2024, 56, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, B.; Yan, Y.T.; Huang, X.Y.; Zhang, E.P. β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling. Int. J. Mol. Sci. 2023, 24, 444. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, J.; Liu, Y.; Li, W.; Lu, D. Glycolysis: An emerging regulator of osteoarthritis. Front. Immunol. 2023, 14, 1327852. [Google Scholar] [CrossRef] [PubMed]
- Rueda, E.M.; Johnson, J.E., Jr.; Giddabasappa, A.; Swaroop, A.; Brooks, M.J.; Sigel, I.; Chaney, S.Y.; Fox, D.A. The cellular and compartmental profile of mouse retinal glycolysis, tricarboxylic acid cycle, oxidative phosphorylation, and ~P transferring kinases. Mol. Vis. 2016, 22, 847–885. [Google Scholar] [PubMed]
- Du, F.; Zhu, X.H.; Qiao, H.; Zhang, X.; Chen, W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn. Reson. Med. 2007, 57, 103–114. [Google Scholar] [CrossRef]
- Frederiks, W.M.; Kümmerlin, I.P.; Bosch, K.S.; Vreeling-Sindelárová, H.; Jonker, A.; Van Noorden, C.J. NADPH production by the pentose phosphate pathway in the zona fasciculata of rat adrenal gland. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 975–980. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Oscai, L.B. Effect of exercise on alpha-glycerophosphate dehydrogenase activity in skeletal muscle. Arch. Biochem. Biophys. 1969, 130, 653–656. [Google Scholar] [CrossRef]
- Xu, R.; Yuan, W.; Wang, Z. Advances in Glycolysis Metabolism of Atherosclerosis. J. Cardiovasc. Transl. Res. 2023, 16, 476–490. [Google Scholar] [CrossRef]
- Gomez, L.S.; Zancan, P.; Marcondes, M.C.; Ramos-Santos, L.; Meyer-Fernandes, J.R.; Sola-Penna, M.; Da Silva, D. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 2013, 95, 1336–1343. [Google Scholar] [CrossRef]
- Wan, H.; Zhu, J.; Wu, C.; Zhou, P.; Shen, Y.; Lin, Y.; Xu, S.; Che, L.; Feng, B.; Li, J.; et al. Transfer of β-hydroxy-β-methylbutyrate from sows to their offspring and its impact on muscle fiber type transformation and performance in pigs. J. Anim. Sci. Biotechnol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Díaz-Piña, D.A.; Rivera-Ramírez, N.; García-López, G.; Díaz, N.F.; Molina-Hernández, A. Calcium and Neural Stem Cell Proliferation. Int. J. Mol. Sci. 2024, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Guo, Z.; Pu, J.L.; Zheng, D.F.; Wei, X.F.; Liu, R.; Tang, C.Y.; Wu, Z.J. Resveratrol preconditioning protects hepatocytes against hepatic ischemia reperfusion injury via Toll-like receptor 4/nuclear factor-κB signaling pathway in vitro and in vivo. Int. Immunopharmacol. 2016, 35, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Feng, C.; Zhao, Y.X.; Liu, X.Y. Camk2b protects neurons from homocysteine-induced apoptosis with the involvement of HIF-1α signal pathway. Int. J. Clin. Exp. Med. 2014, 7, 1659–1668. [Google Scholar] [PubMed]
- Messai, Y.; Noman, M.Z.; Hasmim, M.; Janji, B.; Tittarelli, A.; Boutet, M.; Baud, V.; Viry, E.; Billot, K.; Nanbakhsh, A.; et al. ITPR1 protects renal cancer cells against natural killer cells by inducing autophagy. Cancer Res. 2014, 74, 6820–6832. [Google Scholar] [CrossRef] [PubMed]
- Hochman, A.; Sternin, H.; Gorodin, S.; Korsmeyer, S.; Ziv, I.; Melamed, E.; Offen, D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J. Neurochem. 1998, 71, 741–748. [Google Scholar] [CrossRef]
- Yang, B.; Li, H.; Qiao, Y.; Zhou, Q.; Chen, S.; Yin, D.; He, H.; He, M. Tetramethylpyrazine Attenuates the Endotheliotoxicity and the Mitochondrial Dysfunction by Doxorubicin via 14-3-3γ/Bcl-2. Oxidative Med. Cell. Longev. 2019, 2019, 5820415. [Google Scholar] [CrossRef]
- Palmes, D.; Spiegel, H.U. Animal models of liver regeneration. Biomaterials 2004, 25, 1601–1611. [Google Scholar] [CrossRef]
- Shen, X.; Li, X.; Jia, C.; Li, J.; Chen, S.; Gao, B.; Liang, W.; Zhang, L. HPLC-MS-based untargeted metabolomic analysis of differential plasma metabolites and their associated metabolic pathways in reproductively anosmic black porgy, Acanthopagrus schlegelii. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101071. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Ai, Q. Arginine metabolism and its functions in growth, nutrient utilization, and immunonutrition of fish. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2021, 7, 716–727. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Han, J.; Feng, J.; Guo, T.; Li, Z.; Min, F.; Jin, R.; Peng, X. N-Acetylcysteine Inhibits Patulin-Induced Apoptosis by Affecting ROS-Mediated Oxidative Damage Pathway. Toxins 2021, 13, 595. [Google Scholar] [CrossRef]
- Gill, A.J.; Kolson, D.L. Dimethyl fumarate modulation of immune and antioxidant responses: Application to HIV therapy. Crit. Rev. Immunol. 2013, 33, 307–359. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, R.E. Amino acid cross resistance in Agrobacterium tumefaciens. J. Bacteriol. 1962, 84, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.D.; Harper, A.E.; Elvehjem, C.A. Nutritional improvement of white flour with protein and amino acid supplements. J. Nutr. 1957, 62, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, G.; Chen, J.; Yang, X.; Hua, G.; Han, D.; Li, X.; Feng, D.; Deng, X. Combined transcriptome and metabolome analysis reveals breed-specific regulatory mechanisms in Dorper and Tan sheep. BMC Genom. 2024, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Coggins, A.J.; Powner, M.W. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 2017, 9, 310–317. [Google Scholar] [CrossRef]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Wang, C.; Hao, J.; Liu, X.; Li, C.; Yuan, X.; Lee, R.J.; Bai, T.; Wang, D. Isoforsythiaside Attenuates Alzheimer’s Disease via Regulating Mitochondrial Function Through the PI3K/AKT Pathway. Int. J. Mol. Sci. 2020, 21, 5687. [Google Scholar] [CrossRef]
- Shi, Q.; Zeng, Y.; Xue, C.; Chu, Q.; Yuan, X.; Li, L. Development of a promising PPAR signaling pathway-related prognostic prediction model for hepatocellular carcinoma. Sci. Rep. 2024, 14, 4926. [Google Scholar] [CrossRef]
- Ramanarayanan, P.; Heine, G.; Worm, M. Vitamin A and vitamin D induced nuclear hormone receptor activation and its impact on B cell differentiation and immunoglobulin production. Immunol. Lett. 2023, 263, 80–86. [Google Scholar] [CrossRef]
- Heine, G.; Hollstein, T.; Treptow, S.; Radbruch, A.; Worm, M. 9-cis retinoic acid modulates the type I allergic immune response. J. Allergy Clin. Immunol. 2018, 141, 650–658.e5. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Hardwick, J.P. Promoter activity and regulation of the CYP4F2 leukotriene B(4) omega-hydroxylase gene by peroxisomal proliferators and retinoic acid in HepG2 cells. Arch. Biochem. Biophys. 2000, 378, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H.; Gu, J.; Xu, W.; Yuan, N.; Sun, J.; Li, H. Glabridin inhibits liver fibrosis and hepatic stellate cells activation through suppression of inflammation and oxidative stress by activating PPARγ in carbon tetrachloride-treated mice. Int. Immunopharmacol. 2022, 113, 109433. [Google Scholar] [CrossRef] [PubMed]
- Landini, L.; Dadson, P.; Gallo, F.; Honka, M.J.; Cena, H. Microbiota in anorexia nervosa: Potential for treatment. Nutr. Res. Rev. 2023, 36, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]




| Items | Groups | p-Value | |||
|---|---|---|---|---|---|
| H | H-RES | H-HMB | H-RES-HMB | ||
| Antioxidant | |||||
| GSH-PX (Pmol/mL) | 52.41 ± 0.99 b | 63.41 ± 1.49 b | 62.21 ± 2.31 b | 86.43 ± 6.20 a | 0.001 |
| SOD (pg/mL) | 8.40 ± 0.42 b | 9.32 ± 0.47 b | 10.29 ± 0.11 b | 13.40 ± 2.04 a | 0.048 |
| T-AOC (u/mL) | 10.16 ± 0.68 | 10.21 ± 0.63 | 11.76 ± 0.55 | 10.49 ± 1.06 | 0.406 |
| CAT (ng/L) | 147.19 ± 2.36 | 147.84 ± 4.84 | 148.17 ± 15.93 | 160.59 ± 3.00 | 0.651 |
| MDA (Pg/mL) | 10.34 ± 0.27 a | 8.68 ± 1.19 a | 9.87 ± 1.45 a | 4.35 ± 0.42 b | 0.009 |
| Immunological | |||||
| IgA (μg/mL) | 8.39 ± 0.88 | 9.18 ± 0.19 | 11.69 ± 1.50 | 10.40 ± 0.65 | 0.145 |
| IgG (μg/mL) | 886.11 ± 44.91 | 937.96 ± 108.60 | 962.04 ± 12.96 | 1071.29 ± 138.20 | 0.559 |
| IgM (μg/mL) | 13.30 ± 0.74 b | 15.14 ± 0.78 b | 15.99 ± 0.31 ab | 18.90 ± 1.46 a | 0.016 |
| TNF-α (ng/L) | 665.51 ± 10.01 | 689.87 ± 35.27 | 709.10 ± 102.59 | 523.21 ± 96.19 | 0.326 |
| IL-1β (ng/L) | 84.09 ± 3.81 | 85.86 ± 6.14 | 83.59 ± 0.91 | 74.49 ± 3.51 | 0.266 |
| IL-6 (ng/L) | 117.59 ± 12.05 | 133.25 ± 29.59 | 106.75 ± 35.87 | 87.87 ± 20.65 | 0.673 |
| Glycolytic | |||||
| LDH (ng/L) | 31.51 ± 1.42 a | 23.32 ± 4.71 ab | 27.14 ± 4.57 a | 13.50 ± 2.50 b | 0.038 |
| GLU (μmol/L) | 496.15 ± 40.53 | 529.49 ± 26.84 | 511.54 ± 18.75 | 551.28 ± 10.91 | 0.530 |
| MG (mg/L) | 126.66 ± 4.32 | 128.89 ± 2.89 | 129.79 ± 2.27 | 133.81 ± 10.60 | 0.861 |
| HK (ng/L) | 405.49 ± 18.84 | 426.08 ± 31.98 | 533.59 ± 61.46 | 490.46 ± 96.43 | 0.457 |
| PK (ng/L) | 492.36 ± 59.48 | 558.10 ± 35.08 | 628.01 ± 86.15 | 721.53 ± 22.74 | 0.093 |
| CK (ng/mL) | 81.58 ± 2.39 b | 94.50 ± 3.69 b | 90.08 ± 7.36 b | 116.05 ± 4.79 a | 0.003 |
| MDH (pg/mL) | 462.16 ± 29.52 b | 474.51 ± 22.23 b | 486.85 ± 47.30 b | 694.26 ± 51.86 a | 0.009 |
| SDH (μmol/L) | 368.95 ± 24.41 b | 424.81 ± 45.68 b | 372.04 ± 21.66 b | 557.22 ± 31.62 a | 0.010 |
| LA (μg/L) | 1406.13 ± 64.59 a | 1218.17 ± 23.92 a | 1318.83 ± 66.10 a | 963.17 ± 114.03 b | 0.015 |
| Items | MP |
|---|---|
| Corn | 51.50 |
| Soybean meal | 2.00 |
| Canola meal | 12.80 |
| Cottonseed meal | 2.00 |
| Palm meal | 25.00 |
| NaCl | 1.00 |
| Talcum powder | 1.00 |
| Baking soda | 0.10 |
| Premix (No. 6) | 4.60 |
| Total | 100.00 |
| DE/(MJ·kg−1) | 12.84 |
| Crude ash | 12.13 |
| Ether extract | 3.44 |
| Crude fiber | 11.05 |
| Neutral detergent fiber | 26.04 |
| Acid detergent fiber | 19.11 |
| Ca | 0.84 |
| p | 0.40 |
| Name | Primer Sequence (5′-3′) | Tm (°C) | Product Length |
|---|---|---|---|
| Ncbi:101111913 GLYCTK | TGAGCGGCGGCACTGATG GAAGCGGCAGAAGAAGGTATGTG | 60.0 | 149 bp |
| Ncbi:493968 CYP17A1 | GCCCAGAGCAGGATTCAAAGC ACGAAGGATGGTGTAGCAGGTAG | 60.0 | 135 bp |
| Ncbi:100192423 ODC1 | GCCTGGACCGCATTGTTGAG AATCCATTGAAGGTAGAAGCAGCAG | 60.0 | 115 bp |
| Ncbi:101118747 GOT1 | GTGAGGAAGGTGGAGCAGAGG AGGCGGGAAGCACAGGTTC | 60.0 | 101 bp |
| ncbi_101108953 PDE4D | TTCAGACAGTTGGAACAAGCAGAC TGGAGTCGGCAGAAGTGATAGC | 60.0 | 112 bp |
| MSTRG.15268 TAT | GGAGTTCACGGAGCAGTTAGTTG GCCTCCAGCATCATCACTTCAG | 60.0 | 120 bp |
| ncbi_101102876 HNMT | ATTCTAAGCATTGGCGGTGGTG GTTCAGCACTTGGTTCAACAACTTC | 60.0 | 115 bp |
| ncbi_101108052 HDC | AGCAGACCTTCAGCGTAGACC AGGACCGAATCACGAACCAGAG | 60.0 | 132 bp |
| MSTRG.17338 HAL | GACGACGCCCGCTTCCTC AACCACTTCCACGAACTCATTGTC | 60.0 | 105 bp |
| ncbi_443489 PFKM | ATCCCTGCCACTGTCTCCAAC CTGCCGCTGACTGCTTGATG | 60.0 | 112 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, F.; Raza, S.H.A.; Wu, Z.; Su, Q.; Ji, Q.; He, T.; Zhu, K.; Zhang, Y.; Hou, S.; et al. Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis. Int. J. Mol. Sci. 2024, 25, 9865. https://doi.org/10.3390/ijms25189865
Chen X, Zhang F, Raza SHA, Wu Z, Su Q, Ji Q, He T, Zhu K, Zhang Y, Hou S, et al. Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis. International Journal of Molecular Sciences. 2024; 25(18):9865. https://doi.org/10.3390/ijms25189865
Chicago/Turabian StyleChen, Xuan, Fengshuo Zhang, Sayed Haidar Abbas Raza, Zhenling Wu, Quyangangmao Su, Qiurong Ji, Tingli He, Kaina Zhu, Yu Zhang, Shengzhen Hou, and et al. 2024. "Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis" International Journal of Molecular Sciences 25, no. 18: 9865. https://doi.org/10.3390/ijms25189865
APA StyleChen, X., Zhang, F., Raza, S. H. A., Wu, Z., Su, Q., Ji, Q., He, T., Zhu, K., Zhang, Y., Hou, S., & Gui, L. (2024). Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis. International Journal of Molecular Sciences, 25(18), 9865. https://doi.org/10.3390/ijms25189865






