From In Vitro Promise to In Vivo Reality: An Instructive Account of Infection Model Evaluation of Antimicrobial Peptides
Abstract
1. Introduction
2. Results and Discussion
2.1. Peptides
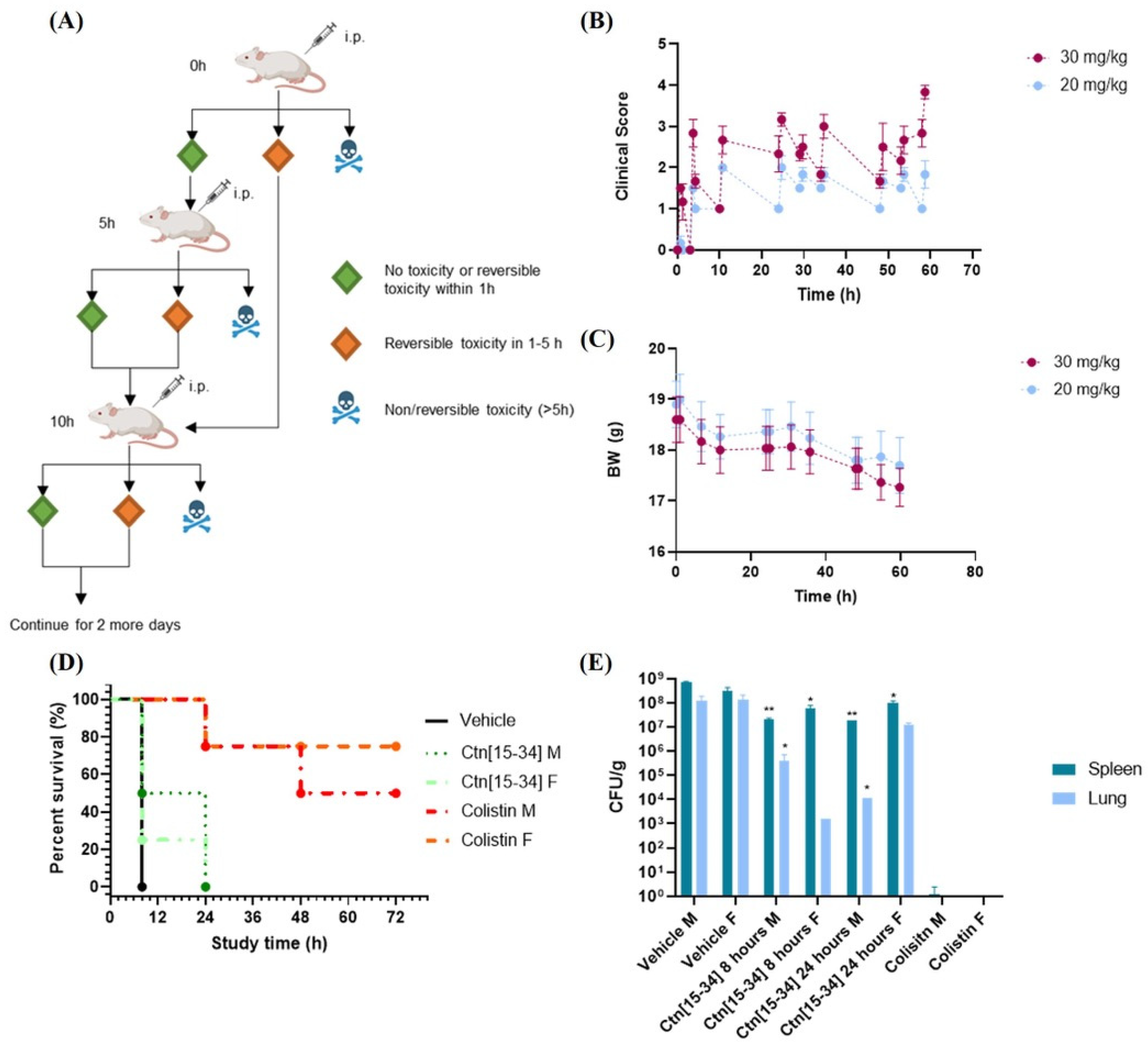
2.2. Treatment with Ctn[15-34] Reduces Bacterial Levels in Females, Whereas Ctn re Does Not Improve the Survival Rate
2.3. Treatment with Ctn[15-34] Thrice a Day Lowers Bacterial Load but Does Not Improve Survival Rate
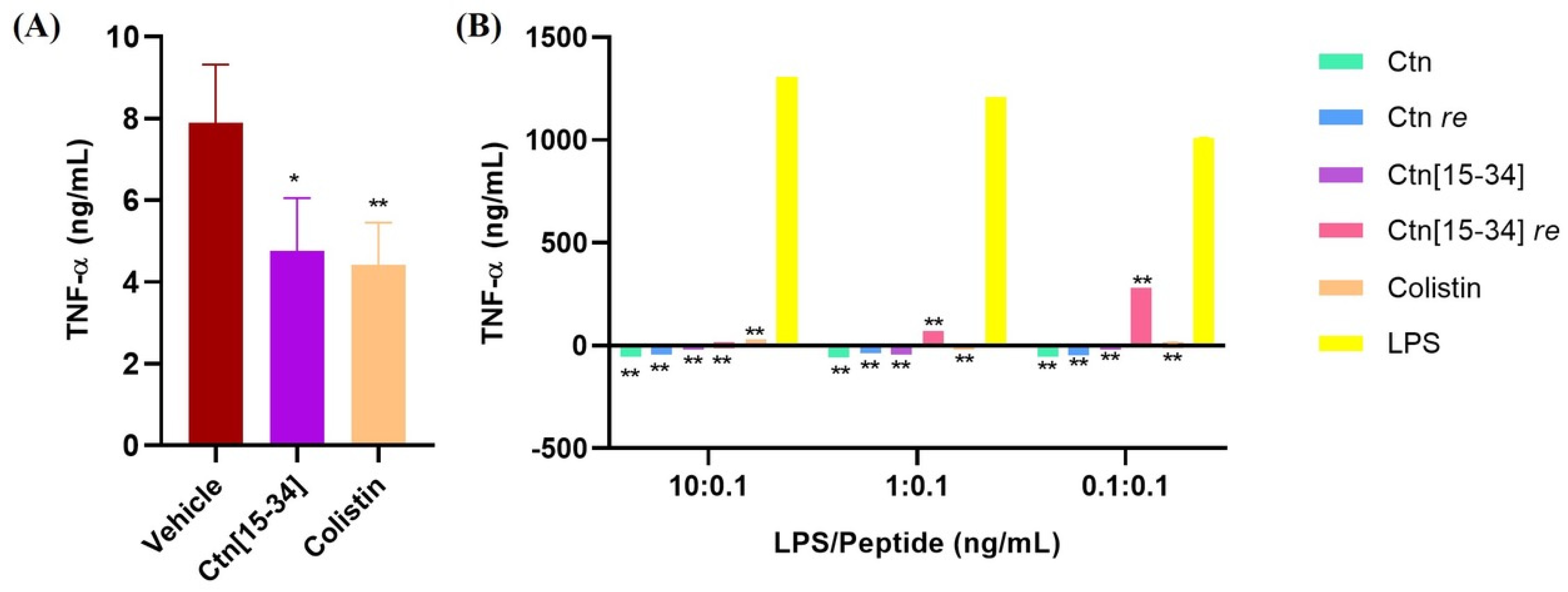
2.4. Ctn[15-34] Reduces Levels of TNF-α in Mice after 8 h and Ctn[15-34] re Increase TNF-α Levels in LPS-Stimulated Human MNC Cells
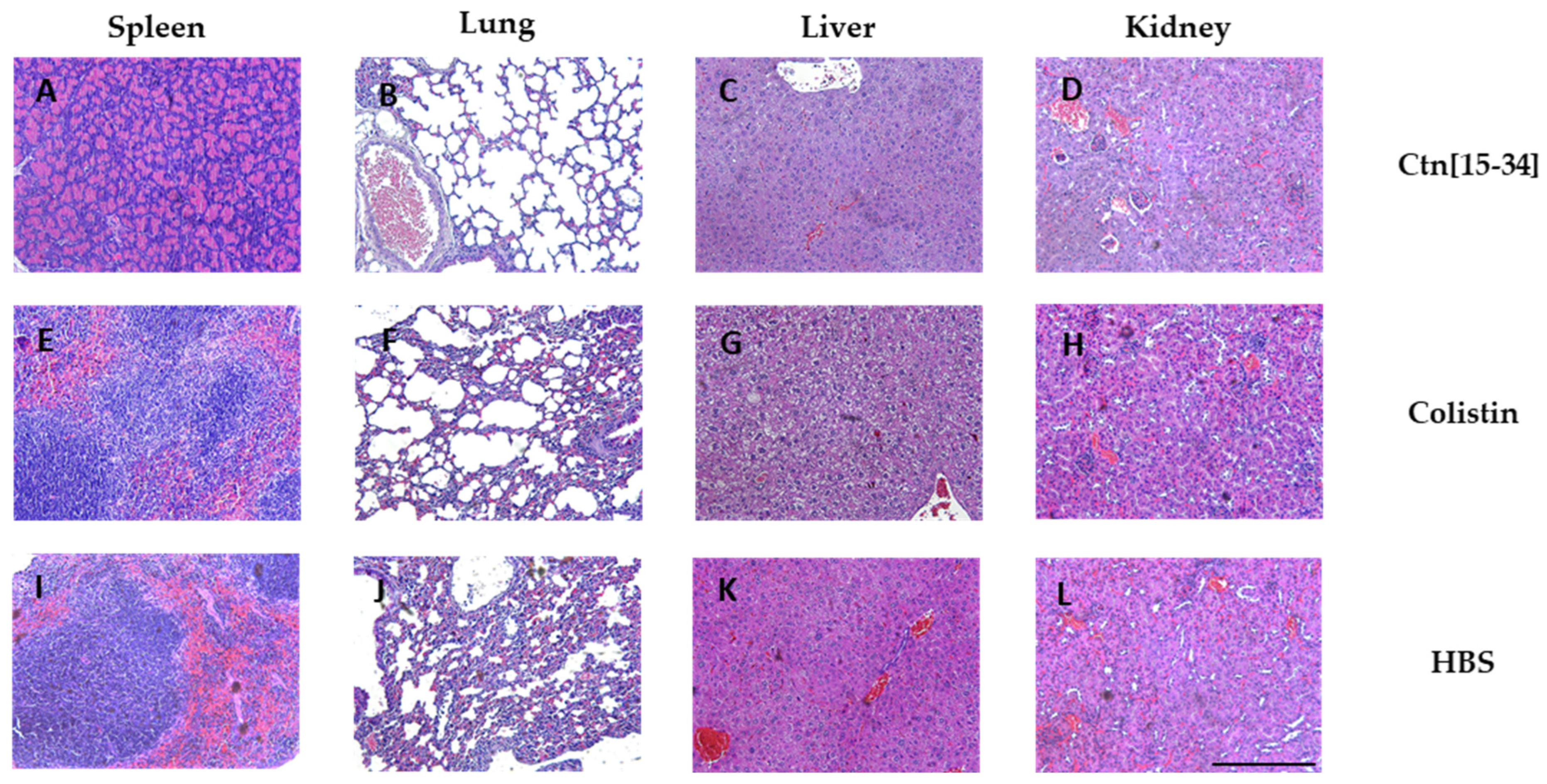
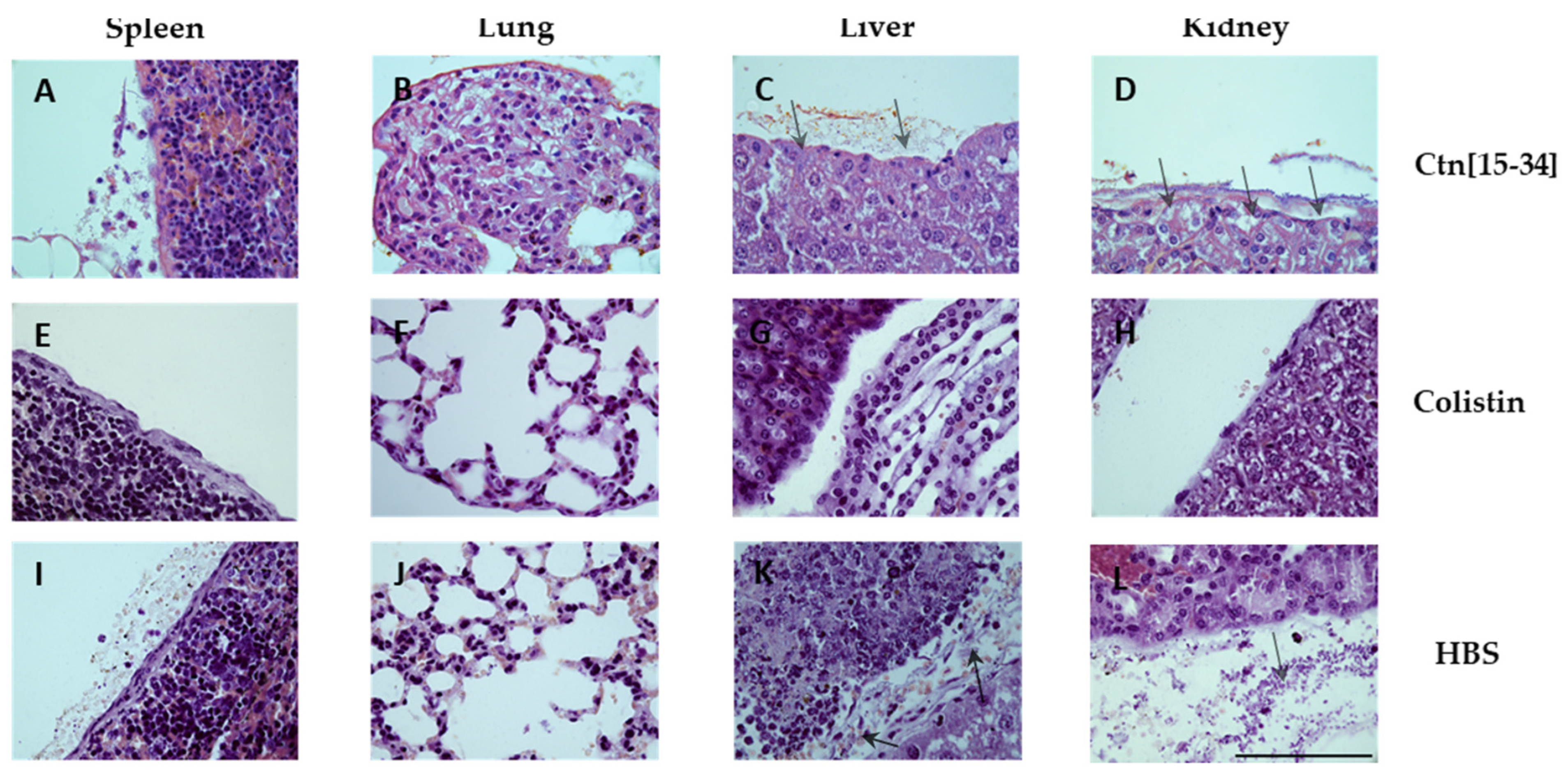
2.5. Organ Staining Consistent with Bacterial Levels at Each Treatment
3. Materials and Methods
3.1. Mice, Bacterial Strains and Other Material
3.2. Peptides
3.3. Minimum Inhibitory Concentration (MIC)
3.4. Peptide Cytotoxicity
3.5. Serum Stability
3.6. Tolerance in Mice
3.7. Mouse Systemic Infection
3.8. Efficacy in Mouse Infection Model
3.9. Evaluation of Body Weight and Clinical Symptoms
3.10. Evaluation of Colony-Forming Units (CFU) in Mouse Tissues
3.11. Quantification of TNF-α in Mouse Sera by ELISA
3.12. Stimulation of Human Mononuclear Cells (MNC) by LPS
3.13. Histopathology, Tissue Gram Stain and Hematoxylin and Eosin Stain
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.P.J.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial Defences: Mechanisms, Evolution and Antimicrobial Resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-Lactam Antibiotics: An Overview from a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A.S.; Embil, J.; Smith, H.; Hoban, D.J. A Critical Review of the Fluoroquinolones: Focus on Respiratory Tract Infections. Drugs 2002, 62, 13–59. [Google Scholar] [CrossRef]
- Rothstein, D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016, 6, a027011. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial Peptides: Application Informed by Evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Tishler, M.; Stokes, J.L.; Trenner, N.R.; Conn, J.B. Some Properties of Gramicidin. J. Biol. Chem. 1941, 141, 197–206. [Google Scholar] [CrossRef]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Yahav, D.; Farbman, L.; Leibovici, L.; Paul, M. Colistin: New Lessons on an Old Antibiotic. Clin. Microbiol. Infect. 2012, 18, 18–29. [Google Scholar] [CrossRef]
- Wilhelm, M.P. Vancomycin. Mayo Clin. Proc. 1991, 66, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagac-Wiens, P.R.S.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New Lipoglycopeptides: A Comparative Review of Dalbavancin, Oritavancin and Telavancin. Drugs 2010, 70, 859–886. [Google Scholar] [CrossRef] [PubMed]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin Is a Peptide Antibiotic with Therapeutic Potential from a Saprophytic Fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Paquette, D.W.; Simpson, D.M.; Friden, P.; Braman, V.; Williams, R.C. Safety and Clinical Effects of Topical Histatin Gels in Humans with Experimental Gingivitis. J. Clin. Periodontol. 2002, 29, 1051–1058. [Google Scholar] [CrossRef]
- Business Review Editor. Micrologix Licenses Anti-Infective to Fujisawa. Pharma Deal. Rev. 2002, 2002. [Google Scholar]
- Wang, G.; Li, X.; Wang, Z. APD2: The Updated Antimicrobial Peptide Database and Its Application in Peptide Design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial CDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Kokryakov, V.N.; Harwig, S.S.L.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O.V.; Korneva, H.A.; Lehrer, R.I. Protegrins: Leukocyte Antimicrobial Peptides That Combine Features of Corticostatic Defensins and Tachyplesins. FEBS Lett. 1993, 327, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Chertov, O.; Lubkowski, J. The Structure of Human β-Defensin-1: New Insights into Structural Properties of β-Defensins. J. Biol. Chem. 2001, 276, 39021–39026. [Google Scholar] [CrossRef] [PubMed]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E.W. Structure of the Bovine Antimicrobial Peptide Indolicidin Bound to Dodecylphosphocholine and Sodium Dodecyl Sulfate Micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef]
- González, C.; Langdon, G.M.; Bruix, M.; Gálvez, A.; Valdivia, E.; Maqueda, M.; Rico, M. Bacteriocin AS-48, a Microbial Cyclic Polypeptide Structurally and Functionally Related to Mammalian NK-Lysin. Proc. Natl. Acad. Sci. USA 2000, 97, 11221–11226. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Hamamoto, K.; Kida, Y.; Zhang, Y.; Shimizu, T.; Kuwano, K. Antimicrobial Activity and Stability to Proteolysis of Small Linear Cationic Peptides with D-Amino Acid Substitutions. Microbiol. Immunol. 2002, 46, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.; Boman, A.; Wåhlin, B.; Drain, C.M.; Andreu, D.; Boman, H.G.; Merrifield, R.B. All-D Amino Acid-Containing Channel-Forming Antibiotic Peptides. Proc. Natl. Acad. Sci. USA 1990, 87, 4761–4765. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B.; Juvvadi, P.; Andreu, D.; Ubach, J.; Boman, A.; Boman, H.G. Retro and Retroenantio Analogs of Cecropin-Melittin Hybrids. Proc. Natl. Acad. Sci. USA 1995, 92, 3449–3453. [Google Scholar] [CrossRef]
- Carrera-Aubesart, A.; Gallo, M.; Defaus, S.; Todorovski, T.; Andreu, D. Topoisomeric Membrane-Active Peptides: A Review of the Last Two Decades. Pharmaceutics 2023, 15, 2451. [Google Scholar] [CrossRef]
- Doti, N.; Mardirossian, M.; Sandomenico, A.; Ruvo, M.; Caporale, A. Recent Applications of Retro-Inverso Peptides. Int. J. Mol. Sci. 2021, 22, 8677. [Google Scholar] [CrossRef] [PubMed]
- Vunnam, S.; Juvvadi, P.; Rotondi, K.S.; Merrifield, R.B. Synthesis and Study of Normal, Enantio, Retro, and Retroenantio Isomers of Cecropin A-Melittin Hybrids, Their End Group Effects and Selective Enzyme Inactivation. J. Pept. Res. 1998, 51, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L. The PyMOL Molecular Graphics System; Version 1.2r3pre; Schrodinger LLC: New York, NY, USA, 2008; 3p. [Google Scholar]
- Falcao, C.B.; Pérez-Peinado, C.; De La Torre, B.G.; Mayol, X.; Zamora-Carreras, H.; Jiménez, M.Á.; Rádis-Baptista, G.; Andreu, D. Structural Dissection of Crotalicidin, a Rattlesnake Venom Cathelicidin, Retrieves a Fragment with Antimicrobial and Antitumor Activity. J. Med. Chem. 2015, 58, 8553–8563. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; Radis-Baptista, G. Crotamine and Crotalicidin, Membrane Active Peptides from Crotalus Durissus Terrificus Rattlesnake Venom, and Their Structurally-Minimized Fragments for Applications in Medicine and Biotechnology. Peptides 2020, 126, 1702342. [Google Scholar] [CrossRef] [PubMed]
- Klaiss-Luna, M.C.; Giraldo-Lorza, J.M.; Jemioła-Rzemińska, M.; Strzałka, K.; Manrique-Moreno, M. Biophysical Insights into the Antitumoral Activity of Crotalicidin against Breast Cancer Model Membranes. Int. J. Mol. Sci. 2023, 24, 16226. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peinado, C.; Defaus, S.; Sans-Comerma, L.; Valle, J.; Andreu, D. Decoding the Human Serum Interactome of Snake-Derived Antimicrobial Peptide Ctn[15-34]: Toward an Explanation for Unusually Long Half-Life. J. Proteomics 2019, 204, 103372. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peinado, C.; Dias, S.A.; Domingues, M.M.; Benfield, A.H.; Freire, J.M.; Rádis-Baptista, G.; Gaspar, D.; Castanho, M.A.R.B.; Craik, D.J.; Henriques, S.T.; et al. Mechanisms of Bacterial Membrane Permeabilization by Crotalicidin (Ctn) and Its Fragment Ctn(15–34), Antimicrobial Peptides from Rattlesnake Venom. J. Biol. Chem. 2018, 293, 1536–1549. [Google Scholar] [CrossRef]
- Carrera-Aubesart, A.; Defaus, S.; Pérez-Peinado, C.; Sandín, D.; Torrent, M.; Jiménez, M.Á.; Andreu, D. Examining Topoisomers of a Snake-Venom-Derived Peptide for Improved Antimicrobial and Antitumoral Properties. Biomedicines 2022, 10, 2110. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Prades, R.; Oller-Salvia, B.; Schwarzmaier, S.M.; Selva, J.; Moros, M.; Balbi, M.; Grazú, V.; De La Fuente, J.M.; Egea, G.; Plesnila, N.; et al. Applying the Retro-Enantio Approach To Obtain a Peptide Capable of Overcoming the Blood–Brain Barrier. Angew. Chem. Int. Ed. 2015, 54, 3967–3972. [Google Scholar] [CrossRef]
- Kumar, P.; Pletzer, D.; Haney, E.F.; Rahanjam, N.; Cheng, J.T.J.; Yue, M.; Aljehani, W.; Hancock, R.E.W.; Kizhakkedathu, J.N.; Straus, S.K. Aurein-Derived Antimicrobial Peptides Formulated with Pegylated Phospholipid Micelles to Target Methicillin-Resistant Staphylococcus Aureus Skin Infections. ACS Infect. Dis. 2019, 5, 443–453. [Google Scholar] [CrossRef]
- Lynn, M.A.; Kindrachuk, J.; Marr, A.K.; Jenssen, H.; Panté, N.; Elliott, M.R.; Napper, S.; Hancock, R.E.; McMaster, W.R. Effect of BMAP-28 Antimicrobial Peptides on Leishmania Major Promastigote and Amastigote Growth: Role of Leishmanolysin in Parasite Survival. PLoS Negl. Trop. Dis. 2011, 5, e1141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Prats-Ejarque, G.; Torrent, M.; Andreu, D.; Brandenburg, K.; Fernández-Millán, P.; Boix, E. In Vivo Evaluation of ECP Peptide Analogues for the Treatment of Acinetobacter Baumannii Infection. Biomedicines 2022, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An Update on the Antibiotic of the 21st Century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of Polymyxins: A Systematic Review of the Evidence from Old and Recent Studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachón, J. Acinetobacter Baumannii: Human Infections, Factors Contributing to Pathogenesis and Animal Models. FEMS Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann, T.; Razquin-Olazarán, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Hornef, M.; Schürholz, T.; Rössle, M.; Sanchez-Gómez, S.; et al. New Antiseptic Peptides to Protect against Endotoxin-Mediated Shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, G.; Müller, M.; Koch, M.H.J.; Brandenburg, K. Interaction of Hemoglobin with Enterobacterial Lipopolysaccharide and Lipid A. Eur. J. Biochem. 2001, 268, 4233–4242. [Google Scholar] [CrossRef] [PubMed]
- Bratthauer, G.L. Processing of Tissue Specimens. In Immunocytochemical Methods and Protocols. Methods in Molecular BiologyTM.; Javois, L.C., Ed.; Humana Press: Totowa, NJ, USA, 1999; Volume 115, pp. 77–84. [Google Scholar]
- Fowler, C.B.; Cunningham, R.E.; O’Leary, T.J.; Mason, J.T. ‘Tissue Surrogates’ as a Model for Archival Formalin-Fixed Paraffin-Embedded Tissues. Lab. Investig. 2007, 87, 836–846. [Google Scholar] [CrossRef]
- Young, D.G. The Gram Stain in Tissue: Increasing the Clarity and Contrast Between Gram-Negative Bacteria and Other Cell Components. J. Histotechnol. 2013, 26, 37–39. [Google Scholar] [CrossRef]
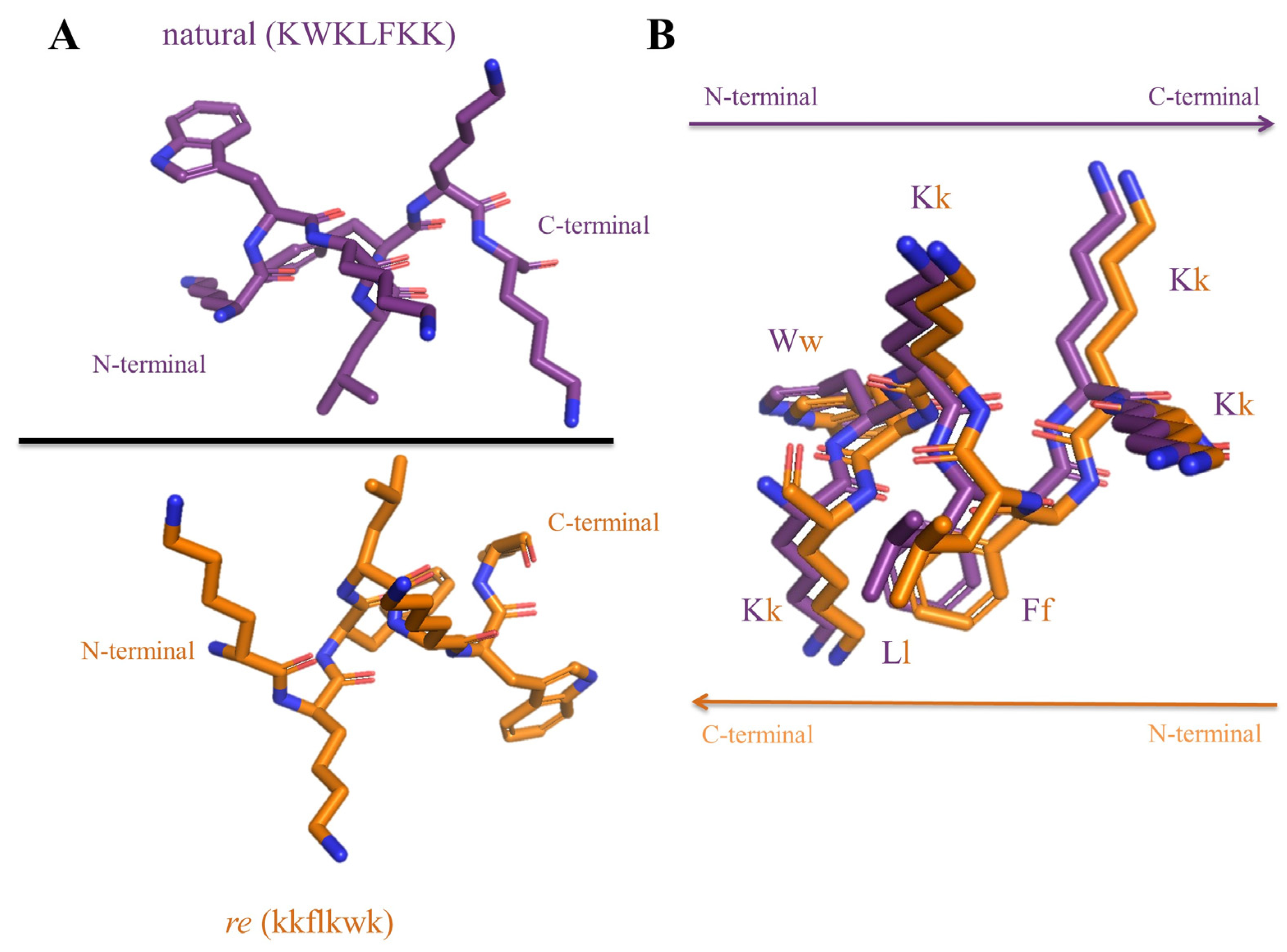

| Peptide | Sequence * | MIC (μM) A. baumannii | IC50 (μM) | t1/2 (min) |
|---|---|---|---|---|
| Ctn | KRFKKFFKKVKKSVKKRLKKIFKKPMVIGVTIPF | 0.78 | 14.32 | 71 |
| Ctn re | fpitvGivmpkkfikklrkkvskkvkkffkkfrk | 1.56 | 4.3 | 211 |
| Ctn[15-34] | KKRLKKIFKKPMVIGVTIPF | 0.78 | >100 | 770 |
| Ctn[15-34] re | fpitvGivmpkkfikklrkk | 0.39 | 43 | 921 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrera-Aubesart, A.; Li, J.; Contreras, E.; Bello-Madruga, R.; Torrent, M.; Andreu, D. From In Vitro Promise to In Vivo Reality: An Instructive Account of Infection Model Evaluation of Antimicrobial Peptides. Int. J. Mol. Sci. 2024, 25, 9773. https://doi.org/10.3390/ijms25189773
Carrera-Aubesart A, Li J, Contreras E, Bello-Madruga R, Torrent M, Andreu D. From In Vitro Promise to In Vivo Reality: An Instructive Account of Infection Model Evaluation of Antimicrobial Peptides. International Journal of Molecular Sciences. 2024; 25(18):9773. https://doi.org/10.3390/ijms25189773
Chicago/Turabian StyleCarrera-Aubesart, Adam, Jiarui Li, Estefanía Contreras, Roberto Bello-Madruga, Marc Torrent, and David Andreu. 2024. "From In Vitro Promise to In Vivo Reality: An Instructive Account of Infection Model Evaluation of Antimicrobial Peptides" International Journal of Molecular Sciences 25, no. 18: 9773. https://doi.org/10.3390/ijms25189773
APA StyleCarrera-Aubesart, A., Li, J., Contreras, E., Bello-Madruga, R., Torrent, M., & Andreu, D. (2024). From In Vitro Promise to In Vivo Reality: An Instructive Account of Infection Model Evaluation of Antimicrobial Peptides. International Journal of Molecular Sciences, 25(18), 9773. https://doi.org/10.3390/ijms25189773









