The Impact of Developmental and Metabolic Cues on Cytoophidium Formation
Abstract
1. Introduction
2. Characteristics of Cytoophidia
3. Mechanism of Cytoophidium Formation
3.1. Cellular Metabolites
3.2. Cellular Stress
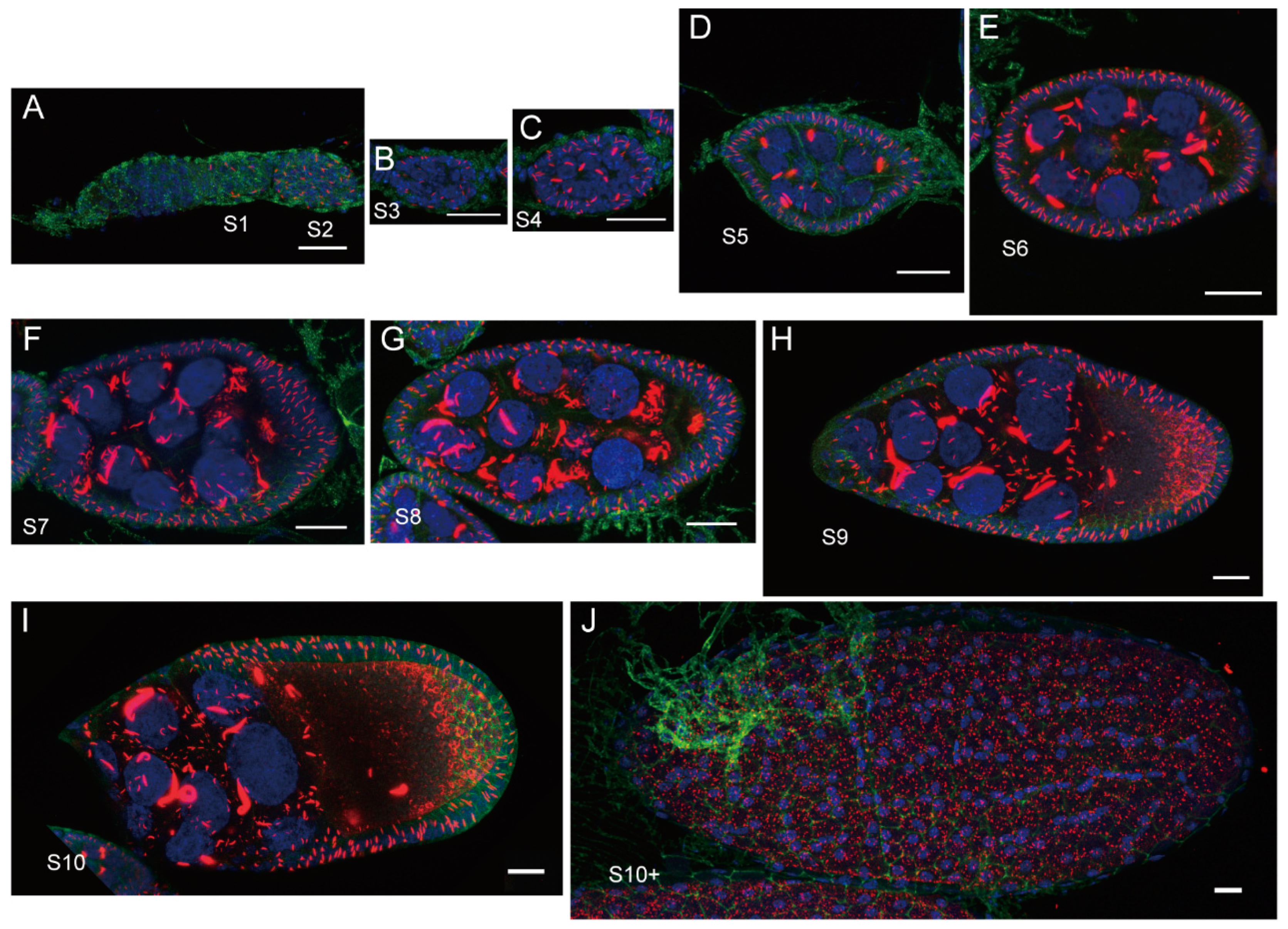
3.3. Developmental Cues
3.4. Proto-Oncogenes
3.5. Other Regulators
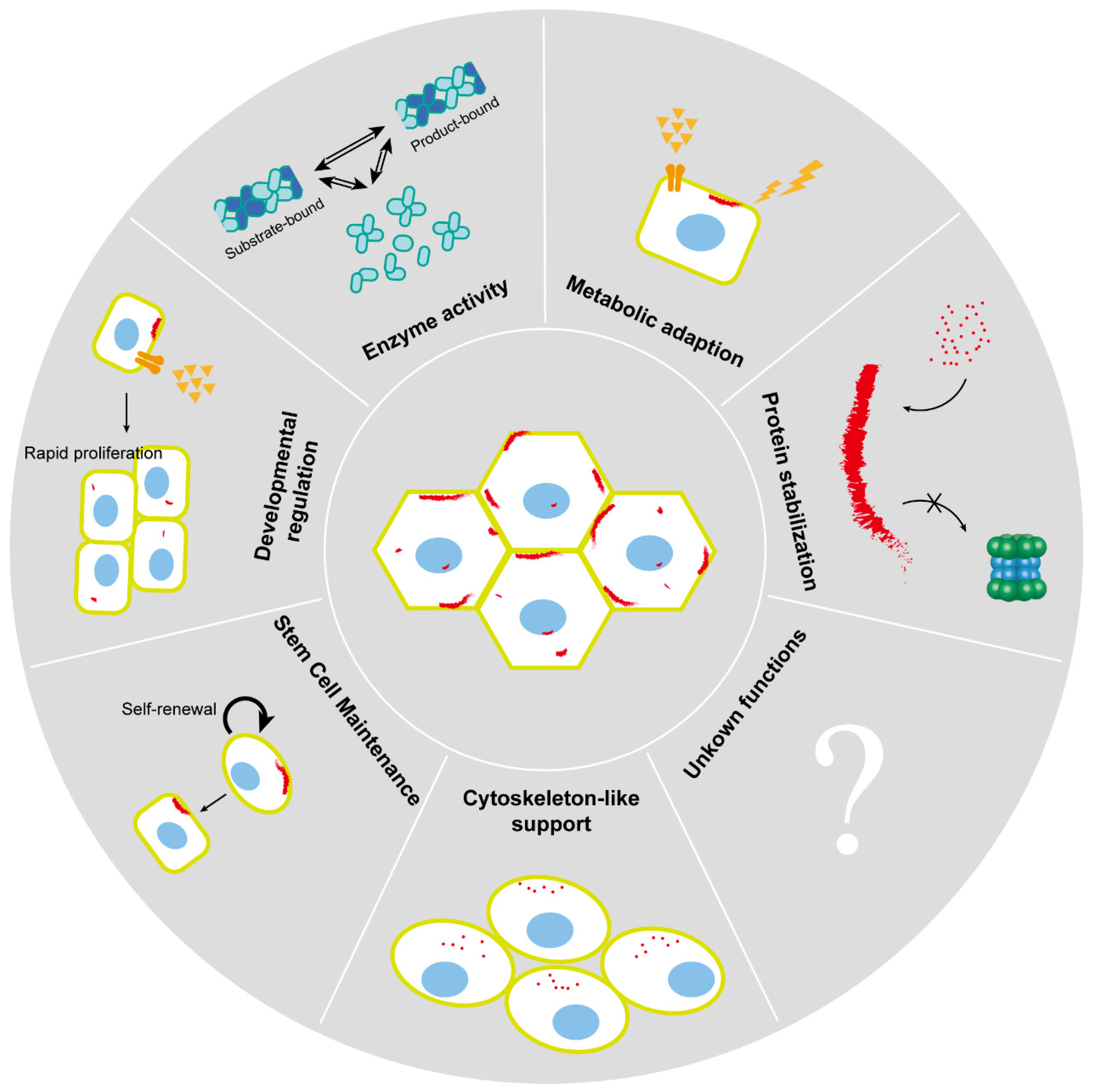
4. Biological Functions of Cytoophidia
4.1. Enzyme Activity
4.2. Metabolic Adaption
4.3. Developmental Regulation
4.4. Stem Cell Maintenance
4.5. Cytoskeleton-like Support
4.6. Protein Stabilization
5. Cytoophidia in Disease
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.L. Intracellular compartmentation of CTP synthase in Drosophila. J. Genet. Genom. = Yi Chuan Xue Bao 2010, 37, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Ingerson-Mahar, M.; Briegel, A.; Werner, J.N.; Jensen, G.J.; Gitai, Z. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat. Cell Biol. 2010, 12, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Noree, C.; Sato, B.K.; Broyer, R.M.; Wilhelm, J.E. Identification of novel filament-forming proteins in Saccharomyces cerevisiae and Drosophila melanogaster. J. Cell Biol. 2010, 190, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Tastan, Ö.Y.; Deussen, Z.A.; Siswick, M.Y.-Y.; Liu, J.-L. Glutamine analogs promote cytoophidium assembly in human and Drosophila cells. J. Genet. Genom. 2011, 38, 391–402. [Google Scholar] [CrossRef]
- Carcamo, W.C.; Satoh, M.; Kasahara, H.; Terada, N.; Hamazaki, T.; Chan, J.Y.; Yao, B.; Tamayo, S.; Covini, G.; von Mühlen, C.A.; et al. Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells. PLoS ONE 2011, 6, e29690. [Google Scholar] [CrossRef]
- Zhang, J.; Hulme, L.; Liu, J.L. Asymmetric inheritance of cytoophidia in Schizosaccharomyces pombe. Biol. Open 2014, 3, 1092–1097. [Google Scholar] [CrossRef]
- Daumann, M.; Hickl, D.; Zimmer, D.; DeTar, R.A.; Kunz, H.H.; Möhlmann, T. Characterization of filament-forming CTP synthases from Arabidopsis thaliana. Plant J. 2018, 96, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Keppeke, G.D.; Antos, C.L.; Peng, M.; Andrade, L.E.C.; Sung, L.Y.; Liu, J.L. CTPS forms the cytoophidium in zebrafish. Exp. Cell Res. 2021, 405, 112684. [Google Scholar] [CrossRef]
- Zhou, S.; Xiang, H.; Liu, J.-L. CTP synthase forms cytoophidia in archaea. J. Genet. Genom. 2020, 47, 213–223. [Google Scholar] [CrossRef]
- Lieberman, I. Enzymatic amination of uridine triphosphate to cytidine triphosphate. J. Biol. Chem. 1956, 222, 765–775. [Google Scholar] [CrossRef]
- Bakovic, M.; Fullerton, M.D.; Michel, V. Metabolic and molecular aspects of ethanolamine phospholipid biosynthesis: The role of CTP: Phosphoethanolamine cytidylyltransferase (Pcyt2). Biochem. Cell Biol. = Biochim. Biol. Cell 2007, 85, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Carman, G.M. CTP synthetase and its role in phospholipid synthesis in the yeast Saccharomyces cerevisiae. Prog. Lipid Res. 2008, 47, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, D.B.; O’Brien, D.J.; Gorman, J.A.; Carman, G.M. Effect of CTP synthetase regulation by CTP on phospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 18992–19001. [Google Scholar] [CrossRef] [PubMed]
- Hatse, S.; De Clercq, E.; Balzarini, J. Role of antimetabolites of purine and pyrimidine nucleotide metabolism in tumor cell differentiation. Biochem. Pharmacol. 1999, 58, 539–555. [Google Scholar] [CrossRef]
- Shridas, P.; Waechter, C.J. Human dolichol kinase, a polytopic endoplasmic reticulum membrane protein with a cytoplasmically oriented CTP-binding site. J. Biol. Chem. 2006, 281, 31696–31704. [Google Scholar] [CrossRef]
- Robertson, J.G. Determination of subunit dissociation constants in native and inactivated CTP synthetase by sedimentation equilibrium. Biochemistry 1995, 34, 7533–7541. [Google Scholar] [CrossRef]
- Liu, J.L. The Cytoophidium and Its Kind: Filamentation and Compartmentation of Metabolic Enzymes. Annu. Rev. Cell Dev. Biol. 2016, 32, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.L.; Zalkin, H. Structural role for a conserved region in the CTP synthetase glutamine amide transfer domain. J. Bacteriol. 1987, 169, 3023–3028. [Google Scholar] [CrossRef]
- Endrizzi, J.A.; Kim, H.; Anderson, P.M.; Baldwin, E.P. Crystal structure of Escherichia coli cytidine triphosphate synthetase, a nucleotide-regulated glutamine amidotransferase/ATP-dependent amidoligase fusion protein and homologue of anticancer and antiparasitic drug targets. Biochemistry 2004, 43, 6447–6463. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Z.; Liu, J.L. Filamentation and inhibition of prokaryotic CTP synthase with ligands. mLife 2024, 3, 240–250. [Google Scholar] [CrossRef]
- Barry, R.M.; Bitbol, A.F.; Lorestani, A.; Charles, E.J.; Habrian, C.H.; Hansen, J.M.; Li, H.J.; Baldwin, E.P.; Wingreen, N.S.; Kollman, J.M.; et al. Large-scale filament formation inhibits the activity of CTP synthetase. eLife 2014, 3, e03638. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Horowitz, A.; Lynch, E.M.; Farrell, D.P.; Quispe, J.; DiMaio, F.; Kollman, J.M. Cryo-EM structures of CTP synthase filaments reveal mechanism of pH-sensitive assembly during budding yeast starvation. eLife 2021, 10, e73368. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, C.J.; Hu, H.H.; Zhong, J.; Sun, Q.; Liu, D.; Zhou, S.; Chang, C.C.; Liu, J.L. Drosophila CTP synthase can form distinct substrate- and product-bound filaments. J. Genet. Genom. = Yi Chuan Xue Bao 2019, 46, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.M.; DiMattia, M.A.; Albanese, S.; van Zundert, G.C.P.; Hansen, J.M.; Quispe, J.D.; Kennedy, M.A.; Verras, A.; Borrelli, K.; Toms, A.V.; et al. Structural basis for isoform-specific inhibition of human CTPS1. Proc. Natl. Acad. Sci. USA 2021, 118, e2107968118. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.M.; Kollman, J.M. Coupled structural transitions enable highly cooperative regulation of human CTPS2 filaments. Nat. Struct. Mol. Biol. 2020, 27, 42–48. [Google Scholar] [CrossRef]
- Lynch, E.M.; Hicks, D.R.; Shepherd, M.; Endrizzi, J.A.; Maker, A.; Hansen, J.M.; Barry, R.M.; Gitai, Z.; Baldwin, E.P.; Kollman, J.M. Human CTP synthase filament structure reveals the active enzyme conformation. Nat. Struct. Mol. Biol. 2017, 24, 507–514. [Google Scholar] [CrossRef]
- Guo, C.-J.; Zhang, Z.; Lu, J.-L.; Zhong, J.; Wu, Y.-F.; Guo, S.-Y.; Liu, J.-L. Structural Basis of Bifunctional CTP/dCTP Synthase. J. Mol. Biol. 2024, 436, 168750. [Google Scholar] [CrossRef]
- Guo, C.J.; Liu, J.L. Cytoophidia and filaments: You must unlearn what you have learned. Biochem. Soc. Trans. 2023, 51, 1245–1256. [Google Scholar] [CrossRef]
- Gou, K.M.; Chang, C.C.; Shen, Q.J.; Sung, L.Y.; Liu, J.L. CTP synthase forms cytoophidia in the cytoplasm and nucleus. Exp. Cell Res. 2014, 323, 242–253. [Google Scholar] [CrossRef]
- Chang, C.C.; Keppeke, G.D.; Sung, L.Y.; Liu, J.L. Interfilament interaction between IMPDH and CTPS cytoophidia. FEBS J. 2018, 285, 3753–3768. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, K.; Shen, Q.J.; Zhao, S.; Liu, J.L. Filamentation of asparagine synthetase in Saccharomyces cerevisiae. PLoS Genet. 2018, 14, e1007737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tastan, Ö.Y.; Zhou, X.; Guo, C.-J.; Liu, X.; Thind, A.; Hu, H.-H.; Zhao, S.; Liu, J.-L. The proline synthesis enzyme P5CS forms cytoophidia in Drosophila. J. Genet. Genom. 2020, 47, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, C.-J.; Zhou, X.; Chang, C.-C.; Yin, B.; Zhang, T.; Hu, H.-H.; Lu, G.-M.; Liu, J.-L. Structural basis of dynamic P5CS filaments. eLife 2022, 11, e76107. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-J.; Zhang, T.; Leng, Q.; Zhou, X.; Zhong, J.; Liu, J.-L. Dynamic Arabidopsis P5CS filament facilitates substrate channelling. Nat. Plants 2024, 10, 80–894. [Google Scholar] [CrossRef]
- Meredith, M.J.; Lane, M.D. Acetyl-CoA carboxylase. Evidence for polymeric filament to protomer transition in the intact avian liver cell. J. Biol. Chem. 1978, 253, 3381–3383. [Google Scholar] [CrossRef]
- Kleinschmidt, A.K.; Moss, J.; Lane, D.M. Acetyl coenzyme A carboxylase: Filamentous nature of the animal enzymes. Science 1969, 166, 1276–1278. [Google Scholar] [CrossRef]
- Ashcraft, B.A.; Fillers, W.S.; Augustine, S.L.; Clarke, S.D. Polymer-protomer transition of acetyl-CoA carboxylase occurs in vivo and varies with nutritional conditions. J. Biol. Chem. 1980, 255, 10033–10035. [Google Scholar] [CrossRef]
- Hunkeler, M.; Hagmann, A.; Stuttfeld, E.; Chami, M.; Guri, Y.; Stahlberg, H.; Maier, T. Structural basis for regulation of human acetyl-CoA carboxylase. Nature 2018, 558, 470–474. [Google Scholar] [CrossRef]
- Shen, Q.J.; Kassim, H.; Huang, Y.; Li, H.; Zhang, J.; Li, G.; Wang, P.Y.; Yan, J.; Ye, F.; Liu, J.L. Filamentation of Metabolic Enzymes in Saccharomyces cerevisiae. J. Genet. Genom. = Yi Chuan Xue Bao 2016, 43, 393–404. [Google Scholar] [CrossRef]
- Ji, Y.; Gu, J.; Makhov, A.M.; Griffith, J.D.; Mitchell, B.S. Regulation of the interaction of inosine monophosphate dehydrogenase with mycophenolic Acid by GTP. J. Biol. Chem. 2006, 281, 206–212. [Google Scholar] [CrossRef]
- Hu, H.H.; Lu, G.M.; Chang, C.C.; Li, Y.; Zhong, J.; Guo, C.J.; Zhou, X.; Yin, B.; Zhang, T.; Liu, J.L. Filamentation modulates allosteric regulation of PRPS. eLife 2022, 11, e79552. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.M.; Hu, H.H.; Chang, C.C.; Zhong, J.; Zhou, X.; Guo, C.J.; Zhang, T.; Li, Y.L.; Yin, B.; Liu, J.L. Structural basis of human PRPS2 filaments. Cell Biosci. 2023, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Noree, C.; Begovich, K.; Samilo, D.; Broyer, R.; Monfort, E.; Wilhelm, J.E. A quantitative screen for metabolic enzyme structures reveals patterns of assembly across the yeast metabolic network. Mol. Biol. Cell 2019, 30, 2721–2736. [Google Scholar] [CrossRef]
- Stoddard, P.R.; Lynch, E.M.; Farrell, D.P.; Dosey, A.M.; DiMaio, F.; Williams, T.A.; Kollman, J.M.; Murray, A.W.; Garner, E.C. Polymerization in the actin ATPase clan regulates hexokinase activity in yeast. Science 2020, 367, 1039–1042. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Levy, M.; Tsechansky, M.; Stovall, G.M.; O’Connell, J.D.; Mirrielees, J.; Ellington, A.D.; Marcotte, E.M. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. USA 2009, 106, 10147–10152. [Google Scholar] [CrossRef] [PubMed]
- Josephs, R.; Borisy, G. Self-assembly of glutamic dehydrogenase into ordered superstructures: Multichain tubes formed by association of single molecules. J. Mol. Biol. 1972, 65, 127–155. [Google Scholar] [CrossRef]
- Olsen, B.R.; Svenneby, G.; Kvamme, E.; Tveit, B.; Eskeland, T. Formation and ultrastructure of enzymically active polymers of pig renal glutaminase. J. Mol. Biol. 1970, 52, 239–245. [Google Scholar] [CrossRef]
- Kemp, R.G. Rabbit liver phosphofructokinase. Comparison of some properties with those of muscle phosphofructokinase. J. Biol. Chem. 1971, 246, 245–252. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.-L. mTOR-S6K1 pathway mediates cytoophidium assembly. J. Genet. Genom. 2019, 46, 65–74. [Google Scholar] [CrossRef]
- Andreadis, C.; Hulme, L.; Wensley, K.; Liu, J.-L. The TOR pathway modulates cytoophidium formation in Schizosaccharomyces pombe. J. Biol. Chem. 2019, 294, 14686–14703. [Google Scholar] [CrossRef]
- Aughey, G.N.; Grice, S.J.; Shen, Q.J.; Xu, Y.; Chang, C.C.; Azzam, G.; Wang, P.Y.; Freeman-Mills, L.; Pai, L.M.; Sung, L.Y.; et al. Nucleotide synthesis is regulated by cytoophidium formation during neurodevelopment and adaptive metabolism. Biol. Open 2014, 3, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Chakraborty, A.; Huang, S.-C.; Wang, P.-Y.; Hsieh, Y.-J.; Chien, K.-Y.; Lee, Y.-H.; Chang, C.-C.; Tang, H.-Y.; Lin, Y.-T.; et al. Histidine-Dependent Protein Methylation Is Required for Compartmentalization of CTP Synthase. Cell Rep. 2018, 24, 2733–2745.e7. [Google Scholar] [CrossRef] [PubMed]
- Aughey, G.N.; Grice, S.J.; Liu, J.L. The Interplay between Myc and CTP Synthase in Drosophila. PLoS Genet. 2016, 12, e1005867. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Liu, J.-L. Connecting Ras and CTP synthase in Drosophila. Exp. Cell Res. 2022, 416, 113155. [Google Scholar] [CrossRef]
- Weng, R.Y.; Zhang, L.; Liu, J.L. Connecting Hippo Pathway and Cytoophidia in Drosophila Posterior Follicle Cells. Int. J. Mol. Sci. 2024, 25, 1453. [Google Scholar] [CrossRef] [PubMed]
- Strochlic, T.I.; Stavrides, K.P.; Thomas, S.V.; Nicolas, E.; O’Reilly, A.M.; Peterson, J.R. Ack kinase regulates CTP synthase filaments during Drosophila oogenesis. EMBO Rep. 2014, 15, 1184–1191. [Google Scholar] [CrossRef]
- Wang, P.Y.; Lin, W.C.; Tsai, Y.C.; Cheng, M.L.; Lin, Y.H.; Tseng, S.H.; Chakraborty, A.; Pai, L.M. Regulation of CTP Synthase Filament Formation During DNA Endoreplication in Drosophila. Genetics 2015, 201, 1511–1523. [Google Scholar] [CrossRef]
- Andreadis, C.; Li, T.; Liu, J.-L. Ubiquitination regulates cytoophidium assembly in Schizosaccharomyces pombe. Exp. Cell Res. 2022, 420, 113337. [Google Scholar] [CrossRef]
- Feng, H.C.; Andreadis, C.; Liu, J.L. Histone transcription regulator Slm9 is required for cytoophidium biogenesis. Exp. Cell Res. 2021, 403, 112582. [Google Scholar] [CrossRef]
- Li, H.; Ye, F.; Ren, J.Y.; Wang, P.Y.; Du, L.L.; Liu, J.L. Active transport of cytoophidia in Schizosaccharomyces pombe. FASEB J. 2018, 32, 5891–5898. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhao, P.A.; Tastan, Ö.Y.; Liu, J.L. Polarised maintenance of cytoophidia in Drosophila follicle epithelia. Exp. Cell Res. 2021, 402, 112564. [Google Scholar] [CrossRef] [PubMed]
- Calise, S.J.; Carcamo, W.C.; Krueger, C.; Yin, J.D.; Purich, D.L.; Chan, E.K. Glutamine deprivation initiates reversible assembly of mammalian rods and rings. Cell. Mol. Life Sci. 2014, 71, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.F.; Li, Y.L.; Li, X.M.; Liu, J.L. Super-Resolution Imaging Reveals Dynamic Reticular Cytoophidia. Int. J. Mol. Sci. 2022, 23, 11698. [Google Scholar] [CrossRef]
- McCluskey, G.D.; Bearne, S.L. Biophysical Analysis of Bacterial CTP Synthase Filaments Formed in the Presence of the Chemotherapeutic Metabolite Gemcitabine-5′-triphosphate. J. Mol. Biol. 2018, 430, 1201–1217. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Wang, P.-Y.; Ye, F.; Liu, J.-L. Data on dynamic study of cytoophidia in Saccharomyces cerevisiae. Data Brief. 2016, 8, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.-L. Temperature-sensitive cytoophidium assembly in Schizosaccharomyces pombe. J. Genet. Genom. 2019, 46, 423–432. [Google Scholar] [CrossRef]
- Li, Y.-L.; Liu, J.-L. Hypoosmolality impedes cytoophidium integrity during nitrogen starvation. Yeast 2021, 38, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.-L. Cytoophidia respond to nutrient stress in Drosophila. Exp. Cell Res. 2019, 376, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Chang, C.C.; Liu, J.L.; Sung, L.Y. CTPS and IMPDH form cytoophidia in developmental thymocytes. Exp. Cell Res. 2021, 405, 112662. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Liu, J.L. Dynamic Cytoophidia during Late-Stage Drosophila Oogenesis. Int. J. Mol. Sci. 2024, 25, 2575. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Zhang, Y.; Liu, J.L. Drosophila intestinal homeostasis requires CTP synthase. Exp. Cell Res. 2021, 408, 112838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Liu, J.L. The atlas of cytoophidia in Drosophila larvae. J. Genet. Genom. = Yi Chuan Xue Bao 2020, 47, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Jeng, Y.M.; Peng, M.; Keppeke, G.D.; Sung, L.Y.; Liu, J.L. CTP synthase forms the cytoophidium in human hepatocellular carcinoma. Exp. Cell Res. 2017, 361, 292–299. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.-L. CTP synthase does not form cytoophidia in Drosophila interfollicular stalks. Exp. Cell Res. 2022, 418, 113250. [Google Scholar] [CrossRef]
- Levitzki, A.; Koshland, D.E., Jr. Ligand-induced dimer-to-tetramer transformation in cytosine triphosphate synthetase. Biochemistry 1972, 11, 247–253. [Google Scholar] [CrossRef]
- Pappas, A.; Yang, W.L.; Park, T.S.; Carman, G.M. Nucleotide-dependent tetramerization of CTP synthetase from Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 15954–15960. [Google Scholar] [CrossRef]
- Bearne, S.L.; Guo, C.J.; Liu, J.L. GTP-Dependent Regulation of CTP Synthase: Evolving Insights into Allosteric Activation and NH(3) Translocation. Biomolecules 2022, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Wang, Q.Q.; Zhou, Y.; Liu, J.L. Fat body-specific reduction of CTPS alleviates HFD-induced obesity. eLife 2023, 12, e85293. [Google Scholar] [CrossRef]
- Dzaki, N.; Wahab, W.; Azlan, A.; Azzam, G. CTP synthase knockdown during early development distorts the nascent vertebral column and causes fluid retention in multiple tissues in zebrafish. Biochem. Biophys. Res. Commun. 2018, 505, 106–112. [Google Scholar] [CrossRef]
- Tastan, Ö.Y.; Liu, J.L. CTP Synthase Is Required for Optic Lobe Homeostasis in Drosophila. J. Genet. Genom. = Yi Chuan Xue Bao 2015, 42, 261–274. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Hei, M.; Tang, J.; Wang, Y.; Förster, E.; Zhao, S. High level of CTP synthase induces formation of cytoophidia in cortical neurons and impairs corticogenesis. Histochem. Cell Biol. 2018, 149, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Simonet, J.C.; Foster, M.J.; Lynch, E.M.; Kollman, J.M.; Nicholas, E.; O’Reilly, A.M.; Peterson, J.R. CTP synthase polymerization in germline cells of the developing Drosophila egg supports egg production. Biol. Open 2020, 9, bio050328. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.K.; Dzaki, N.; Thangadurai, S.; Azzam, G. Ectopic miR-975 induces CTP synthase directed cell proliferation and differentiation in Drosophila melanogaster. Sci. Rep. 2019, 9, 6096. [Google Scholar] [CrossRef]
- Wang, Q.Q.; You, D.D.; Liu, J.L. Cytoophidia Maintain the Integrity of Drosophila Follicle Epithelium. Int. J. Mol. Sci. 2022, 23, 15282. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhou, Y.; Wang, Q.-Q.; Ding, K.; Zhao, S.; Lu, P.; Liu, J.-L. Cytoophidia coupling adipose architecture and metabolism. Cell. Mol. Life Sci. 2022, 79, 534. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.L. Forming cytoophidia prolongs the half-life of CTP synthase. Cell Discov. 2019, 5, 32. [Google Scholar] [CrossRef]
- Azzam, G.; Liu, J.L. Only one isoform of Drosophila melanogaster CTP synthase forms the cytoophidium. PLoS Genet. 2013, 9, e1003256. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, Z.Y.; Zhang, K.; Filipovich, L.; Bleesing, J.J. CTP Synthase 1 Deficiency in Successfully Transplanted Siblings with Combined Immune Deficiency and Chronic Active EBV Infection. J. Clin. Immunol. 2016, 36, 750–753. [Google Scholar] [CrossRef]
- Martin, E.; Minet, N.; Boschat, A.C.; Sanquer, S.; Sobrino, S.; Lenoir, C.; de Villartay, J.P.; Leite-de-Moraes, M.; Picard, C.; Soudais, C.; et al. Impaired lymphocyte function and differentiation in CTPS1-deficient patients result from a hypomorphic homozygous mutation. JCI Insight 2020, 5, e133880. [Google Scholar] [CrossRef]
- Martin, E.; Palmic, N.; Sanquer, S.; Lenoir, C.; Hauck, F.; Mongellaz, C.; Fabrega, S.; Nitschké, P.; Esposti, M.D.; Schwartzentruber, J.; et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 2014, 510, 288–292. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Grandits, A.M.; Asnagli, H.; Schneller, A.; Huber, J.; Zojer, N.; Schreder, M.; Parker, A.E.; Bolomsky, A.; Beer, P.A.; et al. CTPS1 is a novel therapeutic target in multiple myeloma which synergizes with inhibition of CHEK1, ATR or WEE1. Leukemia 2024, 38, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Z.; Wang, Q.Q.; Liu, J.L. Combined Inactivation of CTPS1 and ATR Is Synthetically Lethal to MYC-Overexpressing Cancer Cells. Cancer Res. 2022, 82, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Lu, S.; Wang, S.; Zhang, S. Identification of critical genes associated with human osteosarcoma metastasis based on integrated gene expression profiling. Mol. Med. Rep. 2019, 20, 915–930. [Google Scholar] [CrossRef]
- Chiarelli, L.R.; Mori, G.; Orena, B.S.; Esposito, M.; Lane, T.; de Jesus Lopes Ribeiro, A.L.; Degiacomi, G.; Zemanová, J.; Szádocka, S.; Huszár, S.; et al. A multitarget approach to drug discovery inhibiting Mycobacterium tuberculosis PyrG and PanK. Sci. Rep. 2018, 8, 3187. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; Chiarelli, L.R.; Esposito, M.; Makarov, V.; Bellinzoni, M.; Hartkoorn, R.C.; Degiacomi, G.; Boldrin, F.; Ekins, S.; Ribeiro, A.L.; et al. Thiophenecarboxamide Derivatives Activated by EthA Kill Mycobacterium tuberculosis by Inhibiting the CTP Synthetase PyrG. Chem. Biol. 2015, 22, 917–927. [Google Scholar] [CrossRef]
- Pinto, A.; Tamborini, L.; Cullia, G.; Conti, P.; De Micheli, C. Inspired by Nature: The 3-Halo-4,5-dihydroisoxazole Moiety as a Novel Molecular Warhead for the Design of Covalent Inhibitors. ChemMedChem 2016, 11, 10–14. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, G.; Chen, S.Y. Smooth Muscle Cell Proangiogenic Phenotype Induced by Cyclopentenyl Cytosine Promotes Endothelial Cell Proliferation and Migration. J. Biol. Chem. 2016, 291, 26913–26921. [Google Scholar] [CrossRef]
- McCluskey, G.D.; Mohamady, S.; Taylor, S.D.; Bearne, S.L. Exploring the Potent Inhibition of CTP Synthase by Gemcitabine-5′-Triphosphate. Chembiochem 2016, 17, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Ortiz, H.Y.; Lopez, A.J.; Gupta, N.; Zimmermann, B.H. A CTP Synthase Undergoing Stage-Specific Spatial Expression Is Essential for the Survival of the Intracellular Parasite Toxoplasma gondii. Front. Cell Infect. Microbiol. 2018, 8, 83. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Taher, A. Metabolic drug targets of the cytosine metabolism pathways in the dromedary camel (Camelus dromedarius) and blood parasite Trypanosoma evansi. Trop. Anim. Health Prod. 2020, 52, 3337–3358. [Google Scholar] [CrossRef]
- Ruan, H.; Song, Z.; Cao, Q.; Ni, D.; Xu, T.; Wang, K.; Bao, L.; Tong, J.; Xiao, H.; Xiao, W.; et al. IMPDH1/YB-1 Positive Feedback Loop Assembles Cytoophidia and Represents a Therapeutic Target in Metastatic Tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Keppeke, G.D.; Barcelos, D.; Fernandes, M.; Comodo, A.N.; Guimarães, D.P.; Cardili, L.; Carapeto, F.C.L.; Andrade, L.E.C.; Landman, G. IMP dehydrogenase rod/ring structures in acral melanomas. Pigment. Cell Melanoma Res. 2020, 33, 490–497. [Google Scholar] [CrossRef] [PubMed]

| Enzyme Name | Species | Discovery Year | Reference/Publication |
|---|---|---|---|
| CTP synthase (CTPS) | Drosophila C. crescentus S. cerevisiae | 2010 | Liu, J Genet Genomics, 2010 [1] Ingerson-Mahar et al., Nat Cell Biol, 2010 [2] Noree et al., J Cell Biol, 2010 [3] |
| Inosine monophosphate dehydrogenase (IMPDH) | Homo sapiens | 2006 | Ji et al., J Biol Chem, 2006 [40] |
| PRPP synthase (PRPS) | E. coli Homo sapiens | 2022 2023 | Hu et al., Elife. 2022 [41]; Lu et al., Cell Biosci. 2023 [42] |
| Glycogen debranching enzyme (GDE), thioredoxin peroxidase (TPx), asparagine synthetase (ASNS) | S. cerevisiae | 2016 | Shen et al., J Genet Genomics, 2016 [39] |
| Kynureninase, PRPP synthetase, GDP-mannose pyrophosphorylase | S. cerevisiae | 2019 | Noree et al., Mol Biol Cell, 2019 [43] |
| Delta-1-pyrroline-5-carboxylate synthase (P5CS) | Drosophila | 2020 | Zhang et al., J Genet Genomics, 2020 [32] |
| Glucokinase (GLK) | Yeast | 2020 | Stoddard et al., Science, 2020 [44] |
| Glutamine synthetase (GLN) | Yeast | 2009 | Narayanaswamy et al., Proc Natl Acad Sci, 2009 [45] |
| Glutamic dehydrogenase (GDH) | Bovine | 1972 | Josephs & Borisy, J Mol Biol, 1972 [46] |
| Glutaminase | Pig | 1970 | Olsen et al., J Mol Biol, 1970 [47] |
| Acetyl coenzyme A carboxylase (ACC) | Several animals | 1969 | Kleinschmidt et al., Science, 1969 [36] |
| Phosphofructokinase (PFK) | Rabbit | 1971 | Kemp, J Biol Chem, 1971 [48] |
| Regulators | Functions | Reference/Publication |
|---|---|---|
| Nucleotides and analogs | Directly binding. | / |
| mTORC1/S6K1 | mTOR pathway controls CTPS cytoophidium assembly. | Sun and Liu, J Genet Genomics, 2019 [49]; Andreadis et al., J Biol Chem, 2019 [50] |
| AKT1 | Inactivation of the AKT1 pathway induces cytoophidia formation. | Aughey et al., Biol Open, 2014 [51] |
| GCN2/ATF4/MTHFD2 | Starvation stress and glutamine deficiency activate the GCN2/ATF4/MTHFD2 axis, thus coordinating CTPS filament formation. | Lin et al., Cell Rep, 2018 [52] |
| Myc | CTPsyn acts downstream of Myc. | Aughey et al., PLoS Genet, 2016 [53] |
| Ras | Overexpressing active Ras induces elongate and abundant cytoophidia. | Zhou et al., Exp Cell Res, 2022 [54] |
| Hippo | Inactivation of the Hippo pathway correlates with reduced cytoophidium. | Weng et al., Int J Mol Sci, 2024 [55] |
| Ack kinase | DAck localizes to CTPS filaments. | Strochlic at al., EMBO Rep, 2014 [56] |
| Cbl | Cbl is required for CTPsyn filament formation. | Wang et al., Genetics, 2015 [57] |
| Ubiquitination regulators | Ubiquitination and deubiquitination affect CTPS filamentation. | Andreadis et al., Exp Cell Res, 2022 [58] |
| Histone chaperone Slm9 | Slm9 is required for cytoophidium biogenesis. | Feng et al., Exp Cell Res, 2022 [59] |
| Myo52 | Myo52 is required for the active transport of cytoophidia. | Li et al., FASEB J, 2018 [60] |
| Polarity regulators | Knockdown of apical polarity regulators leads to cytoophidia instability and abnormal distribution. | Wang et al., Exp Cell Res, 2021 [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, J.-L. The Impact of Developmental and Metabolic Cues on Cytoophidium Formation. Int. J. Mol. Sci. 2024, 25, 10058. https://doi.org/10.3390/ijms251810058
Zhang Y, Liu J-L. The Impact of Developmental and Metabolic Cues on Cytoophidium Formation. International Journal of Molecular Sciences. 2024; 25(18):10058. https://doi.org/10.3390/ijms251810058
Chicago/Turabian StyleZhang, Yuanbing, and Ji-Long Liu. 2024. "The Impact of Developmental and Metabolic Cues on Cytoophidium Formation" International Journal of Molecular Sciences 25, no. 18: 10058. https://doi.org/10.3390/ijms251810058
APA StyleZhang, Y., & Liu, J.-L. (2024). The Impact of Developmental and Metabolic Cues on Cytoophidium Formation. International Journal of Molecular Sciences, 25(18), 10058. https://doi.org/10.3390/ijms251810058


_Kim.png)




