Abstract
Despite advances in the treatment of hepatocellular carcinoma (HCC) over the last few decades, treatment opportunities for patients with HCC remain limited. HCC is the most common form of liver cancer, accounting for approximately 90% of all cases worldwide. Moreover, apart from the current pharmacological interventions, hepatic resection and liver transplantation are the mainstay curative approaches for patients with HCC. This systematic review included phase I, II, III, and IV clinical trials (CTs) and randomized controlled trials (RCTs) on current treatments for patients with HCC in Asian populations (2013–2023). A total of 427 articles were screened, and 184 non-duplicate publications were identified. After screening the titles and abstracts, 96 publications were excluded, and another 28 were excluded after full-text screening. The remaining 60 eligible RCTs/CTs were finally included. A total of 60 clinical trials fulfilled our inclusion criteria with 36 drugs used as monotherapy or combination therapy for HCC. Most studies used sorafenib alone or in combination with any of the treatment regimens. Lenvatinib or atezolizumab with bevacizumab was used for HCC after initial sorafenib treatment. Eighteen studies compared the efficacy of sorafenib with that of other drugs, including lenvatinib, cabozantinib, tepotinib, tigatuzumab, linifanib, erlotinib, resminostat, brivanib, tislelizumab, selumetinib, and refametinib. This study provides comprehensive insights into effective treatment interventions for HCC in Asian populations. The overall assessment indicates that sorafenib, used alone or in combination with atezolizumab and bevacizumab, has been the first treatment choice in the past decade to achieve better outcomes in patients with HCC in Asian populations.
1. Introduction
The increasing incidence of hepatocellular carcinoma (HCC) poses a global health challenge [1,2]. According to a recent report by GLOBOCAN 2020, Mongolia has the highest age-standardized rate for both mortality and incidence of HCC. It is also estimated that in Asia, China alone accounts for 62.4% of the cases, followed by Japan (7.0%), India (5.3%), Thailand (4.2%), and Vietnam (4%) [3]. In Asia, liver cancer is the fifth most common cancer after thyroid, stomach, colon, and lung cancers, and it is the second most common cause of malignancy-related deaths in Asia [4]. In Asia, HCC accounts for the highest incidence and mortality among patients with liver cancer [4].
Over the last three decades, the annual crude mortality rate of HCC has increased in Asia. In addition to surgical intervention, several systemic therapies, including chemotherapy, immunotherapy, and molecular target-based therapies, have been proposed for advanced HCC. With technological advancements in research, molecular-targeted therapies are the mainstream approach for treating patients with HCC either alone or in combination with other drugs, especially in Asian populations.
The etiology of HCC varies according to geographical region, as reported by a recently published study [5]. In the Asia–Pacific region, hepatitis virus infection is among the major causes of HCC; 70% of the patients from these regions have chronic hepatitis B virus (HBV) infection, whereas 20% have hepatitis C viral (HCV) infection [5]. A study from the Asia–Pacific region has reported that 75% of the patients with HCC in Japan have HCV infection [6].
The incidence of liver cancer varies among Asian populations. According to statistics from a recently published study, East Asian regions, including China, South Korea, and Japan, and Southeast Asian regions, including the Philippines, demonstrated a sharp decline in the incidence rate of liver cancer [7]. The same study observed a decline in the annual average percent change in the incidence rate of liver cancer in countries, including China (−1.6%), South Korea (−2.2%), and the Philippines (−1.7%), since 1978 [7]. However, a significant increase in the incidence of liver cancer has been reported in southwestern Asian countries, especially Israel [7]. HCC accounts for majority of liver cancer cases and affects 27% of the population in Thailand alone [7]. In recent decades, the incidence of liver cancer has significantly increased in Iran, Afghanistan, Qatar, Iraq, Azerbaijan, and Nepal [3].
Among Asian countries, liver cancer in South Korea is the fourth most common cancer in men and the sixth most common in women. The decrease in the incidence of liver cancer in South Korea is mainly because of the sharp decline in HBV, which is considered a major cause of HCC. Moreover, large-scale HBV vaccination has affected the incidence of HCC in the South Korean population. Despite several pharmaceutical and technological advancements, the advanced stage of HCC at the time of diagnosis in South Korea still requires serious attention. In a previous study, the 5-year survival rate of HCC among Korean patients was relatively lower than that of other cancer types owing to several effective surveillance drives among the high-risk population in South Korea [8].
Sorafenib is among the first Food and Drug Administration (FDA)-approved interventions that are accepted worldwide for the treatment of advanced-stage HCC. It exhibits a molecularly targeted therapeutic approach by targeting and inhibiting several pathways, including vascular endothelial growth factor receptor-2 (VEGFR-2), platelet-derived growth factor receptor (PDGFR), and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase–ERK (MEK)/rapidly accelerated fibrosarcoma (RAF), thereby offering antiproliferative, antiangiogenic, and antiapoptotic effects [9,10]. In the Asia–Pacific phase III clinical trial (CT), sorafenib alone demonstrated a median overall survival of 6.5 months compared to placebo in patients with HCC, and, thereafter, sorafenib was approved as a first-line therapeutic approach in these patients [11].
Another drug known for treating HCC is regorafenib, a multikinase inhibitor that inhibits angiogenesis and oncogenesis, thereby altering the tumor microenvironment. One phase III RESORCE trial has demonstrated regorafenib as a second-line drug for HCC treatment after sorafenib treatment [12]. Similarly, another multikinase inhibitor, lenvatinib, is considered the first-line therapy for patients with unresectable HCC [13]. Sorafenib is among the first-line therapies for advanced-stage HCC in Asia, whereas atezolizumab and bevacizumab are among the second-line therapies for progressive HCC.
Moreover, owing to the high incidence and prevalence of HCC in Asia and the Asia–Pacific region, an extensive approach to the selection of appropriate therapies against HCC is necessary. Currently, the available treatment options are limited in Asia and the Asia–Pacific region; therefore, a reliable first-line therapy, without any side effects, should be selected to treat HCC. Therefore, this study aimed to distinguish between drug therapies among the approaches available for the treatment of HCC in Asian populations.
2. Material and Methods
Search Strategy and Selection Criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis criteria (Figure 1). A systematic search for eligible studies in the EMBASE, MEDLINE (via PubMed), and CENTRAL (via the Cochrane Library) databases was conducted from 2013 to 2023 (Figure 2). A total of 427 articles were screened, and among them, 184 non-duplicate publications were identified. We excluded 96 publications after screening titles and abstracts and another 28 published papers after full-text screening. Finally, the remaining 60 eligible randomized controlled trials (RCTs)/CTs were included in this systematic review.
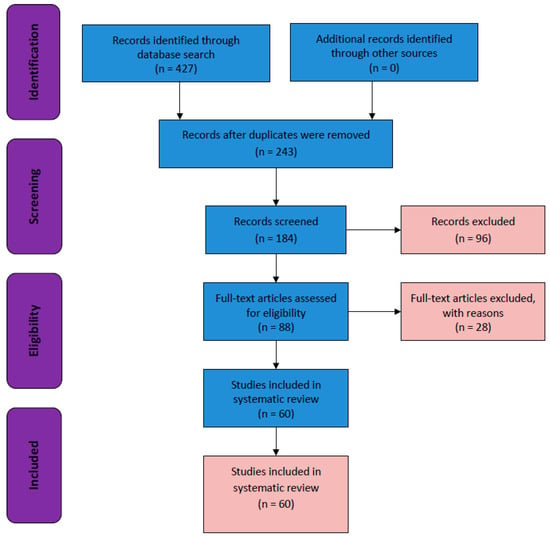
Figure 1.
Flow chart of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Figure 2.
Number of publications in the last 10 years (2013 to 2023), extracted from the PubMed database, on phase I–IV clinical trials related to hepatocellular carcinoma that were conducted in South Korea.
The inclusion criteria for this systematic review were CTs (phases I, II, III, and IV) and RCTs conducted on adult patients (≥18 years), including men and women with all stages of HCC, who received the intervention compared to those who received either placebo or active comparator in Asia or any multicentric trial wherein one study center was located in Asia.
The quality of this systematic review was assessed using the grade system. Briefly, the grade system was divided into four levels: very low, low, moderate, and high. All eligible studies included in this systematic review were screened for imprecision, inconsistency, risk of bias, and publication bias. The validity and authenticity of the included studies were assessed by two independent reviewers using kappa statistics with inter- and intrarater agreements. The outcomes of the extracted studies were noted in the form of majority of the use of particular drugs for the treatment of HCC in Asia.
3. Results
We performed a systematic review of phases I, II, III, and IV CTs and RCTs on current treatments for patients with HCC (2013–2023). A total of 427 articles were screened, and among them, 184 non-duplicate publications were identified. We excluded 96 publications after screening the titles and abstracts and excluded another 28 published papers after full-text screening. The remaining 60 eligible RCTs/CTs were included in this systematic review (Figure 1).
A total of 60 CTs fulfilled our inclusion criteria, with 36 drugs screened for monotherapy or combination therapy for HCC. Most studies used sorafenib alone or in combination with any of the treatment regimens. Lenvatinib or atezolizumab with bevacizumab was used for HCC after initial sorafenib treatment. Eighteen studies compared the efficacy of sorafenib with that of other drugs, including lenvatinib, cabozantinib, tepotinib, tigatuzumab, linifanib, erlotinib, resminostat, brivanib, tislelizumab, selumetinib, and refametinib (Table 1). Three studies reported on the use of a combination of lenvatinib and sorafenib (Table 1). Another three studies reported on the use of nivolumab monotherapy for the pharmacological intervention of HCC, while one study utilized a combination of ipilimumab and sorafenib (Table 1) [14]. Single-arm studies reported on the use of cabozantinib, sorafenib, and immunotherapy using cytokines and enzalutamide (Table 1). Two studies reported on the treatment of HCC using ramucirumab and pembrolizumab (Table 1). This study provides comprehensive insights into effective treatment interventions for HCC in Asian populations. The overall assessment suggests that sorafenib, used alone or in combination with atezolizumab and bevacizumab, has remained the first treatment choice in the past decade for providing better outcomes in patients with HCC in Asian populations. A systematic review of the published articles found consistency in validity appraisal among the two raters, as assessed by a kappa statistic of 0.86. The weighted bar plots of the distribution of the risk of bias judgments within each bias domain are presented in Figure 3. A network visualization of the selected articles is shown in Figure 4. Altogether, these findings suggest that sorafenib, as part of a combination approach with other drugs, is the first-line treatment for patients with HCC in Asian populations.

Table 1.
Eligible studies included in the systematic review showing the application in the treatment of hepatocellular carcinoma.
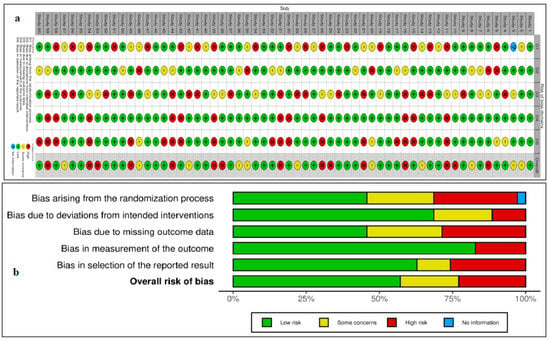
Figure 3.
Plot demonstrating risk of bias (a) Traffic light plots of domain-level judgments for each individual result. (b) Weighted bar plots of the distribution of risk of bias judgments within each bias domain.
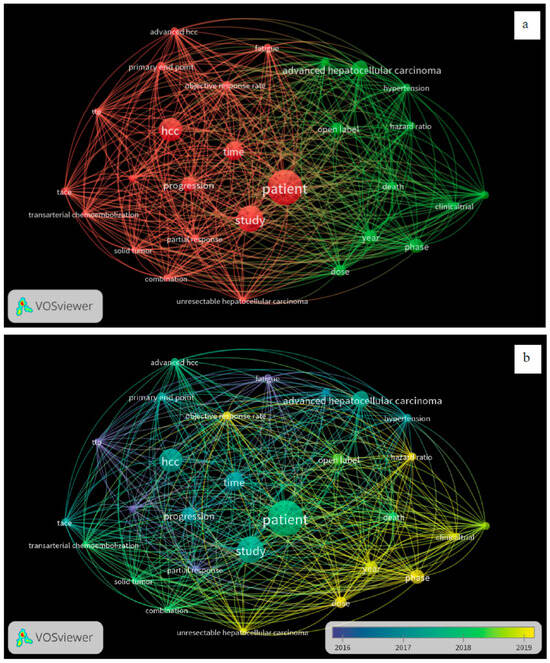
Figure 4.
Visualization of bibliometric networks of eligible articles using VOSviewer version 1.6.16 software (n = 60): (a) network visualization and (b) overlay visualization.
4. Discussion
This review evaluated the drugs used to treat HCC in Asia over the past decade. Sorafenib is a multikinase kinase inhibitor with a molecular weight of 637 g/mol that inhibits protein pathways acting as anticancer agents. Sorafenib acts on RAF, vascular endothelial growth factor (VEGF), and platelet-derived growth factors receptors (PDGFR), as previously demonstrated [72]. RAF is a serine/threonine kinase that initiates the activation of gene transcription responsible for tumor promotion upon activation by the ras protein present on the membrane. Moreover, VEGF is responsible for angiogenesis in both normal and cancerous tissues, which is mediated through endothelial cell division and migration. The interaction of VEGF with VEGFRs 1, 2, and 3 promotes autophosphorylation of tyrosine receptor kinase, resulting in the activation of a cascade of downstream proteins.
Additionally, sorafenib inhibits the activities of VEGFR-2/3, PDGFR-β, Flt3, and c-Kit [73,74]. The precise molecular mechanism underlying the antitumor activity of sorafenib remains unclear, although previously published studies have suggested that sorafenib acts on RAF/MEK/ERK-dependent or -independent protein kinases [75,76,77]. Another study demonstrated that sorafenib inhibits the expression of the β-catenin oncoprotein in HepG2 cells and activates the c-Jun N-terminal kinase (JNK) and p38MAPK pathways [78]. A similar study also observed that sorafenib is actively involved in the downregulation of several DNA repair and recombination genes (XRCC-2, XRCC-5, FANCA, and FANCD2), along with genes involved in cell cycle regulation (CDC45L, CDC6, and CDCA5) that further exert anticancer activities [78].
Sorafenib is associated with common adverse effects, including diarrhea and weight loss, as well as other secondary effects, such as alopecia, anorexia, and voice changes. A previously published study revealed that sorafenib has a significant survival benefit in patients with advanced HCC, although many patients demonstrated disease progression after a reduction in dosage or treatment discontinuation [11,79]. In the Study of Heart and Renal Protection (SHARP) trial, sorafenib exerted primary and acquired resistance, which hampered the survival benefit [80]. Previous studies demonstrated the antitumor activity of sorafenib monotherapy with some limitations, such as drug resistance and adverse effects, discouraging its use as monotherapy. A combination with nivolumab can resolve the problems associated with sorafenib monotherapy. Our results also demonstrated a trend toward the increased use of sorafenib combination therapy.
Nivolumab is a human recombinant monoclonal G4 immunoglobulin with anticancer activity mediated through programmed cell death receptor-1 (PD-1). T-cell response is commonly mediated through the PD-1 mechanism. The blockade of PD-1 receptors present on T-cells inhibits the proliferation of T-cells through a programmed cell death mechanism. In a recently published study, nivolumab was associated with some grade 1–2 adverse events, including the development of colitis and pneumonitis, along with increased alanine aminotransferase and aspartate aminotransferase activities [81].
Another anticancer drug, atezolizumab, acts by targeting PD-L1 on tumor cells, thereby preventing the binding of PD-L1 to its receptors, PD-1 and B7-1. The binding of PD-L1 to its receptor PD-1 inhibits the proliferation of T-cells, along with the inhibition of cytokine production and cytolytic activity, which in turn leads to T-cell inactivation. Similarly, T-cells and antigen-presenting cells (APCs) inhibit immune responses, including T-cell activation and cytokine release, owing to the active binding of PD-L1 to B7-1 present on T-cells and APCs [82,83]. Similar to other FDA-approved PD-1/PD-L1-targeted therapies, atezolizumab is also associated with adverse immune responses, including grade 1–4 immune-mediated colitis, hepatitis, and pneumonitis [84].
Bevacizumab is a recombinant humanized monoclonal immunoglobulin G that binds to the VEGF protein and prevents it from binding to its receptor, thereby exerting a neutralizing effect [85]. HCC is an extensively vascularized solid tumor with immense dense microvessels owing to angiogenesis. Hence, targeting VEGF is a crucial step in preventing tumor angiogenesis. Adverse reactions associated with bevacizumab include hypertension, fatigue, and proteinuria [85]. Bevacizumab can be used in combination with sorafenib to overcome these side effects.
A previously published study reported portal vein tumor invasion in 30% of Korean patients with HCC [86]. A single-center Korean RCT reported that conventional transarterial chemoembolization (cTACE) with radiation therapy had better outcomes than sorafenib monotherapy in HCC patients with portal vein invasion. However, two other RCTs conducted in the Korean population revealed that sorafenib monotherapy did not result in survival gain compared to transarterial radioembolization (TARE) [87,88]. The study concluded that TARE, sorafenib, and cTACE did not result in any survival gains [89].
Despite several drugs being present in the pharmaceutical market, HCC is a highly uncontrollable cancer with a tendency to metastasize to distant organs, including the lungs and stomach. Moreover, the gap between the etiology and genetic mutations contributes to poor treatment outcomes. The current boom in nanotechnology can provide new hope for the early intervention and treatment of HCC without any associated side effects, as in the case of drugs. Nanotechnology offers alternatives to several nanoparticles that have been widely employed in biomedical research related to cancer therapeutics. Nanoparticles improve the accessibility of drugs to human cells and increase their metabolic tendency along with delayed and prolonged therapeutic actions. Their modified surface area offer greater drug loading and mitigate the side effects of drugs. Their enhanced penetration and retention mechanisms, along with active targeting, provide highly specific targeted anticancer therapeutics. Owing to their low or negligible toxicity, enhanced biocompatibility, and biodegradability, anticancer nanoparticles have been the focus of research. In addition to the aforementioned characteristics, these nanoparticles also exhibit anti-inflammatory, antioxidant, and antiangiogenic effects, making them useful as anticancer therapeutics.
The global pharmaceutical companies are steadily manufacturing new and novel molecules in the form of drugs for treatment of hepatocellular carcinoma during past decades. These industries considered Asia–Pacific, North America and Europe as the leading areas for their drug trials [90]. There are several pharmaceutical industries sponsor sites in these regions including Sun Yat-sen University, National Cancer Institute US, FUDAN University and Eastern Hepatobiliary Surgery Hospital. These drug trials for hepatocellular carcinoma (Phase 0 to Phase IV) were sponsored by the company itself in collaboration with the governments, individuals or institutions (Table 2; Figure 5). There are numerous drugs available in the market of USA and Europe to treat hepatocellular carcinoma, which includes pembrolizumab (Keytruda), nivolumab (Opdivo, Opdyta) and bevacizumab (Avastin) [90]. Pembrolizumab (Keytruda) is marketed for the treatment of hepatocellular carcinoma in USA and Europe and is an antineoplastic immunomodulating molecule that antagonist mechanism on Programmed Cell Death Protein 1 (PD1 or CD279 or PDCD1). It was first commercially approved in the year 2014 and launched in the markets of the US, the UK, Australia, France, and Germany by Merck & Co. Inc. and its subsidiaries (Rahway, NJ, USA). Another drug named nivolumab (Opdivo, Opdyta) performs antagonist action on Programmed Cell Death Protein 1 (PD1 or CD279 or PDCD1) and is a human IgG4 anti PD-1 monoclonal antibody that treats hepatocellular carcinoma. This drug was first approved in the year 2014 and launched in the market of the US, the UK, Australia, France and Germany by Bristol Myers Squibb Co and its subsidiaries [90]. According to global data, 26.80% of the clinical trials are Phase II, 20.98% are Phase III, 19.02% Phase I/II, 17.43% Phase I, while Phase II/III and Phase 0 comprised 2.38 and 2.90%, respectively (Table 2).

Table 2.
Ongoing drug trials for HCC.
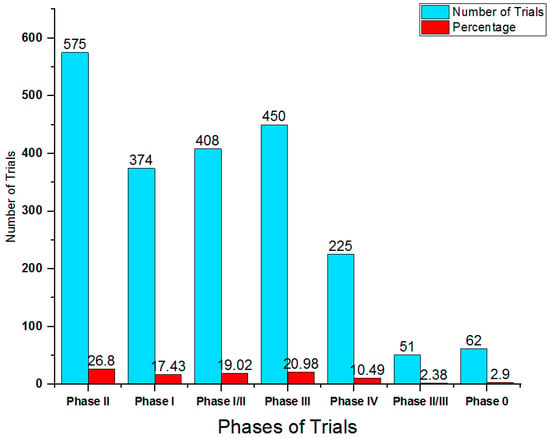
Figure 5.
Bar representation of ongoing drug trails for HCC.
4.1. Pharmacogenetics of Hepatocellular Carcinoma in Asian Population
The Asian population presents diverse pharmacogenetic differences that influence the efficacy of several hepatocellular carcinoma drugs and the adverse drug reactions (ADRs) related to their racial/ethnic backgrounds [91,92,93]. Previous studies have reported that variants with higher frequencies are more common in Asian populations compared to other population types [80,94,95]. The Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US Food and Drug Administration (FDA) have developed guidelines for adjusting treatments based on genetic variations within populations. For example, carbamazepine and clopidogrel have been shown to present ADRs in Asian populations compared to others [96,97]. Impaired gene expression, presentation of spliced variants, gene polymorphism, and mutations are among the important factors associated with poor prognosis and altered drug metabolism in Asian populations in the treatment of liver cancer, which is the fourth most deadly cancer (Figure 6). These factors severely affect the function of genes and in turn lead to a reduction of active drug molecules within intracellular tumor microenvironment along with phenotypic transitions and hampered survival pathways. SLCO, SLC22A and SLC31A are the gene families responsible for the transport of anticancer drugs against HCC. Mutations in these genes could affect the drug-mediated response against HCC. Other SCLO gene families including OATP1B1 (SLCO1B1) and OATP1B3 (SLCO1B3) transport sorafenib and possess redundant substrate specificity [98]. Past studies reported that single nucleotide polymorphisms (SNPs) of OATP1B1 and OATP1B3 severely affect the pharmacokinetics of statins and paclitaxel [99]. Authors of another study reported germline mutations in OATP1B1, c.388A>G (p.Asn130Asp) and c.521T>C (p.Val174Ala), which are associated with emerging side effects of sorafenib in HCC patients [100].
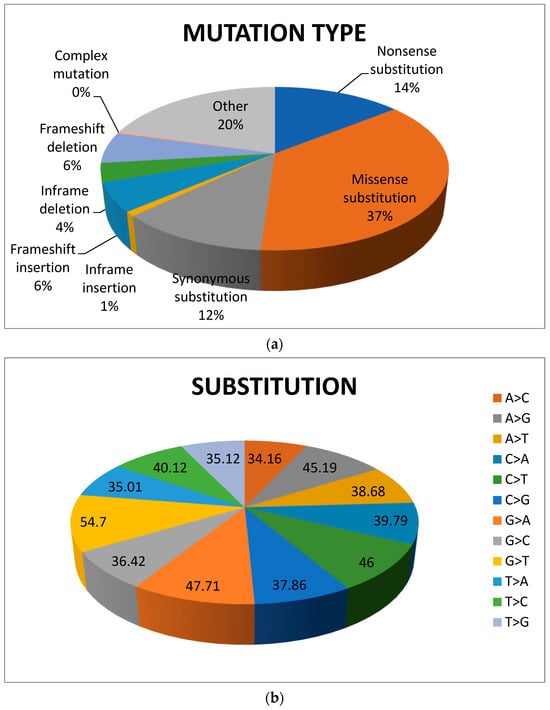
Figure 6.
Pie chart showing (a) mutation and (b) substitution in HCC (data source: https://cancer.sanger.ac.uk/cosmic (accessed on 8 February 2024) (Ref. [101]).
4.2. Drug-Induced Liver Injury (DILI) by Immune Checkpoint Inhibitors (ICIs)
The US FDA has approved eight ICIs for intervention, including anti-programmed death-1 [anti-PD-1] antibodies nivolumab, pembrolizumab, and cemiplimab; anti-programmed death ligand-1 [anti-PD-L1] antibodies avelumab, atezolizumab, and durvalumab; and anti-CTLA-4 antibodies ipilimumab and tremelimumab, targeting three immune checkpoints (PD-1, PD-L1, and CTLA-4) [102] (Table 3). ICIs have been used for many decades in the effective treatment of HCC. Hepatotoxicity is associated with several immune-related adverse events (irAEs) that are different from DILI and is thought to be related to the autoimmunity [102]. A previous study noted that ICI induced liver toxicity is associated with the infiltration of CD8-positive T-cells [102]. The same study reported that the incidence of ICI induced DILI is between 0.8 and 14.6% for CTLA-4 inhibitors like ipilimumab and between 2.7 and 16% for PD-1/PD-L1 inhibitors such as nivolumab [102]. Swenson and co-workers analyzed 112 patients who received durvalumab, an anti-PD-L1 antibody treatment, and observed that 19% of the patients were diagnosed with DILI using RUCAM. It is known that the risk of DILI development is directly proportional and positively correlated with the concomitant use of ICIs and chemotherapy or other ICIs [103]. Another previously published meta-analysis of 122 clinical trials reported a 0.09% mortality due to hepatitis as an irAE and 0% with an anti-CTLA-4 antibody, and 0.13% with the combination of PD-1 antibody/anti-PD-L1 antibody and an anti-CTLA-4 antibody, suggesting a non-fatal effect of combination therapies in liver damage cases [104] (Figure 7). Considering these events, using ICI-enabled treatment options could be explored for HCC cases where no other options or alternative treatments are available or where patients have high DILIs.

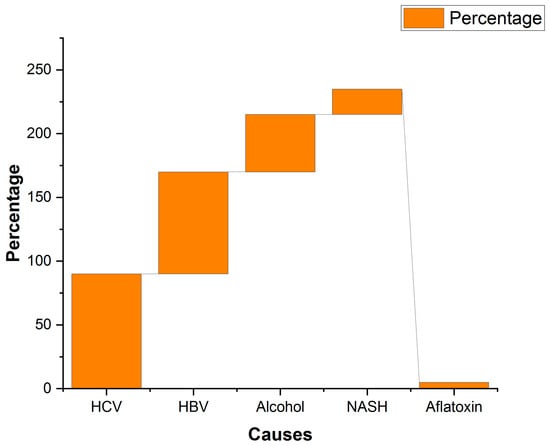

Table 3.
Clinical trials of immunotherapies combinations in locally advanced unresectable and metastatic HCC.
Table 3.
Clinical trials of immunotherapies combinations in locally advanced unresectable and metastatic HCC.
| Trial Identifier | Line | Agents | Primary Endpoints | Patients | Status |
|---|---|---|---|---|---|
| NCT03713593 | First-line | Lenvatinib + pembrolizumab v/s. lenvatinib | PFS, OS | 750 | Ongoing |
| NCT03764293 | First-line | PD-1 antibody SHR-1210 + apatinib mesylate v/s. sorafenib | PFS, OS | 510 | Ongoing |
| NCT03298451 | First-line | Durvalumab v/s. durvalumab + tremelimumab v/s. sorafenib | OS | 1310 | Active, not recruiting |
| NCT03412773 | First-line | BGB-A317 (PD-1 antibody) v/s. sorafenib | OS | 674 | Active, not recruiting |
| NCT03434379 | First-line | Atezolizumab + bevacizumab v/s. sorafenib | OS, PFS | 480 | Active, not recruiting |
| NCT01658878 | First-line | Nivolumab + cabozantinib v/s. nivolumab + ipilimumab + cabozantinib | Safety, tolerability and ORR | 1097 | Active, not recruiting |
| NCT03347292 | First-line | Pembrolizumab + regorafenib | TEAEs, DLTs | 57 | Ongoing, recruiting |
| NCT03439891 | First-line | Nivolumab + sorafenib | MTD, ORR | 40 | Ongoing, recruiting |
PFS, progression free survival; OS, overall survival; ORR, objective response rate; TEAEs, treatment-emergent adverse events; DLTs, dose limiting toxicities; MTD, maximum tolerated dose. (Adopted from Ref. [105] under Creative Commons Attribution-NonCommercial-4.0 International License (CC BY-NC-ND 4.0).)

Figure 7.
Causes and their percentage contributions to HCC in the Asian population. Data Source: Ref. [106] under Creative Commons Attribution 4.0 International License.
5. Conclusions
Sorafenib, used either as a monotherapy or in combination with atezolizumab and bevacizumab has remained the first choice of drug in the past decade for providing better outcomes in patients with HCC in a Asian populations. Other approaches, including cytokine-based immunotherapy, have also been explored in Asia for the treatment of HCC with minimal side effects and significant benefits. However, newer therapeutic approaches, including nanotechnology-based delivery, need to be explored further for the effective treatment of patients with HCC.
Funding
The authors thank the Brain Pool Program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (Grant Number 2022H1D3A2A01096346) for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [CrossRef]
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular carcinoma. Lancet 2003, 362, 1907–1917. [Google Scholar] [CrossRef]
- Umemura, T.; Kiyosawa, K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol. Res. 2007, 37 (Suppl. 2), S95–S100. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/MERK pathway and receptor tyrosine kinases involved in tumour progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Yoon, S.K.; Chun, H.G. Status of hepatocellular carcinoma in South Korea. Chin. Clin. Oncol. 2013, 2, 39. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signalling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- Yau, T.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Kang, Y.K.; Hou, M.M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef]
- Vogel, A.; Qin, S.; Kudo, M.; Su, Y.; Hudgens, S.; Yamashita, T.; Yoon, J.H.; Fartoux, L.; Simon, K.; López, C.; et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: Patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 649–658. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820, Erratum in Lancet Oncol. 2020, 21, e341. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Yoo, C.; Hong, J.Y.; Kim, H.S.; Lee, D.W.; Lee, M.A.; Kim, J.W.; Kim, I.; Oh, S.B.; Hwang, J.E.; et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022, 42, 674–681. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Kim, D.Y.; Bae, S.H.; Paik, S.W.; Lee, Y.J.; Kim, H.Y.; Lee, H.C.; Han, S.Y.; Cheong, J.Y.; et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J. Hepatol. 2019, 70, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Kim, J.Y.; Hong, J.H.; Hur, M.H.; Cho, H.; Park, M.K.; Kim, J.; Lee, Y.B.; Cho, E.J.; Lee, J.H.; et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Chon, H.J.; Bang, Y.; Park, N.H.; Shin, J.W.; Kim, K.M.; Lee, H.C.; Lee, J.; Yoo, C.; Ryoo, B.Y. Real-World Efficacy and Safety of Lenvatinib in Korean Patients with Advanced Hepatocellular Carcinoma: A Multicenter Retrospective Analysis. Liver Cancer 2020, 9, 613–624. [Google Scholar] [CrossRef]
- Yoon, S.M.; Ryoo, B.Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef]
- Hong, J.Y.; Cho, H.J.; Sa, J.K.; Liu, X.; Ha, S.Y.; Lee, T.; Kim, H.; Kang, W.; Sinn, D.H.; Gwak, G.Y.; et al. Hepatocellular carcinoma patients with high circulating cytotoxic T cells and intra-tumoral immune signature benefit from pembrolizumab: Results from a single-arm phase 2 trial. Genome Med. 2022, 14, 1. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Ryoo, B.Y.; Palmer, D.H.; Park, S.R.; Rimassa, L.; Debashis Sarker Daniele, B.; Steinberg, J.; López, B.; Lim, H.Y. Efficacy and Safety Results from a Phase 2, Randomized, Double-Blind Study of Enzalutamide Versus Placebo in Advanced Hepatocellular Carcinoma. Clin. Drug Investig. 2021, 41, 795–808. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; He, A.R.; Lim, H.Y.; Ryoo, B.Y.; Hung, C.H.; Sheen, I.S.; Izumi, N.; Austin, T.; Wang, Q.; et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study. J. Hepatol. 2015, 63, 896–904. [Google Scholar] [CrossRef]
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179, Erratum in J. Clin. Oncol. 2017, 35, 2590. [Google Scholar] [CrossRef]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef]
- Tak, W.Y.; Ryoo, B.Y.; Lim, H.Y.; Kim, D.Y.; Okusaka, T.; Ikeda, M.; Hidaka, H.; Yeon, J.E.; Mizukoshi, E.; Morimoto, M.; et al. Phase I/II study of first-line combination therapy with sorafenib plus resminostat, an oral HDAC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Investig. New Drugs 2018, 36, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Qin, S.; Park, J.W.; Poon, R.T.; Raoul, J.L.; Philip, P.A.; Hsu, C.H.; Hu, T.H.; Heo, J.; Xu, J.; et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.Y.; Yoon, J.H.; Hsu, C.; Rau, K.M.; Poon, R.T.; Yeo, W.; Park, J.W.; et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 5976–5985. [Google Scholar] [CrossRef]
- Chau, I.; Peck-Radosavljevic, M.; Borg, C.; Malfertheiner, P.; Seitz, J.F.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Kudo, M.; Poon, R.; et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur. J. Cancer 2017, 81, 17–25, Erratum in Eur. J. Cancer 2018, 100, 135–136. . [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- Llovet, J.M.; Vogel, A.; Madoff, D.C.; Finn, R.S.; Ogasawara, S.; Ren, Z.; Mody, K.; Li, J.J.; Siegel, A.B.; Dubrovsky, L.; et al. Randomized Phase 3 LEAP-012 Study: Transarterial Chemoembolization with or without Lenvatinib Plus Pembrolizumab for Intermediate-Stage Hepatocellular Carcinoma Not Amenable to Curative Treatment. Cardiovasc. Interv. Radiol. 2022, 45, 405–412. [Google Scholar] [CrossRef]
- Ding, X.; Sun, W.; Li, W.; Shen, Y.; Guo, X.; Teng, Y.; Liu, X.; Zheng, L.; Li, W.; Chen, J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer 2021, 127, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined with Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Qin, S.; Chen, Z.; Liu, Y.; Wang, L.; Zou, J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: Cohort A report in a multicenter phase Ib/II trial. J. ImmunoTherapy Cancer 2021, 9, e002191. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, W.; Qian, X.; Li, X.; Cheng, F.; Wang, K.; Zhang, F.; Zhang, C.; Li, D.; Song, J.; et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: A single-arm, open label, phase II clinical trial. J. Immunother. Cancer 2022, 10, e004656. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Gu, S.; Zhang, Y.; Wu, L.; Wu, J.; Shao, G.; Zhang, Y.; Xu, L.; Yin, T.; et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin. Cancer Res. 2021, 27, 1003–1011. [Google Scholar] [CrossRef]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef]
- Lyu, N.; Wang, X.; Li, J.B.; Lai, J.F.; Chen, Q.F.; Li, S.L.; Deng, H.J.; He, M.; Mu, L.W.; Zhao, M. Arterial Chemotherapy of Oxaliplatin Plus Fluorouracil Versus Sorafenib in Advanced Hepatocellular Carcinoma: A Biomolecular Exploratory, Randomized, Phase III Trial (FOHAIC-1). J. Clin. Oncol. 2022, 40, 468–480. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990, ; Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef]
- Li, Q.J.; He, M.K.; Chen, H.W.; Fang, W.Q.; Zhou, Y.M.; Xu, L.; Wei, W.; Zhang, Y.J.; Guo, Y.; Guo, R.P.; et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J. Clin. Oncol. 2022, 40, 150–160. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yau, T.; Park, J.W.; Lim, H.Y.; Lee, T.Y.; Obi, S.; Chan, S.L.; Qin, S.; Kim, R.D.; Casey, M.; et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann. Oncol. 2015, 26, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Decaens, T.; Raoul, J.L.; Boucher, E.; Kudo, M.; Chang, C.; Kang, Y.K.; Assenat, E.; Lim, H.Y.; Boige, V.; et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 2013, 31, 3509–3516. [Google Scholar] [CrossRef]
- Yau, T.C.C.; Lencioni, R.; Sukeepaisarnjaroen, W.; Chao, Y.; Yen, C.J.; Lausoontornsiri, W.; Chen, P.J.; Sanpajit, T.; Camp, A.; Cox, D.S.; et al. A Phase I/II Multicenter Study of Single-Agent Foretinib as First-Line Therapy in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2017, 23, 2405–2413. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.K.; Lim, H.Y.; Poon, R.T.; Blanc, J.F.; Vogel, A.; Chen, C.L.; et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ryoo, B.Y.; Merle, P.; Park, J.W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020, 5, e000714. [Google Scholar] [CrossRef]
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Tai, W.M.; Yong, W.P.; Lim, C.; Low, L.S.; Tham, C.K.; Koh, T.S.; Ng, Q.S.; Wang, W.W.; Wang, L.Z.; Hartano, S.; et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC). Ann. Oncol. 2016, 27, 2210–2215. [Google Scholar] [CrossRef]
- Toh, H.C.; Chen, P.J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013, 119, 380–387. [Google Scholar] [CrossRef]
- Lim, H.Y.; Merle, P.; Weiss, K.H.; Yau, T.; Ross, P.; Mazzaferro, V.; Blanc, J.F.; Ma, Y.T.; Yen, C.J.; Kocsis, J.; et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 4650–4661. [Google Scholar] [CrossRef]
- Chow, P.K.; Poon, D.Y.; Khin, M.W.; Singh, H.; Han, H.S.; Goh, A.S.; Choo, S.P.; Lai, H.K.; Lo, R.H.; Tay, K.H.; et al. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS ONE 2014, 9, e90909. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Cervello, M.; McCubrey, J.A.; Cusimano, A.; Lampiasi, N.; Azzolina, A.; Montalto, G. Targeted therapy for hepatocellular carcinoma: Novel agents on the horizon. Oncotarget 2012, 3, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Samant, R.S.; Shevde, L.A. Recent advances in anti-angiogenic therapy of cancer. Oncotarget 2011, 2, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Davis, E.M.; Crabtree, T.R.; Habibi, J.R.; Nguyen, T.K.; Dent, P.; Grant, S. The kinase inhibitor sorafenib induces cell death through a process involving the induction of endoplasmic reticulum stress. Mol. Cell. Biol. 2007, 27, 5499–5513. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.T.; Cheng, A.L.; Shiau, C.W.; Huang, H.P.; Huang, J.W.; Chen, P.J.; Chen, K.F. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J. Hepatol. 2011, 55, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, P.; Arienti, C.; Amadori, D.; Fabbri, F.; Carloni, S.; Tesei, A.; Vannini, I.; Silvestrini, R.; Zoli, W. Role of RAF/MEK/ERK pathway, p-STAT-3 and Mcl-1 in sorafenib activity in human pancreatic cancer cell lines. J. Cell. Physiol. 2009, 220, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Cervello, M.; Bachvarov, D.; Lampiasi, N.; Cusimano, A.; Azzolina, A.; McCubrey, J.A.; Montalto, G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle 2012, 11, 2843–2855. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Cammà, C.; Colombo, M.; SOFIA (SOraFenib Italian Assessment) Study Group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, F.; Yang, J.; Fan, H.; Xie, Q.; Jiang, K.; Gong, J.; Gao, B.; Yang, Q.; Lei, Z. Risk of Adverse Events in Cancer Patients Receiving Nivolumab with Ipilimumab: A Meta-Analysis. Front. Oncol. 2022, 12, 877434. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Yang, J.; Riella, L.V.; Chock, S.; Liu, T.; Zhao, X.; Yuan, X.; Paterson, A.M.; Watanabe, T.; Vanguri, V.; Yagita, H.; et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J. Immunol. 2011, 187, 1113–1119. [Google Scholar] [CrossRef]
- Aleem, A.; Shah, H. Atezolizumab. In StatPearls [Internet]; Updated 4 May 2022; StatPearls Publishing: Treasure Island, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567758/ (accessed on 8 February 2024).
- Roviello, G.; Bachelot, T.; Hudis, C.A.; Curigliano, G.; Reynolds, A.R.; Petrioli, R.; Generali, D. The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. Eur. J. Cancer 2017, 75, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Kwak, H.W.; Park, J.W.; Nam, B.H.; Yu, A.; Woo, S.M.; Kim, T.H.; Kim, S.H.; Koh, Y.H.; Kim, H.B.; Park, S.J.; et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J. Gastroenterol. Hepatol. 2014, 29, 820–829. [Google Scholar] [CrossRef]
- Chow, P.K.; Gandhi, M. Phase III multicenter open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: The SIRveNIB study. J. Clin. Oncol. 2017, 35 (Suppl. 15), 4002. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Available online: https://www.globaldata.com/data-insights/healthcare/number-of-ongoing-clinical-trials-for-drugs-involving-hepatocellular-carcinoma-by-phase-503271/ (accessed on 8 February 2024).
- Bruix, J.; Sherman, M.; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef]
- Yao, S.; Johnson, C.; Hu, Q.; Yan, L.; Liu, B.; Ambrosone, C.B.; Wang, J.; Liu, S. Differences in somatic mutation landscape of hepatocellular carcinoma in Asian American and European American populations. Oncotarget 2016, 7, 40491–40499. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236, Erratum in J. Hepatol. 2019, 70, 817. [Google Scholar] [CrossRef] [PubMed]
- Witt-Kehati, D.; Fridkin, A.; Alaluf, M.B.; Zemel, R.; Shlomai, A. Inhibition of pMAPK14 Overcomes Resistance to Sorafenib in Hepatoma Cells with Hepatitis B Virus. Transl. Oncol. 2018, 11, 511–517. [Google Scholar] [CrossRef]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular Carcinoma: Etiology and Current and Future Drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 108–122. [Google Scholar] [CrossRef]
- Zimmerman, E.I.; Hu, S.; Roberts, J.L.; Gibson, A.A.; Orwick, S.J.; Li, L.; Sparreboom, A.; Baker, S.D. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin. Cancer Res. 2013, 19, 1458–1466. [Google Scholar] [CrossRef]
- Gong, I.Y.; Kim, R.B. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet. 2013, 28, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Bins, S.; Lenting, A.; El Bouazzaoui, S.; van Doorn, L.; Oomen-de Hoop, E.; Eskens, F.A.; van Schaik, R.H.; Mathijssen, R.H. Polymorphisms in SLCO1B1 and UGT1A1 are associated with sorafenib-induced toxicity. Pharmacogenomics 2016, 17, 1483–1490. [Google Scholar] [CrossRef]
- Available online: https://cancer.sanger.ac.uk/cosmic (accessed on 8 February 2024).
- Kobayashi, T.; Iwaki, M.; Nogami, A.; Yoneda, M. Epidemiology and Management of Drug-induced Liver Injury: Importance of the Updated RUCAM. J. Clin. Transl. Hepatol. 2023, 11, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.A.; Kassab, I.; Tsung, I.; Schneider, B.J.; Fontana, R.J. Liver injury during durvalumab-based immunotherapy is associated with poorer patient survival: A retrospective analysis. Front. Oncol. 2022, 12, 984940. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728, Erratum in JAMA Oncol. 2018, 4, 1792. [Google Scholar] [CrossRef] [PubMed]
- El Dika, I.; Makki, I.; Abou-Alfa, G.K. Hepatocellular carcinoma, novel therapies on the horizon. Chin. Clin. Oncol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Jafri, W.; Kamran, M. Hepatocellular Carcinoma in Asia: A Challenging Situation. Euroasian J. Hepatogastroenterol. 2019, 9, 27–33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).