Antioxidant and Emulsifying Activity of the Exopolymer Produced by Bacillus licheniformis
Abstract
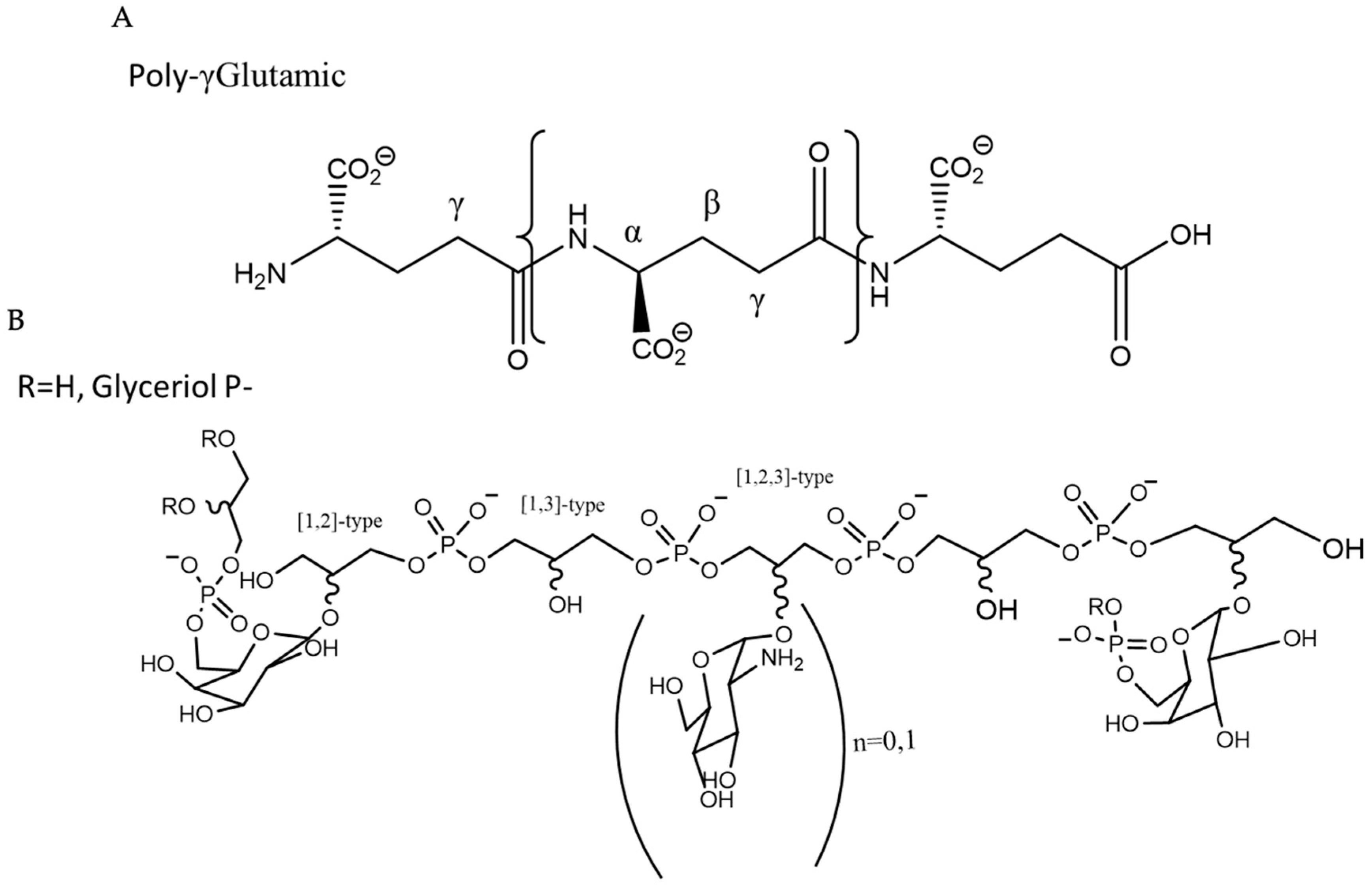
1. Introduction
2. Results and Discussion
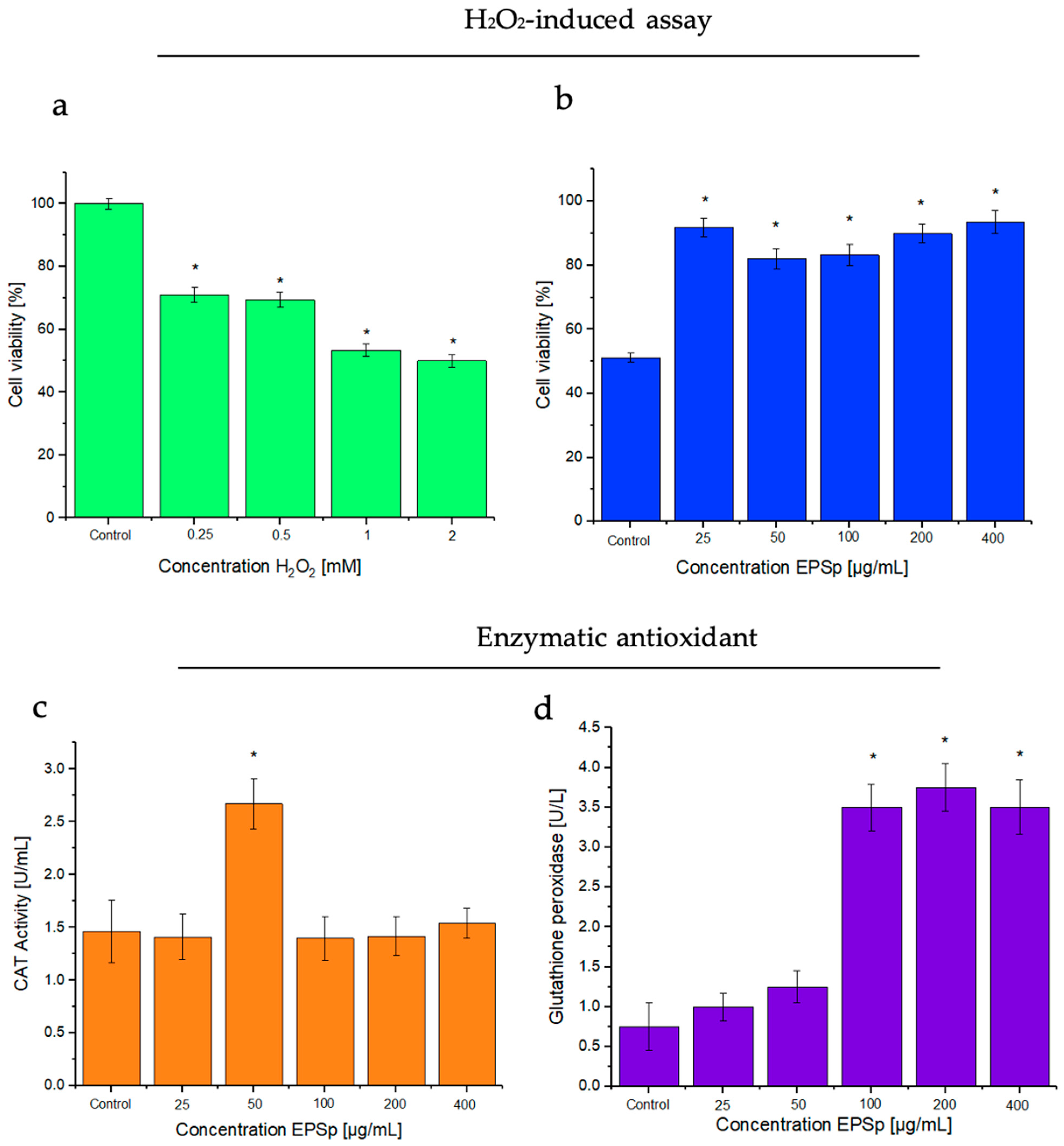
2.1. Cytotoxicity
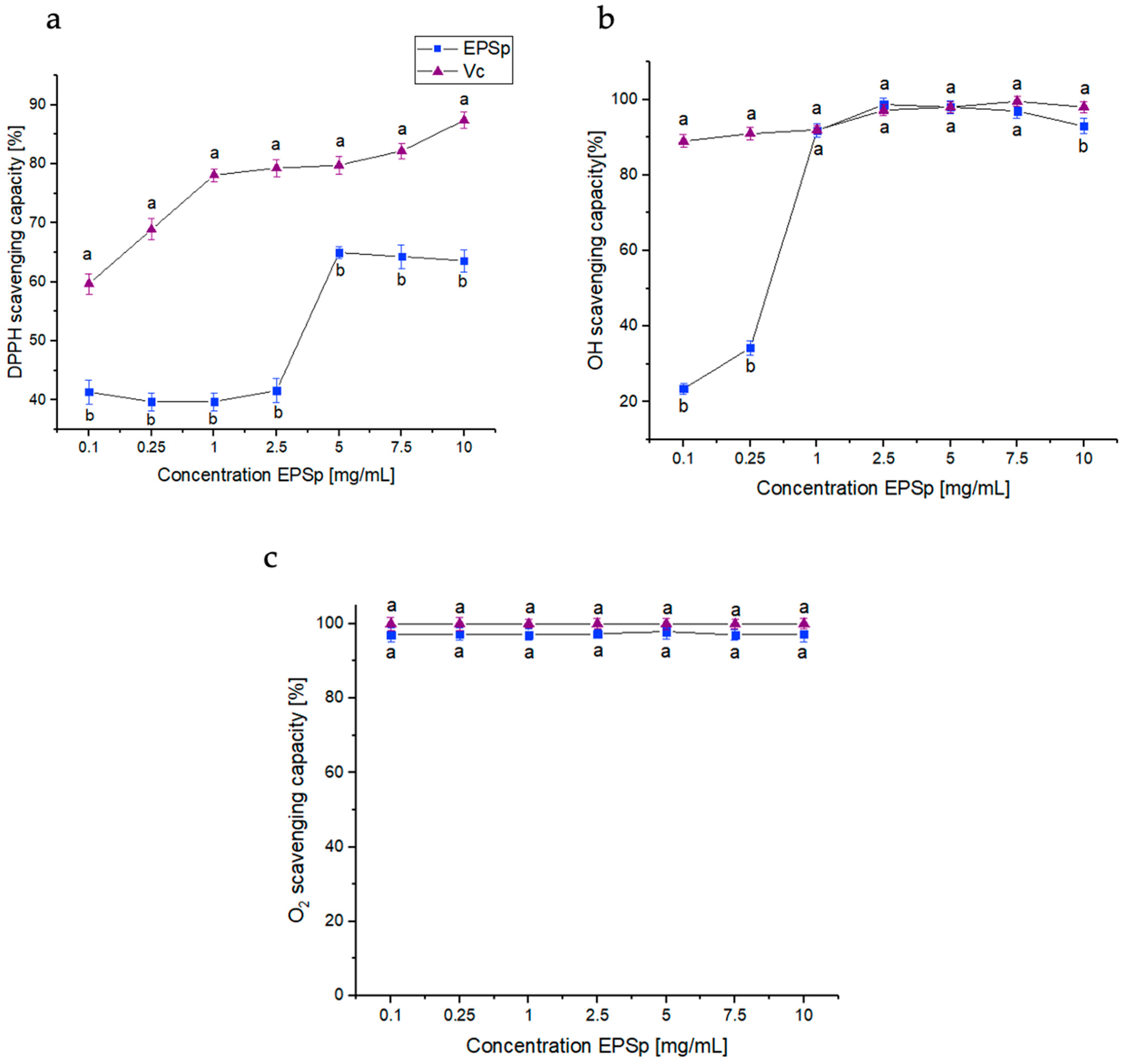
2.2. Free Radical Scavenging Activities
2.3. H2O2-Induced Assay, and Effects of the Exopolymer
2.4. Enzymatic Antioxidant Assays: Catalase (CAT) and Glutathione Peroxidase (GSH-Px)
2.5. The Emulsifying Capacity of EPSp
3. Materials and Methods
3.1. Materials
3.2. Production, Isolation, and Purification of the Exopolymer
3.3. MTT Assay
3.4. Free Radical Scavenging Activities
3.4.1. DPPH (1,1-Diphenyl-2-picryl Hydrazyl Radical) Radical Scavenging Activity
3.4.2. Hydroxyl Radical (OH) Scavenging Activity
3.4.3. Superoxide Anion (O2−) Radical Scavenging Activity
3.5. H2O2-Induced Assay
3.5.1. H2O2-Induced Oxidative Stress
3.5.2. Effects of the Exopolymer against H2O2
3.6. Enzymatic Antioxidant Assays
Catalase (CAT) and Glutathione Peroxidase (GSH-Px) Assay
3.7. Emulsifying Properties
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochhar, N.; Kavya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the Microorganism of Extreme Environments and Their Applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A. Bacillus licheniformis: The Unexplored Alternative for the Anaerobic Production of Lipopeptide Biosurfactants? Biotechnol. Adv. 2022, 60, 108013. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers 2021, 13, 1959. [Google Scholar] [CrossRef]
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 171. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Kotowska, U.; Wydrych, J.; Polińska, W.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Exopolysaccharide Carbohydrate Structure and Biofilm Formation by Rhizobium leguminosarum Bv. Trifolii Strains Inhabiting Nodules of Trifolium Repens Growing on an Old Zn–Pb–Cd-polluted Waste Heap Area. Int. J. Mol. Sci. 2021, 22, 2808. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current Status of Biotechnological Production and Applications of Microbial Exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-león, E.; Huang-lin, E.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers 2023, 15, 1550. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New Emulsifying and Cryoprotective Exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Du, R.; Yu, L.; Sun, M.; Ye, G.; Yang, Y.; Zhou, B.; Qian, Z.; Ling, H.; Ge, J. Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications. Antioxidants 2023, 12, 275. [Google Scholar] [CrossRef]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kurgaeva, I.V.; Efremova, K.V.; Novokuptsev, N.V. Production of Bacterial Exopolysaccharides: Xanthan and Bacterial Cellulose. Int. J. Mol. Sci. 2023, 24, 14608. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Lee, H.; Chea, S.; Lee, K.; Cha, I.; Kim, D. A Report on Five Unrecorded Bacterial Species Belonging to the Phyla Actinomycetota, Bacillota and Pseudomonadota in Korea Isolated in 2020. J. Species Res. 2023, 12, 1–6. [Google Scholar] [CrossRef]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological Applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- He, H.; Yu, Q.; Ding, Z.; Zhang, L.; Shi, G.; Li, Y. Biotechnological and Food Synthetic Biology Potential of Platform Strain: Bacillus licheniformis. Synth. Syst. Biotechnol. 2023, 8, 281–291. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Nicolò, M.S.; Grillo, E.; Caccamo, M.T.; Magazù, S.; Cappello, S.; Gugliandolo, C. Thermophilic Hydrocarbon-Utilizing Bacilli from Marine Shallow Hydrothermal Vents as Producers of Biosurfactants. J. Mar. Sci. Eng. 2022, 10, 1077. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Zhang, W.; Fu, Y.; Jiao, N. A Novel Exopolysaccharide with Metal Adsorption Capacity Produced by a Marine Bacterium Alteromonas sp. JL2810. Mar. Drugs 2017, 15, 175. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and Characterization of an Exopolymer Produced by Bacillus licheniformis: In Vitro Antiviral Activity against Enveloped Viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and Immunoregulatory Effect of a Novel Exopolysaccharide from a Marine Thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef]
- Insulkar, P.; Kerkar, S.; Lele, S.S. Purification and Structural-Functional Characterization of an Exopolysaccharide from Bacillus licheniformis PASS26 with in-Vitro Antitumor and Wound Healing Activities. Int. J. Biol. Macromol. 2018, 120, 1441–1450. [Google Scholar] [CrossRef]
- Song, Y.R.; Song, N.E.; Kim, J.H.; Nho, Y.C.; Baik, S.H. Exopolysaccharide Produced by Bacillus licheniformis Strains Isolated from Kimchi. J. Gen. Appl. Microbiol. 2011, 57, 169–175. [Google Scholar] [CrossRef]
- Nguyen Vu, T.H.; Quach, N.T.; Nguyen, N.A.; Nguyen, H.T.; Ngo, C.C.; Nguyen, T.D.; Ho, P.H.; Hoang, H.; Chu, H.H.; Phi, Q.T. Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis Vtx20. Appl. Sci. 2021, 11, 7055. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohammed Breig, S.J.; Gómez, A.; Sánchez-Arévalo, I.; González-Faune, P.; Sarkar, S.; Bandopadhyay, R.; Vuree, S.; Cornejo, J.; Tapia, J.; et al. Optimization and Characterization of a Novel Exopolysaccharide from Bacillus haynesii CamB6 for Food Applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, S.; Lu, M.; Jiao, Y.; Wang, S. A Novel Method for Promoting Antioxidant Exopolysaccharidess Production of Bacillus licheniformis. Carbohydr. Polym. 2013, 92, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of Antioxidant, Antibacterial and Cytotoxicity Activities of Exopolysaccharide from Enterococcus Strains Isolated from Traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant Activity of an Exopolysaccharide Isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Zheng, L.P.; Zou, T.; Ma, Y.J.; Wang, J.W.; Zhang, Y.Q. Antioxidant and DNA Damage Protecting Activity of Exopolysaccharides from the Endophytic Bacterium Bacillus cereus SZ1. Molecules 2016, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L. Unraveling the Multi-Enzyme-Like Activities of Iron Oxide Nanozyme via a First-Principles Microkinetic Study. J. Phys. Chem. C 2019, 123, 30318–30334. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The Universal Soldier: Enzymatic and Non-enzymatic Antioxidant Functions of Serum Albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Urooj, Y.; Qamar, S.A.; Khalid, N. Improved Exopolysaccharide Production from Bacillus licheniformis MS3: Optimization and Structural/Functional Characterization. Int. J. Biol. Macromol. 2020, 151, 984–992. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Manna, S.; Saha, P.; Basak, R.K.; Sen, R.; Roy, D.; Adhikari, B. Composition Analysis and Material Characterization of an Emulsifying Extracellular Polysaccharide (EPS) Produced by Bacillus megaterium RB-05: A Hydrodynamic Sediment-Attached Isolate of Freshwater Origin. J. Appl. Microbiol. 2011, 111, 1381–1393. [Google Scholar] [CrossRef]
- Song, B.; Zhu, W.; Song, R.; Yan, F.; Wang, Y. Exopolysaccharide from Bacillus vallismortis WF4 as an Emulsifier for Antifungal and Antipruritic Peppermint Oil Emulsion. Int. J. Biol. Macromol. 2019, 125, 436–444. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Sanchez-León, E.; Abrusci, C. Photodegradation and Biodegradation Under Thermophile Conditions of Mulching Films Based on Poly(Butylene Adipate-Co-Terephthalate) and Its Blend with Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 352–363. [Google Scholar] [CrossRef]
- Abrusci, C.; Palomar, J.; Pablos, J.L.; Rodriguez, F.; Catalina, F. Efficient Biodegradation of Common Ionic Liquids by Sphingomonas paucimobilis Bacterium. Green Chem. 2011, 13, 709–717. [Google Scholar] [CrossRef]
- Morro, A.; Abrusci, C.; Pablos, J.L.; Marín, I.; García, F.C.; García, J.M. Inherent Antibacterial Activity and in Vitro Biocompatibility of Hydrophilic Polymer Film Containing Chemically Anchored Sulfadiazine Moieties. Eur. Polym. J. 2017, 91, 274–282. [Google Scholar] [CrossRef]
- Pérez-Blanco, C.; Huang-Lin, E.; Abrusci, C. Characterization, Biodegradation and Cytotoxicity of Thermoplastic Starch and Ethylene-Vinyl Alcohol Copolymer Blends. Carbohydr. Polym. 2022, 298, 120085. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide Production of Pantoea sp. BCCS 001 GH: Physical Characterizations, Emulsification, and Antioxidant Activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Prasad, B.; Rai, A.K.; Velappan, S.P.; Subbanna, M.N.; Narayan, B. In Vitro Antioxidant and Antibacterial Properties of Hydrolysed Proteins of Delimed Tannery Fleshings: Comparison of Acid Hydrolysis and Fermentation Methods. Biodegradation 2011, 22, 287–295. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Abrusci, C.; Amils, R.; Sánchez-león, E. Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide. Polymers 2023, 15, 3974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 Exopolysaccharide Amplifies Its Antioxidant Activities in Vitro and in a Caco-2 Cell Model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Meneghine, A.K.; Moretto, C.; Castellane, T.C.L.; Carareto Alves, L.M. Production, Characterization and Bioemulsifying Activity of an Exopolysaccharide Produced by Sphingomonas sp. Isolated from Freshwater. J. Polym. Environ. 2017, 25, 1080–1086. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enrique, S.-L.; Ricardo, A.; Concepción, A. Antioxidant and Emulsifying Activity of the Exopolymer Produced by Bacillus licheniformis. Int. J. Mol. Sci. 2024, 25, 8249. https://doi.org/10.3390/ijms25158249
Enrique S-L, Ricardo A, Concepción A. Antioxidant and Emulsifying Activity of the Exopolymer Produced by Bacillus licheniformis. International Journal of Molecular Sciences. 2024; 25(15):8249. https://doi.org/10.3390/ijms25158249
Chicago/Turabian StyleEnrique, Sánchez-León, Amils Ricardo, and Abrusci Concepción. 2024. "Antioxidant and Emulsifying Activity of the Exopolymer Produced by Bacillus licheniformis" International Journal of Molecular Sciences 25, no. 15: 8249. https://doi.org/10.3390/ijms25158249
APA StyleEnrique, S.-L., Ricardo, A., & Concepción, A. (2024). Antioxidant and Emulsifying Activity of the Exopolymer Produced by Bacillus licheniformis. International Journal of Molecular Sciences, 25(15), 8249. https://doi.org/10.3390/ijms25158249






